Introduction
As a common malignant tumor of the endocrine system,
thyroid cancer accounts for ~1% of human carcinomas. According to
pathological characteristics, including the extent of
differentiation, thyroid cancer may be divided into papillary
thyroid cancer (PTC), follicular thyroid cancer (FTC) and
anaplastic thyroid cancer (ATC) (1). PTC and FTC are the
well-differentiated types which comprise 95% of the total thyroid
cancer cases. Although ATC accounts for only 5% of thyroid cancer
cases, it is one of the most lethal types of cancer among human
malignant tumors (2). The
prognosis of ATC is poor, which is evidenced by its short survival
time. The median survival of ATC was reported to be between 3 and 5
months following diagnosis (3).
Traditional therapies, including thyroid-stimulating hormone
suppressive therapy and radioiodine ablation therapy, are effective
and curative in PTC and FTC, and not in ATC. According to the
American Joint Commission on Cancer, ATC cases are classified as
stage IV when diagnosed (4). The
malignancy of ATC is characterized by its invasive and metastatic
features. A total of 90% of patients with ATC present with
extra-glandular spread and 75% are identified to have distant
metastasis when diagnosed (5).
TNF receptor-associated factors (TRAFs) are a family
of adaptor proteins which regulate multiple signaling pathways via
their interactions with the intracellular domains of a number of
receptors (6). Through ubiquitin
E3 ligase activity, TRAFs perform interaction-mediated signaling
events. According to previous studies, a number of TRAFs have been
observed to be associated with malignant tumor occurrence,
development and progression (7–9),
suggesting a role for TRAF6 in human cancer. A previous study
indicated thatTRAF6 expression was elevated in tumor cells and
further increased the polyubiquitination of hypoxia inducible
factor (HIF)-1 via direct binding (10), which was an important regulator of
tumor angiogenesis (11,12). Additionally, TRAF6 was indicated to
be correlated with tumor metastasis (8,13). A
recent study demonstrated that the important molecular regulator
CD147 was the cofactor of TRAF6; TRAF6 knockdown with small
interfering RNA significantly decreased the level of CD147-mediated
tumor metastasis (14). These
reports indicated that the role of TRAF6 in tumor malignancy was
characterized by angiogenesis and metastasis.
Emodin, also termed as
1,3,8-trihydroxy-6-methylanthraquinone, is a natural anthraquinone
extracted from rhizome of Rheum palmatum L. Various
biological activities of emodin have been reported previously,
including anti-inflammatory, anti- proliferative, anti-fibrotic and
anti-cancer activities (15).
Emodin was observed to be effective in inhibiting a number of types
of human cancer, including pancreatic cancer, liver cancer, gastric
cancer and leukemia (16–18). However, the anti-cancer activities
of emodin in anaplastic thyroid cancer have rarely been reported
previously. The present study implemented experiments to observe
the anti-cancer activity of emodin in ATC. In addition, the
inhibitory effects of emodin against tumor metastasis and
angiogenesis were investigated. Therefore, it may be hypothesized
that emodin exerts metastasis-and angiogenesis-suppressing
abilities. The present study further demonstrated the
anti-metastatic and anti-angiogenic effects of emodin in nude mice
carrying human ATC in vivo and in cultured human ATC cells
in vitro.
Materials and methods
Cell culture
Human ATC cell lines 8505c and SW1736 were acquired
from the Chinese Academy of Sciences Cell Bank (Shanghai, China)
and used in the present study. Following thawing and recovery,
8505c cells were cultured in Eagle's minimum essential medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) while
SW1736 cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.). The two media were supplemented with
fetal bovine serum (10%; Gibco; Thermo Fisher Scientific, Inc.) and
1% antibiotic mix (penicillin/streptomycin; Invitrogen; Thermo
Fisher Scientific, Inc.). Cells were cultured in an incubator
providing a humidified atmosphere composed of 5% CO2 and
95% fresh air at 37°C.
Cell viability assay
The cell viability of ATC cells was assessed by MTT
assay. Cells were seeded into a 96-well plate at density of
7×103 cells/well and cultured for 12 h. The medium was
replaced with either control saline or emodin solutions
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at incremental
concentrations (0, 10, 15, 20, 25, 30, 35 and 40 µmol/l) for 12 h.
Following washing, MTT solutions were added into each well to
incubate the cells for 4 h at 37°C. Dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA) was added to dissolve the formazan
crystals. A microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to measure the absorbance values at 540
nm. A total of three replicate readings were recorded and used for
further analysis.
ELISA analysis
The concentration of vascular endothelial growth
factor (VEGF) in cell culture supernatants was determined by ELISA
using a Quantkine Human VEGF ELISA kit (cat. no. VDE00; R&D
Systems, Inc., Minneapolis, MN, USA). Procedures were performed
according to the manufacturer's protocol. The recorded absorbance
values and plotted standard curves were used to calculate the
concentration of VEGF in the cell culture supernatants.
Wound healing assay
The migratory ability of ATC cells was evaluated via
a wound healing assay. 5×105 ATC cells were cultured on
a 60-mmdiameter culture dish (Corning Incorporated, Corning, NY,
USA) to reach a confluence of 90%. The wound was made using a 2-mm
razor blade and the injury line was marked. The peeled-off cells
were removed and the remaining cells were cultured and allowed to
migrate to heal the wound for 24 h. Following careful washing,
cells were fixed and subjected to DAPI fluorescence labeling using
a DAPI kit (Beyotime Institute of Biotechnology, Haimen, China),
according to the manufacturer's protocol. The wound closure was
observed with a fluorescence microscope at ×100 magnification.
In vivo studies
The male Balb/c nude mice (6 weeks old; weight,
19–22 g; n=62) used in the present study were acquired from Charles
River Laboratories, Inc. (Wilmington, MA, USA). Mice were kept in
an artificial environment providing a 12-h light/dark cycle, a 50%
humidity and a temperature of 20–24°C in laminar flow cleanrooms.
Animals were had free access to sterile water and standard chow.
All animal experimental procedures were performed according to the
recommended guidelines for the care and use of laboratory animals
issued by the Chinese Council on Animal Research. The protocol was
reviewed and approved by the Animal Ethics Committee of the College
of Medicine, Zhejiang University (Hangzhou, China).
Angiogenesis study
Harvested ATC cells suspended in culture mediums
(1×107 cells in 200 µl per mouse for 10 mice) were
inoculated into the left flank of each mouse. Mice continued to
survive for 28 days. Mice were orally administered with emodin at
100 mg/kg, once per day for 28 days. Immunohistochemical staining
of platelet endothelial cell adhesion molecule (CD31) was used to
mark the neovessels in tumor tissues in the present study. The
tumor tissues were fixed by 10% neutral buffered formaldehyde for
10 h and processed into sections at 5 µm using paraffin-embedding
method. Following blocking by QuickBlock blocking buffer (cat. no.
P0620; Beyotime Institute of Biotechnology, Shanghai, China) at
room temperature for 15 min, the sections were incubated with a
CD31 antibody (cat. no. ab32457; 1:1,000; Abcam, Cambridge, UK) at
4°C for 12 h. Following washing, a secondary antibody (anti-rabbit
IgG HRP-conjugated; cat. no. ab191688; 1:2,000; Abcam, Cambridge,
UK) was used to incubate the slides at room temperature for 20 min.
The images were observed using alight microscope at ×200
magnification. Microvessel density (MVD) was evaluated by counting
the number of CD31-positive vessels in 10 different fields.
Metastasis study
In the lung metastasis experiment, 52 mice were
used. A total of 5 control animals received injection of cell
culture medium. An ATC cell suspension at a density of
2×107 cells/ml was injected into the tail vein of the
mice. Mice continued to survive for 28 days. Mice were orally
administered with emodin at 100 mg/kg, once per day for 28 days.
Lung tissue samples were obtained and then fixed with 10% formalin
at room temperature for 10 h and then embedded in paraffin wax.
Tissues were processed into 5 µm tissue sections. H&E staining
was applied; the slides were stained with hematoxylin for 5 min and
eosin for 30 sec at room temperature. Stained slides were
subsequently observed with a light microscope at ×200
magnification. Metastatic rate was calculated by comparing number
of mice with metastasis to the number of mice without
metastasis.
Western blotting
Total proteins were extracted from tumor tissues and
ATC cells using radioimmunoprecipitation assay lysis buffer (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and a protein extraction
kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. The concentration of proteins was
determined using a bicinchoninic acid assay kit (Beyotime Institute
of Biotechnology). Vertical electrophoresis was performed on
SDS-PAGE gels loaded with protein samples. The gel was 10% and 40
µg protein sample was loaded per lane. Proteins were electro
transferred to polyvinylidene fluoride/nitrocellulose membranes.
Membranes were blocked by 10% defatted milk (in TBST) at 37°C for
30 min. The PVDF membranes were incubated with primary antibodies
against TRAF6 (cat. no. ab33915; 1:2,000; Abcam), HIF-1α (cat. no.
79233; 1:2,000; Cell Signaling Technology, Danvers, MA, USA); VEGF
(cat. no. ab53465; 1:2,000; Abcam); MMP9 (cat. no. sc21733;
1:2,000; Santa Cruz Biotechnology, Inc.); basigin CD147 (cat. no.
13287; 1:2,000; Cell Signaling Technology); GAPDH (cat. no. ab9485;
1:4,000; Abcam) at 4°C for 8 h. Membranes were subsequently washed
with TBST 3 times. Secondary antibodies conjugated to HRP (cat.
nos. 193651 and 99817; 1:2,500; Abcam and cat. no. sc2351; 1:2,500;
Santa Cruz Biotechnology, Inc.) were used to incubate the membranes
at room temperature for 20 min. The immunoblots were visualized
with an Enhanced Chemiluminescence Prime Detection kit (GE
Healthcare, Chicago, IL, USA) and the densities of the immunoblots
were analyzed by ImageJ software version 1.48 (National Institutes
of Health, Bethesda, MD, USA).
Statistics
Data collected in the present study were presented
as the mean ± standard error of the mean. Performed using the
software SPSS (version 18.0; SPSS, Inc., Chicago, IL, USA),
Student's t-test and one-way ANOVA were used to analyze the
significant differences between groups. Post hoc tests were carried
out by SNK tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Emodin inhibits the proliferation of
ATC cells in a concentration-dependent manner
A total of two different human ATC cells, namely
8505c and SW1736 cells, were incubated with emodin solutions at
incremental concentrations. An MTT assay was performed to identify
the non-cytotoxic concentrations. The results are presented in
Fig. 1. Emodin began to inhibit
the proliferation of 8505c and SW1736 cells at 30 µmol/l.
Therefore, concentrations <30 µmol/l (0, 10, 15, 20 and 25
µmol/l) were chosen for the subsequent experiments to avoid the
anti-proliferative effect of emodin on the metastatic and
angiogenic abilities of ATC.
Emodin suppresses VEGF expression by
inhibiting the TRAF6/HIF-1α signaling pathway in cultured ATC cells
in a concentration-dependent manner, within non cytotoxic
concentrations
In the present study, it was observed that in
cultured 8505c and SW1736 cells, the TRAF6/ HIF-1α/VEGF signaling
pathway was activated. As a result, the concentration of VEGF in
the culture supernatant was significantly elevated. VEGF is thought
to be an important effecter in the initiation and promotion of
tumor angiogenesis. Following incubation with emodin at
non-cytotoxic concentrations, however, the activation of
TRAF6/HIF-1α/VEGF was markedly suppressed in a
concentration-dependent manner. Subsequently, the VEGF content in
the culture supernatant was decreased in a concentration-dependent
manner following incubation with emodin. These results are
presented in Fig. 2.
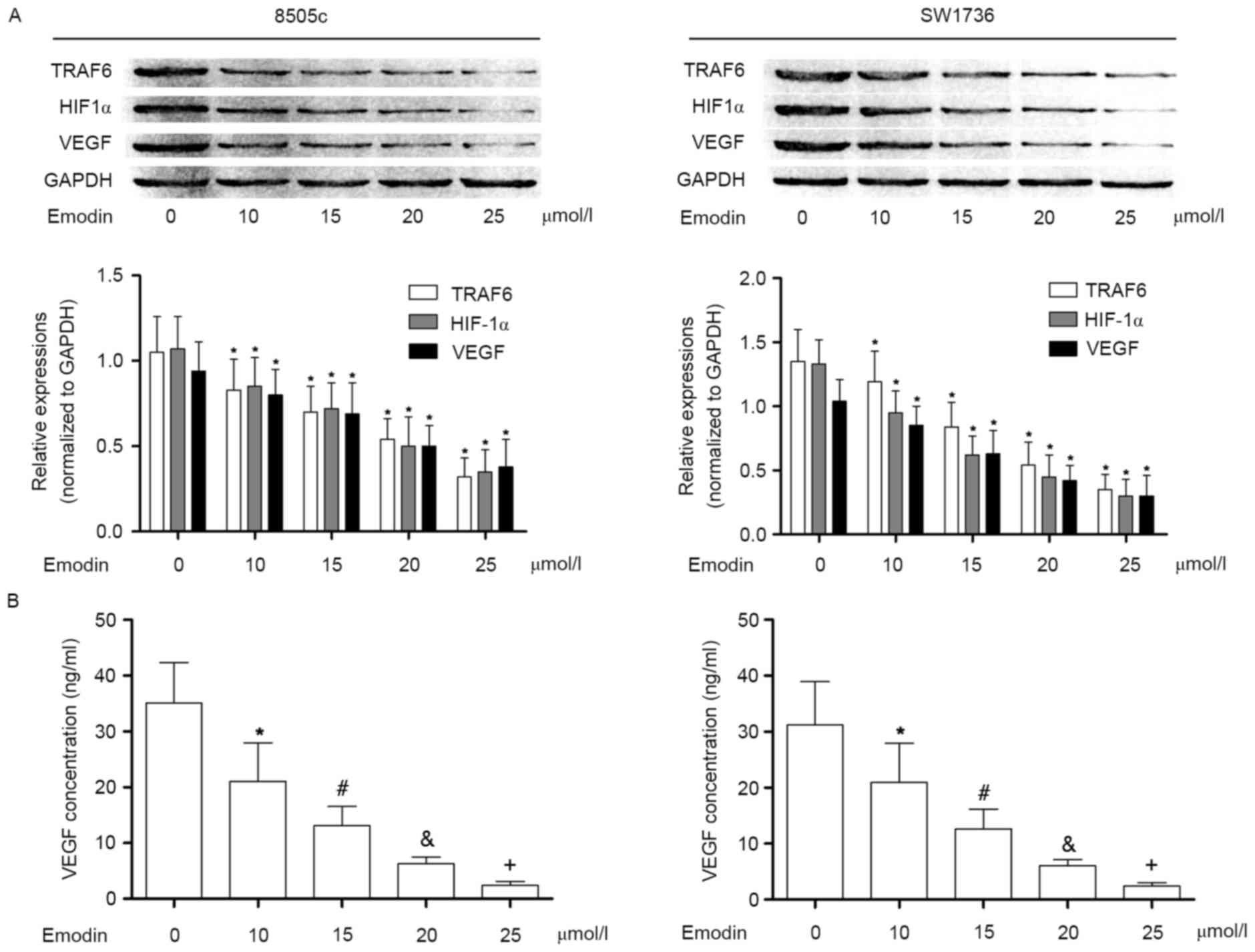 | Figure 2.Effects of emodin on TRAF6/HIF1α/VEGF
pathway in anaplastic thyroid cancer cells. (A) Immunoblotting of
TRAF6, HIF1α, VEGF and GAPDH in 8505c (left) and SW1736 cells
(right) following incubation with emodin at concentrations of 0,
10, 15, 20, 25, 30, 35 and 40 µmol/l, respectively. The lower
panels indicate the relative expression levels of TRAF6, HIF1α and
VEGF (GAPDH was used as an internal reference), in 8505c (left) and
SW1736 cells (right) incubated with emodin at concentrations of 0,
10, 15, 20, 25, 30, 35 and 40 µmol/l, respectively. (B) Results of
ELISA analysis. Columns indicate the detected concentrations of
VEGF in the cell supernatants of 8505c (left) and SW1736 cells
(right) treated with emodin at incremental concentrations.
*P<0.05 vs. 0 µmol/l; #P<0.05 vs. 10 µmol/l;
&P<0.05 vs. 15 µmol/l; +P<0.05 vs.
20 µmol/l. TRAF6, TNF receptor-associated factor 6; HIF1α,
hypoxia-inducible factor 1-α; VEGF, vascular endothelial growth
factor. |
Emodin suppresses the
metastaticability of ATC cells by inhibiting the TRAF6/CD147/MMP9
signaling pathway in cultured ATC cells in a
concentration-dependent manner, within non-cytotoxic
concentrations
As demonstrated in Fig.
3, the TRAF6/CD147/MMP9 signaling pathway was significantly
activated in cultured 8505c and SW1736 cells. However, following
incubation with emodin at non-cytotoxic concentrations, the
activation of TRAF6/CD147/MMP9 signaling was markedly inhibited in
a concentration-dependent manner. Additionally, a wound healing
assay was performed to evaluate the metastatic ability of ATC
cells. Presented in Fig. 3, the
results indicated that incubation with emodin markedly impaired the
motility of 8505c and SW1736 cells in a concentration-dependent
manner.
 | Figure 3.Effects of emodin on the
TRAF6/CD147/MMP9 pathway in anaplastic thyroid cancer cells. (A)
Immunoblotting of TRAF6, CD147, MMP9 and GAPDH in 8505c (left), and
SW1736 cells (right), following incubation with emodin at
concentrations of 0, 10, 15, 20, 25, 30, 35 and 40 µmol/l,
respectively. The lower panels indicate the relative expression
levels of TRAF6, CD147 and MMP9 (GAPDH was used as an internal
reference) in 8505c (left) and SW1736 cells (right) incubated with
emodin at concentrations of 0, 10, 15, 20, 25, 30, 35, 40 µmol/l
respectively. (B) Captured fluorescent images (magnification, ×200)
of the wound healing assay in 8505c (left) and SW1736 cells (right)
following incubation with emodin at incremental concentrations.
Cells were stained with DAPI. Yellow lines indicate the edges of
wound. The lower panels indicate the wound closure in 8505c (left)
and SW1736 cells (right), respectively. *P<0.05 vs. 0 µmol/l;
#P<0.05 vs. 10 µmol/l; &P<0.05 vs.
15 µmol/l; +P<0.05 vs. 20 µmol/l. TRAF6, TNF
receptor-associated factor 6; CD147, basigin; MMP9, matrix
metalloproteinase 9. |
Emodin abates ATC angiogenesis and
metastasis by suppressing TRAF6-mediated signaling pathways in
vivo
In the present study, in vivo experiments
were performed to reinforce the conclusion derived from the in
vitro studies. ATC cells were used to inoculate the nude mice.
In harvested ATC tissues, the TRAF6/HIF-1α/VEGF signaling pathway
was activated. However, following treatment with emodin, the
activation of the TRAF6/HIF-1α/VEGF and TRAF6/CD147/MMP9 signaling
pathways in ATC tissues was significantly inhibited. The MVD assay
demonstrated that emodin administration suppressed angiogenesis in
ATC. In addition, a lung metastasis model was established by tail
vein injection of ATC cells. Emodin administration decreased the
lung metastatic rate. These results are presented in Fig. 4.
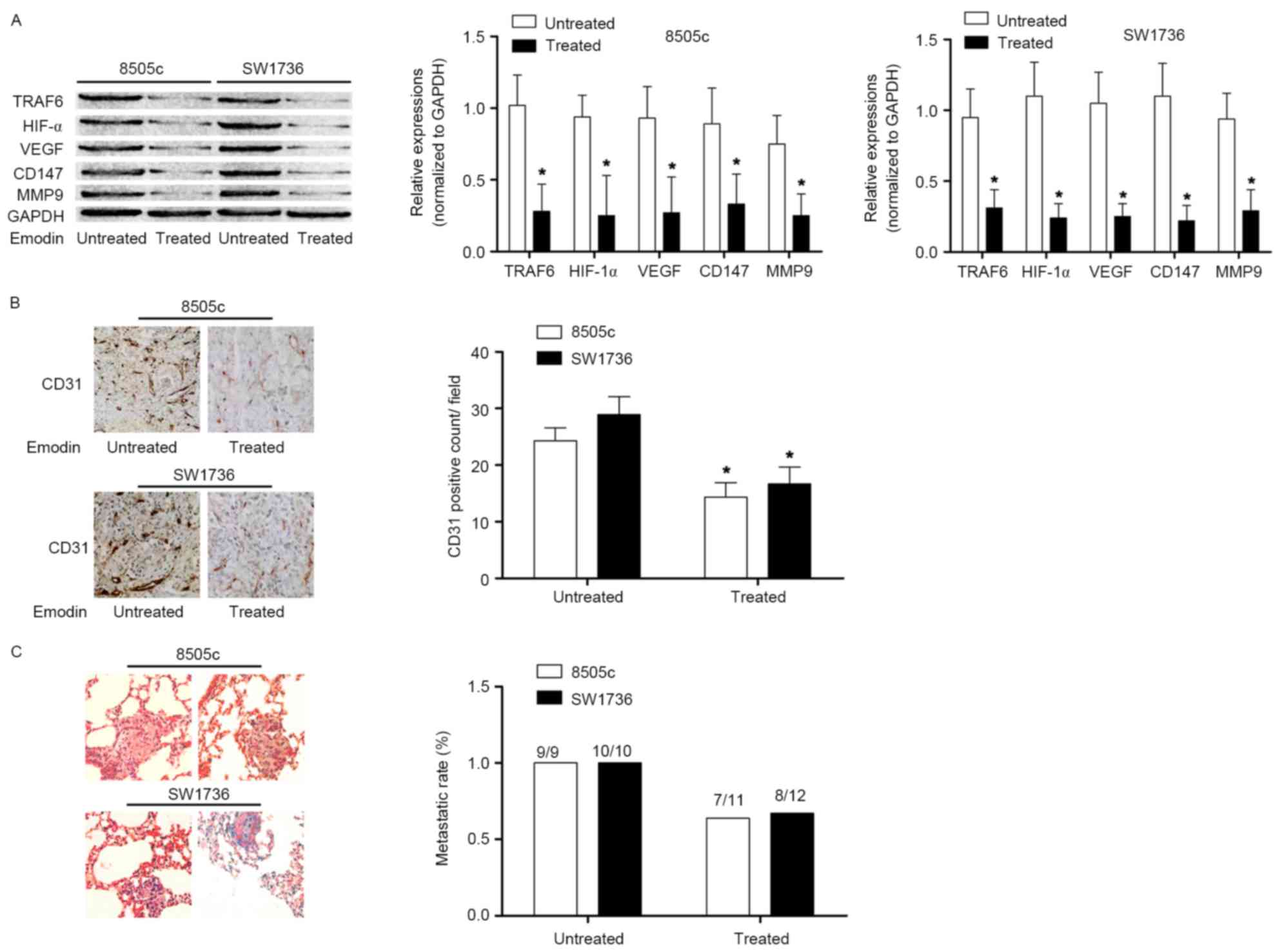 | Figure 4.Effects of emodin on angiogenesis and
metastasis in ATC in vivo. (A) Left Immunoblotting of TRAF6,
HIF1α, VEGF, CD147, MMP9 and GAPDH in ATC xenograft tissues
harvested from nude mice treated or untreated with emodin. The
graphs indicated the relative expression levels of TRAF6, HIF1α,
VEGF, CD147 and MMP9 (normalized to GAPDH) in 8505c and SW1736
×enografts, respectively. (B) Immunohistochemical staining of CD31
in harvested xenograft tissues of nude mice treated or untreated
with emodin. The graph indicates the CD31-positive count. (C)
Captured images (magnification, ×200) of hematoxylin and eosin
staining of lung metastatic lesions of nude mice receiving ATC cell
injections. The graph indicates the metastatic rate of ATC lung
metastasis in nude mice treated or untreated with emodin.
*P<0.05 vs. respective untreated group. ATC, anaplastic thyroid
cancer; TRAF6, TNF receptor-associated factor 6; CD147, basigin;
MMP9, matrix metalloproteinase 9; HIF1α, hypoxia-inducible factor
1-α; VEGF, vascular endothelial growth factor; CD31, platelet
endothelial cell adhesion molecule. |
Discussion
As one of the undifferentiated human carcinomas, ATC
is causes frequent mortality; patients diagnosed with ATC exhibit a
mean survival of ~6 months (1).
ATC is aggressive and characterized by highly invasive behavior and
early distant metastasis (19).
The 5-year survival rate of ATC has been reported to be <5%
(2). Additionally, conventional
therapies have proven to be unable to improve the clinical
outcomes. Thus, investigations into novel anti-cancer agents
against ATC are of clinical significance. In the present study, an
herbal extract termed emodin was used to treat ATC. The results of
the in vitro and in vivo experiments indicated that
emodin inhibited angiogenesis, in addition to suppressing the
metastatic ability of ATC. Further examination demonstrated that
TRAF6-mediated angiogenesis and metastasis were affected by emodin,
indicating that TRAF6 may be the molecular target of emodin.
It has been reported that emodin exerts anti-cancer
activities by inducing cancer cell apoptosis, cell cycle arrest,
and by suppressing the cellular abilities of proliferation,
angiogenesis, migration and invasion (20–22).
Although emodin has been proven to be effective in a number of
human cancer types, the anti-cancer effect of emodin on ATC has
rarely been reported in previous studies. In the present study, two
ATC cell lines, 8505c and SW1736, were treated with serially
diluted emodin solutions. It was observed that the proliferation of
ATC was markedly inhibited when concentrations of emodin were above
30 µmol/l. In addition, the suppressive effect of emodin effects on
the angiogenesis and metastasis of ATC was investigated.
TRAFs are the intracellular protein family which
transduce signals from intracellular part of tumor necrosis factor
receptors. Previous studies have linked one of the family members,
TRAF6, to the oncogenesis, development and metastasis of numerous
types of human cancer (23).
Protein and mRNA overexpression of TRAF6 has been observed in human
cancer tissues (24,25). In vitro, increased and
consistent expression of TRAF6 has been identified in multiple
human cancer cell lines (9). In
the present study, a similar phenomenon was observed: TRAF6 was
highly expressed in two different ATC cell lines in vitro
and their xenografts in vivo. A previous study indicated
that TRAF6 enhanced cancer angiogenesis by upregulating the
expression of HIF-1α in the presence of dimethyloxaloylglycine
(10). Under certain pathological
conditions, HIF-1α translocates to the nucleus and binds to the
hypoxia responsive element, initiating target gene transcription.
VEGF has been demonstrated to be one of the important targets of
HIF-1α and a regulator of cancer angiogenesis (26). In the in vitro part of the
present study, following emodin incubation, TRAF6/HIF-1α signaling
was markedly suppressed in a concentration-dependent manner. As a
result, intra- and extracellular levels of VEGF were decreased. In
the in vivo part, MVD was observed to be decreased following
treatment with emodin, which additionally inhibited
TRAF6/HIF-1α/VEGF in the ATC xenografts.
Evidence has indicated the close association between
TRAF6 and cancer metastasis in a number of human malignant tumors
(8,13). CD147 belongs to the immunoglobulin
family. In a recent study, CD147 was recognized to bean cofactor of
TRAF6 which promoted membrane recruitment of CD147 (14). It was hypothesized that CD147 was
an inducer of MMPs which served an important role in cancer
metastasis and invasion, by degrading the extracellular matrix to
promote cancer cell invasion through vessel walls (27). Overexpression of MMP9 was
demonstrated to be correlated with cancer distant metastasis
(28). In the present study, it
was observed that TRAF6/CD147 signaling was activated in ATC cells
and their xenografts. The wound healing assay revealed that
treatment with emodin impaired the migratory andinvasive abilities
of ATC cells. The results of the in vivo experiments
demonstrated that emodin significantly decreased the lung
metastatic rate of ATC. In addition, it was observed that emodin
significantly suppressed the activation of the TRAF6/CD147/MMP9
pathway in ATC cells in vivo and in vitro.
In conclusion, increased expression of TRAF6 was
observed in ATC cell lines and xenografts. Emodin exerted its
anti-cancer activity against ATC by inhibiting proliferation,
angiogenesis and metastasis. In particular, the results of the
present study indicated that emodin suppressed ATC angiogenesis by
inhibiting the TRAF6/HIF-1α/VEGF pathway; in addition, emodin
suppressed ATC metastasis by inhibiting the TRAF6/CD147/MMP9
pathway. The results of the present study indicated the potential
therapeutic benefits of targeting TRAF6 in ATC.
References
|
1
|
Ranganath R, Shah MA and Shah AR:
Anaplastic thyroid cancer. Curr Opin Endocrinol Diabetes Obes.
22:387–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keutgen XM, Sadowski SM and Kebebew E:
Management of anaplastic thyroid cancer. Gland Surg. 4:44–51.
2015.PubMed/NCBI
|
|
3
|
Glaser SM, Mandish SF, Gill BS,
Balasubramani GK, Clump DA and Beriwal S: Anaplastic thyroid
cancer: Prognostic factors, patterns of care and overall survival.
Head Neck. 38 Suppl 1:E2083–E2090. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Neill JP, Power D, Condron C,
Bouchier-Hayes D and Walsh M: Anaplastic thyroid cancer,
tumorigenesis and therapy. Ir J Med Sci. 179:9–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nambu J, Kobayashi T, Hashimoto M, Tashiro
H, Sugino K, Shimamoto F, Kikuchi A and Ohdan H: h-prune affects
anaplastic thyroid cancer invasion and metastasis. Oncol Rep.
35:3445–3452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walsh MC, Lee J and Choi Y: Tumor necrosis
factor receptor- associated factor 6 (TRAF6) regulation of
development, function and homeostasis of the immune system. Immunol
Rev. 266:72–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun H, Li X, Fan L, Wu G, Li M and Fang J:
TRAF6 is upregulated in colon cancer and promotes proliferation of
colon cancer cells. Int J Biochem Cell Biol. 53:195–201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han F, Zhang L, Qiu W and Yi X: TRAF6
promotes the invasion and metastasis and predicts a poor prognosis
in gastric cancer. Pathol Res Pract. 212:31–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin G, Huang C, Su G, Hu H and Xu H:
Effect of TRAF6 downregulation on malignant biological behavior of
lung cancer cell lines. Zhongguo Fei Ai Za Zhi. 18:661–667.
2015.(In Chinese). PubMed/NCBI
|
|
10
|
Sun H, Li XB, Meng Y, Fan L, Li M and Fang
J: TRAF6 upregulates expression of HIF-1α and promotes tumor
angiogenesis. Cancer Res. 73:4950–4959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weijts BG, Bakker WJ, Cornelissen PW,
Liang KH, Schaftenaar FH, Westendorp B, de Wolf CA, Paciejewska M,
Scheele CL, Kent L, et al: E2F7 and E2F8 promote angiogenesis
through transcriptional activation of VEGFA in cooperation with
HIF1. EMBO J. 31:3871–3884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khromova NV, Kopnin PB, Stepanova EV,
Agapova LS and Kopnin BP: p53 hot-spot mutants increase tumor
vascularization via ROS-mediated activation of the HIF1/VEGF-A
pathway. Cancer Lett. 276:143–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han Q, Yao F, Zhong C and Zhao H: TRAF6
promoted the metastasis of esophageal squamous cell carcinoma.
Tumour Biol. 35:715–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo Z, Zhang X, Zeng W, Su J, Yang K, Lu
L, Lim CB, Tang W, Wu L, Zhao S, et al: TRAF6 regulates melanoma
invasion and metastasis through ubiquitination of Basigin.
Oncotarget. 7:7179–7192. 2016.PubMed/NCBI
|
|
15
|
Dong X, Fu J, Yin X, Cao S, Li X, Lin L,
Huyiligeqi and Ni J: Emodin: A review of its pharmacology, toxicity
and pharmacokinetics. Phytother Res. 30:1207–1218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu JM, Zhou J, Shi J, Xie JS, Huang L, Yip
AY, Loo WT, Chow LW and Ng EL: Emodin affects ERCC1 expression in
breast cancer cells. J Transl Med. 10 Suppl 1:S72012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Yu H, Zhang J, Ge X, Gao J, Zhang
Y and Lou G: Anti-tumor effect of emodin on gynecological cancer
cells. Cell Oncol (Dordr). 38:353–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masaldan S and Iyer VV: Exploration of
effects of emodin in selected cancer cell lines: Enhanced growth
inhibition by ascorbic acid and regulation of LRP1 and AR under
hypoxia-like conditions. J Appl Toxicol. 34:95–104. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurata K, Onoda N, Noda S, Kashiwagi S,
Asano Y, Kawajiri H, Takashima T, Tanaka S, Ohsawa M and Hirakawa
K: Nestin expression as an independent indicator of poor prognosis
for patients with anaplastic thyroid cancer. Oncol Lett.
10:850–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MS, Park MJ, Kim SJ, Lee CH, Yoo H,
Shin SH, Song ES and Lee SH: Emodin suppresses hyaluronic
acid-induced MMP-9 secretion and invasion of glioma cells. Int J
Oncol. 27:839–846. 2005.PubMed/NCBI
|
|
21
|
Huang PH, Huang CY, Chen MC, Lee YT, Yue
CH, Wang HY and Lin H: Emodin and Aloe-Emodin suppress breast
cancer cell proliferation through ER α Inhibition. Evid Based
Complement Alternat Med. 2013:3761232013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ok S, Kim SM, Kim C, Nam D, Shim BS, Kim
SH and Ahn KS, Choi SH and Ahn KS: Emodin inhibits invasion and
migration of prostate and lung cancer cells by downregulating the
expression of chemokine receptor CXCR4. Immunopharmacol
Immunotoxicol. 34:768–778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rong Y, Wang D, Wu W, Jin D, Kuang T, Ni
X, Zhang L and Lou W: TRAF6 is over-expressed in pancreatic cancer
and promotes the tumorigenicity of pancreatic cancer cells. Med
Oncol. 31:2602014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Wang H and Han L: Expression and
clinical significance of tumor necrosis factor receptor-associated
factor 6 in patients with colon cancer. Iran Red Crescent Med J.
18:e239312016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang XL, Dang YW, Li P, Rong MH, Hou XX,
Luo DZ and Chen G: Expression of tumor necrosis factor
receptor-associated factor 6 in lung cancer tissues. Asian Pac J
Cancer Prev. 15:10591–10596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen H, Feng J, Zhang Y, Shen A, Chen Y,
Lin J, Lin W, Sferra TJ and Peng J: Pien Tze Huang inhibits
hypoxia-induced angiogenesis via HIF-1 alpha/VEGF-A pathway in
colorectal cancer. Evid Based Complement Alternat Med.
2015:4542792015.PubMed/NCBI
|
|
27
|
Urbaniak-Kujda D, Kapelko-Slowik K, Prajs
I, Dybko J, Wolowiec D, Biernat M, Slowik M and Kuliczkowski K:
Increased expression of metalloproteinase-2 and −9 (MMP-2, MMP-9),
tissue inhibitor of metalloproteinase-1 and −2 (TIMP-1, TIMP-2) and
EMMPRIN (CD147) in multiple myeloma. Hematology. 21:26–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Wang H, Ke H and Ni S: MiR-129
regulates MMP9 to control metastasis of non-small cell lung cancer.
Tumour Biol. 36:5785–5790. 2015. View Article : Google Scholar : PubMed/NCBI
|


















