Introduction
In the last decade, China has experienced a
substantial increase in the prevalence of type 2 diabetes mellitus
(T2DM) (1), which is characterized
by high morbidity and mortality, due to concomitant cardiovascular
complications. It has previously been revealed that the increased
cardiovascular risk in patients with DM is partly attributed to the
occurrence of endothelial injury, which triggers and accelerates
the pathogenesis of atherosclerotic vascular disease (2). In patients and animals with DM,
preventing endothelial dysfunction helps maintain vascular
homeostasis and diminishes the occurrence of DM-associated vascular
events (3–5).
Circulating endothelial progenitor cells (EPCs),
which are derived from the bone marrow, serve a crucial role in
promoting neovascularization, repairing endothelial damage and
improving endothelial function (6). Numerous risk factors of
cardiovascular disease (CVD), including hypertension, obesity,
dyslipidemia and smoking, are usually concomitant with a decreased
number and activity of circulating EPCs, which may accelerate the
pathophysiological process of endothelial injury (7–10).
However, hyperglycemia appears to be negatively correlated with
circulating EPCs compared with the aforementioned CVD factors. The
reduced number and impaired activity of circulating EPCs can be
observed in patients with T1DM (11) and T2DM (12). Furthermore, reduced levels of
circulating EPCs are correlated not only with glycemic control
(13), but also with severity of
impaired glucose tolerance in subjects with or without T2DM
(14). Although the underlying
mechanism is yet to be fully determined, numerous
pathophysiological mechanisms, including inflammation induced by
hyperglycemia, insulin resistance, oxidative stress and deletion of
nitric oxide (NO), could be involved in these EPC alterations in
patients with DM (12,15–17).
Patients with T2DM have a two- to four-fold
increased risk of CVD (18). In
addition, the deleterious effects of DM on CVD risk also occur in
premenopausal women, in spite of their relatively high estrogen
levels (19). Our previous studies
indicated that, although the number and activity of circulating
EPCs is preserved in prehypertensive premenopausal women compared
with in those without prehypertension (20), DM concomitantly diminishes their
EPC advantages in terms of vascular protection, thus resulting in a
reduced number and impaired activity of EPCs, and even damaged
endothelial function (21).
However, the following issues require further investigation: i)
Whether attenuated endothelial function generally occurs in all
premenopausal women with T2DM and ii) whether the difference in
circulating EPCs between the sexes is influenced by the coexistence
of DM. Certain factors resulting from plasma or cultured medium,
including NO, vascular endothelial growth factor (VEGF) and
granulocyte macrophage-colony-stimulating factor (GM-CSF), are
involved in modulating the number and activity of circulating EPCs
(22–25); however, only NO is involved in
modulating the sex-related differences in circulating EPCs in a
prehypertensive population (20).
The present study aimed to determine whether similar results may be
present in the DM population. The present study evaluated the
number and activity of circulating EPCs in women and men with DM
and normal glucose tolerance (NGT); in addition, NO, VEGF, and
GM-CSF levels in the human plasma, as well as in cultured EPC
media, were measured to identify the possible underlying
mechanisms.
Materials and methods
Characteristics of subjects
A total of 40 premenopausal women (20 with NGT and
20 with T2DM) and 40 age-matched men (20 with NGT and 20 with T2DM;
aged 36–51 years old), were recruited to the present study between
July 2015 and May 2017 in the First Hospital of Chenzhou and
Xiangya Hospital, Central South University (Changsha, China). The
enrolled patients with NGT were diagnosed with a fasting plasma
glucose (FPG) of ≤5.6 mmol/l and 2-h plasma glucose (2-h PG)
following oral glucose challenge of ≤7.8 mmol/l; those with T2DM
were diagnosed with a FPG of ≥7.0 mmol/l and/or 2-h PG of ≥11.1
mmol/l and/or glycated hemoglobin (HbA1c) of ≥6.5% based on the
Experts Committee Reports of the American Diabetes Association
(26). NGT subjects had no
vascular risk factors, including obesity, hypertension, smoking and
dyslipidemia. All subjects had no malignant disease, infection,
inflammatory disorders, known CVD, or ongoing medical treatments
(including with antiplatelet, anti-inflammatory or hypolipidemic
agents). Nursing or pregnant women were excluded from the present
study. The present study was approved by the Ethics committee of
the First Hospital of Chenzhou and Xiangya Hospital, Central South
University, and written informed consent was obtained from all
participants.
Following an overnight fast for 8–12 h, 75 g glucose
solution was administered orally within 5 min. Blood samples (5 ml)
were collected prior to, and at 30 and 120 min post-challenge, and
plasma glucose was measured.
FPG, HbA1c, total cholesterol (TC), triglycerides
(TG), low- and high-density lipoprotein (LDL and HDL) cholesterol
and estrogen levels were measured. Peripheral blood samples were
collected to determine the number and activity of EPCs.
Flow cytometry and cell culture assay
to assess the number of circulating EPCs
The primary circulating EPCs were applied to the
following analysis. EPCs were assessed based on the described
methods in our previous studies (22,27,28).
The peripheral blood mononuclear cells of the participants were
isolated using Ficoll density gradient centrifugation, then
suspended in endothelial cell growth medium 2 (500 ml; Lonza Group,
Ltd., Basel, Switzerland) supplemented with 2% fetal bovine serum
(Sigma-Aldrich; Merck KGaA). The cell suspension
(2.5×106/ml) was added to cell culture flasks (25
cm2; Corning, Inc., Corning, NY, USA), coated with
fibronectin (Clonetics Co., San Diego, CA, USA) and incubated at
37°C in a humidified environment containing 5% CO2.
After 4 days, nonadherent cells were removed and adherent cells
were maintained for another 7 days; these cells were used for
subsequent experiments.
Following 7 days of culture, endothelial marker
proteins were examined by flow cytometry (29). Peripheral blood (100 µl) was
incubated for 40 min at 4°C with phycoerythrin (PE)-labeled
monoclonal mouse anti-human antibodies recognizing cluster of
differentiation (CD)31 (1:10; cat. no. 745290; BD Biosciences, San
Jose, CA, USA), von Willebrand factor (1:10; cat. no. 555849; BD
Biosciences) and kinase-insert domain receptor (KDR; 1:20; cat. no.
FHK309-025; 4A Biotech, Co., Ltd., Beijing, China) or corresponding
immunoglobulin G isotype control (1:10; cat. no. FMCK001K-025IU; 4A
Biotech, Co., Ltd.). Following this, erythrocytes were lysed, and
the remaining cells were washed with PBS and fixed in 2%
paraformaldehyde at 37°C for 10 min prior to further analysis using
a ACEA NovoCyte™ (ACEA Biosciences, San Diego, CA, USA). Cells were
then incubated with monocytic lineage marker CD14 (1:10; cat. no.
555397; BD Biosciences), fluorescein isothiocyanate (FITC)
anti-human CD45 (1:10; cat. no. FHF045-025; 4A Biotech, Co., Ltd.)
and PE-Cy7 anti-human CD34 (1:10; cat. no. FHN034-025; 4A Biotech,
Co., Ltd.) antibodies for 40 min at 4°C. NovoExpress software™
(ACEA Biosciences, San Diego, CA, USA) was used to analyze the
results.
The ratio of CD34+KDR+ cells
per 100 peripheral blood mononuclear cells was used to count the
circulating EPCs. To confirm the EPC phenotype, mononuclear cells
(2.5×106/ml) were plated on cell culture flasks with EBM
and after 7 days culture, the attached EC-like cells were incubated
with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine
perchlorate-labeled acetylated LDL (DiI-acLDL; Molecular Probes;
Thermo Fisher Scientific, Inc.) at 37°C for 1 h. The cells were
then fixed with 4% paraformaldehyde for 30 min at 37°C and
incubated with FITC-labeled lectin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 4 h at 37°C. After staining, the samples
were observed by two independent observers under a phase-contrast
fluorescence microscope (magnification, ×200). Cells demonstrating
double-positive fluorescence were identified as differentiating
EPCs.
Migration and proliferation assay of
EPCs
EPC migration and proliferation assays were
described in our previous studies (22,27,28).
Similar methods were adopted in the present study.
Following harvesting by centrifugation (438 × g at
4°C for 5 min), EPCs were suspended in 500 µl EBM and migration was
then analyzed. A total of 2×104 EPCs were placed in the
upper chamber of a modified Boyden chamber, which was placed in a
24-well culture dish containing EBM and human recombinant VEGF (50
ng/ml). Following incubation at 37°C for 24 h, the lower side of
the filter was washed with PBS and fixed with 2% paraformaldehyde
at 37°C for 10 min. For quantification, cell nuclei were stained
with DAPI. Cells migrating into the lower chamber were counted
manually in three random fields using a fluorescence
microscope.
The proliferation of EPCs was analyzed as follows.
After culturing for 7 days, EPCs were digested using 0.25% trypsin,
and were cultured in serum-free medium in 96-well culture plates
(200 µl/well) for 24 h. Subsequently, EPCs were supplemented with
10 µl MTT (5 g/l; Fluka; Honeywell International, Inc., Shanghai,
China) and incubated for a further 4 h. The supernatant was
discarded by aspiration and the EPC preparation was agitated with
200 µl dimethyl sulfoxide for 10 min, prior to the measurement of
optical density at 490 nm.
Measurement of NO, VEGF and GM-CSF
levels in the plasma and secreted by EPCs
Nitrite, the stable metabolite of NO, was measured
in plasma using the Greiss method, as described in our previous
studies (22,27–29).
The results were presented as mmol NOx of
NO3−/NO2− per liter of
medium. High-sensitivity ELISA assays (VEGF, cat. no. DVE00;
GM-CSF, cat. no. DGM00; R&D Systems, Europe, Ltd., Abingdon,
UK) were used to measure plasma levels (which were isolated from
blood samples via centrifugation at 291 × g for 5 min at room
temperature) of VEGF and GM-CSF in accordance with the
manufacturer's protocol.
After transferring the cultured EPCs
(2×105/ml cells for the determination NO and VEGF
levels, and 1×105/ml cells for the determination of
GM-CSF levels) to Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.)/20% fetal bovine serum (Sigma-Aldrich;
Merck KGaA) for 48 h, NO, VEGF and GM-CSF levels were measured in
the conditioned media, using the Griess method and ELISA assays
according to the aforementioned protocol.
Measurement of flow-mediated dilation
(FMD)
As previously described (30,31),
higher solution ultrasonography using a 5–12 MHz linear transducer
on an HDI 5,000 system (Philips Healthcare, Andover, MA, USA) was
used to measure the brachial artery FMD. Participants were placed
in a supine position, a sphygmomanometer cuff was placed at the
brachial artery on the upper-forearm (20–100 mm proximal to the
antecubital fossa) and the pressure was increased to 250 mmHg for 5
min. FMD was calculated as the percentage increase in mean
diastolic diameter following a reactive hyperemia of 55–65 sec,
followed by deflation to the baseline. After a further 15 min, 400
µg sublingual glyceryltrinitrate was injected and the diastolic
diameter was measured again after 5 min to determine
endothelial-independent dilatation.
Statistical analysis
SPSS v11.0 (SPSS Inc., Chicago, IL, USA) was used
for all statistical analyses. Quantitative variables with normal
distribution were presented as the means ± standard deviation.
Two-factor analysis of variance followed by the Least Significant
Difference post hoc test was used to compare the four groups to
determine sex-related differences, and differences between
individuals with DM and NGT. Correlation coefficients were analyzed
using Pearson's correlation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
Age and body mass index were comparable in the four
groups (Table I). HbA1c, FPG and
2-h PG levels were significantly increased in premenopausal women
and men with DM compared with in normoglycemic premenopausal women
and men, respectively (P<0.05). Furthermore, women with NGT and
DM had significantly higher estrogen levels than age- and
glycemia-matched men (P<0.05). Although TC levels were increased
in men with DM compared with in normoglycemic men, LDL and HDL
cholesterol, TG, systolic blood pressure, diastolic blood pressure
and high-sensitivity C-reactive protein levels were comparable
between these groups.
 | Table I.Clinical and biochemical
characteristics. |
Table I.
Clinical and biochemical
characteristics.
|
Characteristics | NGT women | Diabetic women | NGT men | Diabetic men |
|---|
| No. | 20 | 20 | 20 | 20 |
| Age (years) | 42.2±4.6 | 42.7±4.8 | 43.7±4.4 | 44.3±4.0 |
| Height (cm) | 162.0±5.9 | 162.4±5.8 |
167.4±6.0b |
168.1±6.0b |
| Weight (kg) | 60.2±5.3 | 62.0±5.8 |
65.2±4.9b |
66.0±4.6b |
| BMI
(kg/cm2) | 23.0±1.9 | 23.5±1.9 | 23.3±1.4 | 23.6±1.9 |
| Systolic blood
pressure (mmHg) | 118.7±9.9 | 114.7±9.5 | 119.9±12.3 | 116.4±10.6 |
| Diastolic blood
pressure (mmHg) | 73.4±5.8 | 71.5±5.1 | 75.0±6.4 | 72.4±5.8 |
| Heart rate
(beats/min) | 75.4±9.0 | 78.1±8.4 | 80.7±7.7 | 79.3±8.7 |
| AST (mmol/l) | 26.4±5.6 | 25.2±5.5 | 27.4±5.4 | 28.2±4.6 |
| ALT (mmol/l) | 23.5±6.5 | 22.2±5.2 | 24.2±4.5 | 24.4±4.7 |
| BUN (mmol/l) | 4.8±1.0 | 4.5±0.9 | 4.9±0.9 | 4.6±0.8 |
| Cr (mmol/l) | 60.5±10.6 | 55.6±11.7 | 61.1±12.3 | 57.5±12.9 |
| LDL (mmol/l) | 2.8±0.3 | 3.0±0.4 | 2.9±0.3 | 2.8±0.5 |
| TC (mmol/l) | 4.7±0.4 | 4.78±0.5 | 4.85±0.3 |
4.93±0.4a |
| HDL (mmol/l) | 1.3±0.2 | 1.3±0.2 | 1.3±0.2 | 1.3±0.2 |
| TG (mmol/l) | 1.5±0.2 | 1.5±0.1 | 1.5±0.2 | 1.5±0.1 |
| FPG (mmol/l) | 4.7±0.7 |
8.9±1.2a | 4.5±0.6 |
8.7±1.1a |
| 2-h PG
(mmol/l) | 6.3±0.7 |
10.8±1.7a | 6.2±0.7 |
10.3±1.6a |
| HbA1c (%) | 5.3±0.6 |
8.5±1.5a | 5.2±0.6 | 82±1.5a |
| hsCRP (mmol/l) | 1.0±0.5 | 1.1±0.6 | 2.0±0.9 | 1.8±0.9 |
| Estradiol
(pmol/l) | 206.0±26.2 | 216.5±37.0 |
102.5±20.1b |
108.1±19.9b |
| FMD (%) | 9.8±1.6 |
8.3±1.2a |
9.1±1.9b |
7.2±1.2a,b |
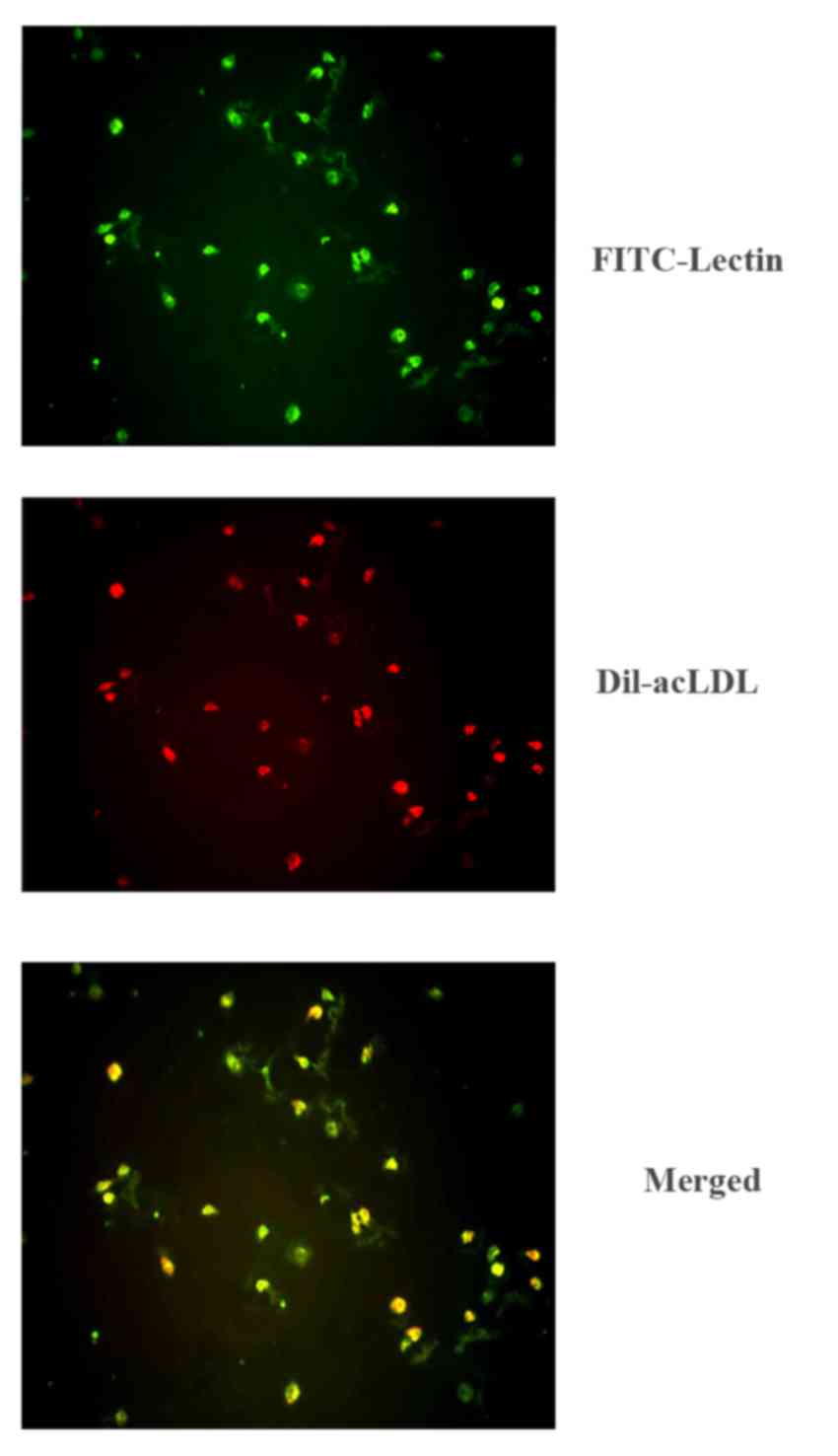
Characterization of early EPCs
Prior to being cultured in EGM, the peripheral blood
mononuclear cells suspended in the medium demonstrated a
monocyte-like appearance. Following 7 days of culture, the
peripheral blood mononuclear cells presented a typical
‘spindle-shaped’ appearance. Fluorescent immunocytochemistry was
used to observe adherent cells that were stained for both DiI-acLDL
and FITC-lectin (Fig. 1).
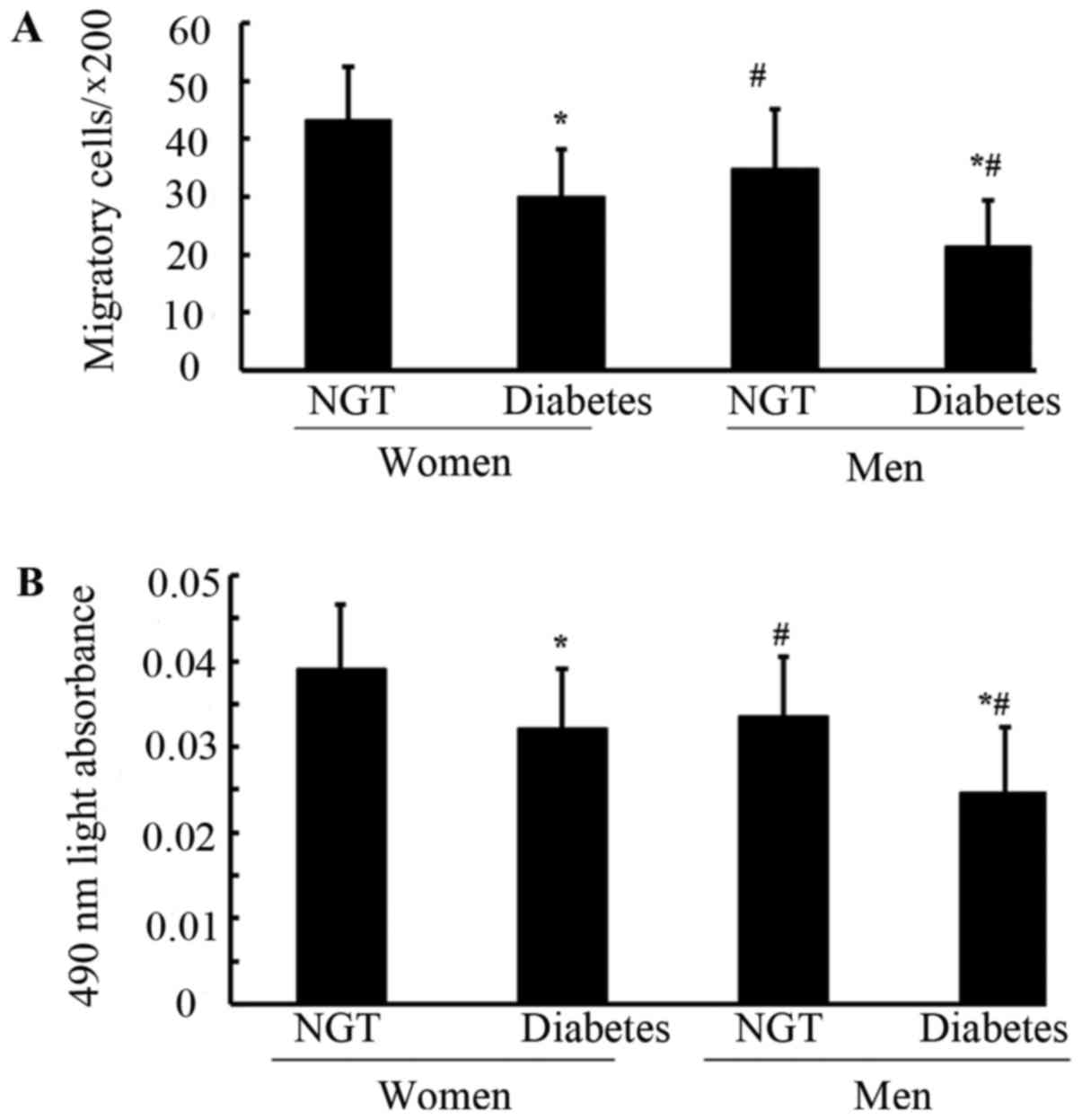
Number and activity of circulating
EPCs in the four groups
As shown in Fig. 2A and
B, the number of circulating EPCs among the four groups was
evaluated using flow cytometric analysis and cell culture assay.
Premenopausal women and men with DM exhibited a significantly
decreased number of EPCs compared with the corresponding NGT
patients (P<0.05). The male population with and without DM had a
significantly lower number of EPCs compared with in the
glycemia-matched female population (P<0.05). The two methods
exhibited a similar trend when comparing the number of EPCs among
the four groups.
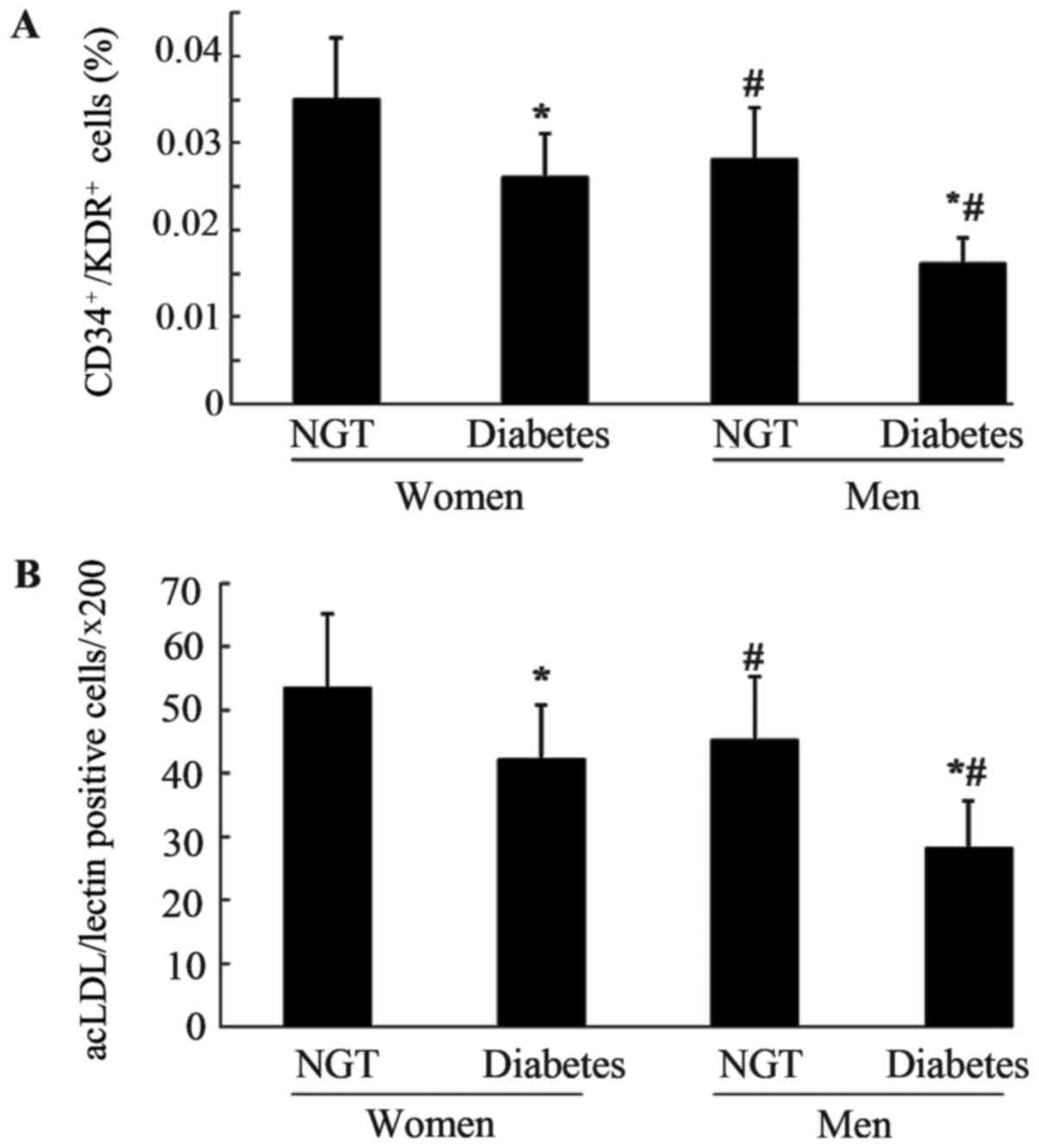 | Figure 2.Number of circulating EPCs in the
four groups. Circulating EPCS were evaluated by (A) flow cytometric
analysis and (B) phase-contrast fluorescent microscopy; the number
of circulating EPCs in men with NGT was decreased compared with in
women with NGT (A, P=0.004; B, P=0.001) and significant reduction
of the number of circulating EPCs was also observed in men with DM
compared with premenopausal women with DM (A, P<0.0001; B,
P=0.002). The number of circulating EPCs in with women and men DM
was decreased compared with in women and men with NGT (all
P<0.0001). Data are presented as the means ± standard deviation.
*P<0.05 vs. NGT; #P<0.05 vs. premenopausal women.
acLDL, acetylated LDL low-density lipoprotein; CD, cluster of
differentiation; DM, diabetes mellitus; KDR, kinase-insert domain
receptor; NGT, normal glucose tolerance; EPCs, endothelial
progenitor cells. |
The results demonstrated that the migratory and
proliferative activity of EPCs was significantly decreased in
premenopausal women and men with DM compared with in normoglycemic
premenopausal women and men (P<0.05; Fig. 3A and B). Furthermore, migratory and
proliferative activity of EPCs was significantly lower in the male
population with NGT or T2DM compared with in women with NGT and DM
(P<0.05).
FMD, plasma NO, VEGF and GM-CSF levels
in the four groups
Differences between the sexes with regards to FMD
and plasma NO levels were detected, indicating that premenopausal
women with NGT and DM exhibited significantly higher FMD and plasma
NO levels compared with in the age- and glycemia-matched men
(P<0.05; Table I and Fig. 4A). However, the plasma NO levels in
premenopausal women with DM were significantly decreased compared
with in the NGT group, which was similar to the difference between
men with NGT and DM (P<0.05). Plasma VEGF and GM-CSF levels
exhibited no significant differences between the groups (P>0.05;
Fig. 4B and C).
NO, VEGF and GM-CSF secretion by EPCs
in the four groups
The difference in NO secretion based on cultured
EPCs between the NGT and DM groups was in line with that of plasma
NO levels; NO secretion was significantly reduced in patients with
T2DM compared with in the NGT group, and significantly reduced in
the male population when compared with the female population
(P<0.05; Fig. 5A). However, the
secretion of VEGF and GM-CSF from cultured EPCs was comparable
among all of the groups (P>0.05; Fig. 5B and C).
Correlation between FMD and
circulating EPCs
A significant positive correlation was detected
between the number of circulating EPCs, as evaluated by flow
cytometry (r=0.49, P<0.05) and cell culture (r=0.56, P<0.05),
and FMD (Fig. 6A and B).
Similarly, the migratory and proliferative activity of EPCs was
significantly positively correlated with FMD (r=0.64, P<0.05 and
r=0.57, P<0.05, respectively; Fig.
6C and D).
Correlation between FMD and NO levels
in the plasma and secreted by EPCs
In addition, the correlations between FMD and plasma
NO levels, and between FMD and NO secretion by EPCs were analyzed.
Both plasma NO levels and NO secretion by EPCs were significantly
positively correlated with FMD (r=0.68, P<0.05 and r=0.51,
P<0.05; Fig. 6E and F).
Discussion
The present study demonstrated that the number and
activity of circulating EPCs were lower, and endothelial function
was impaired in premenopausal women with T2DM compared with in
women with NGT, which was at least partly attributable to decreases
in NO production. It was therefore hypothesized that attenuated
endothelial function, which may be induced by impaired circulating
EPCs, may be associated with an increased CVD risk, to a certain
extent, in patients with DM. Differences between the sexes in the
number and activity of EPCs were also observed both in the
population with and without T2DM.
In the present study, DM was reported to impair the
capability of endothelial cells to repair and it was suggested that
it may lead to endothelial dysfunction in premenopausal women with
DM, which was at least partly attributable to impaired NO
production. These results provided novel evidence on the important
role of endothelial protective interventions in premenopausal women
with DM.
DM is widely regarded as an important risk factor
for CVD pathogenesis, and contributes to cardiovascular morbidity
and mortality (18). Even in
premenopausal women with DM, cardiovascular risk is increased
compared with in age-matched women without DM, thus suggesting that
DM may increase the CVD risk in young premenopausal women due to
certain underlying mechanisms (19). Previously, numerous studies
indicated that the number and functional ability of circulating
EPCs were reduced and weakened, due to the effects of impaired
endothelial repair capacity during DM development or DM-associated
vascular dysfunction (11,13,14,31–33).
The subjects in these studies were either patients with type 2 DM
and a mean age of >50 years old (13,14)
or patients with type 1 DM and a mean age of <30 years old
(31), as age has been reported to
possibly influence the number of circulating EPCs (34). In prehypertensive premenopausal
women, hyperglycemia weakens the preservation of the number and
functional activity of EPCs and damages endothelial diastolic
function (21). However, whether
the impaired endothelial repair capability resulting from T2DM is
present in the general premenopausal female population remains
unclear. The present study detected a reduced number of circulating
EPCs with impaired activity in premenopausal women with T2DM, but
not in women without T2DM. FMD, an index used to assess endothelial
function, was also decreased in these women, which was positively
associated with the number and activity of EPCs. Therefore, it was
hypothesized that the high CVD risk in premenopausal women with DM
may at least partly result from suppressed endothelial repair
capability; this finding differed from the results of our previous
study, which indicated that prehypertensive premenopausal women
possessed preserved endothelial repair capabilities (20). When compared with prehypertension,
DM hyperglycemia may have a more severe effect on endothelial
repair capability and even on endothelial diastolic function.
Therefore, intensive endothelial protective actions should be
adopted in premenopausal women with DM.
In the male population of the present study, DM
reduced the number, as well as the functional activity of
circulating EPCs, which was consistent with the results in
premenopausal women. Furthermore, sex-related differences in the
number and activity of circulating EPCs were consistent in patients
with and without DM, thus suggesting that the DM male population
may be at risk for CVD due to dual impairment (low estrogen levels
and DM) in their endothelial repair capability.
Although reductions in the number and functional
activity of EPCs were observed, the mechanism underlying impairment
or insufficient endothelial repair capability in patients with DM,
and in the overall male population, remains unclear. NO, VEGF and
GM-CSF are regarded as key factors in modulating the number and
function of EPCs (22–25). In a previous study, it was revealed
that NO bioavailability decreases in patients with DM due to
impaired endothelial nitric oxide synthase (eNOS) activity,
resulting from hyperglycemia (34). However, it remains to be determined
as to whether reductions in the number and functional activity of
circulating EPCs in premenopausal women with DM are correlated with
alterations in NO, VEGF and GM-CSF. To investigate this, the plasma
levels of NO, VEGF and GM-CSF were evaluated. Plasma NO levels were
decreased in patients with DM, and differed between male and female
populations. In addition, NO levels were positively correlated with
FMD; these findings indicated that reduced systemic production of
NO may lead to endothelial dysfunction in premenopausal women with
DM and in male populations. Alterations in plasma VEGF and GM-CSF
levels were not significant, indicating that these factors may not
be associated with impaired endothelial repair capacity in patients
with DM.
According to the results of the present study, in
addition to plasma NO levels, reduced endogenous NO biosynthesis
may contribute to the decreased number and activity of EPCs and
endothelial diastolic dysfunction, both in premenopausal women with
DM and in the overall male population. Endogenous NO biosynthesis
serves a crucial role in maintaining the functional activity of
EPCs (35). Chen et al
(34) demonstrated that high
glucose levels decreased eNOS, FoxO1 and Akt phosphorylation levels
as well as levels of bioavailable NO in both early and late EPCs.
The present study observed that, consistent with the alterations in
plasma NO levels, NO production was reduced in premenopausal women
with DM and in the overall male population compared with in
premenopausal women with NGT and in the female population,
respectively. Furthermore, NO production by EPCs and FMD were
positively correlated. Therefore, these results suggested that
efforts to enhance endogenous NO biosynthesis may be beneficial in
preventing endothelial dysfunction.
Several mechanisms, which are involved in
hyperglycemia, are associated with NO production by either vascular
endothelium or EPCs. Advanced glycation end products have been
reported to reduce NO production and eNOS expression (36), and hyperglycemia-activated protein
kinase C increases eNOS gene expression and triggers eNOS
uncoupling, thus leading to decreased bioavailability of NO
(37). Hyperglycemia may also
impair eNOS serine phosphorylation, leading to decreased
EPC-derived NO production (34).
NO is regarded as a crucial component in promoting mobilization,
proliferation and migration of circulating EPCs, which promotes the
number and activity of circulating EPCs (35). Therefore, it was inferred that
DM-induced decreased NO production, either by the vascular
endothelium or EPCs, may serve a role in hyperglycemia-impaired
endothelial repair capability in the young population.
The present study provided several important
guidelines for research regarding DM-induced vascular disease.
Firstly, the results revealed that since premenopausal women with
DM had impaired endothelial repair capability compared with women
with NGT, and thus the corresponding intervention should be
considered in a timely fashion. Since NO production may contribute
to endothelial dysfunction, appropriate therapies, including statin
usage, exercise and certain anti-hyperglycemic agents that increase
NO production, should be considered in a clinical setting.
Secondly, sex-related differences in circulating EPCs existed in
the DM population, thus suggesting that men with DM may have
difficulties in maintaining normal endothelial function; therefore,
strict interventions to improve endothelial repair capacity should
be implemented immediately. In addition, the mechanism underlying
the differences between the sexes with regards to circulating EPCs
remains to be clarified, particularly with regards to the role of
estrogen in modulating the number and activity of circulating EPCs
in a relatively young population. Another limitation associated the
present study was that it was only conducted using data from
Chinese subjects; therefore, these findings need to be confirmed in
other populations.
In conclusion, the present study demonstrated that
the number and activity of circulating EPCs in premenopausal women
with DM were significantly lower compared with in women without DM,
which was possibly associated with reduced NO production. Impaired
endothelial repair capability in premenopausal women with DM may be
an important mechanism underlying the increased relative risk of
CVD. Furthermore, since sex-related differences were detected in
circulating EPCs in the DM population, vascular protection should
be carefully considered in men with DM.
Acknowledgements
We are grateful to all participants, research staff
and faculty staff members who participated in the present study. We
would also thank Professor Jinxin Zhang from the Department of
Medical Statistic and Epidemiology, School of Public Health, Sun
Yat-sen University (Guangzhou, China) for his contribution to
statistical analysis.
Funding
The present study was financially supported by
grants from the National Natural Scientific Foundation of China
(grant nos. 81670220, 31270992 and 30800215), the project of
Guangdong Province Science and Technology Plan (grant nos.
2015A020212013 and 2013B021800275), the Guangdong Natural Science
Foundation (grant no. 2014A030313086), the Fundamental Research
Funds for Central Universities in Sun Yat-Sen University (grant
nos. 17ykzd18 and 13ykpy24), the Science and Technology Program of
Guangzhou City (grant no. 201803010008), the International
Scientific and Technological Cooperation project of Guangzhou
Economic and Technological Development Zone (grant no. 2017GH13),
the Industrial Technology Research and Development funding projects
of Guangdong Province (grant no. 2014A020212436), and the Medical
Scientific Research Foundation of Guangdong Province (grant no.
A2015127).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY and HH designed the present study, supervised the
writing of the manuscript and provided the final approval of the
version to be published. JuL and DH designed the study, performed
the experiments, wrote and revised the manuscript and provided
final approval of the manuscript version for publication. HY, XA
and ZR performed the experiments. JiL and HZ were involved the
analysis and interpretation of data and contributed to the revision
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the First Hospital of Chenzhou and Xiangya Hospital,
Central South University (Changsha, China).
Patient consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
Men and Women in China. N Engl J Med. 362:1090–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Avogaro A, de Kreutzenberg SV and Fadini
G: Endothelial dysfunction: Causes and consequences in patients
with diabetes mellitus. Diabetes Res Clin Pract. 82(Suppl 2):
S94–S101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berezin AE: Endothelial progenitor cells
dysfunction and impaired tissue reparation: The missed link in
diabetes mellitus development. Diabetes Metab Syndr. 11:215–220.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ali M, Mehmood A, Anjum MS, Tarrar MN,
Khan SN and Riazuddin S: Diazoxide preconditioning of endothelial
progenitor cells from streptozotocin-induced type 1 diabetic rats
improves their ability to repair diabetic cardiomyopathy. Mol Cell.
Biochem. 410:267–279. 2015.
|
|
5
|
Jin P, Li T, Li X, Shen X and Zhao Y:
Suppression of oxidative stress in endothelial progenitor cells
promotes angiogenesis and improves cardiac function following
myocardial infarction in diabetic mice. Exp Ther Med. 11:2163–2170.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aicher A, Zeiher AM and Dimmeler S:
Mobilizing endothelial progenitor cells. Hypertension. 45:321–325.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giannotti G, Doerries C, Mocharla PS,
Mueller MF, Bahlmann FH, Horvàth T, Jiang H, Sorrentino SA,
Steenken N, Manes C, et al: Impaired endothelial repair capacity of
early endothelial progenitor cells in prehypertension: Relation to
endothelial dysfunction. Hypertension. 55:1389–1397. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mandraffino G, Sardo MA, Riggio S,
D'Ascola A, Loddo S, Alibrandi A, Saitta C, Imbalzano E,
Mandraffino R, Venza M, et al: Smoke exposure and circulating
progenitor cells: Evidence for modulation of antioxidant enzymes
and cell count. Clin Biochem. 43:1436–1442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Y, Wu Y, Choi HC and Wang S: Diabetic
endothelium dysfunction, cardiovascular complications, and
therapeutics. J Diabetes Res 2016. 53498012016.
|
|
10
|
Tsai TH, Chai HT, Sun CK, Yen CH, Leu S,
Chen YL, Chung SY, Ko SF, Chang HW, Wu CJ and Yip HK: Obesity
suppresses circulating level and function of endothelial progenitor
cells and heart function. J Transl Med. 10:1372012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hörtenhuber T, Rami-Mehar B, Satler M,
Nagl K, Höbaus C, Höllerl F, Koppensteiner R, Schernthaner G,
Schober E and Schernthaner GH: Endothelial progenitor cells are
related to glycemic control in children with type 1 diabetes over
time. Diabetes care. 36:1647–1653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fadini GP, Boscaro E, de Kreutzenberg S,
Agostini C, Seeger F, Dimmeler S, Zeiher A, Tiengo A and Avogaro A:
Time Course and Mechanisms of circulating progenitor cell reduction
in the natural history of type 2 diabetes. Diabetes care.
33:1097–1102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Pascale MR, Bruzzese G, Crimi E,
Grimaldi V, Liguori A, Brongo S, Barbieri M, Picascia A, Schiano C,
Sommese L, et al: Severe type 2 diabetes induces reversible
modifications of endothelial progenitor cells which are ameliorate
by glycemic control. Int J Stem Cells. 9:137–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yue WS, Lau KK, Siu CW, Wang M, Yan GH,
Yiu KH and Tse HF: Impact of glycemic control on circulating
endothelial progenitor cells and arterial stiffness in patients
with type 2 diabetes mellitus. Cardiovasc Diabetol. 10:1132011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheetz MJ and King GL: Molecular
understanding of hyperglycemia's adverse effects for diabetic
complications. JAMA. 288:2579–2588. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cubbon RM, Mercer BN, Sengupta A and
Kearney MT: Importance of insulin resistance to vascular repair and
regeneration. Free Radical Bio Med. 60:246–263. 2013. View Article : Google Scholar
|
|
17
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fox CS: Cardiovascular disease risk
factors, type 2 diabetes mellitus, and the Framingham Heart Study.
Trends Cardiovasc Med. 20:90–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garcia NH, Perez HA, Spence JD and Armando
LJ: Risk of vascular disease in premenopausal women with diabetes
mellitus. Clin Ther. 36:1924–1934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhen Y, Xiao S, Ren Z, Shen HW, Su H, Tang
YB and Zeng H: Increased endothelial progenitor cells and nitric
oxide in young prehypertensive women. J Clin Hypertens (Greenwich).
17:298–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng H, Jiang Y, Tang H, Ren Z, Zeng G and
Yang Z: Abnormal phosphorylation of Tie2/Akt/eNOS signaling pathway
and decreased number or function of circulating endothelial cells
in prehypertensive premenopausal women with diabetes mellitus. BMC
Endocr Disord. 16:132016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Z, Wang JM, Chen L, Luo CF, Tang AL
and Tao J: Acute exercise-induced nitric oxide production
contributes to upregulation of circulating endothelial progenitor
cells in healthy subjects. J Hum Hypertens. 21:452–460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonafè F, Guarnieri C and Muscari C:
Nitric oxide regulates multiple functions and fate of adult
progenitor and stem cells. J Physiol Biochem. 71:141–153. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue J, Du G, Shi J, Li Y, Yasutake M, Liu
L, Li J, Kong Y, Wang S, Yun F and Li W: Combined treatment with
erythropoietin and granulocyte colony-stimulating factor enhances
neovascularization and improves cardiac function after myocardial
infarction. Chin Med J (Engl). 127:1677–1683. 2014.PubMed/NCBI
|
|
25
|
Shurygin MG, Shurygina IA, Dremina NN and
Kanya OV: Endogenous progenitors as the source of cell material for
ischemic damage repair in experimental myocardial infarction under
conditions of changed concentration of vascular endothelial growth
factor. Bull Exp Biol Med. 158:1–531. 2015. View Article : Google Scholar
|
|
26
|
Expert Committee on the Diagnosis and
Classification of Diabetes Mellitus: Report of the expert committee
on the diagnosis and classification of diabetes mellitus. Diabetes
Care. 26(Suppl 1): S5–S20. 2003.PubMed/NCBI
|
|
27
|
Yang Z, Chen L, Su C, Xia WH, Wang Y, Wang
JM, Chen F, Zhang YY, Wu F, Xu SY, et al: Impaired endothelial
progenitor cell activity is associated with reduced arterial
elasticity in patients with essential hypertension. Clin Exp
Hypertens. 32:1–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Z, Tao J, Wang JM, Tu C, Xu MG, Wang
Y and Pan SR: Shear stress contributes to t-PA mRNA expression in
human endothelial progenitor cells and nonthrombogenic potential of
small diameter artificial vessels. Biochem Biophys Res Commun.
342:1–584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z, Xia WH, Zhang YY, Xu SY, Liu X,
Zhang XY, Yu BB, Qiu YX and Tao J: Shear stress-induced activation
of Tie2-dependent signaling pathway enhances reendothelialization
capacity of early endothelial progenitor cells. J Mol Cell Cardiol.
52:1–1163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corretti MC, Anderson TJ, Benjamin EJ,
Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H,
Gerhard-Herman M, Herrington D, et al: Guidelines for the
ultrasound assessment of endothelial-dependent flow-mediated
vasodilation of the brachial artery: A report of the International
Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 39:1–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sibal L, Aldibbiat A, Agarwal SC, Mitchell
G, Oates C, Razvi S, Weaver JU, Shaw JA and Home PD: Circulating
endothelial progenitor cells, endothelial function, carotid
intima-media thickness and circulating markers of endothelial
dysfunction in people with type 1 diabetes without macrovascular
disease or microalbuminuria. Diabetologia. 52:1464–1473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsukada S, Masuda H, Jung SY, Yun J, Kang
S, Kim DY, Park JH, Ji ST, Kwon SM and Asahara T: Impaired
development and dysfunction of endothelial progenitor cells in type
2 diabetic mice. Diabetes Metab. 43:154–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fadini GP, Sartore S, Agostini C and
Avogaro A: Significance of endothelial progenitor cells in subjects
with diabetes. Diabetes Care. 30:1305–1313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR,
Huang PH, Liu PL, Chen YL and Chen JW: High glucose impairs early
and late endothelial progenitor cells by modifying nitric
oxide-related but not oxidative stress-mediated mechanisms.
Diabetes. 56:1559–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aicher A, Heeschen C and Dimmeler S: The
role of NOS3 in stem cell mobilization. Trends Mol Med. 10:421–425.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen C, Li Q, Zhang YC, Ma G, Feng Y, Zhu
Q, Dai Q, Chen Z, Yao Y, Chen L, et al: Advanced glycation
endproducts increase EPC apoptosis and decrease nitric oxide
release via MAPK pathways. Biomed Pharmacother. 64:35–43. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cosentino F, Eto M, De Paolis P, van der
Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H,
Volpe M and Lüscher TF: High glucose causes upregulation of
cyclooxygenase-2 and alters prostanoid profile in human endothelial
cells: Role of protein kinase C and reactive oxygen species.
Circulation. 107:1017–1023. 2003. View Article : Google Scholar : PubMed/NCBI
|