Introduction
Pancreatic islet transplantation is a potential
treatment option for type 1 diabetes mellitus; however, the
shortage of human pancreas donors continues to restrict clinical
transplantation. The pig represents an alternative source of
unlimited organs and tissue, making xenotransplantation a potential
strategy for use in humans. However, xenogeneic rejection mediated
by T cell responses remains a major limitation to its clinical
application (1,2). Long-term survival of xenogeneic
islets in large animal models has been achieved with
immunosuppression (3,4); however, the high dose of
immunosuppressive agents required, accompanied by their side
effects (5,6), limits clinical application.
Regulatory T cells (Tregs), are critically important for
maintaining tolerance and controlling autoimmunity (7–9),
therefore they may represent an alternative and novel strategy for
achieving transplant tolerance. Previous studies have indicated
that adoptive transfer with ex vivo polyclonally expanded
human Tregs prevents islet xenograft rejection by suppressing
effector T cell responses (10),
and in vitro polyclonally expanded human Tregs maintain
their suppressive function in CD4+CD25−
effector T cells in a xenogeneic-stimulated mixed lymphocyte
reaction (11). These findings
indicate a possible strategy for overcoming cellular xenoresponses
in vitro and in vivo. However, a major limitation to
using polyclonally expanded Tregs is that they can cause
pan-immunosuppressive effects, leading to opportunistic infections
and tumor growth, due to their non-specific suppressive functions.
Studies in human and animal models have demonstrated that small
numbers of alloantigen-specific Tregs exhibit high efficiency to
prevent allograft rejection with fewer side effects (12–14).
Therefore, antigen-specific Tregs may hold immense promise for
human immunotherapy.
The present study investigated whether ex
vivo expanded human Tregs receiving xenoantigen stimulation are
more potent than polyclonally expanded Tregs in protecting against
islet xenograft rejection in NOD-SCID interleukin (IL)-2 receptor
(IL2r)γ−/− mice.
Materials and methods
Animals
A total of 3 newborn pigs (1 to 3 days old) supplied
by Chongqing Enservier Biological Technology Co., Ltd. (Chongqing,
China) were used to isolate neonatal porcine islet cell clusters
(NICC). A total of 2 adult landrace pigs (male, 18 months old,
Chongqing Enservier Biological Technology Co., Ltd.) were used to
isolate porcine peripheral blood mononuclear cell as xenoantigen,
and were housed in separate cages at 20–26°C, 12-h light/dark cycle
with fresh air, and fed pig chow twice a day with free access to
water. NOD-SCID IL2rγ−/− mice (age, 6–8 weeks, weight,
25–30 g) were obtained from Chengdu Dashuo experimental animals Co.
Ltd. (Chengdu, Sichuan, China) and housed under specific
pathogen-free conditions (20–26°C, relative humidity, 40–70%, free
access to sterile feeds and sterile water and 12-h light/dark
cycle) in the approved Experimental Animal Center at Sichuan
University (Chengdu, China). The mice were used for porcine islet
transplantation. The procedures described in this study were
conducted according to the guidelines set by the Institute of
Laboratory Animals Resources Guide for the Care and Use of
Laboratory Animals (Institutional Animal Care and Use Committee
Guidebook) (15).
Porcine islet isolation and
transplantation
NICC were isolated from the pancreas of 1–3 day old
piglets and cultured for 6 days, as previously described (16). The NICC were pooled and 5,000
clusters (10) were transplanted
under the renal capsule of both kidneys of NOD-SCID
IL2rγ−/− mice.
Peripheral blood mononuclear cell
(PBMC) isolation and human Treg isolation
Human PBMCs were isolated from the blood of 4
healthy donors (age, 28–58; gender, 2 male and 2 female) by density
gradient centrifugation using Lymphoprep™ (STEMCELL
Technologies China Co., Ltd, Shanghai, China).
CD4+CD25+CD127lo T cells were
isolated from PBMCs using the
CD4+CD25+CD127dim/− Regulatory T
Cell Isolation kit II (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany), according to the manufacturer's protocol. The purity of
CD4+CD25+CD127lo T cells was ≥98%.
Porcine PBMCs were isolated from heparinized whole blood of adult
landrace pigs by density gradient centrifugation using
Lymphoprep™ (STEMCELL Technologies China Co., Ltd.) and
used as xenogeneic stimulator cells. Human and animal studies were
approved by the Sichuan University Medical Ethics Committee and
Animal Research Ethics Communities. Written informed consent was
obtained from all donors.
In vitro expansion of human Tregs with
xenoantigen stimulation
To obtain large numbers of functional human Tregs
with xenoantigen specificity (Xeno-Treg) from
CD4+CD25+CD127lo T cells, cells
were harvested after 7 days of polyclonal stimulation and further
expanded for two subsequent cycles (7 days per cycle) by
stimulating with irradiated xenogeneic PBMCs. Polyclonal Tregs
(Poly-Treg) were solely expanded using CD3/CD28 beads.
CD4+CD25+CD127lo T
cells were expanded in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% human AB
serum (Gibco; Thermo Fisher Scientific, Inc.), 2 mM glutamine
(Gibco; Thermo Fisher Scientific, Inc.), 50 µM 2-mercaptoethanol
(2-ME) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 100 U/ml
penicillin (Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and 100 nM
rapamycin (Sigma-Aldrich; Merck KGaA) at 37°C and 5%
CO2, in the presence of 400 U/ml IL-2 (Novartis
Corporation, East Hanover, NJ, USA) and Human T-Activator CD3/CD28
beads (Dynabeads®; Invitrogen; Thermo Fisher Scientific,
Inc.) in 96-well U-bottom plates (BD Biosciences, Franklin Lakes,
NJ, USA). After 7 days of expansion, Tregs were harvested and used
to induce Xeno-Treg. For both cycles of xenoantigen stimulation,
5×104 Tregs were cultured with 2×105
irradiated (30 Gy) porcine PBMCs (xenogeneic PBMC:Treg, 4:1), in
the presence of 5×104 Dynabeads®. The cells
were split and fresh medium was added every 3 days. After two
cycles of expansion, Treg were harvested for all subsequent
experiments.
Flow cytometry
Single-cell suspensions were obtained from mouse
spleen or peripheral blood at 4-weeks, 9-weeks and 12-weeks
following NICC transplantation and were processed using red blood
cell lysis buffer (BioLegend, Inc., San Diego, CA, USA), according
to the manufacturer's protocol. Human antigen CD45 (368503;
BioLegend, Inc.) was used for the flow cytometric analysis of human
leukocyte engraftment in the mouse spleen or peripheral blood cell
suspension. Human cells were surface stained with fluorescently
labeled antibodies specific for the human antigens CD4 (cat. nos.
317407 and 317425), CD25 (cat. nos. 302605 and 302613), CD127 (cat.
no. 351323), CD62L (cat. no. 304821), glucocorticoid-induced tumor
necrosis factor receptor-related protein (GITR; cat. no. 371223)
and HLA-DR (cat. no. 307617) (all from BioLegend, Inc.), in
staining buffer at 4°C for 30 min in the dark, followed by fixation
and permeabilization (Fix/Perm buffer; BioLegend, Inc.).
Intracellular staining was conducted with fluorescently labeled
anti-forkhead box P3 (Foxp3) (cat. no. 320105) and -cytotoxic
T-lymphocyte antigen-4 (CTLA-4; cat. no. 369603) antibodies (both
BioLegend, Inc.) for 30 min at room temperature. Flow cytometric
data were acquired using an LSRII flow cytometer (BD
Biosciences).
IL-10 analyses
Total RNA was extracted from Tregs using the RNeasy
Mini kit (Qiagen GmbH, Hilden, Germany), according to the
manufacturer's protocol, followed by cDNA synthesis using the
SuperScript™ III First-Strand Synthesis system (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) was performed on the Bio-Rad CFX Connect Real-Time system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) using the Platinum
SYBR Green qPCR Supermix-UDG (Thermo Fisher Scientific, Inc.). The
reaction was 50°C for 2 min and 95°C for 2 min followed by 40
cycles of 95°C for 15 sec and 65°C for 35 sec. PCR primers specific
for human IL-10 were used: Sense 5′-GCCTAACATGCTTCGAGATC-3′ and
antisense 5′-GGGTTCAGGTACCGCTTCTC-3′. Human GAPDH primer (Sense
5′-TGCACCACCAACTGCTTAGC-3′ and antisense
5′-GGCATGGACTGTGGTCATGAG-3′) was used as an internal reference gene
and gene expression was normalized to GAPDH expression levels in
each PCR reaction (17).
IL-10 in the supernatants collected from Xeno-Treg
and Poly-Treg stimulation cultures was measured by ELISA, using the
IL-10 Human ELISA kit (cat. no. BMS215-2TEN; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
TCR Vβ CDR3 spectratyping
CDR3 spectratyping was performed as previously
described (18). Briefly, PCR
amplification of expanded human Tregs with xenoantigen stimulation
of polyclonal stimulation was performed until a plateau was
reached. For the 29 human TCR Vβ families, this required 36 PCR
amplification cycles, 29 TCR Vβ primers and a Fam-labeled Cβ
reverse primer (18). All the TCR
Vβ family primers were provided by Professor Stephen I. Alexander
(Centre for Kidney Research, The Children's Hospital at Westmead,
Sydney, NSW, Australia; Table I).
The PCR product (1 µl) from this reaction was mixed with 12 µl
Hi-Di™ Formamide and 0.2 µl GeneScan™ 500
TAMRA™ dye Size Standard in a 0.5 ml Genetic Analyzer sample tube
(all Thermo Fisher Scientific, Inc.). The sample was denatured by
heating at 95°C for 10 min and then rapidly cooled on ice. The
sample was then electrophoresed on the ABI Prism® 310
Genetic Analyzer (Thermo Fisher Scientific, Inc.). An
electropherogram of the GeneScan-500 Size Standard was generated
under denaturing conditions on the ABI Prism® 310
Genetic Analyzer. Fragments were run using the POP-4™
Polymer (Thermo Fisher Scientific, Inc.) at 60°C. When the size of
the PCR product was <500 bp, a capillary with dimensions of 47
cm × 50 µm i.d. (Thermo Fisher Scientific, Inc.) was used. If the
signal was too strong, the sample injection time or voltage was
decreased; or the sample was further diluted. The data were sized
and quantified using ABI Prism 310 Genetic Analyzer with built in
software (ABI Prism 310 collection; Thermo Fisher Scientific,
Inc.).
 | Table I.Primer sequences of TCR Vβ
families. |
Table I.
Primer sequences of TCR Vβ
families.
| Gene name | Oligonucleotide
sequence |
|---|
| BV2 |
GAAATCTCAGAGAAGTCTGAAATATTCG |
| BV3 |
CCTAAATCTCCAGACAAAGCTCACT |
| BV4 |
CCTGAATGCCCCAACAGC |
| BV5 |
ACCTGATCAAAACGAGAGGACAG |
| BV6 |
CTCTCCTGTGGGCAGGTCC |
| BV7-2 |
GTTTTTAATTTACTTCCAAGGCAACA |
| BV7-3 |
CAAGGCACGGGTGCG |
| BV7-6 |
ACTTACTTCAATTATGAAGCCCAACA |
| BV7-7 |
GAGTCATGCAACCCTTTATTGGTAT |
| BV7-8 |
AGGGGCCAGAGTTTCTGACTTAT |
| BV7-9 |
CTCAACTAGAAAAATCAAGGCTGCT |
| BV9 |
AACAGTTCCCTGACTTGCACTCT |
| BV10 |
TTCTTCTATGTGGCCCTTTGTCT |
| BV11 |
GGCTCAAAGGAGTAGACTCCACTCT |
| BV12 |
GGTGACAGAGATGGGACAAGAAGT |
| BV13 |
CATCTGATCAAAGAAAAGAGGGAAAC |
| BV14 |
AGAGTCTAAACAGGATGAGTCCGGTAT |
| BV15 |
AGAGTCTAAACAGGATGAGTCCGGTAT |
| BV16 |
AAACAGGTATGCCCAAGGAAAGA |
| BV18 |
CAGCCCAATGAAAGGACACAGT |
| BV19 |
GGGCAAGGGCTGAGATTGAT |
| BV20 |
AACCATGCAAGCCTGACCTT |
| BV23 |
TGTACCCCCGAAAAAGGACATAC |
| BV24 |
CAGTGTCTCTCGACAGGCACA |
| BV25 |
CTCAAACCATGGGCCATGA |
| BV27 |
CCAGAACCCAAGATACCTCATCAC |
| BV28 |
GGCTACGGCTGATCTATTTCTCA |
| BV29 |
GACGATCCAGTGTCAAGTCGATAG |
| BV30 |
CTGAGGTGCCCCAGAATCTCT |
| BC for BV |
CTGCTTCTGATGGCTCAAACA |
| BC for BV
probe |
6-FAMCACCCGAGGTCGCTMGBNFQ |
| BC forward |
TCCAGTTCTACGGGCTCTCG |
| BC reverse |
AGGATGGTGGCAGACAGGAC |
| BC probe |
6-FAMACGAGTGGACCCAGGATAGGGCCAA NFQ |
| GAPDH forward |
TGCACCACCAACTGCTTAGC |
| GAPDH reverse |
GGAAGGCCATGCCAGTGA |
| GAPDH probe |
VICCCTGGCCAAGGTCATCCATGACAACTTTAMRA |
In vitro Treg suppression assay
The suppressive capacity of Tregs was assessed by
measuring inhibition of proliferation in mixed leukocyte reaction
(MLR) assays. Proliferation was evaluated using the
CellTrace™ Carboxyfluorescein succinimidyl ester (CFSE)
Cell Proliferation kit (Invitrogen; Thermo Fisher Scientific,
Inc.). Responder cells (human PBMCs) were labeled with 0.5 µM CFSE
(Molecular Probes; Thermo Fisher Scientific, Inc.) prior to
stimulation. CFSE-labeled responder cells (1×105) from
autologous Treg donors were stimulated with 2×105
irradiated xenogeneic stimulator PBMCs or purified anti-human CD3
antibody (BD Biosciences). Tregs were titrated into the cultures at
different ratios. After 7 days of culture, the proliferation of
responder cells was analyzed by flow cytometry (LSR II; BD
Biosciences).
Adoptive transfer of human cells
Adoptive transfer of human cells was performed as
previously described (10). Human
PBMCs were isolated from the blood of healthy donors. A total of
1×107 CD25+ cell-depleted PBMCs with or
without 1×106 autologous ex vivo expanded human
Xeno-Treg or Poly-Treg were injected intravenously into NOD-SCID
IL2rγ−/− mice 3 days after NICC transplantation.
Peripheral blood, spleen and NICC grafts were collected from
recipient mice at predetermined time points to analyze human
leukocyte engraftment and NICC graft survival. Graft rejection was
defined as no visible intact graft observed by histological
examination (19).
Histology and
immunohistochemistry
Histology and immunohistochemistry of cryostat
section (6–8 µm) were undertaken as described previously (10). Porcine endocrine cells were
detected using anti-porcine insulin antibody (IR00261-2 insulin;
1:100; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) and
the VECTASTAIN® ABC kit (Vector Laboratories, Inc.,
Burlingame, CA, USA). Graft-infiltrating human leukocytes were
stained using anti-human CD45 antibody (14-9457-82; 1:200;
eBioscience; Thermo Fisher Scientific, Inc.), followed by
incubation with horseradish peroxidase-conjugated secondary rabbit
anti-mouse antibody (31451; 1:200, Thermo Fisher Scientific, Inc.),
then analyze using a Zeiss microscope (AX10; Zeiss AG, Oberkochen,
Germany).
Statistical analysis
Results comparisons involving two groups were
analyzed using Student's t-test (two-tailed) and those involving
multiple groups were analyzed using one-way analysis of variance
with the Tukey multiple comparison test by SPSS version 22 (IBM
Corp., Armonk, NY, USA). Graft survival was evaluated using
Kaplan-Meier analysis. The data were presented as the means ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Human Tregs expanded ex vivo with
xenoantigen stimulation retain Treg phenotype and secrete
IL-10
The phenotype of Xeno-Treg was examined by flow
cytometry. After two cycles of xenoantigen stimulation, Xeno-Treg
retained the classic Treg phenotype, as did Poly-Treg, which is
characterized by high levels of CD25, Foxp3, CTLA-4, CD62L and GITR
expression, and very low or undetectable CD127 expression (Fig. 1). However, compared with Poly-Treg,
Xeno-Treg expressed more HLA-DR, which has been described as an
effector marker of Treg (20,21)
(Fig. 1).
 | Figure 1.Phenotypic characterization of
expanded Tregs. Gates were set on CD4+CD25+
cells. Cell surface expression of CD25, CD127, CD62L, GITR and
HLA-DR, and intracellular expression of Foxp3 and CTLA-4 in
Xeno-Treg or Poly-Treg are presented as the percentage of
CD4+CD25+ cells co-expressing individual Treg
markers examined. Data presented in figures represent the
percentage of CD4+CD25+ cells co-expressing
individual Treg markers tested. Data are representative of three
independent experiments. CD, cluster of differentiation; CTLA-4,
cytotoxic T-lymphocyte antigen-4; FoxP3, forkhead box P3; GITR,
glucocorticoid-induced tumor necrosis factor receptor-related
protein; HLA-DR, human leukocyte antigen-DR; Poly-Treg, polyclonal
Treg; Tregs, regulatory T cells; Xeno-Treg, Treg with xenoantigen
specificity. |
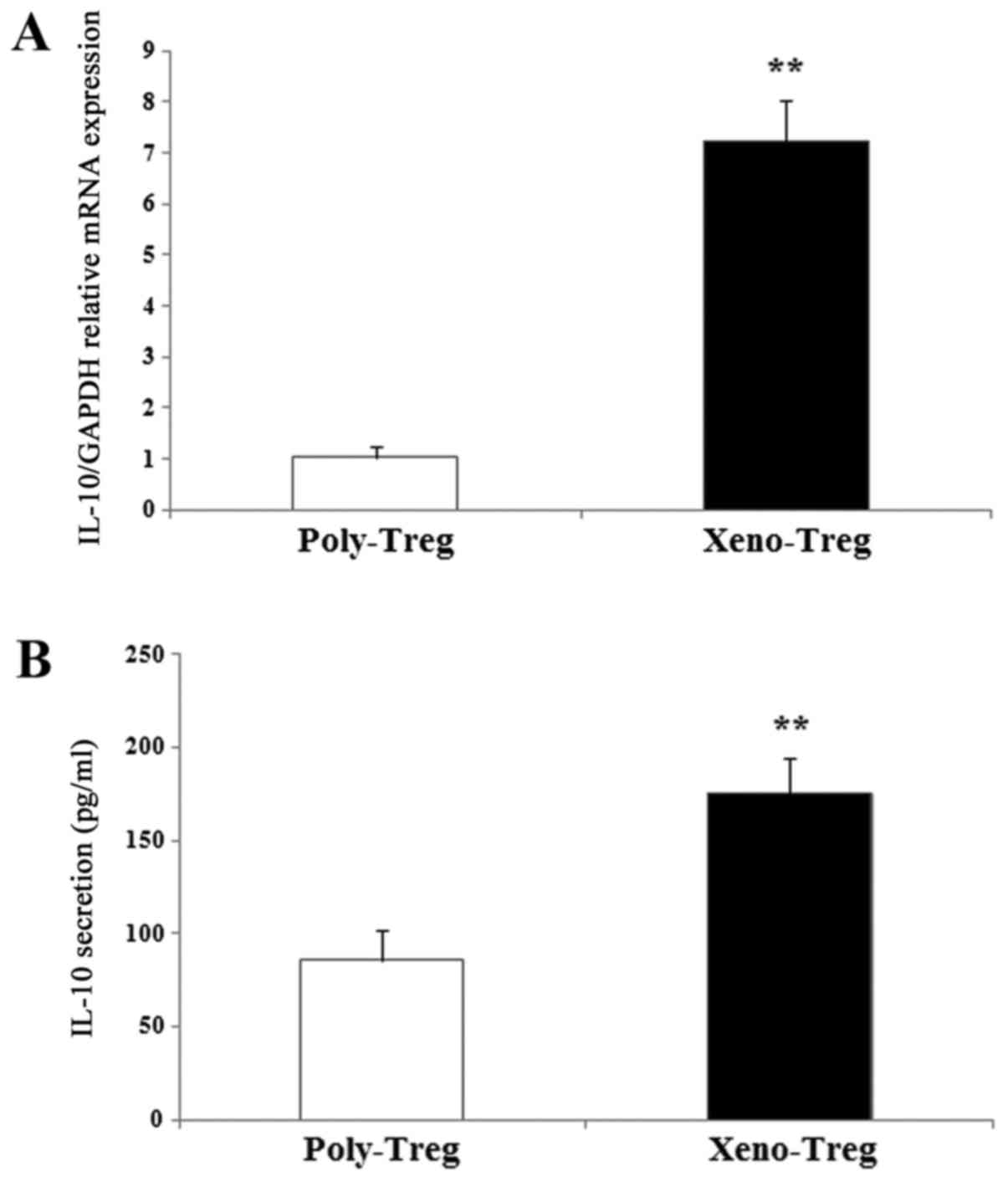
IL-10 expression in Tregs was assessed by RT-qPCR.
Xenoantigen stimulation led to an upregulation of IL-10 expression,
with expression levels 7-fold higher compared with Poly-Treg
(Fig. 2A; P<0.01). In addition,
IL-10 secretion was measured in the cell culture supernatants of
Tregs receiving xenoantigen or polyclonal stimulation. Consistent
with mRNA expression, IL-10 secretion by Xeno-Treg was enhanced
compared with Poly-Treg (Xeno-Treg: 175±18.9 pg/ml vs. Poly-Treg:
86±16.4 pg/ml; Fig. 2B;
P<0.01). These results demonstrated that Xeno-Treg may retain a
Treg phenotype, but secrete higher levels of IL-10 compared with
Poly-Treg, which may result in greater suppressive potency.
Tregs exhibit restricted TCR Vβ
repertoire following xenoantigen stimulation
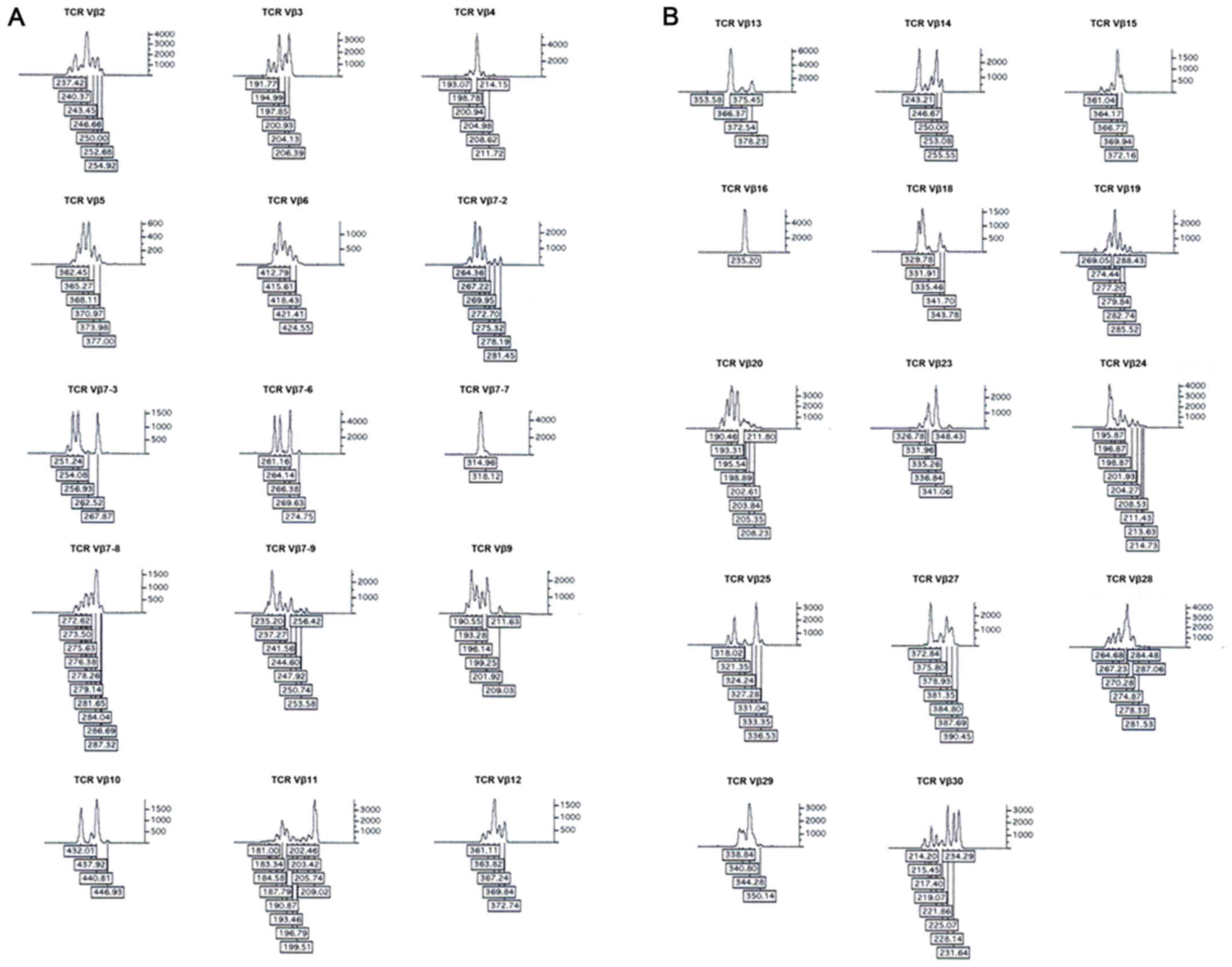
Spectratyping was used to analyze the TCR Vβ
families at the CDR3 level and screen for clonal expansion of
specific T cells (22–24). All 29 TCR Vβ families were detected
in Xeno-Treg or Poly-Treg, and in control CD4+ T cells.
However, there were altered TCR Vβ repertoires in both Xeno-Treg
and Poly-Treg compared with in CD4+ T cells. Increased
expression of TCR Vβ4, Vβ7-9, Vβ20, Vβ28 (>5% in repertoire) and
TCR Vβ10 and Vβ18 were observed in Xeno-Treg and Poly-Treg compared
with in CD4+ T cells (Fig.
3). In addition, expression levels of TCR Vβ4, Vβ10, Vβ18 and
Vβ20 were markedly increased in Xeno-Treg compared with in
Poly-Treg (Fig. 3).
Overall spectratyping of PCR products revealed a
restricted TCR Vβ repertoire in Xeno-Treg and Poly-Treg compared
with in CD4+ T cells, which possessed a diverse TCR Vβ
repertoire. Nearly all the TCR Vβ families exhibited a Gaussian
distribution, with the exception of TCR Vβ3, Vβ7-7, Vβ19 and Vβ23
(Fig. 4). Spectratypes of TCR Vβ4,
Vβ20 and Vβ28 (>5% of the TCR Vβ repertoire and increased
expression) in Xeno-Treg and Poly-Treg demonstrated restriction and
expanded clone at size 205, 196 and 274, respectively (Figs. 5 and 6). Spectratypes of TCR Vβ7–9 (>5% of
the TCR Vβ repertoire and increased expression) exhibited
restriction and expanded clone at size 234 in Poly-Treg and at size
237 in Xeno-Treg. In addition, spectratypes of TCR Vβ10 (<5% of
the TCR Vβ repertoire and increased expression) possessed
restriction and expanded clone at size 432 in Poly-Treg and at size
432 and 441 in Xeno-Treg.
Human Tregs expanded ex vivo with
xenoantigen stimulation exhibit enhanced suppressive capacity
To determine whether Xeno-Treg possessed more potent
and xenoantigen-specific suppressive capacity against xenoimmune
responses compared with Poly-Treg, their suppressive function was
assessed in an MLR assay using CFSE-labeled PBMCs as responder
cells. In a xenoantigen-driven MLR (Xeno MLR) assay, Xeno-Treg
exhibited an enhanced xenoantigen-specific suppressive capacity
compared with Poly-Treg, as evidenced by the ~55 and 45%
suppression of responder cell proliferation at low responder cell:
Treg ratios of 1:1/8 and 1:1/16, respectively (Fig. 7A). Even at a higher responder cell:
Treg ratio of 1:1/32, Xeno-Treg still demonstrated >35%
suppression of responder cell proliferation, which was 2.5-fold
higher compared with Poly-Treg (Fig.
7A). These data revealed that Xeno-Treg possess enhanced and
xenoantigen-specific suppressive capacity. However, both Xeno- and
Poly-Treg demonstrated similar ability to suppress responder cell
proliferation in a polyclonally-stimulated MLR (Poly MLR) assay
(Fig. 7B), thus suggesting that
xenoantigen stimulation did not alter the capacity to suppress
polyclonally-stimulated responses.
Human Treg expanded ex vivo with
xenoantigen stimulation prevent rejection of porcine islet
xenografts
To determine the in vivo suppressive capacity
of ex vivo expanded Xeno-Treg, a total of 1×107
CD25+ cell-depleted PBMCs with or without
1×106 autologous ex vivo expanded Xeno-Treg or
Poly-Treg were injected intravenously into NOD-SCID
IL2rγ−/− mice 3 days after NICC transplantation.
Nonreconstituted mice were used as a control. Mice that were
reconstituted with human PBMCs, rejected their xenografts
completely within 28 days of transplantation, whereas NICC grafts
survived for ≥84 days in nonreconstituted recipients (Fig. 8A). In mice reconstituted with
Xeno-Treg and PBMCs (Xeno-Treg:PBMC ratio of 1:10), 75% of NICC
xenografts survived beyond 84 days (Fig. 8A). In contrast, in mice
reconstituted with Poly-Treg and PBMCs (Poly-Treg:PBMC ratio of
1:10), 75% of NICC xenografts survived ≥48 days, 25% of NICC
xenografts survived until day 56, and all xenografts were rejected
by day 63 (Fig. 8A). These results
suggested that Xeno-Treg may prolong NICC xenograft survival.
 | Figure 8.Xeno-Treg suppress rejection of islet
xenografts in humanized mice. (A) Percentage of graft survival in
mice administered 1×107 CD25+ cell-depleted
human PBMCs with or without 1×106 Poly-Treg or
Xeno-Treg. Graft survival was monitored 18, 21, 28, 48, 56, 63 and
84 days post cell transfer. (B) Flow cytometric analysis of the
percentage of human leukocyte engraftment in the spleen and
peripheral blood of NOD-SCID interleukin-2 receptor γ−/−
mice after PBMC plus Treg adoptive transfer. Data were acquired on
day 63 for Poly-Treg or day 84 for Xeno-Treg. Data are presented as
the means ± standard deviation of three independent experiments.
CD, cluster of differentiation; PBMC, peripheral blood mononuclear
cell; Poly-Treg, polyclonal Treg; Tregs, regulatory T cells;
Xeno-Treg, Treg with xenoantigen specificity. |
Human leukocyte engraftment was confirmed by flow
cytometry. Following human Poly-Treg and PBMC adoptive transfer,
the spleen and peripheral blood was engrafted with 27.3±6.3 and
14.7±4.2% of human CD45+ cells respectively, by day 63
(Fig. 8B). However, following
human Xeno-Treg and PBMC adoptive transfer, the spleen and
peripheral blood was engrafted with 15.2±3.8 and 10.5±3.2% of human
CD45+ cells respectively, by day 84 (Fig. 8B). Decreased engraftment of human
CD45+ cells in mice reconstituted with Xeno-Treg and
PBMCs may indicate that graft survival is as a result of
xenoantigen-specific Treg-mediated suppression and not due to
engraftment failure.
Immunohistochemistry of NICC grafts from
nonreconstituted recipients revealed intact insulin-positive cells
with no CD45+ cells infiltration (Fig. 9A-C). Numerous graft-infiltrating
human CD45+ cells were detected in the rejected
xenografts from PBMC-reconstituted mice; however, no
insulin-positive cells were visible (Fig. 9D-F). Long-term surviving grafts
from Xeno-Treg- and PBMC-reconstituted mice contained intact
insulin-secreting cells surrounded by a small number of human
CD45+ cells (Fig.
9G-I). On day 63, immunohistochemistry of NICC grafts from
Poly-Treg- and PBMC-reconstituted mice revealed small, damaged and
insulin-positive cells with numerous graft-infiltrating human
CD45+ cells (Fig.
9J-L). These results suggested that adoptive transfer of ex
vivo expanded Xeno-Treg may possess a greater capacity to
reduce xenograft damage and prevent rejection of porcine islet
xenografts compared with Poly-Treg.
 | Figure 9.Histology and immunohistochemical
analysis of NICC xenografts. Representative hematoxylin and eosin
staining images; and immunohistochemical staining images of porcine
insulin and human CD45 in NICC xenograft samples from mice
receiving (A-C) no human cells (NICC alone 84 days
post-transplantation), (D-F) only human PBMCs (NICC + PBMC 28 days
post-PBMC transfer), (G-I) human PBMCs and Xeno-Treg (NICC + PBMC +
Xeno-Treg 84 days post-cell transfer) or (J-L) human PBMCs and
Poly-Treg (NICC + PBMC + Poly-Treg 63 days post-cell transfer). (A,
B, E-I, K and L) Magnification, ×200; (C, D and J) magnification,
×100. CD, cluster of differentiation; NICC, neonatal porcine islet
cell clusters; PBMC, peripheral blood mononuclear cell; Poly-Treg,
polyclonal Treg; Tregs, regulatory T cells; Xeno-Treg, Treg with
xenoantigen specificity. |
Discussion
The use of efficient antigen-specific Tregs may
reduce the number of Tregs required for therapy and lower the risk
of systemic immunosuppression (25,26).
Numerous studies have investigated strategies for large-scale
expansion of alloantigen-specific human Tregs (27–29),
and ex vivo alloantigen-specific Tregs were shown to possess
enhanced suppressive capacity in allogeneic responses in
vitro and in vivo (30,31).
In the present study, a strategy using one cycle of polyclonal
stimulation followed by two subsequent cycles of xenoantigen
stimulation was developed to selectively expand Xeno-Treg.
Following stimulation, spectratyping was conducted to analyze the
TCR Vβ families of Tregs at the CDR3 level and screen for clonal
expansion of specific T cells. CDR3 spectratyping is a
well-described method for measuring oligoclonality within T cell
populations (32). Normal PBMC
samples of a single Vβ family display a Gaussian distribution of
6–11 peaks, each separated by three nucleotides. Each peak
corresponds to a TCR transcript with a given CDR3 length that may
contain numerous sequences (32).
The number of TCR transcripts with a specific CDR3 length is
proportional to the area under each peak. An increase in the height
and area of a size peak typically indicates oligoclonal or
monoclonal expansion in the polyclonal T cell background.
Oligoclonal T cells give fewer peaks in a restricted distribution.
Single clones give a single peak. This method has been used
previously to screen PCR products in an efficient manner for
possible T cell clonal expansion following MLR, renal biopsies and
urine at the time of rejection (22,33).
The present study identified an increase in the expression of TCR
Vβ4, TCR Vβ10, TCR Vβ18 and TCR Vβ20 families for Xeno-Treg
compared with Poly-Treg. In addition, spectratypes of TCR Vβ4,
Vβ10, Vβ18 and Vβ20 in Xeno-Treg demonstrated restriction and
expanded clone at size 205, 441, 332 and 196, respectively, which
indicated that Treg acquired xenoantigen specificity following
xenoantigen stimulation by identifying the specific expanded clones
in TCR Vβ families. Furthermore, Xeno-Treg were acquired and showed
enhanced suppressive capacity in the xenoimmune response detected
in a MLR.
The mechanisms underlying human xenoantigen-specific
Treg suppressive functions in vivo remain largely unknown.
Previous studies have revealed that IL-10 serves a critical role in
Treg-mediated suppression of xenogeneic responses in vivo
and in vitro (10,34,35).
In the present study, Xeno-Treg upregulated the expression of IL-10
and produced more IL-10, compared with Poly-Treg, in the culture
medium. Therefore, it may be hypothesized that the suppressive
functions of Xeno-Treg in the Xeno MLR assay are mediated by IL-10.
Furthermore, the results demonstrated that Xeno-Treg expressed
increased levels of HLA-DR, thus suggesting that IL-10 secretion of
Xeno-Treg was associated with the upregulated effector marker. Yi
et al demonstrated that adoptive transfer with expanded
autologous Tregs prevents islet xenograft rejection in human
PBMC-reconstituted mice, by inhibiting graft infiltration of
effector cells and their function via IL-10 (10). In the present study, NOD-SCID
IL2rγ−/− mice reconstituted with Xeno-Treg and PBMCs at
a Xeno-Treg:PBMC ratio of 1:10; 75% of NICC xenografts contained
intact insulin-secreting cells, which survived beyond 84 days
compared with the Poly-Treg- and PBMC-reconstituted group, in which
75% of NICC xenografts survived to day 48 and by day 63 all
xenografts showed small, damaged and insulin positive-staining
cells, with a large number of graft-infiltrating human
CD45+ cells. These findings suggested that adoptively
transferred ex vivo expanded Xeno-Treg may display a greater
capacity to reduce xenograft damage and prevent porcine islet
xenograft rejection compared with Poly-Treg. However, the
mechanisms of human xenoantigen-specific Treg suppressive function
in vivo remain largely unknown. Further studies are required
to explore whether IL-10 has an important role in human
xenoantigen-specific Treg suppressive function in vivo.
In conclusion, the present study demonstrated that
adoptive transfer with ex vivo expanded Xeno-Treg exhibited
a greater capacity to prevent islet xenograft rejection in
humanized NOD-SCID IL2rγ−/− mice compared with
Poly-Treg, thus suggesting a novel strategy for adoptive Treg cell
therapy for immunomodulation in islet xenotransplantation that may
minimize systemic immunosuppression.
Acknowledgements
The authors would like to thank Professor Shounan Yi
from the Centre for Transplant and Renal Research of Westmead
Millennium Institute in the University of Sydney for his expert
technical assistance.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81501602
and 81271712), the Science and Technology Project of the Health
Planning Committee of Sichuan (grant no. 17PJ483) and the Hunan
Provincial Natural Science Foundation of China (grant no.
14JJ2039).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XJ contributed to the design of the present study,
data analysis, and helped draft the manuscript. MH conducted the
TCR Vβ CDR3 spectratyping and analyzed the data. LG contributed to
the animal work and performed the immunohistochemistry. HFL and YW
performed the flow cytometry. MJ participated in the Treg
suppression assay and revised the article. HL helped to design the
present study and with the data analysis, and participated in
reviewing and revising the article.
Ethics approval and consent to
participate
All human and animal studies were approved by the
Sichuan University Medical Ethics Committee and Animal research
Ethics communities. All donors provided informed consent.
Patient consent for publication
Written informed consent was obtained from all
donors.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vadori M and Cozzi E: The immunological
barriers to xenotransplantation. Tissue Antigens. 86:239–253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butler JR, Wang ZY, Martens GR, Ladowski
JM, Li P, Tector M and Tector AJ: Modified glycan models of
pig-to-human xenotransplantation do not enhance the human-anti-pig
T cell response. Transpl Immunol. 35:47–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin JS, Kim JM, Min BH, Yoon IH, Kim HJ,
Kim JS, Kim YH, Kang SJ, Kim J, Kang HJ, et al: Pre-clinical
results in pig-to-non-human primate islet xenotransplantation using
anti-CD40 antibody (2C10R4)-based immunosuppression.
Xenotransplantation. 25:2018. View Article : Google Scholar
|
|
4
|
Lee JI, Kim J, Choi YJ, Park HJ, Park HJ,
Wi HJ, Yoon S, Shin JS, Park JK, Jung KC, et al: The effect of
epitope-based ligation of ICAM-1 on survival and retransplantation
of pig islets in nonhuman primates. Xenotransplantation. 25:2018.
View Article : Google Scholar
|
|
5
|
Qiu F, Liu H, Liang CL, Nie GD and Dai Z:
A new immunosuppressive molecule emodin induces both
CD4+FoxP3+ and
CD8+CD122+ regulatory T cells and suppresses
murine allograft rejection. Front Immunol. 8:15192017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garakani R and Saidi RF: Recent progress
in cell therapy in solid organ transplantation. Int J Organ
Transplant Med. 8:125–131. 2017.PubMed/NCBI
|
|
7
|
Marek-Trzonkowska N, Myśliwiec M,
Iwaszkiewicz-Grześ D, Gliwiński M, Derkowska I, Żalińska M,
Zieliński M, Grabowska M, Zielińska H, Piekarska K, et al: Factors
affecting long-term efficacy of T regulatory cell-based therapy in
type 1 diabetes. J Transl Med. 14:3322016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kasper IR, Apostolidis SA, Sharabi A and
Tsokos GC: Empowering regulatory T cells in autoimmunity. Trends
Mol Med. 22:784–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu L, Barbi J and Pan F: The regulation of
immune tolerance by FOXP3. Nat Rev Immunol. 17:703–717. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi S, Ji M, Wu J, Ma X, Phillips P,
Hawthorne WJ and O'Connell PJ: Adoptive transfer with in vitro
expanded human regulatory T cells protects against porcine islet
xenograft rejection via interleukin-10 in humanized mice. Diabetes.
61:1180–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin X, Wang Y, Hawthorne WJ, Hu M, Yi S
and O'Connell P: Enhanced suppression of the xenogeneic T-cell
response in vitro by xenoantigen stimulated and expanded regulatory
T cells. Transplantation. 97:30–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dawson NAJ, Vent-Schmidt J and Levings MK:
Engineered tolerance: Tailoring development, function, and
antigen-specificity of regulatory T cells. Front Immunol.
8:14602017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore C, Tejon G, Fuentes C, Hidalgo Y,
Bono MR, Maldonado P, Fernandez R, Wood KJ, Fierro JA, Rosemblatt
M, et al: Alloreactive regulatory T cells generated with retinoic
acid prevent skin allograft rejection. Eur J Immunol. 45:452–463.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sagoo P, Lombardi G and Lechler RI:
Relevance of regulatory T cell promotion of donor-specific
tolerance in solid organ transplantation. Front Immunol. 3:1842012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
The OLAW office of NIH, . Institutional
animal care and use committee guidebook. 2nd. 2002
|
|
16
|
Korbutt GS, Elliott JF, Ao Z, Smith DK,
Warnock GL and Rajotte RV: Large scale isolation, growth, and
function of porcine neonatal islet cells. J Clin Invest.
97:2119–2129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walters G and Alexander SI: T cell
receptor BV repertoires using real time PCR: A comparison of SYBR
green and a dual-labelled HuTrec fluorescent probe. J Immunol
Methods. 294:43–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi S, Hawthorne WJ, Lehnert AM, Ha H, Wong
JK, van Rooijen N, Davey K, Patel AT, Walters SN, Chandra A and
O'Connell PJ: T cell-activated macrophages are capable of both
recognition and rejection of pancreatic islet xenografts. J
Immunol. 170:2750–2758. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baecher-Allan C, Wolf E and Hafler DA: MHC
class II expression identifies functionally distinct human
regulatory T cells. J Immunol. 176:4622–4631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fountoulakis S, Vartholomatos G, Kolaitis
N, Frillingos S, Philippou G and Tsatsoulis A: HLA-DR expressing
peripheral T regulatory cells in newly diagnosed patients with
different forms of autoimmune thyroid disease. Thyroid.
18:1195–1200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gagne K, Brouard S, Giral M, Sebille F,
Moreau A, Guillet M, Bignon JD, Imbert BM, Cuturi MC and Soulillou
JP: Highly altered V beta repertoire of T cells infiltrating
long-term rejected kidney allografts. J Immunol. 164:1553–1563.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baron C, McMorrow I, Sachs DH and LeGuern
C: Persistence of dominant T cell clones in accepted solid organ
transplants. J Immunol. 167:4154–4160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walters G, Habib AM, Reynolds J, Wu H,
Knight JF and Pusey CD: Glomerular T cells are of restricted
clonality and express multiple CDR3 motifs across different Vbeta
T-cell receptor families in experimental autoimmune
glomerulonephritis. Nephron Exp Nephrol. 98:e71–e81. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adair PR, Kim YC, Zhang AH, Yoon J and
Scott DW: Human tregs made antigen specific by gene modification:
The power to treat autoimmunity and antidrug antibodies with
precision. Front Immunol. 8:11172017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma B, Yang JY, Song WJ, Ding R, Zhang ZC,
Ji HC, Zhang X, Wang JL, Yang XS, Tao KS, et al: Combining exosomes
derived from immature DCs with donor antigen-specific treg cells
induces tolerance in a rat liver allograft model. Sci Rep.
6:329712016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng J, Liu Y, Qin G, Chan PL, Mao H, Lam
KT, Lewis DB, Lau YL and Tu W: Efficient induction and expansion of
human alloantigen-specific CD8 regulatory T cells from naive
precursors by CD40-activated B cells. J Immunol. 183:3742–3750.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Veerapathran A, Pidala J, Beato F, Yu XZ
and Anasetti C: Ex vivo expansion of human Tregs specific for
alloantigens presented directly or indirectly. Blood.
118:5671–5680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheraï M, Hamel Y, Baillou C, Touil S,
Guillot-Delost M, Charlotte F, Kossir L, Simonin G, Maury S, Cohen
JL and Lemoine FM: Generation of human alloantigen-specific
regulatory T cells under good manufacturing practice-compliant
conditions for cell therapy. Cell Transplant. 24:2527–2540. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peters JH, Hilbrands LB, Koenen HJ and
Joosten I: Ex vivo generation of human alloantigen-specific
regulatory T cells from CD4(pos)CD25(high) T cells for
immunotherapy. PLoS One. 3:e22332008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boardman DA, Philippeos C, Fruhwirth GO,
Ibrahim MA, Hannen RF, Cooper D, Marelli-Berg FM, Watt FM, Lechler
RI, Maher J, et al: Expression of a chimeric antigen receptor
specific for donor HLA class I enhances the potency of human
regulatory T cells in preventing human skin transplant rejection.
Am J Transplant. 17:931–943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fozza C, Barraqueddu F, Corda G, Contini
S, Virdis P, Dore F, Bonfigli S and Longinotti M: Study of the
T-cell receptor repertoire by CDR3 spectratyping. J Immunol
Methods. 440:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alvarez CM, Opelz G, Giraldo MC, Pelzl S,
Renner F, Weimer R, Schmidt J, Arbeláez M, García LF and Süsal C:
Evaluation of T-cell receptor repertoires in patients with
long-term renal allograft survival. Am J Transplant. 5:746–756.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li M, Eckl J, Abicht JM, Mayr T, Reichart
B, Schendel DJ and Pohla H: Induction of porcine-specific
regulatory T cells with high specificity and expression of IL-10
and TGF-β1 using baboon-derived tolerogenic dendritic cells.
Xenotransplantation. 25:2018. View Article : Google Scholar
|
|
35
|
Li M, Eckl J, Geiger C, Schendel DJ and
Pohla H: A novel and effective method to generate human
porcine-specific regulatory T cells with high expression of IL-10,
TGF-β1 and IL-35. Sci Rep. 7:39742017. View Article : Google Scholar : PubMed/NCBI
|