Introduction
Aconitum plants including Chuan-wu (Crude Radix
Aconiti) which have analgesic, diuretic and anti-inflammatory
effects, have been widely used to treat various diseases, including
chronic neuralgia, rheumatoid arthritis, acute myocardial
infarction, coronary heart disease and angina pectoris in China for
thousands of years (1,2). Thus, numerous herbal medicines have
been formulated containing aconitum plant extracts as the main
ingredient. However, the apparent toxicity of these compounds has
severely limited their clinical use (3,4). The
toxic effects predominantly involve the central nervous and
cardiovascular systems, particularly the cardiovascular system
where aconitum plant extracts can cause polymorphous arrhythmias.
Patients often succumb from arrhythmia and respiratory center
paralysis. However, little information is available regarding the
mechanisms of aconite poisoning.
The exact mechanisms involved in aconite-induced
cellular damage are extremely complex and have not been fully
elucidated. Aconitine (AC), a major bioactive diterpenoid alkaloid
derived from aconitum plants, stimulates the majority of the
cardiotoxic effects of aconitum plants (5). A previous study has shown that AC can
increase the excitability of ectopic rhythms and lead to
tachyarrhythmias (6). At the
cellular level, AC has been shown to bind to voltage-gated
Na+ channels and prolong their open state, favoring the
entry of a large quantity of Na+ into the cytosol, which
is accompanied by Ca2+ overload via sequential
activation of electrogenic Na+-Ca2+
exchangers or L-type Ca2+ channels, eventually inducing
delayed afterdepolarization (DAD) and triggered activity (TA)
(7,8). Additionally, L-type Ca2+
channel inhibition contributes to the arrhythmic effects of AC in
human cardiomyocytes (9).
Therefore, it is important to identify more effective strategies
against AC-induced cardiovascular complications, and search for
therapeutic targets that could counteract the myocardial toxicity
induced by AC.
Berberine (Ber), one of the main alkaloids extracted
from Rhizoma coptidis, has been extensively used to treat
various parasitic and fungal infections, and has a long history of
use for treating diarrhea in traditional Chinese medicine (10). Additionally, it also exhibits
positive inotropic, negative chronotropic and anti-arrhythmic
properties (11,12). It has been reported that Ber
improves cardiac function in patients with severe congestive heart
failure (13). Ber is extracted
from Rhizoma coptidis, and the latter has also been used to
treat diabetes mellitus in China for >1,400 years (14). Additionally, it has also been
demonstrated that Ber alleviates the acute cardiotoxicity and
hepatorenal toxicity resulting from doxorubicin treatment in rats
(15,16). However, to the best of our
knowledge, no study to date has been performed concerning the
protective effects of Ber on aconite-induced myocardial toxicity.
Therefore, the present study was designed to investigate whether
Ber has an antagonistic effect on myocardial damage and the
associated arrhythmias caused by AC, and to elucidate the potential
mechanisms of action in isolated guinea pig papillary muscle.
Materials and methods
Drugs and reagents
Chuan-wu was obtained from the Anguo medicine market
(Shijiazhuang, China). AC was obtained from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Ber was obtained from Acros Organics
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Pentobarbital
was obtained from Sigma-Aldrich (Merck KGaA). Creatine kinase (CK)
and CK-MB isoenzyme diagnostic kits were all from Sysmex
Corporation (Kobe, Japan). Lactate dehydrogenase (LDH) assay kit
was obtained from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China).
Animals
Sprague Dawley (SD) rats and guinea pigs were
obtained from the Experimental Animal Center of Hebei Medical
University [license no. SCXK (Hebei) 2013-003]. All experiments
were approved by the Ethics Committee for the Use of Experimental
Animals at Hebei Medical University (Shijiazhuang, Hebei,
China).
Experimental protocol of Chuan-wu
induced cardiac injury
Male SD rats (8-week-old; certificate no. 1109105),
weighing 250–300 g, were housed under controlled atmosphere (12-h
light/dark cycle, relative humidity 50–60%, and 24±3°C) and food
and water were provided ad libitum. Rats (n=40) were
randomly divided into Control group (A), AC group (B), AC + Ber (8
mg/kg/d) group (C), and AC + Ber (16 mg/kg/d) group (D). Ber was
administered intragastrically once a day for 7 consecutive days in
groups C and D. Group A and B rats were administrated with an equal
volume of distilled water. Chuan-wu (0.08 mg/kg) was administrated
from day 4 during the Ber administration period by dissolving the
Chuan-wu powder into distilled water; this was performed once a day
for 3 days, while an equal volume of distilled water was given to
the control group.
Electrocardiogram (ECG) analysis
On days 1 and 3 following Chuan-wu administration,
30 min following Ber administration, the rats were anesthetized
with 45 mg/kg pentobarbital sodium via an intraperitoneal injection
(i.p.). The electrodes were inserted into the subcutaneous tissue
of the rat limb, and the ECG parameters (day 1) and the different
types of arrhythmia (day 3) were recorded in each group using an RM
6240CD multi-channel physiological signal acquisition and
processing system (Chengdu Instrument Factory, Chengdu, China) via
standard limb lead II.
Determination of serum biochemical
indicators
On day 3 following Chuan-wu administration, blood
samples were collected from the abdominal aorta and stored on ice
prior to centrifugation at 2,100 × g for 10 min within 1 h of
collection. Subsequently, the supernatant was used for the
determination of biochemical indicators. The levels of LDH, CK and
CK-MB in the serum were assayed as sensitive indicators of
myocardial damage, according to the manufacturer's protocol of the
diagnostic kits (cat. nos., LDH, R1 1000331, R2 1000312; CK, R1
1000531, R2 1000512; CK-MB R1 1001611 and R2 1001612; Sysmex
Corporation) using a CHEMIX-180 automatic biochemistry analyzer
(Sysmex Corporation).
Histopathological analysis
At day 3 after Chuan-wu administration, the heart
was rapidly removed and rinsed with ice-cold saline following the
collection of blood samples. Heart samples were fixed in 4%
formaldehyde immediately following excision for 7 days at room
temperature, then dehydrated in an ascending series of ethanol (70,
80, 96 and 100%). Following paraffin embedding, transverse slides
(5 µm thickness) were stained with hematoxylin and eosin at room
temperature for 2 min respectively, and histological examination
was conducted using a light microscope (Olympus BX-50; Olympus
Corporation, Tokyo, Japan).
Effects of Ber on AC-induced
arrhythmias in rats
Male SD rats (n=40, certificate no.1107071),
weighing 250–300 g were randomly divided into 4 groups: Control
group (A), AC group (B), AC + Ber (8 mg/kg/d) group (C), and AC +
Ber (16 mg/kg/d) group (D). Ber was administered intragastrically
once a day for 7 consecutive days in groups C and D. Rats in groups
A and B received an equal volume of distilled water. On day 7, 30
min following Ber administration, the rats were anesthetized with
45 mg/kg pentobarbital sodium (i.p.). The surface lead II ECG was
recorded continuously with three subcutaneous limb electrodes using
the RM 6240CD multi-channel physiological signal acquisition and
processing system. The femoral vein was cannulated for
administration of AC (10 µg/ml) at a rate of 0.08 ml/min in groups
B, C and D, while an equal volume of normal saline was given to
group A. The time onset of the first ventricular premature
contraction discrete (VPCD), ventricular premature contraction
bigeminy (VPB), successive ventricular tachycardia (VT),
ventricular fibrillation (VF) and mortality (DD) was recorded, and
the amount of AC required to induce arrhythmia was calculated.
Effects of Ber and AC on papillary
muscle action potential
Male guinea pigs (4–5 weeks of age, n=6), weighing
200–250 g, were housed under controlled atmosphere (12-h light/dark
cycle, relative humidity 50~60% and 22±2°C), and food and water
were provided ad libitum. The animals were anesthetized with
40 mg/kg pentobarbital sodium (i.p), then the heart was rapidly
removed and put into a preparation dish containing ice-cold K-H
solution (140 mmol/l NaCl, 5.4 mmol/l KCl, 1 mmol/l
MgCl2, 2 mmol/l CaCl2, 10 mmol/l HEPES and 10
mmol/l glucose). The right ventricle was opened and columnar
papillary muscles were removed. The papillary muscles were then
transferred into the tissue chamber and perfused with K-H solution,
which was continually gassed with 100% O2. The
temperature was maintained at 36°C. A conventional microelectrode
recording technique was used to record the action potential of the
papillary muscle of guinea pigs under the stimulation frequency of
1 Hz. The following action potential parameters were calculated:
Action potential amplitude (APA), duration of 30% repolarization
(APD30) and duration of 90% repolarization
(APD90). The hearts were divided into two groups (n=3 in
each group): Control and Ber treatment groups. Once a normal action
potential was continuously recorded in the control group, Tyrode's
solution containing AC (3×10−7 mol/l) was perfused, and
DAD and TA by AC were observed. While in the Ber treatment group,
once a normal action potential was continuously recorded, solution
containing Ber (1×10−5 mol/l) was perfused. Following 5
min, the action potential was recorded. Then, AC (3×10−7
mol/l) was perfused, and the changes in action potential following
AC perfusion were recorded. If no change appeared, 15 min later,
1×10−6 mol/l AC was added to observe the change in the
action potential.
Statistical analysis
All values are presented as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance and Dunnett's test and SPSS v11.0 software
(SPSS, Inc., Chicago, IL, USA). Comparisons of the same parameters
prior to and following administration were performed using a paired
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of Ber on ECG in rats with
Chuan-wu-induced cardiac injury
During the experiments, no animals succumbed in the
control or Ber 16 mg/kg treatment groups; whereas, 3 animals in the
Chuan-wu group and 1 animal in the Ber 8 mg/kg group perished.
On day 1 following Chuan-wu administration, the
analysis of ECG parameters revealed that the heart rate was
decreased (P<0.05) and the P-R, QRS and QT intervals were all
prolonged in the Chuan-wu group (P<0.05 or P<0.01), compared
with that observed in control group. The effects of Chuan-wu on
heart rate, P-R, QRS and QT intervals in the 8 and 16 mg/kg Ber
treatment groups were all reversed by different degrees when
compared with the Chuan-wu group (P<0.05 or P<0.01; Fig. 1). On day 3 following Chuan-wu
administration, there was no ECG abnormality in the control group.
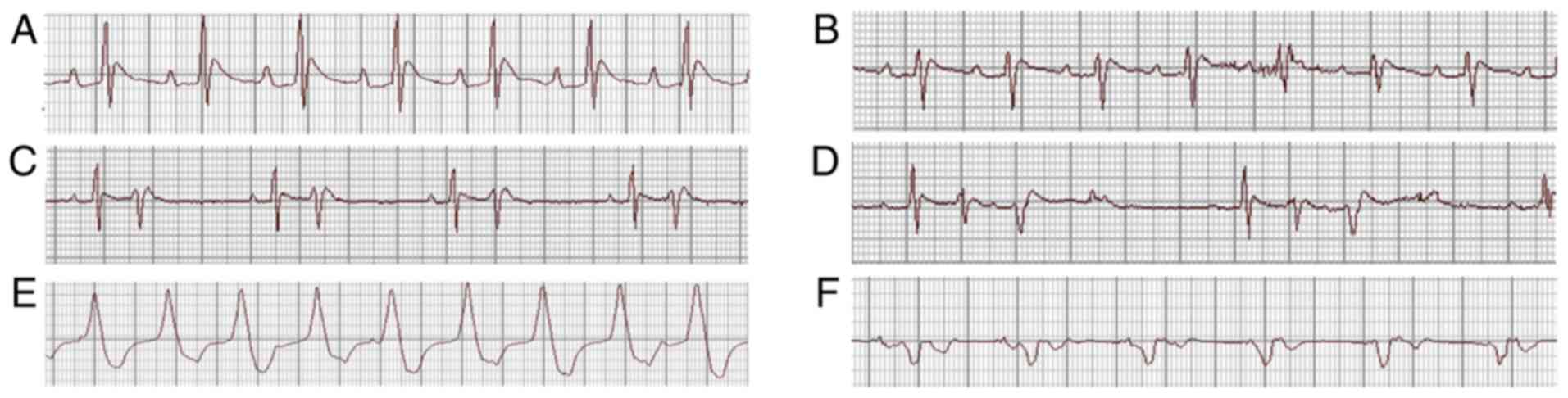
However, in the Chuan-wu group, ventricular premature beat,
bigeminies or trigeminies were observed in 3 rats, typical
ventricular tachycardia was observed in 4 rats, and 3 rats
exhibited ventricular tachycardia and then succumbed following
ventricular fibrillation. Additionally, in the Ber 8 mg/kg group, 4
rats exhibited ventricular premature beat-bigeminies, 3 rats
presented typical ventricular tachycardia, and 1 rat perished from
ventricular fibrillation. In the Ber 16 mg/kg group, 2 rats
exhibited ventricular premature beat, bigeminies or trigeminies,
and 1 rat presented with typical ventricular tachycardia, with no
evident abnormalities. Typical arrhythmias are presented in
Fig. 2. The summaries of the
arrhythmias and mortalities in each group are presented in Table I.
 | Table I.Influence of Berberine on the
occurrence rate of arrhythmias and mortality rate on day 3
following Chuan-wu administration in rats. |
Table I.
Influence of Berberine on the
occurrence rate of arrhythmias and mortality rate on day 3
following Chuan-wu administration in rats.
| Group | Normal (no. of
cases) | VPC (no. of
cases) | VT (no. of
cases) | Mortalities (no. of
cases) |
|---|
| Control | 10 | 00 | 00 | 00 |
| Chuan-wu | 0 | 33 | 4 | 3 |
| Ber 8 mg/kg | 2 | 4 | 3 | 1 |
| Ber 16 mg/kg | 7 | 2 | 1 | 0 |
Effects of Ber on cardiac function
indexes in rats
Compared with the control group, the levels of serum
CK, CK-MB and LDH were significantly increased in the Chuan-wu
group (B; P<0.05 or P<0.01). Whereas, in the 8 and 16 mg/kg
Ber treatment groups (C and D), the levels of serum CK, CK-MB and
LDH were all markedly reduced when compared with the Chuan-wu
group, particularly in the Ber 16 mg/kg group (P<0.05 or
P<0.01; Fig. 3).
Effects of Ber on the heart
histopathology of rats with Chuan-wu induced cardiac injury
Histopathological examination of the heart tissue
revealed that there were no evident abnormalities in the myocardium
of the control group (Fig. 4A).
Whereas, following Chuan-wu administration for 3 days, small vein
dilation, congestion, ventricular dilatation and congestion were
observed in myocardial tissue. Additionally, some muscle fibers
exhibited mild atrophy and inflammatory cell infiltration was
observed in the muscle. The representative images of congestion in
veins and slight inflammatory cell infiltration are shown in
Fig. 4B. Compared with the
Chuan-wu group, the congestion was reduced in the Ber 8 and 16
mg/kg groups. Notably, in the Ber 16 mg/kg group, no marked
congestion was observed and only minor infiltration of inflammatory
cells in myocardial tissues was exhibited (Fig. 4C).
Effects of Ber on AC-induced
arrhythmias in rats
In the control group, an ECG was recorded
continuously for 120 min with no evident abnormalities observed. In
all of the other groups, no abnormal ECG features were observed
prior to the administration of AC. However, in the AC group, VPCD,
VPB, VT, VF and DD occurred successively following the
administration of AC. Compared with the AC group, the occurrence
time of VT, VF and DD was significantly prolonged in the Ber 8
mg/kg pretreatment group (P<0.05). Ber 16 mg/kg pretreatment
also significantly prolonged the occurrence of all types of
arrhythmias mentioned above (P<0.05 or P<0.01; Fig. 5A). Therefore, pretreatment with Ber
8 and 16 mg/kg increased the dosage of AC required to induce
arrhythmias (P<0.05 or P<0.01; Fig. 5B).
Effects of Ber and AC on papillary
muscle action potential
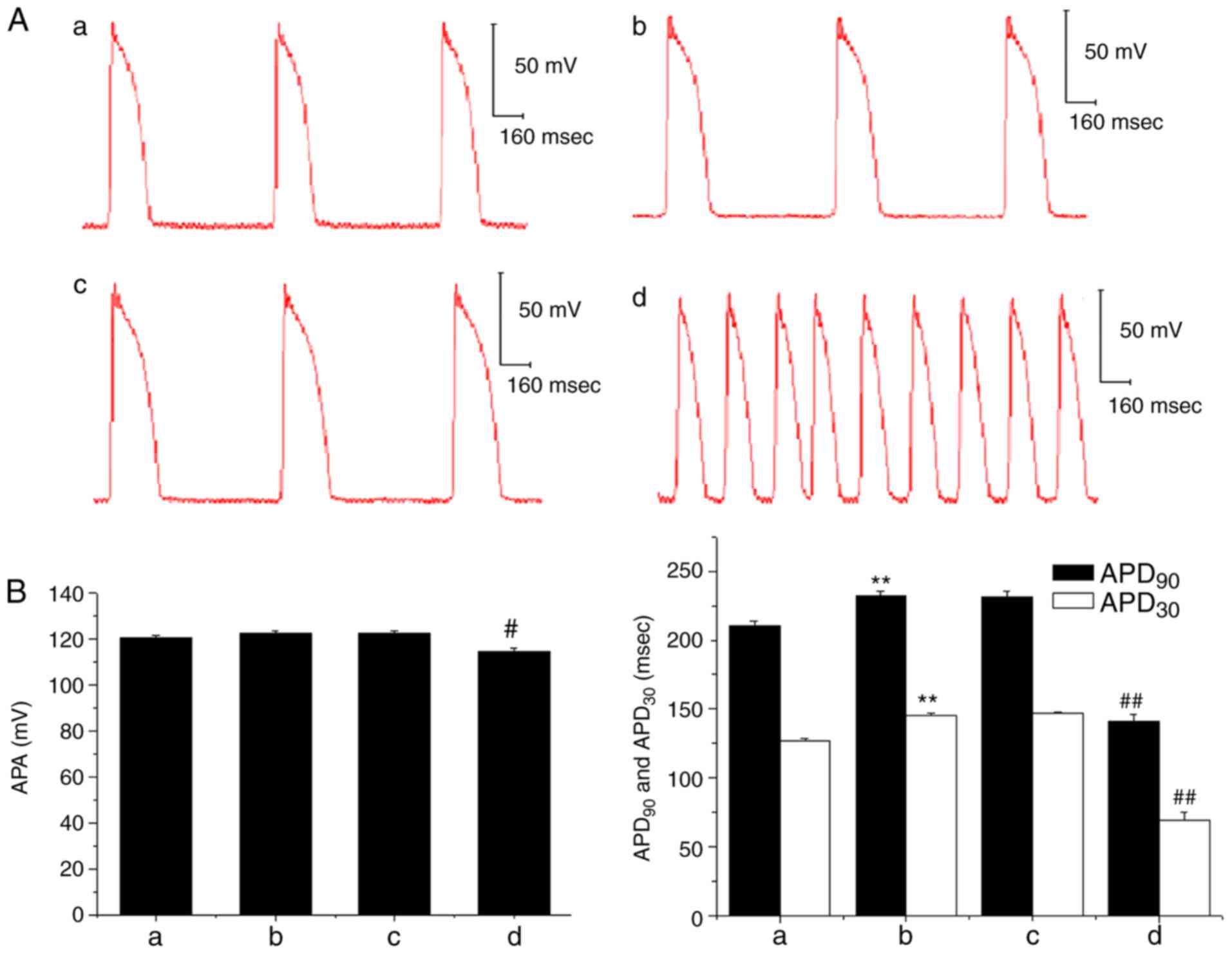
In the ex vivo experiment, the APA of the
papillary muscle action potential was decreased following the
administration of 3×10−7 mol/l AC, and the
APD30 and APD90 were also significantly
shortened when compared with prior to AC treatment; DAD and TA was
also subsequently observed (Fig.
6). However, when Ber 1×10−5 mol/l
pre-administration was performed, there were no marked changes in
APA, APD30 and APD90, and no DAD occurred
following AC perfusion. Subsequently, when the concentration of AC
was increased to 1×10−6 mol/l, a decrease in APA and a
shortening in APD90 and APD30 were induced
(P<0.01); and DAD and TA were also observed (Fig. 7).
Discussion
In recent years, increasing attention has been paid
to the study of toxicity-reducing effects that can be achieved by
co-administration of Chinese herbal medicines. Aconitum drugs have
served an important role in the treatment of acute myocardial
infarction, coronary heart disease and angina pectoris (1,2).
However, the therapeutic and toxic doses of this type of medicine
are very close, which can easily lead to damaging effects in
patients (17). Arrhythmias are a
major side-effect of Aconitum drugs (18).
Berberine hydrochloride, also known as Ber, is the
main active ingredient of Rhizoma coptidis. Previous studies
have revealed that Ber has an antagonistic effect on
isoproterenol-induced myocardial damage and the ischemic arrhythmia
caused by ligation of the anterior descending branch of the left
coronary artery in rat models (19,20).
In the present study, the antagonistic effects of Ber on
Chuan-wu-induced acute myocardial injury were observed in rats. The
results revealed that ventricular arrhythmias, including premature
ventricular bigeminy, trigeminy or ventricular tachycardia, were
all observed in rats following treatment with Chuan-wu for 3 days.
In addition, the serum levels of CK, CK-MB and LDH, which are
indicators of cardiac function, were all increased following
Chuan-wu administration. The serum levels of CK, CK-MB and LDH are
quantitative indicators of loss of membrane integrity during
myocardial damage (21). The
increase in CK, CK-MB and LDH suggested that Chuan-wu can affect
the integrity of myocardial cell membranes. The histological
findings of the myocardium also revealed dilation of small veins
and the ventricular lumen, congestion of the heart cavity and some
atrophy of muscle fibers appeared following the administration of
Chuan-wu for 3 days, which is consistent with the literature
(22,23). The results suggested that Chuan-wu
disrupts the integrity of myocardial cell membranes and causes cell
damage. Ber significantly reduced the degree of arrhythmia induced
by Chuan-wu, and improved the survival rate of rats. Analysis of
myocardial enzymes and histopathological examination demonstrated
marked improvements in Ber pretreatment groups. This result
indicated that Ber may have clear beneficial effects against
Chuan-wu-induced myocardial injury; however, this requires further
confirmation.
The toxic components of Chuan-wu include AC and
mesaconitine. AC can stimulate the vagus nerve, and inhibit the
sinoatrial node and conduction system, resulting in a slowed heart
rate and conduction block (24,25).
In addition, AC can act directly on myocardial cells, promoting the
opening of Na+ channels, accelerating the ion influx,
inducing cell membrane depolarization and improving the
automaticity of fast response cells, including atrioventricular
bundles and Purkinje fibers, thereby causing one-source or
multifocal ectopic rhythms (26,27).
It was also demonstrated that AC significantly increased
Ca2+ influx, inhibited outward K+ currents,
prolonged repolarization, and produced DAD and re-entry, resulting
in arrhythmias (28). Therefore,
the arrhythmia caused by Chuan-wu in rats may be associated with
the presence of AC-type alkaloids.
As AC is a common arrhythmogenic toxin within
Aconitum drugs, the present study investigated whether Ber has
antagonistic effects on AC-induced arrhythmia. The in vivo
ECG recording experiments demonstrated that AC administration
decreased the heart rate, and prolonged the S-T and Q-T intervals,
which lead to ventricular premature beat, coupled rhythm, trigeminy
and tachycardia; these ultimately induced ventricular fibrillation
and rat mortality. These observations are consistent with the
literature (6). Pre-administration
of Ber effectively delayed the onset of ventricular premature beat
and coupled rhythm, and in turn prolonged the survival time of
rats.
Considering guinea pig papillary muscle is a
suitable preparation for the assessment of action potential in
drug-induced Torsades De Pointes liability in humans (29), the present study observed the
effects of Ber and AC on action potentials to investigate the
mechanism of the anti-arrhythmic effects of Ber with papillary
muscle in guinea pigs. The results revealed that AC decreased the
APA of papillary muscle action potentials, shortened the
APD90 and APD30, and DAD and TA were
observed. However, Ber pretreatment completely inhibited the DAD
induced by the same concentration of AC. However, when the dose of
AC was increased, DAD and TA still occurred, even when Ber was
pre-administered. From these results it was concluded that Ber may
competitively counteract DAD and TA induced by AC. At the molecular
level, it is generally believed that AC can accelerate the influx
of Na+ ions into cardiomyocytes, promote the
depolarization of the cell membrane and accelerate the automaticity
of the pacemaker (30). It has
also been reported that the cardiac toxicity of AC is associated
with an increase in free radicals caused by oxidative stress
(8). Additionally, Ca2+
overload and apoptosis via p38 signaling were also involved in the
cardiotoxicity of AC (31).
Therefore, AC-induced arrhythmias may be the result of a
combination of multiple mechanisms. Modulation of apoptosis or cell
signaling may also be involved in the underlying mechanisms of
Ber's protective effects; the complete molecular mechanisms still
require validation in further experiments. Therefore, a lack of a
more thorough mechanistic analysis of the underlying pathway was a
limitation of the present study.
In conclusion, Ber had preventive effects on
Chuan-wu-induced acute myocardial injury, alleviated the symptoms
of arrhythmia and improved the animal survival rate. Additionally,
Ber also exhibited marked antagonistic effects on AC-induced
arrhythmias, which may be associated with the inhibition of DAD and
TA induced by AC. Thus, combination treatment of Ber with Aconitum
plants is a novel strategy that has the potential for protection
against Aconite-induced myocardial injury in clinical practice.
Acknowledgements
We would like to thank Professor Yongli Wang,
Professor Hailin Zhang and Professor Yanfang Xu (all from the
Department of Pharmacology, Hebei Medical University) for providing
general support.
Funding
The present study was supported by the Natural
Science Foundation of China (grant nos. 81773828, 81273600 and
81473292) and the Natural Science Foundation of Hebei Province
(grant nos. H2018206297, C2011206145 and H2013206147).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Study design: SS and QM; study conduct and data
collection: HG, QL, KX, XC, YZ, XZ and QM; data analysis: QL, XC,
ZZ and HL; manuscript writing: XC, QL and SS; manuscript review and
editing: SS, QM and XC. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee for the Use of Experimental Animals at Hebei Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou G, Tang L, Zhou X, Wang T, Kou Z and
Wang Z: A review on phytochemistry and pharmacological activities
of the processed lateral root of Aconitum carmichaelii Debeaux. J
Ethnopharmacol. 160:173–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin CC, Phua DH, Deng JF and Yang CC:
Aconitine intoxication mimicking acute myocardial infarction. Hum
Exp Toxicol. 30:782–785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan TY: Aconitum alkaloid content and the
high toxicity of aconite tincture. Forensic Sci Int. 222:1–3. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan TY: Incidence and causes of aconitum
alkaloid poisoning in Hong Kong from 1989 to 2010. Phytother Res.
29:1107–1111. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheth S, Tan EC, Tan HH and Tay L:
Herb-induced cardiotoxicity from accidental aconitine overdose.
Singapore Med J. 56:e116–e119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu M, Dong YH, Han F, Qin JM, Zhang HN,
Du JX, Hao XM and Yang YM: Influence of total flavonoids derived
from Choerospondias axillaris folium on aconitine-induced
antiarrhythmic action and hemodynamics in Wistar rats. J Toxicol
Environ Health A. 79:878–883. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou YH, Piao XM, Liu X, Liang HH, Wang
LM, Xiong XH, Wang L, Lu YJ and Shan HL: Arrhythmogenesis toxicity
of aconitine is related to intracellular ca(2+) signals. Int J Med
Sci. 10:1242–1249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang YJ, Chen BS, Lin MW, Lin AA, Peng H,
Sung RJ and Wu SN: Time-dependent block of ultrarapid-delayed
rectifier K+ currents by aconitine, a potent cardiotoxin, in
heart-derived H9c2 myoblasts and in neonatal rat ventricular
myocytes. Toxicol Sci. 106:454–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu J, Wang X, Chung YY, Koh CH, Liu Z, Guo
H, Yuan Q, Wang C, Su S and Wei H: L-type calcium channel
inhibition contributes to the proarrhythmic effects of aconitine in
human cardiomyocytes. PLoS One. 12:e01684352017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Derosa G, Maffioli P and Cicero AF:
Berberine on metabolic and cardiovascular risk factors: An analysis
from preclinical evidences to clinical trials. Expert Opin Biol
Ther. 12:1113–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lau CW, Yao XQ, Chen ZY, Ko WH and Huang
Y: Cardiovascular actions of berberine. Cardiovasc Drug Rev.
19:234–244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahmoudvand H, Ayatollahi Mousavi SA,
Sepahvand A, Sharififar F, Ezatpour B, Gorohi F, Saedi Dezaki E and
Jahanbakhsh S: Antifungal, antileishmanial and cytotoxicity
activities of various extracts of berberis vulgaris (Berberidaceae)
and its active principle berberine. ISRN Pharmacol.
2014:6024362014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Pan Y, Kan M, Xiao X, Wang Y, Guan
F, Zhang X and Chen L: Hepatoprotective effects of berberine on
liver fibrosis via activation of AMP-activated protein kinase. Life
Sci. 98:24–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren LM, Zhuo YJ, Hao ZS, He HM, Lu HG and
Zhao D: Berberine improves neurogenic contractile response of
bladder detrusor muscle in streptozotocin-induced diabetic rats. J
Ethnopharmacol. 150:1128–1136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong C, Wu YZ, Zhang Y, Wu ZX, Chen XY,
Jiang P, Guo HC, Xie KR, Wang KX and Su SW: Protective effect of
berberine on acute cardiomyopathy associated with
doxorubicin-treatment. Oncol Lett. 15:5721–5729. 2018.PubMed/NCBI
|
|
16
|
Chen X, Zhang Y, Zhu Z, Liu H, Guo H,
Xiong C, Xie K, Zhang X and Su S: Protective effect of berberine on
doxorubicin-induced acute hepatorenal toxicity in rats. Mol Med
Rep. 13:3953–3960. 2016 May; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kasahara Y, Itou T, Numazawa T and Wada A:
Aconitine analogues in wild Aconitum plants: Contents toxicity to
mice and decrease by boiling. Shokuhin Eiseigaku Zasshi.
54:364–369. 2013.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu L, Wu J, Zhao M, Song W, Qi X, Wang Y,
Lu L and Liu Z: Mdr1a plays a crucial role in regulating the
analgesic effect and toxicity of aconitine by altering its
pharmacokinetic characteristics. Toxicol Appl Pharmacol. 320:32–39.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang T, Yang S and Du J: Protective
effects of berberine on isoproterenol-induced acute myocardial
ischemia in rats through regulating HMGB1-TLR4 axis. Evid Based
Complement Alternat Med. 2014:8497832014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang LH, Li XL, Li Q, Fu Y, Yu HJ, Sun YQ,
Zhang L and Shan HL: Berberine alleviates ischemic arrhythmias via
recovering depressed I(to) and I(Ca) currents in diabetic rats.
Phytomedicine. 19:206–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gautam PL, Luthra N, Kaur M, Singh J,
Wander GS, Tandon R and Gautam N: Evaluation of myocardial injury
using standard diagnostic tools and tissue doppler imaging in blunt
trauma chest. J Clin Diagn Res. 11:OC33–OC36. 2017.PubMed/NCBI
|
|
22
|
Ge YB, Jiang Y, Zhou H, Zheng M, Li J,
Huang XJ and Gao Y: Antitoxic effect of Veratrilla baillonii on the
acute toxicity in mice induced by Aconitum brachypodum, one of the
genus Aconitum. J Ethnopharmacol. 179:27–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai Y, Gao Y, Tan G, Wu S, Dong X, Lou Z,
Zhu Z and Chai Y: Myocardial lipidomics profiling delineate the
toxicity of traditional Chinese medicine Aconiti Lateralis radix
praeparata. J Ethnopharmacol. 147:349–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ono T, Hayashida M, Tezuka A, Hayakawa H
and Ohno Y: Antagonistic effects of tetrodotoxin on
aconitine-induced cardiac toxicity. J Nippon Med Sch. 80:350–361.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung BC, Lee SH, Cho YK, Park HS, Kim YN,
Lee YS and Shin DG: Role of the alternans of action potential
duration and aconitine-induced arrhythmias in isolated rabbit
hearts. J Korean Med Sci. 26:1576–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wright SN: Comparison of
aconitine-modified human heart (hH1) and rat skeletal (mu1) muscle
Na+ channels: An important role for external
Na+ ions. J Physiol. 538:759–771. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki K, Matsumoto A, Nishida H, Reien Y,
Maruyama H and Nakaya H: Termination of aconitine-induced atrial
fibrillation by the KACh-channel blocker tertiapin: Underlying
electrophysiological mechanism. J Pharmacol Sci. 125:406–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitamura M, Horie S, Sakaguchi M, Someya
A, Tsuchiya S, Van de Voorde J, Murayama T and Watanabe K:
Mesaconitine-induced relaxation in rat aorta: Involvement of Ca2+
influx and nitric-oxide synthase in the endothelium. Eur J
Pharmacol. 436:217–225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ducroq J: Sensitivity and specificity of
the in vitro guinea pig papillary muscle action potential duration
for the assessment of drug-induced torsades de pointes liability in
humans. Handb Exp Pharmacol. 229:205–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coulson JM, Caparrotta TM and Thompson JP:
The management of ventricular dysrhythmia in aconite poisoning.
Clin Toxicol (Phila). 55:313–321. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun GB, Sun H, Meng XB, Hu J, Zhang Q, Liu
B, Wang M, Xu HB and Sun XB: Aconitine-induced Ca2+ overload causes
arrhythmia and triggers apoptosis through p38 MAPK signaling
pathway in rats. Toxicol Appl Pharmacol. 279:8–22. 2014. View Article : Google Scholar : PubMed/NCBI
|