Introduction
Sarcomatoid carcinoma (SC) or carcinosarcoma (CS) is
a rare and complicated malignant neoplasm that consists of both
malignant epithelial components and atypical spindle cells that
express an epithelial phenotype (1,2). SC
or CS primarily occurs in the lungs, esophagus, breast, larynx, and
gallbladder (3). SC of the
pancreas (SCP) is extremely rare. Its clinical presentation is
similar to that of pancreatic ductal adenocarcinoma. In most cases,
the diagnosis is made on histopathological examination of the
resected specimen. SCP is associated with poor prognosis. Surgery
remains the mainstay of treatment. Due to rarity of the disease, no
specific adjuvant therapy is available. Here, we report a patient
with obstructive jaundice who was suspected to have distal
cholangiocarcinoma and underwent pancreaticoduodenectomy (PD); the
SCP diagnosis was made according to a detailed pathological
examination.
Case report
A 63-year-old man was admitted to our hospital with
the chief complaints of epigastralgia and jaundice of one month's
duration. Significant past history included a weight loss of 10 kg
in two months and a left nephrectomy due to left renal cancer one
year before. Physical examination showed tenderness in the
epigastrium. Laboratory blood tests revealed elevated serum liver
enzymes levels, including alkaline phosphatase and γ-glutamyl
transferase levels. The level of total serum bilirubin was 244
µmol/l (normal range: 5–21 µmol/l), with a direct serum bilirubin
of 140 µmol/l. Tumor marker levels were within normal ranges with
the exception of mildly elevated CA72-4 levels (10.38 U/ml, normal
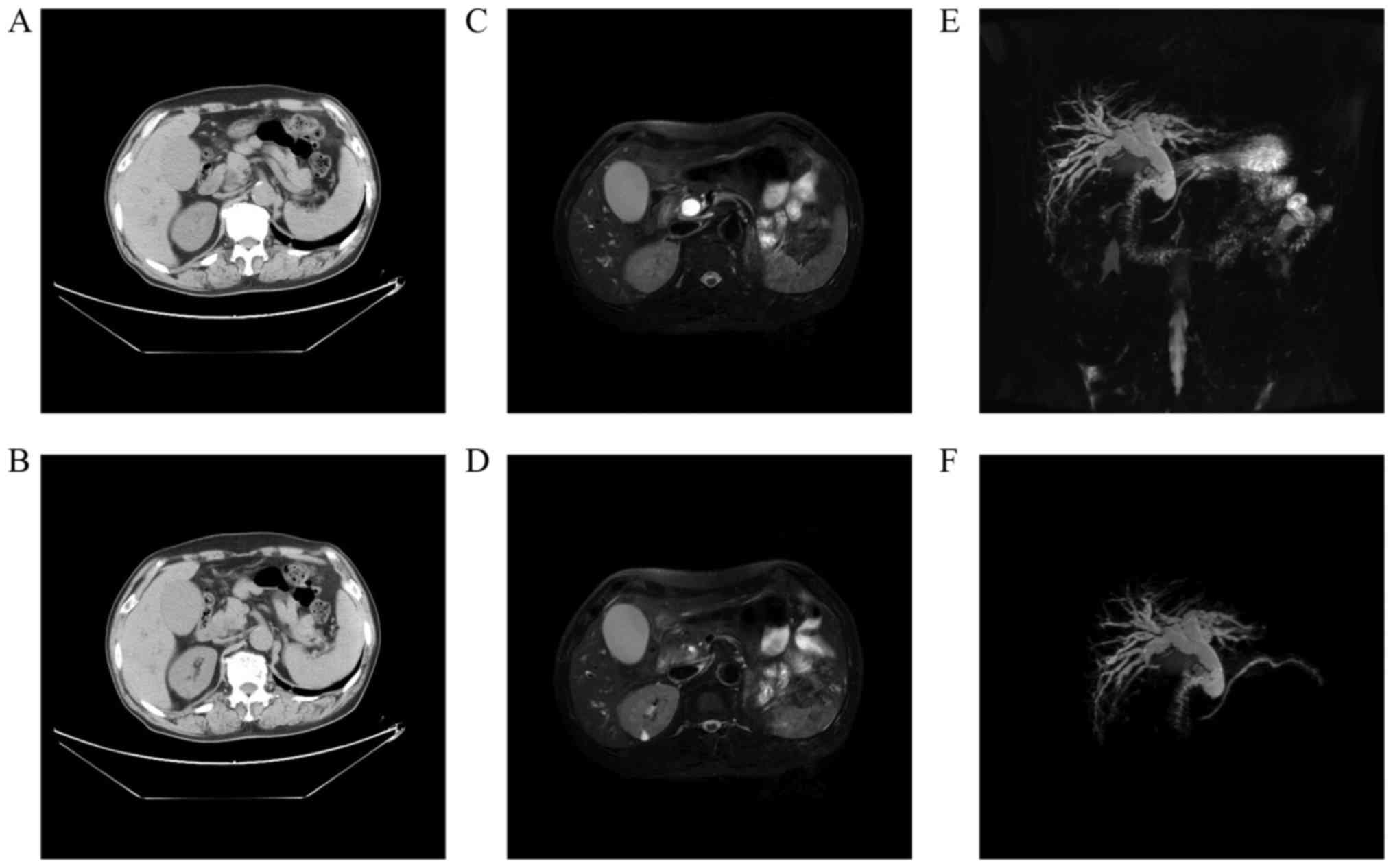
range: 0.2–6.9 U/ml). Abdominal computed tomography (CT) showed
gross dilation of the bile ducts and gallbladder (Fig. 1A) with abrupt narrowing at the
distal common bile duct (CBD) (Fig.
1B). Magnetic resonance cholangiopancreatography (MRCP)
confirmed the above CT findings (Fig.
1C and D) and showed a beaklike change in the distal CBD
(Fig. 1E and F). However, no
definite mass lesion was visible. Based on the clinicoradiological
findings, distal bile duct cancer was suspected, and the patient
was prepared for surgery. Informed written consent was taken from
the patient. Because there was no cholangitis, preoperative biliary
drainage was not performed. During the surgery, a mass in the
pancreatic head, measuring 2.5×2×1.8 cm, was detected without any
vascular invasion, and a PD was therefore performed.
Gross examination of the specimen showed a grayish
white mass with variegated areas of necrosis and invasion of the
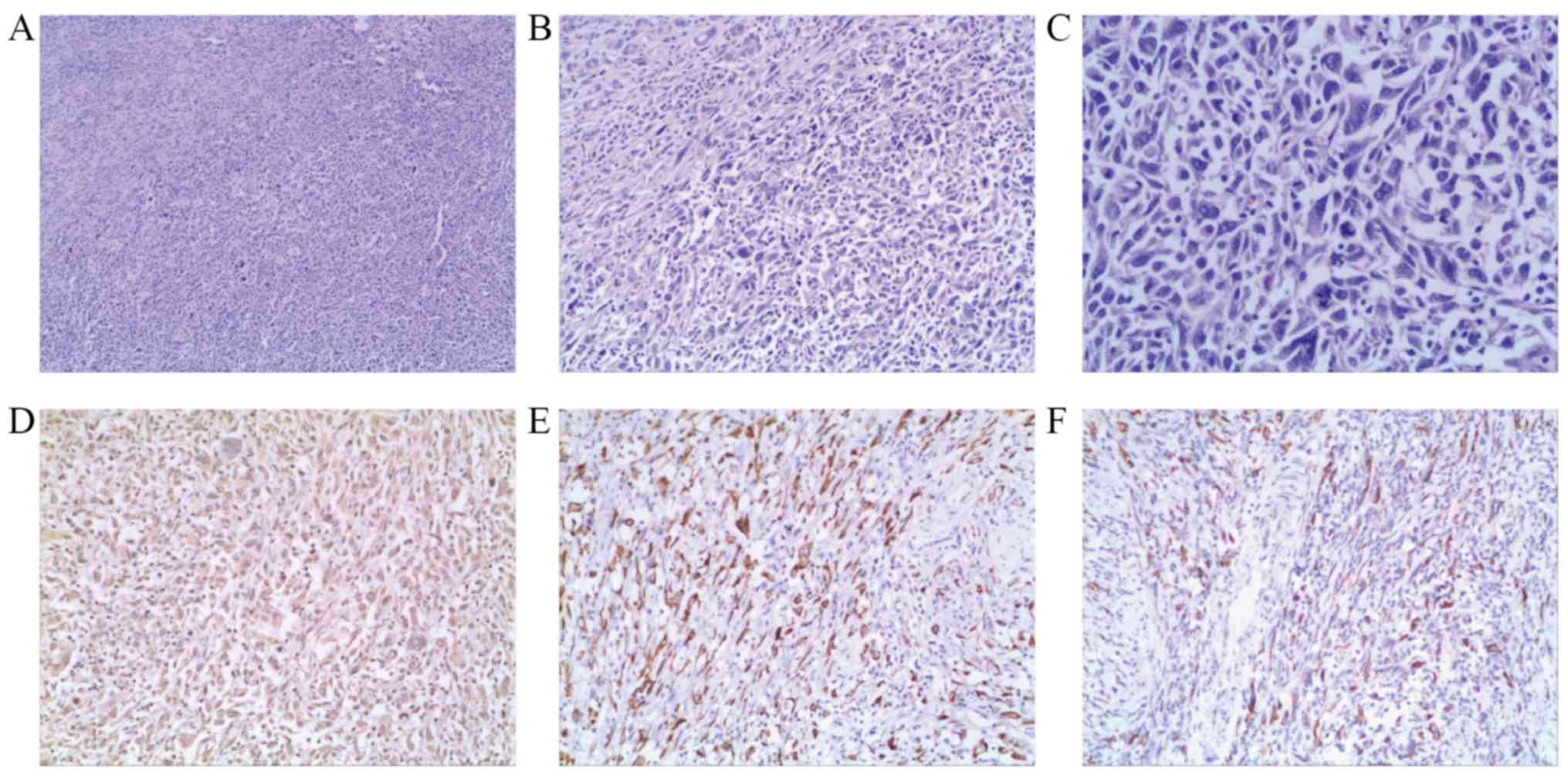
distal CBD. Under the microscope, the cut specimen primarily
consisted of staggered spindle cells with apparent atypia and
frequent mitotic activities (Fig.
2A-C). Pleomorphic giant cells were also observed amid the
spindle cells (Fig. 2A-C).
However, we also observed some malignant epithelial components, and
local invasion of the peripheral nerves. The lymph nodes, blood
vessels and resection margins were free from tumor tissue.
Immunohistochemistry showed that the spindle cells were positive
for vimentin (Fig. 2D), CK7
(Fig. 2E) and CK19 (Fig. 2F). Thus, a diagnosis of SCP was
confirmed.
The patient received thymopeptides (1 mg per day)
which is extracted from the traditional Chinese medicine for 15
days after surgery, to enhance immunity. His postoperative course
was uneventful, and he was discharged from the hospital 10 days
after surgery. At 16 months after surgery, the patient was found to
have multiple hepatic metastases (Fig.
3). Because the patient's general condition was poor, no
chemoradiation was offered. Palliative care and thymopeptides (1 mg
twice per week) were given. He died 18 months after surgery.
Discussion
SCP is an exocrine neoplasm that originates from
pancreatic ducts and acini (4).
The most recent WHO classification of exocrine pancreatic tumors
categorizes spindle cell carcinoma, SC and CS under a common
heading of undifferentiated (anaplastic) carcinoma (5) because the spindle cells commonly
express an epithelial immunohistochemical phenotype and/or genetic
alterations in pancreatic ductal adenocarcinoma (6). Although several histogenetic
mechanisms have been suggested for SC or CS, the exact mechanism is
unclear. One proposed mechanism is that the mesenchymal components
of SC include metaplasia from carcinoma under the influence of
transforming growth factor beta (TGFβ) (3,7). CS
formation is also suggested to occur when a monoclonal stem cell
differentiates in two different directions (epithelial and
mesenchymal components) under the stimulation of oncogenic factors
(3).
SCP is commonly observed in older men with the
average age of 60 years and its incidence is twice as high in males
as in females according to previous data (4,8,9). We
collected and analyzed data from 23 patients with SC or CS of the
pancreas (Table I) and found that
the average age at diagnosis is 63.30±14.61 years old. However,
incidence rates between males and females are almost the same (10
male patients and 13 female patients). The most common symptoms of
SCP include epigastralgia, poor appetite, abdominal distension,
indigestion, diarrhea and weight loss. Vomiting and hematochezia
may occur when the tumor invades the duodenum. Jaundice develops
when the tumor infiltrates and obstructs the common bile duct.
Tenderness in the epigastrium, with or without a mass, may be found
via abdominal palpation. In the present case, the patient had
obvious jaundice because of the partial displacement and
obstruction of the distal CBD by the tumor.
 | Table I.Clinicopathological characteristics of
cases of sarcomatoid carcinoma of the pancreas reported in the
English literature. |
Table I.
Clinicopathological characteristics of
cases of sarcomatoid carcinoma of the pancreas reported in the
English literature.
| Sr. no. | Author, year | Age in
years/gender | Tumor location | Tumor size in cm | US/EUS | CT/enhanced CT | MRI/enhanced MRI | PTC | MRCP | ERCP | Treatment | Carcinoma | Sarcomatoid
component | Follow-up in
months/outcome | (Refs.) |
|---|
| 7 | De la Riva et
al, 2006 | 72/female | Vater papilla | NA | +/- | +/+ | −/− | – | – | + | Papillotomy | Not identified, but
associated with choledochal with choledochal cyst | SC; IHC: CK and
vimentin (F+) | 9/succumbed to SC
metastatic to the liver | (1) |
| 13 | Kane et al,
2014 | 85/male | PB | 3.3×3.0× 2.6 | −/+ | +/+ | −/− |
| – | – | Distal
pancreatectomy, splenectomy and partial gastrectomy | PD1 adeno | SC; IHC: diffuse
pan-CK, CK52, p53 (D+), synaptophysin, chromogranin, calponin,
S100, SMA, CK19, MUC1, nuclear β-Catenin, p63, EMA and CD10
(−) | 26/alive and
well | (2) |
| 15 | Ren et al,
2013 | 48/male | PT | 9.3× 9.4×7.5 | +/- | +/- | −/− |
| – | – | NA | MD adeno | SC; IHC: CK19,
vimentin, α-1-antichymot- rypsin (+), CD68 (−) | 36/alive and
well | (7) |
| 22 | Mszyco et
al, 2017 | 85/male | PH | NA | +/+ | +/+ | −/− | + | – | – | PD2 | NA | NA | NA | (10) |
| 17 | Lu et al,
2014 | 58/female | PT | 16×18 | −/− | +/+ | +/- |
| – | – | Resection of the
pancreatic tail, fundus of the stomach and spleen | WD squamous | SC; IHC: CK7 and
vimentin (+) | 5/alive but hepatic
metastases were found | (12) |
| 20 | Jia et al,
2017 | 44/female | PH | 2.9×1.6 | +/- | +/+ | +/- |
| + | + | PD2 | MD adeno | CS; CK7 and
vimentin (+) | 31/alive and
well | (13) |
| 6 | Kim et al,
2006 | 73/female | PB, PT | 20.0×
15.0×13.0 | −/− | +/- | −/− | – | – | – | Distal
pancreatectomy, splenectomy, partial gastrectomy and colectomy | PD1 adeno | SC, vimentin (D+),
CD68 (F+), CK (−) | 3/succumbed to
cachexia with generalized tumor extension | (18) |
| 19 | Lee et al,
2015 | 24/female | PT | 4.7×3.5 | −/− | +/+ | −/− |
| – | – | Distal
pancreate-ctomy and splenectomy | NA | CS; CK and vimentin
(+) | NA | (21) |
| 8 | Gelos et al,
2008 | 61/female | PH | 7×6×3.5 | −/− | +/+ | −/− |
| – | – | PD2 | MD adeno | CS; vimentin (D+),
PDGF (F+), Ki-67 (10%+),CD117 and CK(−) | 11/recurrence | (27) |
| 1 | Cresson et
al, 1987 | 69/male | PH, PT | NA | −/− | −/− | −/− | – | – | – | Chemotherapy and
radiotherapy | NA | CS, tubular
structures, desmosomes and hemijunctions under electron
microscope | 5/hemorrhage after
jejunum resection | (28) |
| 2 | Higashi et
al, 1999 | 74/male | PH | 4.5×4.0×3.0 | +/- | +/+ | −/− | – | – | + | PPPD | PD1 adeno | SC; IHC: CK AE1,
variable CK AE3, EMA, MUC1-ARA (D+), S100, SMA (F+), desmin,
vimentin, NSE and CEA (−) | 3/succumbed to
diffuse peritoneal carcinomatosis | (29) |
| 3 | Darvishian et
al, 2002 | 74/male | PH | 4.0×3.0 | −/− | +/- | −/− | – | – | + | PD2 | MD adeno | SC; IHC: vimentin
(D+), CK (F+), CEA, SMA, DESMIN and CD68 (−) | 4/alive and
well | (30) |
| 4 | Barkatullah et
al, 2005 | 67/female | PH | 2.5×2.5×2.0 | +/+ | +/- | −/− | – | – | – | PPPD | MD adeno | SC, separate focus
of OGC; IHC (SC): CK8/18 vimentin (D+) | 8/NA | (31) |
| 5 | Bloomston et
al, 2006 | 67/female | PH | 4×4×3 | −/− | +/- | −/− | – | – | + | PPPD | PD adeno with a
focus of malignant squamous cell carcinoma | CS; vimentin (D+),
CK, EMA, CD117, S100, SMA and desmin (−) | 4/metastatic
disease of the liver and peritoneum | (32) |
| 8 | Nakano et
al, 2008 | 82/female | PH | 18.0× 11.0×
10.0 | −/− | +/+ | −/− | + | – | – | PD2 | WD adeno | SC, foci of OGC
around hemorrhage; IHC (SC): vimentin, CD10 (D+), CK AE1/AE3 (F+),
CK7, CK20, CEA, EMA, SMA and S100 (−) | O/succumbed to DIC
on post-operative day 13 | (33) |
| 10 | Kim et al,
2011 | 48/male | PT | 3.5×2.5 ×1.5 | −/− | +/- | +/- |
| – | – | Pancreatectomy with
splenectomy and colonic segmental resection | Mucinous cyst,
adeno and anaplastic carcinoma | SC, scattered OGC;
IHC (SC): vimentin (D+), pan-CK, CK7, CK8/18, EMA, CEA, CD34, CD56,
CD68, CD117, desmin, SMA, myogenin, S100, ER and PR (−) | 4/succumbed to
hepatic and peritoneal metastases | (34) |
| 11 | Shen et al,
2010 | 72/female | PH | 5×4×4 | +/- | +/+ | −/− |
| – | – | PD2, left hepatic
lobe resection and local resection of the gastric mass | PD1 adeno | CS; vimentin
(D+) | 2/multiple
metastatic masses in the whole liver and recurrence at tail of the
pancreas | (35) |
| 12 | Zhu et al,
2012 | 53/female | PH | 5×4×3 | +/- | −/− | +/- |
| – | – | PD2 | PD1 adeno | CS; SMA (D+), CK18,
EMA, and S-100 (−) | 20/NA | (36) |
| 14 | Yao et al,
2013 | 48/male | PT | 10.0× 8.0×5.0 | +/- | +/- | −/− |
| – | – | Spleen-preserving
left pancreatectomy | PD1 adeno | SC; IHC: CK (D+),
vimentin (−) | 3/succumbed to
recurrence | (37) |
| 16 | Oymaci et
al, 2013 | 66/male | PH | 3.5× 2.0×1.5 | −/− | +/+ | −/− |
| – | + | PD2 | MD adeno | CS; vimentin (D+)
and SMA (F+) | 20
days/gastrointestinal bleeding complication | (38) |
| 18 | Shi et al,
2015 | 74/female | PT | 9×6×3 | −/− | +/+ | −/− |
| – | – | Distal
pancreate-ctomy and splenectomy | Columnar
mucin-producing epithelial cells | CS; vimentin
(D+) | NA | (39) |
| 21 | Salibay et
al, 2017 | 49/female | PT | NA | −/− | −/− | −/− |
| – | – | Chemotherapy and
radiotherapy | MD adeno | CS; CK AE1+3, CK7,
CDX2, CD10, desmin and SMA (+) | 10/succumbed to
disease progression | (40) |
| 23 | Present study | 63/male | PH | 2.5×2×1.8 | −/− | +/- | −/− | – | + | – | PD2 | MD adeno | SC; IHC: CK19, CK7
and vimentin (D+) p53 (50%+), Ki67 (40%+), VEGF, C-erbB-2, K-ras
(−) | 18/succumbed to
cachexia with multiple hepatic metastases | – |
Many imaging examinations are helpful for the
diagnosis of SCP. Ultrasonography (US) is a convenient, noninvasive
method for the initial screening of pancreatic tumors. SCP shows a
low or mixed echo with a well-defined margin under US. Some
indirect signs, such as the dilation of the common bile duct, may
also be seen. However, disturbance of gas in the stomach and
intestines limits US in diagnosing SCP. Endoscopic ultrasonography
(EUS) overcomes this defect of US, with higher imaging resolution
and diagnostic accuracy. Fine needle aspiration may also be
performed during EUS, for biopsies, which are crucial for the
preoperational diagnosis of SCP.
CT and enhanced CT are also widely used. SCP shows
several manifestations on CT images. First, SCP primarily occurs in
the pancreatic head and tail with an average diameter of 7.20±5.44
cm, according to our data (pancreatic head: n=13, tail:
n=9, body: n=2; papilla: n=1; Table I). Pancreatic head SCP can cause
slight to moderate obstruction of the bile and pancreatic ducts. No
atrophy of the pancreatic tail occurs. Second, nonenhanced CT
images show well-circumscribed, hypodense or heterogeneous masses
with cystic or solid lesions (10). Third, necrosis often occurs due to
insufficient vessels and a solid-cystic mixed SCP structure
(11). Fourth, small nodules of
calcification may be observed in SCP. Fifth, enhanced CT images may
show heterogeneous enhancement in the peripheral solid part of SCP,
while the internal unilocular cystic part is often not enhanced
(10). Last, due to tumor
invasion, the peripancreatic lymph nodes, splenic artery, adjacent
duodenum, liver and colon may be displaced and partly or entirely
destroyed. In the present case, the small mass (which was invading
the bile duct) was not clearly shown by radiography; however, the
dilation of the bile ducts and gallbladder provided indirect
evidence for the tumor. In magnetic resonance imaging (MRI), SCP
shows abnormal signals, such as high or low signals in T2 weighted
imaging (12,13). Enhanced MRI is valuable for
estimating the extent of local malignant involvement. Together with
CT, MRI can clearly increase the diagnostic rate of pancreatic
neoplasms, while preventing unnecessary injury during surgery by
providing comprehensive imaging. Percutaneous transhepatic
cholangiography (PTC) can clearly display the degree of bile duct
dilation, thus facilitating diagnosis of pancreatic-head neoplasms.
However, this invasive examination could be replaced by a
noninvasive technique: MRCP, which provides a comprehensive display
of the bile and pancreatic ducts at different levels. In the
present case, dilation of the bile and pancreatic ducts indirectly
indicated the existence of a periampullary tumor. Endoscopic
retrograde cholangiopancreatography (ERCP) not only provides clear
observation of periampullary neoplasms under direct vision, but
also shows the dilation extent of the pancreatic and bile ducts
through injection of contrast medium. Furthermore, tumors can be
biopsied after papillotomy, thus providing important pre-surgical
evidence for the diagnosis of SCP.
Histologically, SC consists of both malignant
epithelial and mesenchymal components (14,15),
primarily mesenchymal components. The epithelial components can be
adenocarcinoma or squamous cell carcinoma. According to our data
(Table I), adenocarcinoma is the
most common type (poorly differentiated: n=7, moderately
differentiated: n=8, well-differentiated: n=2).
Intersecting bundles of spindle cells with apparent atypia and
frequent mitotic activities constituted the mesenchymal components.
Ordinarily, the proportion of the sarcomatoid part should be
greater than 50% to receive a diagnosis of SC (16). A study by Alguacil-Garcia
classified SC into four histological subtypes based on light
microscopy (4): (a) spindle cell
carcinoma, (b) osteoclastic giant cell tumors, (c) pleomorphic
giant cell carcinoma, and (d) round cell anaplastic carcinoma.
Additionally, some SC specimens show sarcomatoid constituents in
metastasized lymph nodes, which show the tumorous nature of the
sarcomatoid region rather than reacting hyperplasia of the
tissue.
Immunohistochemistry, electron microscopy and
genetic studies are of great value in diagnosing SC. Generally, the
mesenchymal components of SC express both mesenchymal and
epithelial markers, which is critical for SC diagnosis. In the
present case, the neoplasm primarily consisted of spindle cells
with few epithelial components; however, expression of both
mesenchymal and epithelial markers of the spindle cells supported a
diagnosis of SC. Vimentin is the most common mesenchymal marker.
Other myogenic markers (such as SMA, actin, desmin and myoglobin),
neurogenic markers and osteogenic markers may also be positive in
related components. Among epithelial markers, CK and EMA may be
expressed in epithelial and/or sarcomatoid regions. Electron
microscopy may show chitin mother cell granules, tonofibrils,
desmosomes and melanin granules in SC spindle cells (17). Among genetic studies, p53
overexpression in both components of SC was found in a Korean case
report (18). Furthermore,
Almoguera found a KRAS mutation at codon 12 of exon 2 in
spindle cells of two SCP cases (19). The identical genetic mutation of
epithelial and mesenchymal components provides strong evidence for
an EMT mechanism.
Radical resection of the neoplasm is the primary
treatment for SCP (20). PD and
pylorus-preserving PD (PPPD) are the major procedures for SCP of
the pancreatic head, whereas SCP of the pancreatic body and tail is
commonly treated with partial resection of the pancreas with
splenectomy. Chemotherapy is necessary for cases with metastases in
the lymph nodes (3). The prognosis
of SCP is extremely poor (21–23)
and may be associated with the tumor's volume and histological
type, and the extent of local lymph node metastases. The average
life expectancy is 2–3 months (24,25),
with a 3-year survival rate of less than 3% (26). According to a report by Gelos, the
average post-operative survival interval was 6 months and the
longest living patient survived for 15 months (27). According to our statistics of SCP
reported in English literature (Table
I), the average post-operative survival of SCP is 10.15±10.23
months. In this case, the patient lived for 18 months after
surgery, which suggests that early, radical surgery extends
survival for patients with SCP.
SCP is extremely rare, and its clinical presentation
is similar to that of pancreatic ductal adenocarcinoma. Diagnosis
is made on histopathological examination of the resected specimen.
SCP has a poor prognosis, and is primarily treated with surgery; no
specific adjuvant therapy is available.
Acknowledgements
The authors would like to thank Mrs Zhehui Wang for
providing the pathological section and associated figure
legends.
Funding
The present study was supported by grants from the
Finance Department of Jilin Province (grant nos. SCZSYZ01502,
SCZSYZ01521 and SCZSYZ01522).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
YJX and YX designed the study and wrote the
manuscript. YY and XZ contributed to the manuscript revision. DZ,
XY and JS contributed to the collection of clinical information and
associated literature of SCP.
Ethics approval and consent to
participate
The research was approved and consented by the
Ethics Committee of the Second Hospital of Jilin University.
Patient consent for publication
The written consent to publish was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SC
|
sarcomatoid carcinoma
|
|
CS
|
carcino-sarcoma
|
|
SCP
|
sarcomatoid carcinoma of the
pancreas
|
|
PD
|
pancreaticoduodenectomy
|
|
CT
|
computed tomography
|
|
CBD
|
common bile duct
|
|
MRCP
|
magnetic resonance
cholangiopancreatography
|
|
TGFβ
|
transforming growth factor beta
|
|
US
|
ultrasonography
|
|
EUS
|
endoscopic ultrasonography
|
|
MRI
|
magnetic resonance imaging
|
|
PTC
|
percutaneous transhepatic
cholangiography
|
|
ERCP
|
endoscopic retrograde
cholangiopancreatography
|
|
PPPD
|
pylorus-preserving PD
|
References
|
1
|
De la Riva S, Muñoz-Navas MA, Betés M,
Súbtil JC, Carretero C and Sola JJ: Sarcomatoid carcinoma of the
pancreas and congenital choledochal cyst. Gastrointest Endosc.
64:1005–1006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kane JR, Laskin WB, Matkowskyj KA, Villa C
and Yeldandi AV: Sarcomatoid (spindle cell) carcinoma of the
pancreas: A case report and review of the literature. Oncol Lett.
7:245–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang XH: New arguments on carcinosarcoma
(CS) and sarcomatoid carcinoma (SC). Cancer Res Clinic. 17:138–139.
2005.(In Chinese).
|
|
4
|
Alguacil-Garcia A and Weiland LH: The
histologic spectrum, prognosis, and histogenesis of the sarcomatoid
carcinoma of the pancreas. Cancer. 39:1181–1189. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukushima N, Hruban RH and Kato Y: Ductal
adenocarcinoma variants and mixed neoplasms of the pancreas. WHO
Classific Tumors Digest System. 1–299. 2010.
|
|
6
|
Hruban RH, Pitman MB and Klimstra DS:
Adenocarcinoma variantsTumors of the Pancreas. (AFIP Atlas of Tumor
Pathology: Series 4). American Registry of Pathology; Washington,
DC: pp. 165–190. 2007
|
|
7
|
Ren CL, Jin P, Han CX, Xiao Q, Wang DR,
Shi L, Wang DX and Chen H: Unusual early-stage pancreatic
sarcomatoid carcinoma. World J Gastroenterol. 19:7820–7824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shirobe T, Yamamoto T and Mori T: A case
of spindle cell carcinoma of the pancreas. Suizo. 10:387–392.
1995.
|
|
9
|
Wolfman NT, Karstaedt N and Kawamoto EH:
Pleomorphic carcinoma of the pancreas: Computed-tomographic,
sonographic, and pathologic findings. Radiology. 154:329–332. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mszyco S, Teng L, Annunziata J and Hartman
MS: Pancreatic carcinosarcoma: A case report highlighting computed
tomography characteristics. Curr Probl Diagn Radiol. 46:342–345.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Husain AN, Colby TV, Ordóñez NG, Krausz T,
Borczuk A, Cagle PT, Chirieac LR, Churg A, Galateau-Salle F, Gibbs
AR, et al: Guidelines for pathologic diagnosis of malignant
mesothelioma: A consensus statement from the International
Mesothelioma Interest Group. Arch Pathol Lab Med. 133:1317–1331.
2009.PubMed/NCBI
|
|
12
|
Lu BC, Wang C, Yu JH, Shen ZH and Yang JH:
A huge adenosquamous carcinoma of the pancreas with sarcomatoid
change: An unusual case report. World J Gastroenterol.
20:16381–16386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia Z, Zhang K, Huang R, Zhou X and Jiang
L: Pancreatic carcinosarcoma with rare long-term survival: Case
report and review of the literature. Medicine (Baltimore).
96:e59662017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu W, Wang Y, Jiang Y, Zhang W and Li Y:
Distinct immunophenotypes and prognostic factors in renal cell
carcinoma with sarcomatoid differentiation: A systematic study of
19 immunohistochemical markers in 42 cases. BMC Cancer. 17:2932017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue H, Takahashi H, Hashimura M, Eshima
K, Akiya M, Matsumoto T and Saegusa M: Cooperation of Sox4 with
β-catenin/p300 complex in transcriptional regulation of the Slug
gene during divergent sarcomatous differentiation in uterine
carcinosarcoma. BMC Cancer. 16:532016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding HY and Liao SL: Carcinosarcoma (CS)
and sarcomatoid carcinoma (SC). Chin J Diagn Pathol. 6:56–57.
1999.(In Chinese).
|
|
17
|
Lichtiger B, Mackay B and Tessmer CF:
Spindle-cell variant of squamous carcinoma. A light and electron
microscopic study of 13 cases. Cancer. 26:1311–1320. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KH, Kang DY, Lee MK, Yang HW and Han
HY: Sarcomatoid carcinoma of the pancreas: A case report. Kor J
Pathol. 40:306–310. 2006.
|
|
19
|
Almoguera C, Shibata D, Forrester K,
Martin J, Arnheim N and Perucho M: Most human carcinomas of the
exocrine pancreas contain mutant c-K-ras genes. Cell. 53:549–554.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dall'Oglio MF, Lieberknecht M, Gouveia V,
Sant'Anna AC, Leite KR and Srougi M: Sarcomatoid differentiation in
renal cell carcinoma: Prognostic implications. Int Braz J Urol.
31:10–16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee J, Hyun JJ and Lee HS: A Rare cause of
abdominal pain by pancreatic mass in a young female patient.
Carcinosarcoma of the pancreas. Gastroenterology. 149:e3–e5. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goto T, Hirotsu Y, Mochizuki H, Nakagomi
T, Oyama T, Amemiya K and Omata M: Stepwise addition of genetic
changes correlated with histological change from
‘well-differentiated’ to ‘sarcomatoid’ phenotypes: A case report.
BMC Cancer. 17:652017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schaefer IM, Sahlmann CO, Overbeck T,
Schweyer S and Menke J: Blastomatoid pulmonary carcinosarcoma:
Report of a case with a review of the literature. BMC Cancer.
12:4242012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurihara K, Nagai H, Kasahara K, Kawai T,
Saito K and Kanazawa K: Pleomorphic carcinoma of the pancreas with
massive lymphocytic stromal infiltration and long-term survival
after resection. Int J Pancreatol. 27:241–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tschang TP, Garza-Garza R and Kissane JM:
Pleomorphic carcinoma of the pancreas: An analysis of 15 cases.
Cancer. 39:2114–2126. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamisawa T, Tabata I, Isawa T, Tsuruta K,
Okamoto A and Koike M: A case of pleomorphic carcinoma of the
pancreas showing sequential histological change by
immunohistochemical study. Int J Pancreatol. 18:67–70.
1995.PubMed/NCBI
|
|
27
|
Gelos M, Behringer D, Philippou S and Mann
B: Pancreatic carcinosarcoma. Case report of multimodal therapy and
review of the literature. JOP. 9:50–55. 2008.PubMed/NCBI
|
|
28
|
Cresson DH and Reddick RL: Sarcomatoid
carcinoma of the pancreas presenting as gastric carcinoma:
Clinicopathologic and ultrastructural findings. J Surg Oncol.
36:268–274. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higashi M, Takao S and Sato E: Sarcomatoid
carcinoma of the pancreas: A case report with immunohistochemical
study. Pathol Int. 49:453–456. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Darvishian F, Sullivan J, Teichberg S and
Basham K: Carcinosarcoma of the pancreas: A case report and review
of the literature. Arch Pathol Lab Med. 126:1114–1117.
2002.PubMed/NCBI
|
|
31
|
Barkatullah SA, Deziel DJ, Jakate SM,
Kluskens L and Komanduri S: Pancreatic carcinosarcoma with unique
triphasic histological pattern. Pancreas. 31:291–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bloomston M, Chanona-Vilchis J, Ellison
EC, Ramirez NC and Frankel WL: Carcinosarcoma of the pancreas
arising in a mucinous cystic neoplasm. Am Surg. 72:351–355.
2006.PubMed/NCBI
|
|
33
|
Nakano T, Sonobe H, Usui T, Yamanaka K,
Ishizuka T, Nishimura E and Hanazaki K: Immunohistochemistry and
K-ras sequence of pancreatic carcinosarcoma. Pathol Int.
58:672–677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HS, Joo SH, Yang DM, Lee SH, Choi SH
and Lim SJ: Carcinosarcoma of the pancreas: A unique case with
emphasis on metaplastic transformation and the presence of
undifferentiated pleomorphic high-grade sarcoma. J Gastrointestin
Liver Dis. 20:197–200. 2011.PubMed/NCBI
|
|
35
|
Shen ZL, Wang S, Ye YJ, Wang YL, Sun KK,
Yang XD and Jiang KW: Carcinosarcoma of pancreas with liver
metastasis combined with gastrointestinal stromal tumour of the
stomach: Is there a good prognosis with the complete resection? Eur
J Cancer Care (Engl). 19:118–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu WY, Liu TG and Zhu H: Long-term
recurrence-free survival in a patient with pancreatic
carcinosarcoma: A case report with a literature review. Med Oncol.
29:140–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao J, Qian JJ, Zhu CR, Bai DS and Miao Y:
Laparoscopic left pancreatectomy for pancreatic sarcomatoid
carcinoma: A case report and review of the literature. Oncol Lett.
6:568–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oymaci E, Argon A, Coşkun A, Uçar AD,
Carti E, Erkan N and Yildirim M: Pancreatic carcinosarcoma: Case
report of a rare type of pancreatic neoplasia. JOP. 14:212–215.
2013.PubMed/NCBI
|
|
39
|
Shi HY, Xie J and Miao F: Pancreatic
carcinosarcoma: First literature report on computed tomography
imaging. World J Gastroenterol. 21:1357–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salibay CJ, Rewerska J, Gupta S and Ree N:
Primary carcinosarcoma of the pancreas with CD10-positive sarcoma
component. J Investig Med High Impact Case Rep.
5:23247096177409062017.PubMed/NCBI
|