Introduction
Osteoarthritis (OA) is one of the most common types
of joint disease, particularly in the elderly, with ~3 million
newly diagnosed cases each year (1,2).
Owing to limited information about the pathophysiological process
of OA, joint replacement remains the main treatment for patients
with advanced OA (3), and only
limited effective disease-modifying OA drugs have been used for
treatment (4). Further
understanding of the mechanism underlying OA may provide insights
for the development of novel treatments.
Previous studies have demonstrated that inflammation
contributes to the pathogenesis and the progression of OA (5,6), and
is a major factor associated with cartilage loss and disease
symptoms, including joint pain, swelling and stiffness, as well as
synovitis indications, such as joint tenderness and abscesses
(7). Infiltration of mononuclear
cells into the synovial membrane and production of proinflammatory
mediators, such as interleukin 1β (IL-1β), tumor necrosis factor-α
(TNF-α) and chemokines, may initiate and exacerbate OA (7). Exploration of the mechanisms and
identification of the key mediators involved in the inflammatory
process, particularly to those associated with IL-1β and TNF-α,
will aid in prevention and management of OA.
S100B is a 21 kDa EF hand-type cytosolic
calcium-binding protein that has been demonstrated to serve a
stimulatory role for inflammatory responses in multiple cells, such
as macrophages, lymphocytes, endothelial cells, vascular smooth
muscle cells and cardiomyocytes (8,9).
According to previous studies (10,11),
S100B has been revealed to be expressed in human articular
cartilage, and the expression of S100B is upregulated in diseased
tissue. In addition, a previous study suggested that extracellular
S100B is a pro-catabolic and pro-inflammatory factor that promotes
cartilage degradation (11).
However, the exact role of S100B in OA as well its association with
important inflammatory factors, such as IL-1β and TNF-α, have not
been investigated. Therefore, the aim of the present study was to
explore the role of S100B in the inflammation process during OA.
Furthermore, lipopolysaccharides (LPS), an important
proinflammatory product of the microbiome, has a role in the
pathogenesis of OA, and both systemic and local LPS burden is
considered to be associated with knee OA (12). Therefore, LPS was as the stimulator
in the cell culture system used in the present study (12).
Materials and methods
Ethics approval of the study
protocol
All research involving human participants was
approved by the Institutional Review Board of Soochow University
(Suzhou, China). Written informed consent was provided by all
participants. The animal study protocol was approved by the
Institutional Animal Care and Use Committee of Soochow University
and was conducted following the international guidelines for animal
experimentation (13).
OA cartilage samples
OA cartilage samples (n=23; age 74.0±3.9 years old;
sex, 21 females and 2 males) were collected between January and
December 2016 during total knee arthroplasty in the Department of
Orthopaedics at The First Affiliated Hospital of Soochow
University. Inclusion criteria were as follows: Patients were
diagnosed with OA in accordance with the definition and
classification provided by the Diagnostic and Therapeutic Criteria
Committee of the American Rheumatism Association (14); patients with rheumatoid arthritis
and other autoimmune diseases, as well as chondrodysplasias and
posttraumatic OA were excluded from the study. For RNA extraction,
cartilage samples (2–3 g) were collected in 1.5 ml centrifugation
tube, grinded with liquid nitrogen, mixed with 1 ml
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and stored in −80°C in the absence of extraction
prior to subsequent use. For immunohistochemical analyses,
cartilage samples were fixed with 10% neutral formalin prior to
further processing. A total of 20 samples of articular cartilage
were obtained from patients (age, 71.7±3.6 years old; sex, 18
females and 2 males) with tibial plateau fracture and without OA
were also collected as control between January and December 2016 at
the same hospital. Written informed consent was obtained from all
OA and control subjects. The patient demographic data, including
age, sex, disease duration and Kellgren-Lawrence grading are listed
in Table I.
 | Table I.Clinicopathological characteristics
of the patients with OA used in the present study. |
Table I.
Clinicopathological characteristics
of the patients with OA used in the present study.
| Characteristic | OA | Control | P-value |
|---|
| Patients (n) | 23 | 20 |
|
| K/L Grading |
|
|
|
| Grade
2+3 | 4 |
|
|
| Grade
4 | 19 |
|
|
| Age (years) | 74.0±3.9 | 71.7±3.6 | 0.24 |
| Sex (F/M) | 21/2 | 18/2 | 0.70 |
| Disease duration
(months) | 140.6±80.8 |
|
|
Rabbit OA model establishment
Female adult New Zealand white rabbits (n=9, 6
rabbits were used to establish the OA model and 3 rabbits were used
as control; age, 4 months; weight, 3.2–3.8 kg) were purchased from
Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China), housed
in an air-conditioned room at 22°C with a 12/12 h light/dark cycle
and 40–60% humidity. Rabbits were permitted free access to standard
laboratory food and water, and underwent right knee immobilization
at 180° of extension using orthopedic casting tape (Nanjing
Shuangwei Biotech Co., Ltd., Jiangsu, China). Normal weight bearing
was carried out in all immobilized rabbits. Following 4 weeks
immobilization, the tape was removed and cartilage samples were
collected for experimentation. Successful establishment of the OA
model was confirmed by visual observation of roughness of articular
cartilage at 4 weeks in 5 rabbits and the efficacy was 83.3% (5 out
of 6 rabbits were successfully established into to OA model;
roughness of articular cartilage was not observed in the remaining
rabbit, which suggested that OA was not successfully established).
In addition, 3 rabbits without right knee immobilization were
employed as normal Control. Therefore, 5 and 3 rabbits were used as
the OA model and the control group, respectively.
Sample collection and histological
assessment
The intermediate region of the medial femoral
condyles of the OA and control osteochondral samples obtained from
humans and rabbits were fixed with 10% neutral formalin at 22°C for
24 h, followed by 5% nitric acid decalcification for 4 days,
dehydration in a graded ethanol series at 22°C (70, 80, 90 and
100%; each for 10 min), and then xylene clearance was performed
using the following steps: Incubation at 60°C for 10 min and then
two incubations at 22°C for 10 min. Following this, sections were
embedded in paraffin at 22°C, and then cut into sections (5 µm).
Sections were stained with hematoxylin and eosin (H&E) for
general examination and Safranin-O/Fast-Green (SO) for
glycosaminoglycan distribution was performed on the sections.
Staining results in four visual fields were evaluated separately
using a light microscope (Nikon Corporation, Tokyo, Japan). OA
severity was evaluated by the Mankin and Osteoarthritis Research
Society International scoring system (15).
Synovial fluid was obtained from the knee joint of
OA and Control rabbits by injecting 0.5 ml saline solution followed
by aspiration, which was performed three times. Synovial fluid
(~0.8–1 ml) was extracted and samples were centrifuged at 2,200 × g
for 10 min. The supernatant was collected and stored at −70°C until
further use.
Immunohistochemical evaluation
Human OA and control sections were treated for 30
min with proteinase K (Kangchen BioTech Co., Ltd., Shanghai,
China), 10 min with 3% hydrogen peroxide/methanol and 30 min with
10% goat serum (Vector Laboratories, Inc., Burlingame, CA, USA).
Sections were incubated with mouse primary antibodies against TNF-α
(1:100; cat. no. sc-52746; Santa Cruz Biotechnology Inc., Dallas,
TX, USA); IL-1β (1:100; cat. no. sc-7884; Santa Cruz Biotechnology
Inc.), type II collagen (1:100; cat. no. NB600-844; Novus
Biologicals, LLC, Littleton, CO, USA) and S100B (1:100; cat. no.
HPA015768; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 90
min. Subsequently, the sections were incubated with a secondary
goat anti-mouse antibody (1:200; cat. no. KGAA37; Nanjing KeyGen
Biotech. Co., Ltd., Nanjing, China) for 30 min at room temperature,
followed by 5 min 3,3′-diaminobenzidine tetrahydrochloride solution
staining, 8 min hematoxylin counterstaining and mounted using
neutral balsam. A light microscope was used for evaluation and four
distinct visual fields were observed. Digital images were
subsequently analyzed using ImageJ software version 1.42q (National
Institute of Health, Bethesda, MD, USA), according to previous
studies (16,17), and the results are expressed as
relative intensity of staining.
Human synovial fibroblast isolation
and culture
Knee synovial tissues were obtained from normal
patients with traumatic injury (as aforementioned) and human
synovial fibroblasts were prepared using outgrowth technology, as
described in a previous study (16). Briefly, synovial tissue samples
were washed twice with PBS, minced into 1 mm pieces and placed in a
35 mm tissue culture dish and incubated in Ham's F12 medium
supplemented with 10% FBS and penicillin/streptomycin (100 U/ml;
all purchased from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The medium was changed every 3–4 days. Following
confluency (80–90%), cells were detached with 0.05% trypsin/EDTA
solution and subcultured at a ratio of 1:3 (one plate was split
into three plates). Human synovial fibroblasts were used between
passage four and eight.
Lentiviral short interfering (si)RNA
and overexpression vectors
Recombinant lentiviral particles overexpressing
S100B or siRNA against S100B or fibroblast growth factor receptor 1
(FGFR1), and the controls (empty vector or Scrambled siRNA;
sequences are provided in Table
II). pLVX-IRES-ZsGreen1 was used as the overexpression vector,
whereas pLVX-shRNA2 was used as the siRNA vector (both obtained
from GenePharma Inc., Shanghai, China). Cells were grown to 40%
confluency and transduced with complete medium containing
lentiviral particles overexpressing S100B or siRNA against S100B or
FGFR1 at concentrations of 1×108 transducing units/ml
[multiplicity of infection (MOI) of 20] (18) at 37°C for 48 h. Polybrene (cat. no.
H9268; Sigma-Aldrich; Merck KGaA) at a concentration of 8 µg/ml was
added simultaneously to increase infection efficiency. No adverse
effects were observed on the cell viability by siRNA or Polybrene
(data not shown). The siRNAs had no off-target effects, and did not
affect cell adherence, shape and viability at the MOI of 20,
according to manufacturer's protocol and treatment duration.
 | Table II.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction and siRNA
sequences. |
Table II.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction and siRNA
sequences.
| Gene | Primer sequence
(5′-3′) |
|---|
| S100B | F:
CTGGAGAAGGCCATGGTTGC |
|
| R:
CTCCAGGAAGTGAGAGAGCT |
| TNF-α | F:
GGAGAAGGGTGACCGACTCA |
|
| R:
CTGCCCAGACTCGGCAA |
| IL-1β | F:
AAGCTGATGGCCCTAAACAG |
|
| R:
AGGTGCATCGTGCACATAAG |
| FGF1 | F:
ACAAGGGACAGGAGCGAC |
|
| R:
TCCAGCCTTTCCAGGAACA |
| FGFR1 | F:
TTCCTCATCTCCTGCATGGT |
|
| R:
GTGGTGCTGAGTGTGCAAAT |
| GAPDH | F:
CAAAGCCAGAGTCCTTCAGA |
|
| R:
GATGGTCTTGGTCCTTAGCC |
|
| Gene | siRNA sequence
(5′-3′) |
|
| S100B |
GGAATTCATGGCCTTTGTT |
| FGF |
CCACAGAATTGGAGGCTACAA |
| Scramble |
CCGTATCGTAAGCAGTACTTT |
Cell treatment and LPS simulation
To observe the effects of S100B on inflammatory
cytokines, human synovial fibroblasts were infected with S100B
overexpression, S100B siRNA, Scrambled siRNA or empty vector
lentivirus as aforementioned, and subjected to LPS stimulation at
20 µg/l for 48 h, followed by cell lysing using 200–500 µl
radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of
Biotechnology, Haimen, China) at 4°C and subsequently subjected to
centrifugation at 14,000 × g for 15 min at 4°C to separate the
total protein. In addition, condition medium was collected
following further centrifugation at 1,500 × g for 15 min at 4°C. To
observed the effects FGFR1 on inflammatory cytokines, human
synovial fibroblasts were infected with S100B overexpression or
empty vector lentivirus alone or co-transfected with FGFR1 siRNA or
scramble siRNA lentivirus, stimulated with 20 µg/l LPS for 48 h
before cell lysis and condition medium collection, as
aforementioned.
ELISA
TNF-α and IL-1β expression levels from OA model and
Control rabbit synovial fluid or condition medium of human synovial
fibroblasts following cell treatment and LPS simulation were
detected using the following ELISA kits purchased from R&D
Systems, Inc. (Minneapolis, MN, USA): Rabbit TNF-α kit (cat. no.
DY5670), human TNF-α kit (cat. no. DY210-05), rabbit IL-1β kit
(cat. no. DY7464) and human IL-1β kit (cat. no. DY201-05).
Following this, absorbance values were measured at 450 nm to
determine the concentrations, according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from human synovial
fibroblasts (1×106) that had previously undergone
different treatments, or osteochondral samples from control and OA
rabbits, using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). One Step SYBR PrimeScript RT-PCR kit
(Takara Biotechnology Co., Ltd., Dalian, China) and an iQ5
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) were used for PCR. The temperature protocol used
for RT was 42°C for 5 min followed by 95°C for 10 sec. The
following thermocyling conditions were used for qPCR: 40 cycles at
95°C for 5 sec followed by 60°C for 30 sec; followed by a final
termination step at 4°C for 10 min. Relative gene expression was
determined by the 2−ΔΔCq method (19) and expression level of the GAPDH
gene from the same samples was used as an internal control.
Specific oligonucleotide primers for S100B, TNF-α, IL-1β, FGFR1 and
GAPDH are listed in Table II.
Western blot analysis
Human synovial fibroblasts or cells
(1×107 cells) in cartilage tissue (2–3 g) from OA model
or Control rabbits were lysed in radioimmunoprecipitation assay
buffer (RIPA; Beyotime Institute of Biotechnology, Nantong, China),
and extracted by supernatant collection following high speed
centrifugation at 14,000 × g for 15 min at 4°C, followed by
bicinchoninic acid assay for protein quantification. Cellular
proteins (20 µg) were separated by 10% SDS-PAGE gel and transferred
onto polyvinylidene difluoride membranes. Subsequently, the
membranes were blocked with non-fat milk and incubated with primary
monoclonal antibodies at 4°C overnight against S100B (cat. no.
9550), FGF1 (cat. no. 3139) and FGFR1 (cat. no. 9740; all from Cell
Signaling Technology, Inc., Danvers, MA, USA); and anti-β-actin
antibody (Santa Cruz Biotechnology Inc.) was used as the loading
control. Protein bands were incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. 7074; 1:2,000;
Cell Signaling Technology, Inc.) at room temperature for 1 h and
developed with SuperSignal Ultra Chemiluminescent Substrate
(Pierce; Thermo Fisher Scientific, Inc.) and exposed on X-ray films
(Kodak, Rochester, NY, USA). Image J software version 1.42 q
(National Institutes of Health) was used for densitometric
analysis, and protein expression levels were normalized against
β-actin.
Statistical analysis
All experiments were performed at least three times.
SPSS v18 (SPSS, Inc., Chicago, IL, USA) was used for statistical
analysis. Data are presented as the mean ± standard deviation.
Between-group comparisons were performed by using Student's t-test
or one-way analysis of variance followed by Tukey's post hoc test.
Correlation analysis was performed with Pearson's correlation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
S100B, TNF-α and IL- expression in
human OA tissues
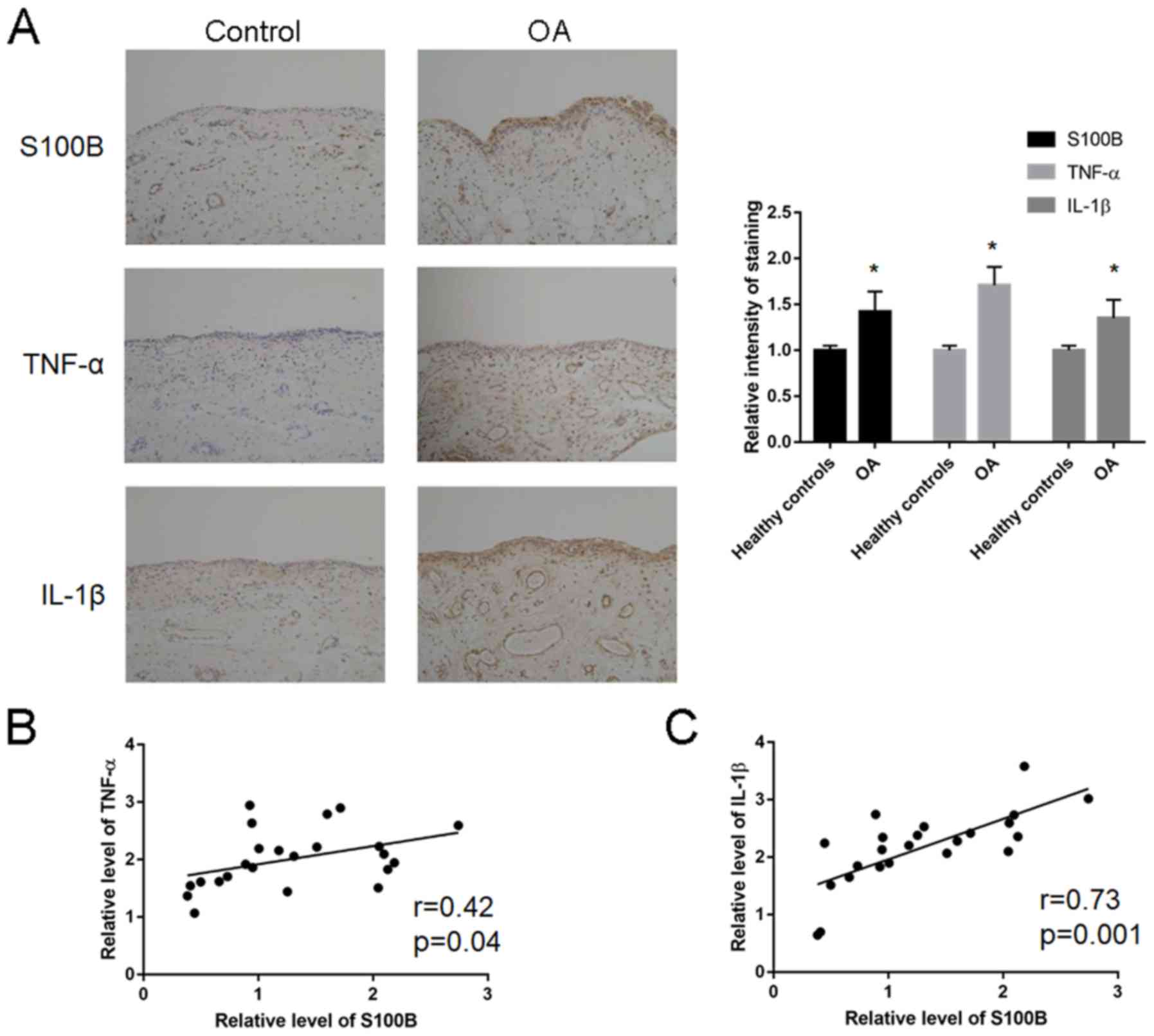
Expression analysis of S100B, TNF-α and IL-1β was
performed in human OA tissues (Fig.
1A; Table III). The relative
staining intensity of S100B (1.28±0.66 vs. 0.42±0.31; P=0.01),
TNF-α (2.17±0.63 vs. 1.02±0.61; P=0.02) and IL-1β (2.01±0.50 vs.
1.11±0.50; P=0.02) expression levels were significantly increased
in the patients with OA compared with Control patients. Correlation
analysis revealed a significant correlation between S100B and TNF-α
(r=0.42; P=0.04; Fig. 1B) and
between S100B and IL-1β (r=0.73; P=0.001; Fig. 1C).
 | Table III.Quantification of mRNA expression
levels of S100B, TNF-α and IL-1β in human OA tissues. |
Table III.
Quantification of mRNA expression
levels of S100B, TNF-α and IL-1β in human OA tissues.
| Gene name | OA (n=23) | Control (n=20) | P-value |
|---|
| S100B | 1.28±0.66 | 0.42±0.31 | 0.01 |
| TNF-α | 2.17±0.63 | 1.02±0.61 | 0.02 |
| IL-1β | 2.01±0.50 | 1.11±0.50 | 0.02 |
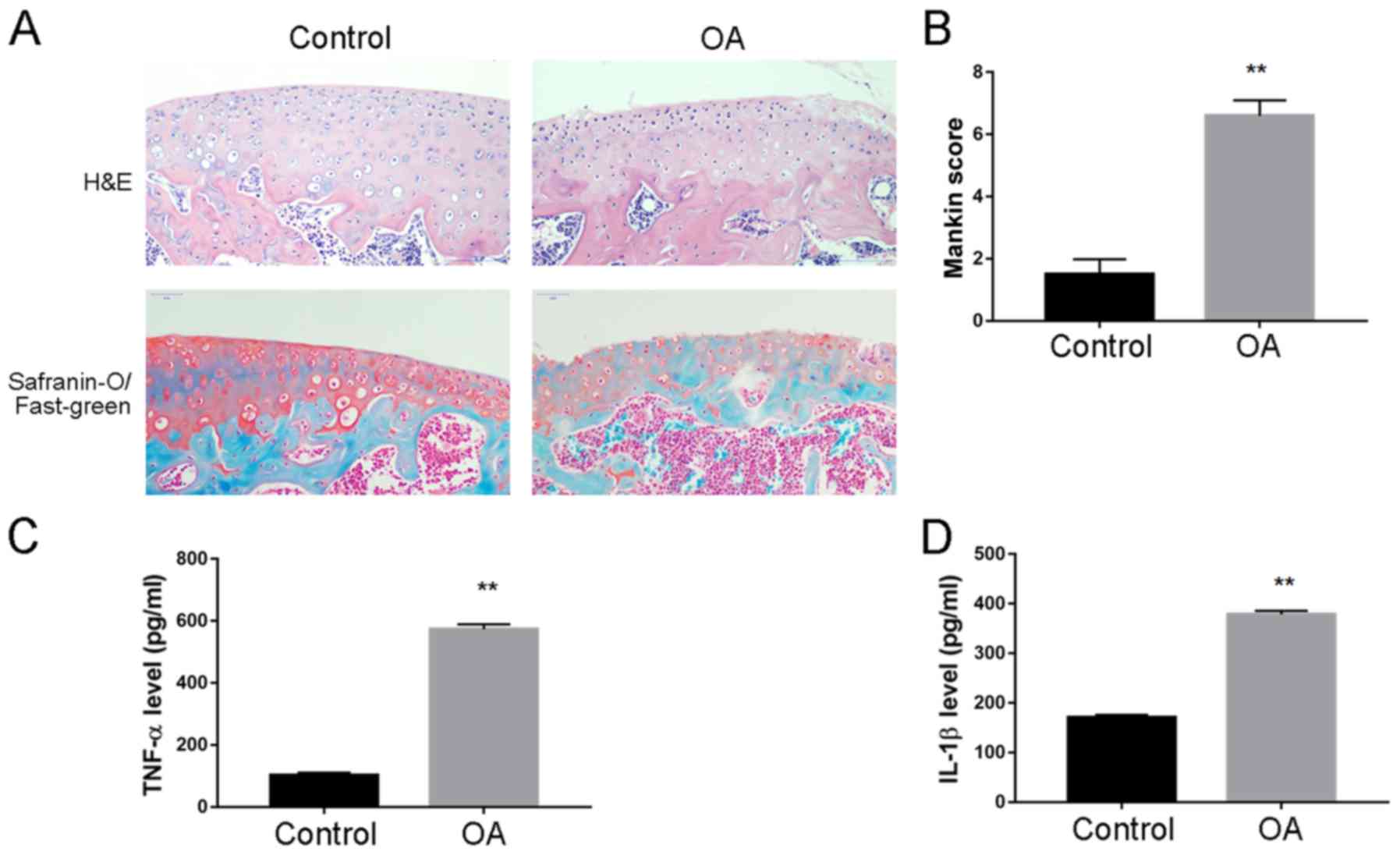
Increased expression levels of
inflammatory cytokines in synovial joint fluid in OA model
rabbits
A rabbit model of OA was established and the
cartilage tissue and synovial fluid were collected for disease
assessment. H&E staining revealed an increased cartilage tissue
destruction; there was decreased SO staining of the superficial
cartilage layer, as well as increased Mankin score, which indicated
successful establishment of OA model (Fig. 2A and B, respectively). ELISA
analysis was performed to quantify the level of inflammatory
cytokines in synovial fluid, and it was demonstrated that TNF-α
(573.3±15.4 vs. 102.6±8.7 pg; Fig.
2C) and IL-1β (378.6±7.2 vs. 170.1±5.8 pg; Fig. 2D) expression levels were
significantly increased in OA compared with Control rabbits.
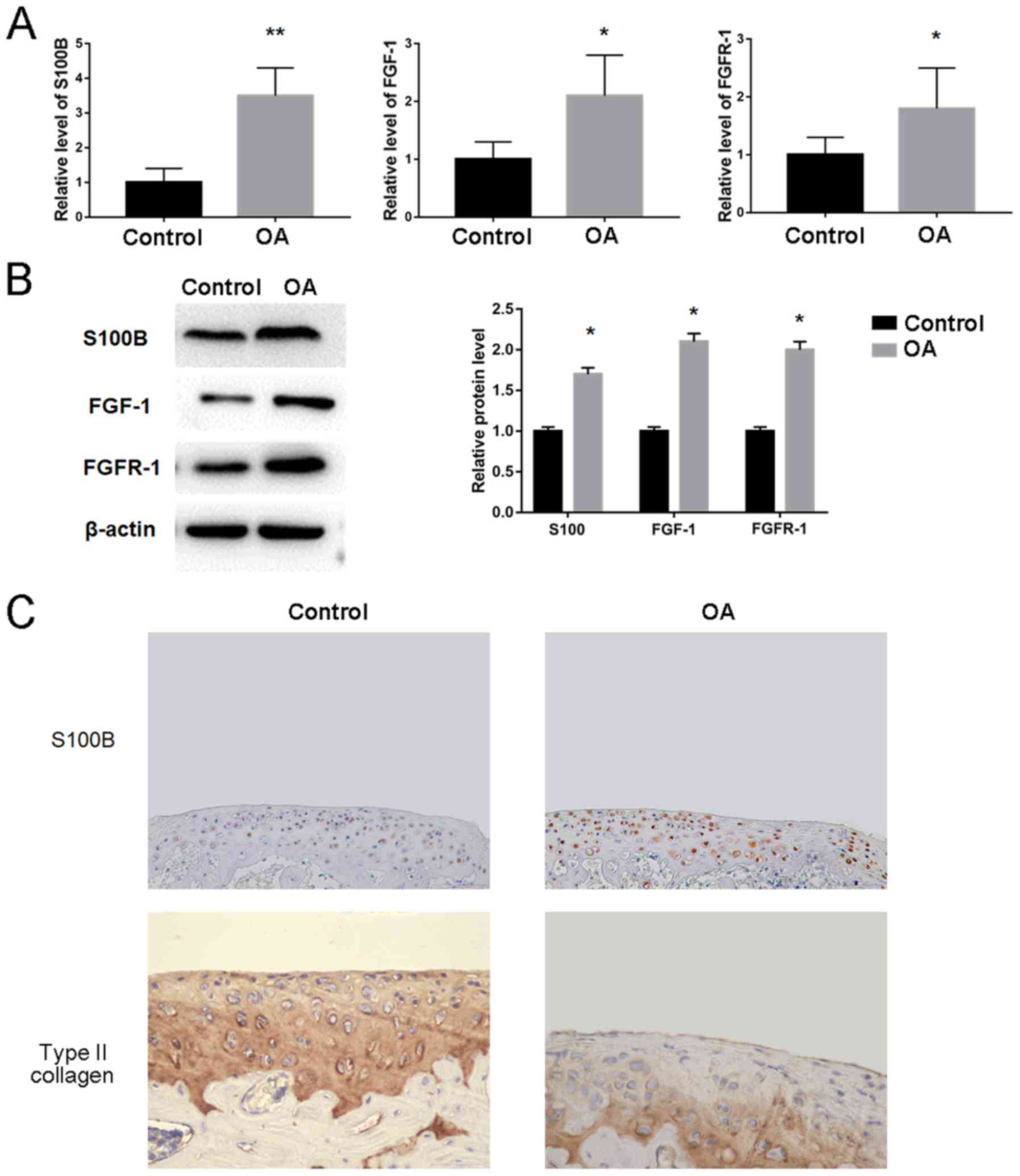
S100B expression levels are increased
in OA rabbits
Owing to the increased expression levels of
inflammatory cytokines in OA rabbits, the possible changes of the
genes including S100B, FGF1 and FGFR1 were investigated using OA
and Control cartilage tissue. The results demonstrated that S100B
mRNA (3.5±0.8 vs. 1.0±0.4) and protein (1.7-fold) expression levels
were significantly increased in OA compared with Control rabbits
(Fig. 3A and B, respectively).
Significant increases in expression were also observed for FGF1
mRNA (2.1±0.7 vs. 1.0±0.3) and protein (2.1-fold) levels, as well
as FGFR1 mRNA (1.8±0.7 vs. 1.0±0.3) and protein (2.0-fold) levels
in OA compared with the Control rabbits (Fig. 3A and B). In addition,
immunohistochemical staining confirmed that the expression levels
of S100B were increased and the level of type II collagen was
decreased in OA compared with the Control rabbits (Fig. 3C).
S100B regulates inflammatory cytokine
secretion in human synovial fibroblasts
Owing to the increased expression levels of S100B
and some inflammatory cytokines in OA rabbits, synovial fibroblasts
were isolated from normal human cartilage tissues and the
expression levels of inflammatory cytokines were analyzed in the
condition medium following manipulation of S100B expression by
overexpression vector or siRNA transfection (Fig. 4). The results indicated that S100B
overexpression significantly increased TNF-α (623.8±23.8 vs.
214.5±14.8 pg; Fig. 4B) and IL-1β
(1101.3±29.6 vs. 617.5±37.5 pg; Fig.
4C) expression levels in LPS-stimulated human synovial
fibroblasts compared with Control transfected cells, whereas S100B
knockdown decreased TNF-α (186.2±16.7 vs. 214.5±14.8 pg; Fig. 4E) and IL-1β (404.1±15.99 vs.
617.5±37.5 pg; Fig. 4F) expression
levels in the LPS-stimulated human synovial fibroblasts compared
with the Controls.
FGFR1 is involved in the
S100B-mediated inflammation response in LPS-treated synovial
fibroblasts
As S100B is an intracellular protein and
FGFR1-mediated signaling pathway is involved in the inflammatory
effects in synovial tissues (20),
the roles of S100B and FGFR1 were investigated. FGFR1 mRNA and
protein expression levels (Fig. 5A and
B, respectively) were increased in human synovial fibroblasts
cells overexpressing S100B and were significantly decreased in
cells treated with S100B siRNA knockdown, compared with Control
cells. Furthermore, FGFR1 knockdown was performed (Fig. 5C), and the results of the RT-qPCR
analyses revealed significantly decreased levels of FGF1 mRNA in
knockdown cells, which confirmed that transfection was successfully
performed. In addition, the change of inflammatory cytokine
expression into the condition medium of LPS stimulated human
synovial fibroblasts was investigated, and the results revealed
that increased FGFR1 expression induced by LPS and S100B
overexpression was significantly attenuated following treatment
with FGFR1 siRNA (Fig. 5D and E).
The results demonstrated that FGFR1 knockdown significantly
decreased TNF-α (197.9±16.7 vs. 631±33 pg; Fig. 5F) and IL-1β (588.7±33.1 vs. 1173±30
pg; Fig. 5G) secretion levels in
LPS-stimulated S100B-overexpressing human synovial fibroblasts.
 | Figure 5.FGFR1 mediates the inflammatory
effects of S100B. (A) FGFR1 mRNA expression levels were increased
or decreased in S100B-overexpressing and S100B-siRNA synovial
fibroblasts, respectively, as measured by RT-qPCR. (B) FGFR1
protein expression levels were increased or decreased in
S100B-overexpressing and S100B-siRNA synovial fibroblasts,
respectively, as measured by western blotting. (C) Validation of
the FGFR1 knockdown FGFR1, by RT-qPCR. (D) RT-qPCR and (E) western
blotting were used to determine FGFR1 expression levels in
LPS-simulated S100B overexpressed synovial fibroblasts. Increased
FGFR1 expression induced by LPS and S100B overexpression was
significantly attenuated by FGFR1 siRNA. (F) TNF-α expression
levels were decreased following FGFR1 knockdown in LPS-simulated,
S100B-overexpressing synovial fibroblasts. (G) IL-1β expression
levels were decreased following FGFR1 knockdown in LPS-simulated,
S100B-overexpressing synovial fibroblasts. **P<0.01 vs. LPS +
Empty vector or LPS + Scrambled siRNA; †P<0.05 vs.
LPS + Empty vector; $P<0.05 vs. LPS + Scrambled
siRNA; #P<0.05 vs. LPS + S100B + Scrambled siRNA.
IL-1β, interleukin 1β; FGFR, fibroblast growth factor receptor;
LPS, lipopolysaccharide; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; siRNA, small
interfering RNA; TNF-α, tumor necrosis factor. |
Discussion
In the present study, cartilage tissue and synovial
fluid from patients with OA and a rabbit model of OA, as well as
human synovial fibroblasts were used to investigate the role of
S100B in the inflammatory response during OA. The results
demonstrated that increased expression levels of the inflammatory
cytokines TNF-α and IL-1β in OA rabbits were accompanied with
significantly increased expression of S100B, FGF1 and FGFR1 at the
mRNA and protein level. Furthermore, S100B overexpression and
knockdown revealed its regulatory role on the production of TNF-α
and IL-1β in LPS-stimulated human synovial fibroblasts. In
addition, it was also demonstrated that FGFR1-mediated signaling
pathway may be involved in the effects of S100B on inflammatory
cytokines. To the best of our knowledge, this is the first study to
explore the role of S100B in inflammatory response regulation
during OA.
The S100 protein family includes 24 members that
function as intracellular and/or extracellular regulators (21). Within cells, S100 proteins serve
important roles in multiple biological processes, including cell
proliferation, differentiation, apoptosis, Ca2+
homeostasis, energy metabolism, inflammation and migration/invasion
through interactions with a variety of target proteins (such as
enzymes, cytoskeletal subunits, receptors, transcription factors
and nucleic acids). S100B is expressed in chondrocytes (22) and synovial fibroblasts (23). S100B has also been associated with
chronic inflammation conditions such as rheumatoid arthritis,
diabetes and cystic fibrosis (24); however, its role in inflammation
regulation during OA has not yet been explored. In the present
study, it was demonstrated that S100B mRNA and protein expression
levels were increased in OA rabbits and S100B overexpression and
knockdown experiments indicated a regulatory role on the production
of TNF-α and IL-1β in LPS-stimulated human synovial
fibroblasts.
FGFRs contain an extracellular ligand-binding domain
and an intracellular tyrosine kinase domain, and are single-pass
transmembrane receptors that belong to the receptor tyrosine
kinases (RTK) family (25).
Activation of RTKs by their corresponding ligands (such as FGFs)
activate kinases that subsequently initiate intracellular signaling
networks that regulate key cellular processes such as cell
proliferation, growth, differentiation, migration and survival
(25,26). Furthermore, the spatial and
temporal expression of ligands and receptors, as well as the
binding specificity between ligands and receptors may affect FGF
signaling pathway regulation (27,28).
Specificity of FGFR-mediated signaling depends on the cell type and
the maturation stage of the cell, and a number of different
signaling pathways are involved, including the mitogen-activated
protein kinase pathway (extracellular signal-regulated kinases 1
and 2, p38, and c-Jun N-terminal kinases), the phosphoinositide
3-kinase/Akt pathway, and the phospholipase Cγ pathway (29). As FGF ligands are involved in
articular cartilage maintenance, it is reasonable to postulate that
FGFRs may also serve a crucial role in articular cartilage
homeostasis. Notably, highly expressed FGFR1 and FGFR3 in human
articular chondrocytes have been implicated in cartilage metabolism
(30). In myoblasts, extracellular
S100B was demonstrated to modulate differentiation, stimulate
proliferation and reduce apoptosis in vitro by engaging its
multi-ligand receptor, receptor for advanced glycosylation
end-products, or enhancing basic-FGF (bFGF)-FGFR1 signaling,
depending on myoblast density (31,32).
In early acute injuries, muscles release S100B prior to bFGF and
high mobility group box protein (HMGB)-1, and this initial
expression diminishes over time, suggesting that S100B may regulate
the initial phases of the regeneration process (24). According to a previous study,
synovial macrophages and fibroblasts were demonstrated to actively
secrete HMGB1 in collagen-induced arthritis and in hypoxic
conditions (33). These
similarities between myoblasts and bone tissues may indirectly
support the association between S100B and FGFR1 in synovial
macrophages. The present results indicated that FGF1 and FGFR1
expression levels were significantly increased during OA and were
consistent with the pattern of increased S100B expression.
Furthermore, a previous study demonstrated that silencing of FGFR1
in adult mouse articular chondrocytes inhibits cartilage
degeneration progression (34).
Knockdown FGFR1 in S100B-overexpressing human synovial fibroblasts
in the present study demonstrated that FGFR1 knockdown may
attenuate the inflammatory effects exerted by S100B. These data
provided a potential link between FGFR1, S100B and inflammatory
effects. However, owing to the lack of a purified S100B, whether
S100B may also interact with bFGF extracellularly, as previously
described (35), were not explored
in the present study.
Furthermore, spontaneous OA models, which were not
induced via gene modification methods such as knockout mice or
animals, would provide the best models to study the aging phenotype
as they represent primary OA (36). In the present study an OA model
rabbit was established, which is also a spontaneous OA model.
Compared with the more expensive genetically modified models (fit
for specific gene function investigation) and large animal models
(suitable for therapy test) (36),
the present model could mimic OA disease progression condition with
a lower cost.
In conclusion, it was demonstrated that S100B may be
involved in the FGFR1-mediated inflammatory response during OA,
which may be considered as a potential therapeutic target; however,
further studies are needed to confirm the present findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by Science and
Technology Plan of Suzhou Municipal Government (grant. no.
SYS201502, to Lifan Zhu) and Ke Jiao Xing Wei Plan of Wujiang
District (grant no. wwk201704, to Pengcheng Shen).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, ZW, PS and HY analyzed and interpreted the
patient data. LZ, ZW, PS, JZhou, JZen, FW, XZ and HY performed the
experiments and analyzed the data. LZ, ZW, PS and HY contributed to
the writing of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Soochow University; written informed consent was
provided by all participants. The animal study protocol was
approved by the Institutional Animal Care and Use Committee of
Soochow University and was conducted following the international
guidelines for animal experimentation.
Patient consent for publication
Patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palazzo C, Nguyen C, Lefevre-Colau MM,
Rannou F and Poiraudeau S: Risk factors and burden of
osteoarthritis. Ann Phys Rehabil Med. 59:134–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prieto-Alhambra D, Judge A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lohmander LS and Roos EM: Clinical update:
Treating osteoarthritis. Lancet. 370:2082–2084. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karsdal MA, Michaelis M, Ladel C, Siebuhr
AS, Bihlet AR, Andersen JR, Guehring H, Christiansen C, Bay-Jensen
AC and Kraus VB: Disease-modifying treatments for osteoarthritis
(DMOADs) of the knee and hip: Lessons learned from failures and
opportunities for the future. Osteoarthritis Cartilage.
24:2013–2021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sellam J and Berenbaum F: The role of
synovitis in pathophysiology and clinical symptoms of
osteoarthritis. Nat Rev Rheumatol. 6:625–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sorci G, Bianchi R, Riuzzi F, Tubaro C,
Arcuri C, Giambanco I and Donato R: S100B protein, a
damage-associated molecular pattern protein in the brain and heart,
and beyond. Cardiovasc Psychiatry Neurol. 2010:6564812010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sorci G, Riuzzi F, Arcuri C, Tubaro C,
Bianchi R, Giambanco I and Donato R: S100B protein in tissue
development, repair and regeneration. World J Biol Chem. 4:1–12.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yammani RR: S100 proteins in cartilage:
Role in arthritis. Biochim Biophys Acta. 1822:600–606. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yammani RR, Carlson CS, Bresnick AR and
Loeser RF: Increase in production of matrix metalloproteinase 13 by
human articular chondrocytes due to stimulation with S100A4: Role
of the receptor for advanced glycation end products. Arthritis
Rheum. 54:2901–2911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang ZY, Stabler T, Pei FX and Kraus VB:
Both systemic and local lipopolysaccharide (LPS) burden are
associated with knee OA severity and inflammation. Osteoarthritis
Cartilage. 24:1769–1775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferdowsian HR and Beck N: Ethical and
scientific considerations regarding animal testing and research.
PLoS One. 6:e240592011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R and
Hochberg M: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee. Diagnostic and therapeutic criteria committee of the
American rheumatism association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogura N, Tobe M, Sakamaki H, Kujiraoka H,
Akiba M, Abiko Y and Nagura H: Interleukin-1beta induces
interleukin-6 mRNA expression and protein production in synovial
cells from human temporomandibular joint. J Oral Pathol Med.
31:353–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji Y, Strawn TL, Grunz EA, Stevenson MJ,
Lohman AW, Lawrence DA and Fay WP: Multifaceted role of plasminogen
activator inhibitor-1 in regulating early remodeling of vein bypass
grafts. Arterioscler Thromb Vasc Biol. 31:1781–1787. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang J, Tang L, Lei H, Zhang XG, Zuo Z,
Huang W and Fu H: Effects of lentiviral infection of mesenchymal
stem cells on the expression of octamer transcription factor 4. Mol
Med Rep. 10:2249–2254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta AA, Chou RH, Li H, Yang LW and Yu C:
Structural insights into the interaction of human S100B and basic
fibroblast growth factor (FGF2): Effects on FGFR1 receptor
signaling. Biochim Biophys Acta. 1834:2606–2619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donato R, Cannon BR, Sorci G, Riuzzi F,
Hsu K, Weber DJ and Geczy CL: Functions of S100 proteins. Curr Mol
Med. 13:24–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donato R, Sorci G, Riuzzi F, Arcuri C,
Bianchi R, Brozzi F, Tubaro C and Giambanco I: S100B's double life:
Intracellular regulator and extracellular signal. Biochim Biophys
Acta. 1793:1008–1022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bo GP, Zhou LN, He WF, Luo GX, Jia XF, Gan
CJ, Chen GX, Fang YF, Larsen PM and Wu J: Analyses of differential
proteome of human synovial fibroblasts obtained from arthritis.
Clin Rheumatol. 28:191–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hofmann MA, Drury S, Fu C, Qu W, Taguchi
A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al: RAGE
mediates a novel proinflammatory axis: A central cell surface
receptor for S100/calgranulin polypeptides. Cell. 97:889–901. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schlessinger J: Cell signaling by receptor
tyrosine kinases. Cell. 103:211–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turner N and Grose R: Fibroblast growth
factor signalling: From development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dailey L, Ambrosetti D, Mansukhani A and
Basilico C: Mechanisms underlying differential responses to FGF
signaling. Cytokine Growth Factor Rev. 16:233–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ornitz DM and Itoh N: The fibroblast
growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol.
4:215–266. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang J, Su N, Zhou S, Xie Y, Huang J, Wen
X, Wang Z, Wang Q, Xu W, Du X, et al: Fibroblast growth factor
receptor 3 inhibits osteoarthritis progression in the knee joints
of adult mice. Arthritis Rheumatol. 68:2432–2443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riuzzi F, Beccafico S, Sagheddu R,
Chiappalupi S, Giambanco I, Bereshchenko O, Riccardi C, Sorci G and
Donato R: Levels of S100B protein drive the reparative process in
acute muscle injury and muscular dystrophy. Sci Rep. 7:125372017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Juban G and Chazaud B: Metabolic
regulation of macrophages during tissue repair: Insights from
skeletal muscle regeneration. FEBS Lett. 591:3007–3021. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bertheloot D and Latz E: HMGB1, IL-1α,
IL-33 and S100 proteins: Dual-function alarmins. Cell Mol Immunol.
14:43–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weng T, Yi L, Huang J, Luo F, Wen X, Du X,
Chen Q, Deng C, Chen D and Chen L: Genetic inhibition of fibroblast
growth factor receptor 1 in knee cartilage attenuates the
degeneration of articular cartilage in adult mice. Arthritis Rheum.
64:3982–3992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Riuzzi F, Sorci G and Donato R: S100B
protein regulates myoblast proliferation and differentiation by
activating FGFR1 in a bFGF-dependent manner. J Cell Sci.
124:2389–2400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuyinu EL, Narayanan G, Nair LS and
Laurencin CT: Animal models of osteoarthritis: Classification,
update, and measurement of outcomes. J Orthop Surg Res. 11:192016.
View Article : Google Scholar : PubMed/NCBI
|