Introduction
Ovarian cancer is the most lethal malignant
gynecologic cancer (1). More than
70% of ovarian cancer patients are diagnosed at an advanced stage
due to a lack of early-stage symptoms, and the overall 5-year
survival rates are less than 30% (2). Even after receiving primary
cytoreductive surgery and platinum-based chemotherapy, 60–70% of
EOC patients eventually undergo relapse, which is largely due to
chemotherapy resistance. Unfortunately, the specific mechanism of
chemoresistance remains unclear.
Dicer belongs to the RNase III family of enzymes
whose function is central to the processing of miRNA precursors
into mature miRNAs in the cytoplasm (3). Dicer directly affects the levels and
activity of miRNAs (4). Numerous
recent studies have revealed the importance of Dicer in cancer
development, chemoresistance and progression, but its expression
levels are not consistent in different types of tumors. There are
tissue-specific effects associated with the aberrant expression of
Dicer (5). Recent research has
shown that Dicer is associated with chemoresistance in colon cancer
cells (6), breast cancer (7) and lung cancer cells (8). In ovarian cancer, it was reported
that the deletion of Dicer could promote epithelial ovarian cancer
(EOC) progression by increasing PDIA3 expression levels (9). In addition, several studies indicated
that low levels of Dicer expression were significantly associated
with high-grade histologic features and poor responses to
chemotherapy (10,11). Although these findings support the
hypothesis that Dicer plays an essential role in ovarian cancer
chemoresistance, the underlying molecular mechanism is unclear.
Therefore, in the present study, we aimed to
demonstrate whether downregulating Dicer can mediate cell apoptosis
and regulate cisplatin resistance in ovarian cancer cells in
vitro.
Materials and methods
Cell lines and culture conditions
The human EOC cell lines A2780 and CAOV3 were
purchased from the China Center for Type Culture Collection (CCTCC,
Wuhan University, China). A cisplatin-resistant subline (A2780/DDP)
was generated and maintained by our laboratory (12). Cisplatin was purchased from
Shandong Qilu Pharmaceutical Factory. All cell lines were cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal calf serum (FCS; Gibco; Thermo
Fisher Scientific, Inc.). Cells were maintained in a 37°C
humidified incubator with an atmosphere of 5% CO2.
A2780/DDP cells were cultured without cisplatin for more than 1
month prior to use in this study to exclude stress reactions
mediated by drug treatment.
Dicer shRNA stable transfection
The Dicer-shRNA and control-shRNA vectors were
purchased from Shanghai GenePharma (Shanghai, China). The target
sequence for Dicer-shRNA was 5′-GCCAAGGAAATCAGCTAAATT-3′. A2780 and
CAOV3 cells were seeded in a six-well plate at a concentration of
5×105 cells per well. Transfection was performed via
lentivirus infection according to the manufacturer's instructions.
Then, the cells were selected by 2 µg/ml puromycin until resistant
cell colonies were obtained. The stably transfected cells were
harvested 48 h after transfection and used for further assays. To
verify the knockdown efficiency, mRNA and protein levels in the
stably transfected cells were analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot assays.
RT-qPCR
Total RNA was extracted from the cell lines using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and
cDNA was synthesized using an RT kit (Toyobo Life Science, Osaka,
Japan) according to the manufacturer's protocol. Dicer and β-actin
were measured by a SYBR Green (Takara Bio, Inc., Otsu, Japan) qPCR
assay. The sequences of the Dicer primers were as follows:
Upstream, 5′-GTGGTTCGTTTTGATTTGCCC-3′ and downstream,
5′-CGTGTTGATTGTGACTCGTGGA-3′ (NM_001195573.1). β-actin was used for
normalization, and the primer sequences were as follows: Upstream,
5′-GCCAACACAGTGCTGTCTGG-3′ and downstream,
5′-GCTCAGGAGGAGCAATGATCTTG-3′. Dicer was amplified under the
following qPCR reaction conditions: Initial denaturation at 95°C
for 1 min, followed by 40 cycles of denaturation at 95°C for 15
sec, and annealing at 62°C for 1 min. All reactions were run on an
Applied Biosystems 7500 Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The 2−ΔΔCq method was
used to calculate relative changes in gene expression (13).
Western blotting
Cellular proteins were lysed in NP40 Cell Lysis
Buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing 1 mM protease inhibitor cocktail (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 4°C for 30 min. The protein
concentrations were determined using the bicinchoninic acid (BCA)
method. Fifty micrograms of total protein from each sample was
resolved by 8% (Dicer) or 10% (P53, P63, P73, caspase-9 and
caspase-3) SDS-PAGE and then was transferred onto polyvinylidene
difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Membranes were incubated with mouse anti-Dicer (1:500
dilution; Abcam, Cambridge, MA, USA), rabbit anti-P53 (1:1,000
dilution; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit anti-P63 (1:500 dilution; Abcam), rabbit anti-P73 (1:500
dilution; Abcam), rabbit anti-caspase-9 (1:1,000 dilution; Cell
Signaling Technology, Inc.), rabbit anti-caspase-3 (1:2,000
dilution; Cell Signaling Technology, Inc.), rabbit anti-P21
(1:1,000 dilution; Cell Signaling Technology, Inc.) and mouse
anti-β-actin (1:5,000 dilution; Wuhan Boster Biological Technology,
Ltd., Wuhan, China). β-Actin was used as a loading control. The
primary antibodies were detected using HRP-conjugated secondary
antibody (1:5,000 dilution; Wuhan Boster Biological Technology,
Ltd.). The final immunoblots were detected by using an enhanced
chemiluminescence system (Pierce; Thermo Fisher Scientific, Inc.).
Protein bands were quantitated after scanning using Quantity One
software (Bio-Rad Laboratories, Inc.).
MTT assays
Chemoresistance to cisplatin was assessed using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays. Briefly, cells were plated in triplicate in a 96-well plate
at a density of 5000 cells/well. Cells were treated with cisplatin
at various concentrations ranging from 2.5 to 100 µM for an
additional 48 h. After that, 20 µl of MTT (Sigma-Aldrich; Merck
KGaA) at a final concentration of 0.5 mg/ml was added to each well
and incubated at 37°C for 6 h. Then, the cells were dissolved in
100 µl of DMSO by incubating overnight at 37°C. The absorbance at a
wavelength of 570 nm was measured using an iMark microplate reader
(serial no. 10601; Bio-Rad Laboratories, Inc.). The percentage of
cell survival at each dose was calculated as the absorbance ratio
of treated to untreated cells. The 50% inhibitory concentration
(IC50) values were calculated by linear interpolation.
The data shown are representative of three independent
experiments.
Hoechst 33342 staining
Cells were plated in triplicate in a six-well plate
at a density of 1×105 cells/well and incubated for 24 h.
After that, A2780 cells were treated with 40 µM cisplatin, and
CAOV3 cells were treated with 30 µM cisplatin. After a 48 h
incubation at 37°C, the cells were fixed in 4% paraformaldehyde for
10 min at room temperature. Finally, 1 µg/ml Hoechst 33342
(Invitrogen; Thermo Fisher Scientific, Inc.) was added for 5 min.
Then, images of the six-well plates mounted with emission
fluorescence were acquired with a fluorescence microscope (model
IX70; Olympus Corporation, Tokyo, Japan) and Image-Pro Plus
software (Media Cybernetics, Inc., Rockville, MD, USA). The Hoechst
reagent was taken up by nuclei, and the nuclei of apoptotic cells
exhibited bright blue fluorescence. Each experiment was repeated
separately three times.
Flow cytometric assays
Cells were plated in triplicate in a six-well plate
at a density of 1×105 cells/well and incubated for 24 h.
After that, A2780 cells were treated with 40 µM cisplatin, and
CAOV3 cells were treated with 30 µM cisplatin. After a 48 h
incubation at 37°C, the cells were harvested, trypsinized and
washed with cold PBS. Subsequently, the cells were stained with APC
Annexin V and propidium iodide (cat. no. 640932; BioLegend, Inc.,
San Diego, CA, USA). The stained cells were immediately analyzed by
an LSR flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA),
and the data were analyzed using ModFit LT software (Verity
Software House, Inc., Topsham, ME, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
(n≥3). Statistical comparisons between groups were estimated by
two-tailed Student's t-tests or one-way analysis of variance
followed by Dunnett's post hoc test. Data analyses were carried out
using the SPSS v.13.0 statistical software package (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Dicer expression levels are decreased
in cisplatin-resistant ovarian cancer cells
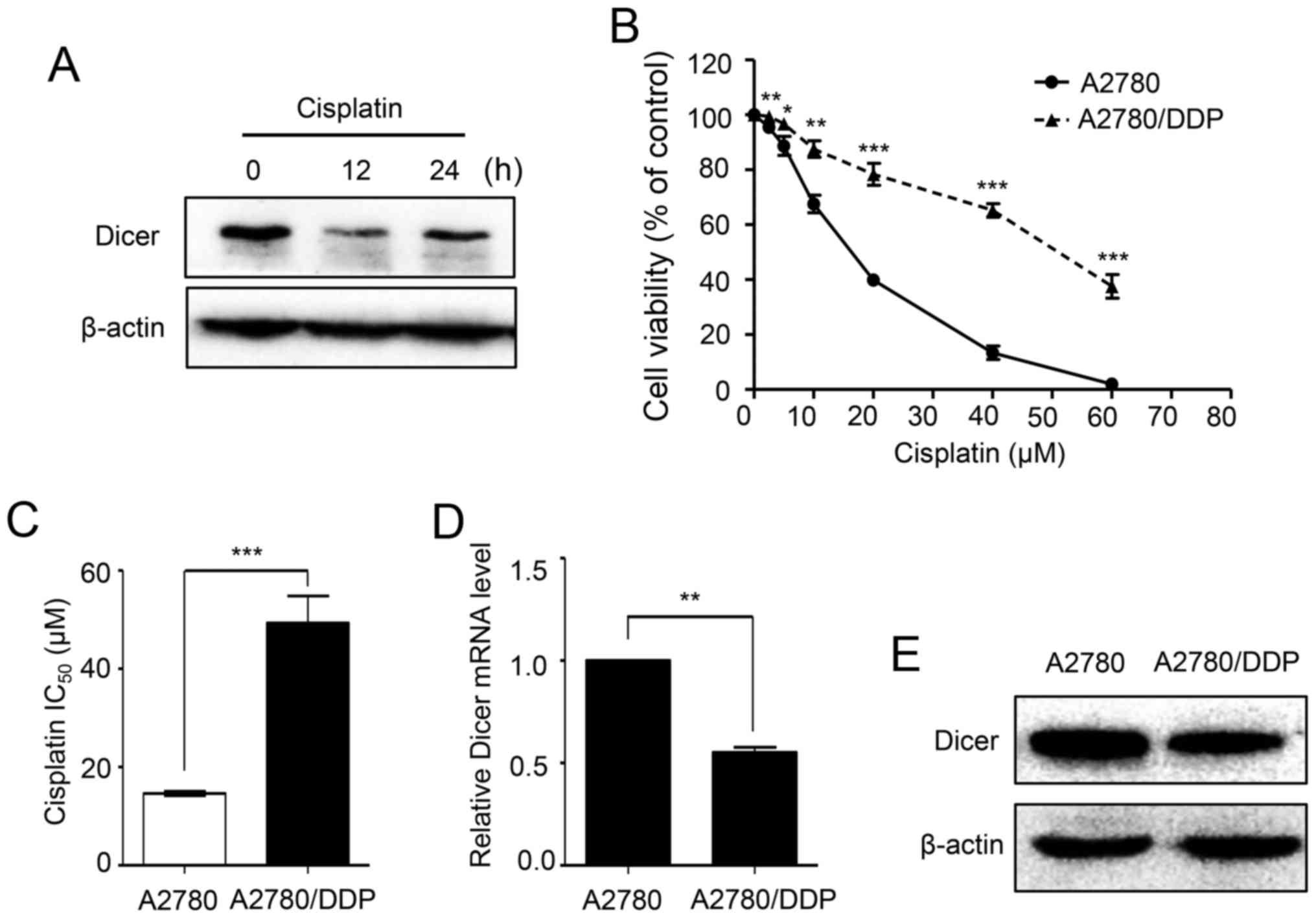
To investigate whether the expression of Dicer is
affected by cisplatin, we treated A2780 cells with 20 µM cisplatin
for different periods of time. Western blot analyses showed that
the protein expression levels of Dicer were dramatically decreased
after cisplatin treatment for 12 and 24 h (Fig. 1A). To identify the role of Dicer in
cisplatin resistance, first, the cisplatin sensitivities of
parental A2780 cells and A2780/DDP were assessed using MTT assays.
As demonstrated in Fig. 1B, the
A2780/DDP cells dose-survival curve was shifted to the right, and
the cells became more resistant to cisplatin. The cisplatin
IC50 value for the A2780/DDP cells was 3.38-fold higher
than that of A2780 cells (49.31 vs. 14.61 µM; P<0.001; Fig. 1C). Then, the expression of Dicer in
A2780 and A2780/DDP cells was analyzed by RT-qPCR and western blot
assays. The results showed that Dicer mRNA and protein levels were
significantly lower in A2780/DDP cells than in parental A2780 cells
(Fig. 1D and E).
Dicer knockdown increases cisplatin
resistance in ovarian cancer cells
To study the effects of Dicer knockdown on cisplatin
sensitivity, first, A2780 and CAOV3 cells were transfected with a
shRNA plasmid against Dicer (shDicer) or a scrambled control
plasmid (shNC). Compared with those in the shNC cells, Dicer mRNA
levels were decreased by 77.3 and 73.09% in A2780/shDicer and
CAOV3/shDicer cells, respectively (P<0.01; Fig. 2A). Similarly, western blot analyses
confirmed Dicer knockdown after shDicer transfection (Fig. 2B). Then, the sensitivities of
parental control, shNC and shDicer cells to cisplatin were assessed
by MTT assays. The results showed that the dose-survival curves in
the A2780/shDicer and CAOV3/shDicer cells were shifted to the
right, and the cells became more resistant to cisplatin than the
shNC cells (Fig. 2C and D). In
addition, compared with those of the corresponding shNC cells, the
IC50 values for cisplatin were increased by 2.37- and
1.68-fold in the A2780/shDicer (14.42 vs. 34.2 µM; P<0.001,
Fig. 2D) and CAOV3/shDicer cells
(20.34 vs. 34.2 µM; P<0.001, Fig.
3D), respectively.
Dicer knockdown inhibits
cisplatin-induced cell apoptosis in ovarian cancer cells
Cisplatin is primarily believed to kill caner cells
by inducing cell apoptosis. To explore the effects of Dicer on
cisplatin-induced cancer cell apoptosis, A2780 and CAOV3 cells were
treated with cisplatin for 48 h at concentrations of 40 and 30 µM,
respectively, and Hoechst 33342 staining was then performed.
According to fluorescence microscopy images, the shDicer cells had
significantly less nuclear fragmentation than the parental control
cells and the shNC cells in both A2780 ((5.22±0.41), (13.14±0.84)
and (13.33±0.69)% apoptosis rates for shDicer, parental control and
shNC cells, respectively, P<0.001) and CAOV3 cells ((5.14±0.46),
(12.17±0.79) and (12.39±0.73)% apoptosis rates for shDicer,
parental control and shNC cells, respectively, P<0.001; Fig. 3A and B). Consistently, Annexin
V/propidium iodide dual staining followed by flow cytometric
analyses showed that compared with the shNC cells, the proportion
of apoptotic shDicer cells was decreased by 1.88-, 3.11- and
6.95-fold in A2780 cells after treatment with cisplatin at
concentrations of 0, 20 and 40 µM, respectively (Fig. 3C and D); the proportion of
apoptotic shDicer cells was decreased by 2.26-, 4.27- and
10.17-fold in CAOV3 cells after treatment with cisplatin at
concentrations of 0, 15 and 30 µM, respectively (Fig. 3C and E).
Dicer knockdown decreases
apoptosis-related protein expression levels
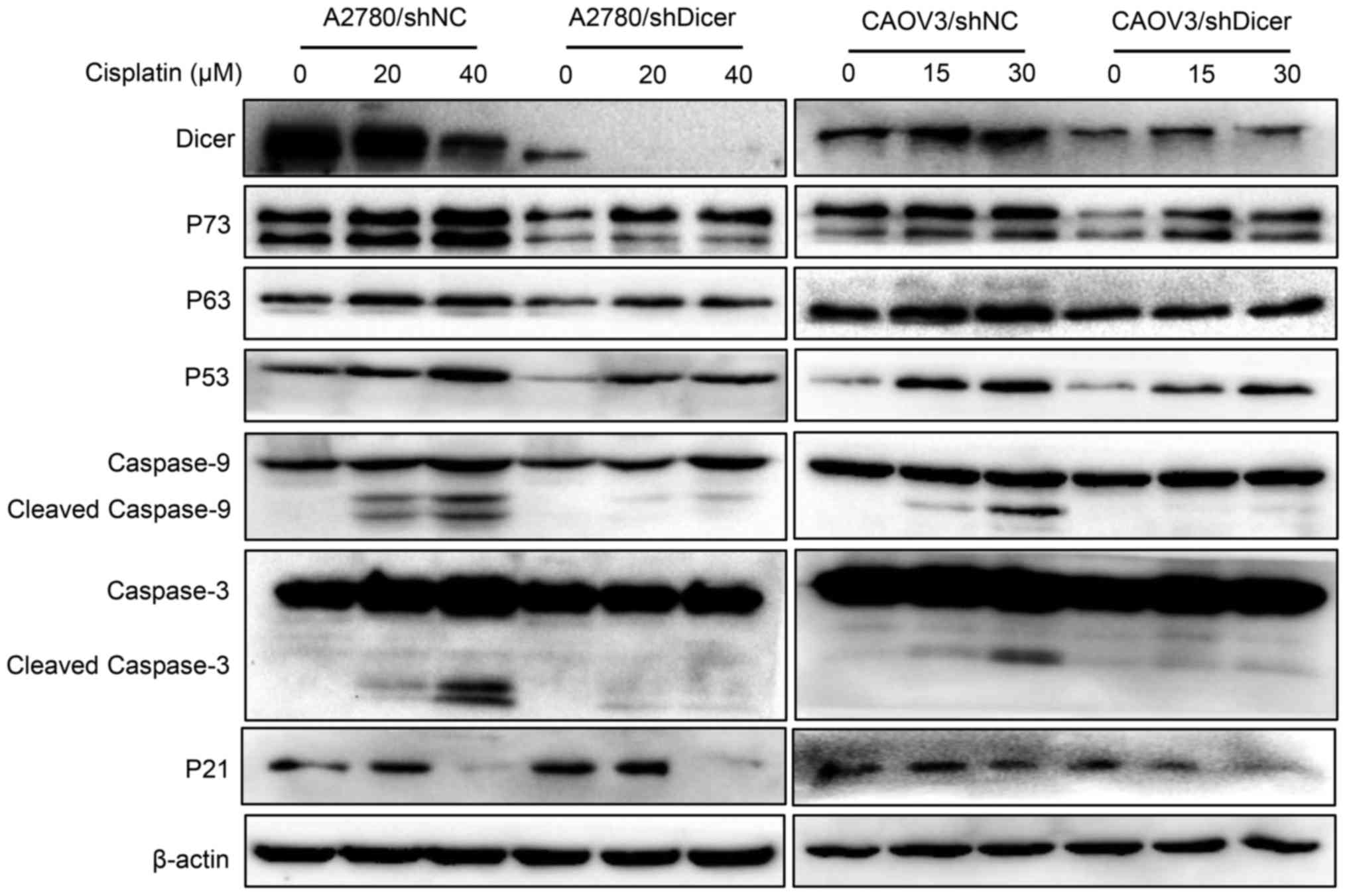
To investigate whether Dicer affects the expression
of apoptosis-related proteins in cancer cells exposed to cisplatin,
P73, P63, P53, cleaved caspase-9, cleaved caspase-3 levels and P21
were measured in ovarian cancer cells that were treated with
cisplatin for 48 h. The treatment concentrations of cisplatin were
0, 20 and 40 µM for A2780 cells and 0, 15 and 30 µM for CAOV3
cells. Western blot analyses showed that the cisplatin-mediated
increases in the expression levels of P73, P63, P53, P21, cleaved
caspase-9 and cleaved caspase-3 were concentration-dependent in
both shNC and shDicer cells; however, these expression levels were
generally lower in the shDicer cells, especially the active
fragments of caspase-9 and caspase-3 (Fig. 4).
Discussion
In the present study, we showed that decreased Dicer
expression levels contributed to cisplatin resistance in ovarian
cancer cells. Our results indicate that Dicer knockdown could
protect ovarian cancer cells from cisplatin-induced apoptosis by
decreasing the expression levels of proteins involved in apoptosis
signaling pathways, including P73, P63, P53, caspase-9 and
caspase-3. These findings provide novel insight into the mechanism
of chemoresistance in ovarian cancer.
In our study, we found a dramatic decrease in Dicer
protein level 1 h after cisplatin treatment. Previous study
reported that cisplatin-induced proteasome degradation cause rapid
down-regulation of hCTR1 (human copper transporter 1) expression in
human ovarian carcinoma cells (14). In addition, another research
indicated that cisplatin could induce EGFR phosphorylation and
subsequent ubiquitination and degradation in head and neck cancer
(15). Whether the instant
downregulation of Dicer after exposure to cisplatin is caused by
cisplatin-induced protein degradation or not need to be further
explored.
A previous study revealed that reduced expression
levels of Dicer in ovarian cancer were associated with increased
tumor cell proliferation, enhanced migration ability and increased
cisplatin resistance (11). In our
previous study, we found that downregulation of Dicer could promote
proliferation, colony formation, and migration ability of A2780,
CAOV3, and SKOV3 ovarian cancer cells. Besides, proteomic profiling
assay showed that Dicer knockdown promoted ovarian cancer
progression by elevating PDIA3 expression (9). Our study further revealed that the
downregulation of Dicer could increase cisplatin resistance in
ovarian cancer cells through inhibiting cisplatin-induced apoptosis
and decreasing the levels of cleaved caspase-3 and caspase-9.
Similarly, several recent reports have proven that decreased Dicer
levels play an important role in drug resistance in different
cancer types. In cervical cancer, a study showed that EZH2
inhibition could effectively increase the cisplatin sensitivity of
HeLa/DDP cells by upregulating Dicer expression (16). In addition, the knockdown of Dicer
resulted in resistance to 5-FU-based chemoradiotherapy and a poor
prognosis in patients with oral squamous cell carcinoma (17). In addition, the upregulation of
miR-18a decreased Dicer expression levels and conferred paclitaxel
resistance in triple-negative breast cancer (7). These findings are consistent with our
results. However, some studies have obtained the opposite results
regarding the relationship between Dicer expression and drug
resistance. In breast cancer, Bu et al reported that the
suppression of Dicer could result in G1 arrest in MCF-7 cells and
increase their sensitivity to cisplatin (18). It has also been reported that
downregulating Dicer could increase gefitinib sensitivity in human
lung cancer cells (8). These
opposite results may be attributed to the complicated structure and
function of Dicer.
Previous studies have demonstrated that Dicer might
promote cell apoptosis through regulating apoptosis-related genes
(19,20). It is well-known that P53
superfamily proteins including P53, P63 and P73 function in
mediating apoptosis in the presence of many stress stimuli,
including activation of oncogenes and DNA damage (21,22).
As is known, p53 plays a central role in tumor suppression, while
which is the most frequently mutated gene in human cancer, and over
half of human cancers contain p53 mutations. Although the critical
role of wild-type p53 in tumor suppression has been firmly
established, mounting evidence has demonstrated that many
tumor-associated mutant p53 proteins not only lose the
tumor-suppressive function of wild-type p53 but also gain new
activities to promote tumorigenesis independently of wild-type p53,
termed gain-of-function. Mutant p53 protein often accumulates to
very high levels in tumors, contributing to malignant progression
(21). In addition, caspase-9 and
caspase-3 can be activated in response to cisplatin-induced
apoptosis (23). Our findings
indicate that the expression levels of proteins involved in
apoptosis signaling pathways, including P73, P63, P53, caspase-9
and caspase-3, were decreased in ovarian cancer cells after Dicer
knockdown. Therefore, our results indicate that Dicer might play a
specific role in cisplatin-induced apoptosis in ovarian cancer
cells. Besides, we found that the p21 protein expression was not
affected by Dicer down-regulation in A2780 and CAOV3 cells, while
it was decreased under cisplatin treatment. The cisplatin induced
reduction of P21 was similar in the A2780 cells with or without
Dicer depletion, but more intensive in the CAOV3 cells with Dicer
depletion compared with control, which may be due to the presence
of P53 mutations in CAOV3, but not in A2780 (24). Recently, it has been reported that
tumor-associated mutant p53 promoted cancer cell survival upon
glutamine deprivation through p21 induction (25), which is consistent with our
results. As is known, Dicer plays an important role in the
maturation of miRNAs. Emerging evidence has indicated that low
levels of some miRNAs are associated with drug resistance in cancer
cells. For example, low miRNA-107 and low miRNA-204 were linked to
chemo-resistance in non-small cell lung cancers and prostate
cancers, respectively, because they target the mRNAs of
antiapoptotic factors Bcl-w and zinc-finger E-box-binding homeobox
1 (ZEB1) (26,27). These findings suggest the potential
roles of microRNAs in regulating Dicer-mediated chemotherapy
responses in tumor cells. However, whether the role of Dicer in
cisplatin resistance in ovarian cancer is mediated by corresponding
microRNAs needs to be further studied.
In summary, we found that low expression levels of
Dicer can inhibit cisplatin-induced apoptosis in ovarian cancer
cells, which in turn resulted in resistance to cisplatin. However,
the present study also has several limitations. First, Dicer
upregulation in the cisplatin resistant ovarian cancer cells was
not performed because of technical difficulty. Second, only one
cisplatin-resistant cell line was used. Third, the mechanisms
through which Dicer regulates cell apoptosis remain unclear. Given
that Dicer functions as an enzyme that catalyzes miRNA maturation
and that several miRNAs have been identified as regulators of cell
apoptosis, multiple miRNAs might be involved in Dicer-mediated
cisplatin resistance in ovarian cancer. Moreover, our results
suggest that the upregulation of Dicer might be a promising
strategy for treating recurrent, cisplatin-resistant EOC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Hubei Province (grant no. 2014CFB996) and the
Health and Family Planning Commission of Wuhan Municipality (grant
no. WX17C10).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
ZW and HC designed the present study. ZW and JC
supervised the project. XW worked out almost all of the technical
details and performed the functional experiments. QH and YW
performed the experiments regarding molecular mechanisms. ZH
provided technical support. XY, PJ and HC analyzed and interpreted
the data. YW and JC collected the data, and drafted the manuscript
in consultation with PJ and ZW. JC revised the manuscript
substantively. All authors contributed to producing the manuscript
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentate ribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bahubeshi A, Tischkowitz M and Foulkes WD:
miRNA processing and human cancer: DICER1 cuts the mustard. Sci
Transl Med. 3:111ps462011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Li WF, Wu X, Zhang HC, Chen L,
Zhang PY, Liu LY, Ma D, Chen T, Zhou L, et al: Dicer regulates
non-homologous end joining and is associated with chemosensitivity
in colon cancer patients. Carcinogenesis. 38:873–882. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sha LY, Zhang Y, Wang W, Sui X, Liu SK,
Wang T and Zhang H: MiR-18a upregulation decreases Dicer expression
and confers paclitaxel resistance in triple negative breast cancer.
Eur Rev Med Pharmacol Sci. 20:2201–2208. 2016.PubMed/NCBI
|
|
8
|
Chen JC, Su YH, Chiu CF, Chang YW, Yu YH,
Tseng CF, Chen HA and Su JL: Suppression of Dicer increases
sensitivity to gefitinib in human lung cancer cells. Ann Surg
Oncol. 21 Suppl 4:S555–S563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Y, Cai L, Guo J, Chen N, Yi X, Zhao Y,
Cai J and Wang Z: Depletion of Dicer promotes epithelial ovarian
cancer progression by elevating PDIA3 expression. Tumour Biol.
37:14009–14023. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Merritt WM, Lin YG, Han LY, Kamat AA,
Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick
AM, et al: Dicer, Drosha, and outcomes in patients with ovarian
cancer. N Engl J Med. 359:2641–2650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuang Y, Cai J, Li D, Han Q, Cao J and
Wang Z: Repression of Dicer is associated with invasive phenotype
and chemoresistance in ovarian cancer. Oncol Lett. 5:1149–1154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu S, Yu L, Li Z, Shen Y, Wang J, Cai J,
Xiao L and Wang Z: Overexpression of EZH2 contributes to acquired
cisplatin resistance in ovarian cancer cells in vitro and in vivo.
Cancer Biol Ther. 10:788–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holzer AK and Howell SB: The
internalization and degradation of human copper transporter 1
following cisplatin exposure. Cancer Res. 66:10944–10952. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahsan A, Hiniker SM, Ramanand SG, Nyati S,
Hegde A, Helman A, Menawat R, Bhojani MS, Lawrence TS and Nyati MK:
Role of epidermal growth factor receptor degradation in
cisplatin-induced cytotoxicity in head and neck cancer. Cancer Res.
70:2862–2869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai L, Wang Z and Liu D: Interference with
endogenous EZH2 reverses the chemotherapy drug resistance in
cervical cancer cells partly by up-regulating Dicer expression.
Tumour Biol. 37:6359–6369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawahara K, Nakayama H, Nagata M, Yoshida
R, Hirosue A, Tanaka T, Nakagawa Y, Matsuoka Y, Kojima T, Takamune
Y, et al: A low Dicer expression is associated with resistance to
5-FU-based chemoradiotherapy and a shorter overall survival in
patients with oral squamous cell carcinoma. J Oral Pathol Med.
43:350–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bu Y, Lu C, Bian C, Wang J, Li J, Zhang B,
Li Z, Brewer G and Zhao RC: Knockdown of Dicer in MCF-7 human
breast carcinoma cells results in G1 arrest and increased
sensitivity to cisplatin. Oncol Rep. 21:13–17. 2009.PubMed/NCBI
|
|
19
|
Nakagawa A, Shi Y, Kage-Nakadai E, Mitani
S and Xue D: Caspase-dependent conversion of Dicer ribonuclease
into a death-promoting deoxyribonuclease. Science. 328:327–334.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swahari V, Nakamura A, Baran-Gale J,
Garcia I, Crowther AJ, Sons R, Gershon TR, Hammond S, Sethupathy P
and Deshmukh M: Essential function of dicer in resolving DNA damage
in the rapidly dividing cells of the developing and malignant
cerebellum. Cell Rep. 14:216–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aubrey BJ, Kelly GL, Janic A, Herold MJ
and Strasser A: How does p53 induce apoptosis and how does this
relate to p53-mediated tumour suppression? Cell Death Differ.
25:104–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mollereau B and Ma D: The p53 control of
apoptosis and proliferation: Lessons from Drosophila. Apoptosis.
19:1421–1429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gonzalez VM, Fuertes MA, Alonso C and
Perez JM: Is cisplatin-induced cell death always produced by
apoptosis? Mol Pharmacol. 59:657–663. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Domcke S, Sinha R, Levine DA, Sander C and
Schultz N: Evaluating cell lines as tumour models by comparison of
genomic profiles. Nat Commun. 4:21262013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tran TQ, Lowman XH, Reid MA,
Mendez-Dorantes C, Pan M, Yang Y and Kong M: Tumor-associated
mutant p53 promotes cancer cell survival upon glutamine deprivation
through p21 induction. Oncogene. 36:1991–2001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu C, Xie Z and Peng Q: MiRNA-107 enhances
chemosensitivity to paclitaxel by targeting antiapoptotic factor
Bcl-w in non small cell lung cancer. Am J Cancer Res. 7:1863–1873.
2017.PubMed/NCBI
|
|
27
|
Wu G, Wang J, Chen G and Zhao X:
microRNA-204 modulates chemosensitivity and apoptosis of prostate
cancer cells by targeting zinc-finger E-box-binding homeobox 1
(ZEB1). Am J Transl Res. 9:3599–3610. 2017.PubMed/NCBI
|