Introduction
Apoptosis and necrosis are two principal mechanisms
of eukaryotic cell death that can be distinguished biochemically
and morphologically. Apoptosis is a highly regulated and controlled
process, requiring ATP and the activation of specific proteases and
caspases. Meanwhile, necrosis results from membrane damage either
directly or indirectly due to energy depletion. Therefore, necrosis
is strongly pro-inflammatory in vivo, whereas apoptotic
cells are rapidly phagocytosed and thus generate minimal
inflammation (1). In addition to
apoptosis and necrosis, necroptosis as a programmed necrosis has to
come to light. Necroptosis is generally initiated by the engagement
of TNFR1, a receptor for tumor-necrosis factor (TNF), which in turn
activates receptor interacting protein 1 (RIP1) and 3 (RIP3), and
leads to necrotic cell death (2,3).
Salmonella-induced cytotoxicity occurring in
murine macrophages had been previously described as apoptosis,
because the cells exhibited characteristic features of apoptosis
(4). On the other hand, other
reports revealed that Salmonella-infected macrophages were
killed by an unusual caspase-1-dependent mechanism of necrosis
(5,6), suggesting that
Salmonella-induced macrophage death could share features of
both apoptosis and necrosis. However, another study showing
Salmonella-induced necroptosis of macrophages by production
of type I interferon (IFN) suggested the importance of necroptosis
on macrophage death and pathogenesis of the infection (7,8).
Therefore, the precise mechanism of macrophage death by
Salmonella infection remains to be elucidated.
MicroRNAs (miRNAs) are short (21–25 nt), endogenous,
and non-coding RNA molecules, which regulate gene expression
post-translationally by imperfect binding to the target sequences.
In mammals, miRNAs are estimated to regulate approximately 50% of
all protein-coding genes and play important roles in several
biological processes, such as cell proliferation and
differentiation, signal transduction, metabolism, tumorigenesis,
and progression (9,10). Moreover, accumulating evidences
have suggested that some miRNAs play an important role in cell
survival and death (11,12). During the initial stages of
infection, innate immune responses effectively control the
replication and survival of Salmonella. A microarray study
revealed that miRNA-155 (miR-155) was a sole miRNA that was
substantially up-regulated by both polyriboinosinic:
polyribocytidylic acid and IFN-γ stimuli, suggesting that miR-155
can act as a component of the primary macrophage response to
different types of inflammatory mediators (13). Furthermore, modulation of
miR-146a/b, miR-155, and miR-21 was reported in Salmonella
infection, with NF-κB-dependent miRNAs significantly induced upon
infection in mouse macrophages (14,15).
Although miR-155 was first discovered in children
with Burkitt lymphoma and further found to act as an oncogene or a
tumor suppressor in different types of cancer (16,17),
growing evidences have suggested that miR-155 has been considered
as an important pleiotropic regulator of cell homeostasis and a
typical multifunctional miRNA that regulates multiple
pathophysiological pathways (18,19).
In 2011, Liu et al (20)
showed that miR-155 was expressed in growing human cardiomyocyte
progenitor cells (CMPCs) and attenuated the CMPC necrosis induced
by oxidative stress via targeting RIP1, a death domain protein
required for the activation of necroptosis (21). Recently, other reports revealed
that lipopolysaccharide (LPS)-induced miR-155 prevented apoptosis
through CASP-3 mRNA down-regulation in RAW 264.7 macrophages, as a
prerequisite to maintain their crucial function in inflammation
(22). It was also revealed that
miR-155 was up-regulated in a number of osteosarcoma cell lines and
the inhibition of this miRNA led to cancer cell death through
MAP3K10 as a target (23). Thus,
this suggests that miR-155 can prevent cell death through different
targets with cell-type specificity.
In this study, we report the possible role of
miR-155 being highly up-regulated in Salmonella-infected
murine macrophages. Differing from previous studies, our results
display that the up-regulation of miR-155 in RAW 264.7 macrophages
by Salmonella infection enhances cell death by necroptosis
via targeting both RIP1/3 and poly(ADP-ribose) polymerase-1
(PARP-1).
Materials and methods
Cell culture and Salmonella
infection
Murine Raw 264.7 macrophages were routinely cultured
in DMEM supplemented with 10% heat-inactivated fetal bovine serum
and 1% penicillin-streptomycin, and maintained at 37°C in a
humidified incubator (5% CO2). Gram-negative
Salmonella enterica serovar Typhimurium SL1344 strain
(hereafter referred to as Salmonella) was cultured in low
salt Luria Bertani broth at 37°C with aeration.
Raw 264.7 cells (1×106 cells/ml) were
infected with Salmonella (3×107 CFU/ml) at a
multiplicity of infection (MOI) of 10. Cells were washed with
phosphate-buffered saline (PBS) 1 h after infection, followed by
the addition of gentamicin (100 µg/ml) for the remainder of the
infection. At the indicated times, cells were imaged using
microscopy (IX71 instrument, ×20; Olympus Corporation, Tokyo,
Japan) and were harvested for further analysis.
MiRNA screening and analysis
MiRNA expression in mock infection control and
Salmonella-infected macrophages was assessed using
GeneChip® miRNA 3.0 Array (miRBase v17; Affymetrix;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) as previously
described (24). The collected
data were analyzed by performing Robust MultiArray Average (RMA)
and detection above background (DABG) analysis using Affymetrix
default analysis settings and were normalized by global scaling.
Variations in miRNA expression were analyzed for predicting target
miRNAs using TargetScan v7.1 (www.targetscan.org/vert_71/), with a context score
percentile of over 90 for determining differentially expressed
miRNAs.
MiRNAs identified from primary screening were
further analyzed to determine secondary target miRNAs. Firstly,
high reproducibility under the repetitive test, secondly, a base
log value of >7.0 for both mock-infected and
Salmonella-infected macrophages, and finally, log ratio of
>1.0 between Salmonella-infected and mock infection
control macrophages were the cut-off values of miRNA used for the
selection of secondary target miRNAs.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for miRNA-155-5p detection
Total DNA-free RNAs were isolated with TRI Reagent
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the
manufacturer's instructions. RT-PCR was performed at 37°C for 1 h
then at 85°C for 5 min to inactivate the enzymes. Expression of
miRNAs was analyzed using Mir-X miRNA RT-qPCR SYBR kit (Clontech
Laboratories, Inc., Mountainview, CA, USA) according to the
manufacturer's protocol. A 5′ primer specific for mmu-miR-155-5p
detection (accession no. MIMAT0000165,
5′-UUAAUGCUAAUUGUGAUAGGGGU-3′) was used in RT-qPCR. The
thermocycling conditions for qPCR were as follows: 95°C for 10 sec
followed by 40 cycles of 95°C for 5 sec and 60°C for 20 sec. Level
of miRNA-155-5p was normalized using the U6 small nuclear RNA
(NR_003027; Clontech Laboratories, Inc.) as an internal control and
the relative quantity was determined using the 2−∆∆Cq
method (25).
MiRNA transfection
Non-targeting control miRNA (nc-miR), anti-miR
inhibitor for non-targeting control miRNA (anti-nc-miR), miR-155-5p
miRNA (miR-155), and anti-miR inhibitor for miR-155-5p
(anti-miR-155) were synthesized by Cosmogenetech (Cosmogenetech
Co., Ltd., Seoul, South Korea). Raw 264.7 cells were transfected
with 30 nM of individual miRNAs with Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Transfection efficiency of the miRNAs was confirmed
by RT-PCR.
Cytotoxicity assays
Raw 264.7 macrophages were seeded onto a 96-well
plate at a density of 3×104 cells per well and were
infected with Salmonella at an MOI of 10
(Salmonella), along with mock infection (mock).
Simultaneously, cells in other wells were transfected with 30 nM of
synthetic miRNAs. At the indicated time points (6, 18, 24, and 48
h) after infection or transfection, cell culture supernatants were
collected for lactate dehydrogenase (LDH)-release assay, and the
remaining cells in each well were used in neutral red uptake (NRU)
assay (26). Cell death was
quantified by the colorimetric method using LDH Cytotoxicity
Detection kit (Takara Bio, Inc., Otsu, Japan). Cell viability was
assessed using NRU assay. Briefly, NRU assay consisted of 2 h
incubation with neutral red (40 µg/ml) followed by extraction with
a mixture of acetic acid, ethanol, and water (1:50:49). Absorbance
was measured at 540 nm.
Western blot analysis
Cells were rinsed twice with PBS, and proteins were
extracted in cold lysis buffer containing 1% protease inhibitor
mixture. Protein concentration was determined by BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.). Equal amounts of protein were
resolved by SDS-PAGE, and transferred onto a nitrocellulose
membrane. The membrane was blocked with 5% non-fat milk in T-PBS
(0.1% Tween-20) for 1 h, and then incubated with the indicated
primary antibody overnight at 4°C. After 1 h incubation with
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody (cat. no: 7074; Cell Signaling Technology, Inc., Danvers,
MA, USA), signals were visualized with Pierce ECL Western blotting
substrate (Thermo Fisher Scientific, Inc.). The primary antibodies
used were as follows: RIP 1 antibody (1:1,000, rabbit mAb, cat. no:
3493; Cell Signaling Technology, Inc.), RIP3 antibody (1:1,000,
rabbit mAb, cat. no: 14401; Cell Signaling Technology, Inc.) and
PARP-1 antibody (1:1,000, rabbit polyclonal, sc-7150; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA).
Statistical analysis
Statistical analyses were performed with SPSS v24.0
(IBM Corp., Armonk, NY, USA). Measurement data were presented as
the mean ± standard deviation. Data were analyzed by a Student's
t-test or one-way analysis of variance with Dunnett's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
MiR-155 was highly up-regulated upon
Salmonella infection
Secondary target miRNA analysis identified 18 miRNAs
which were up-regulated in Salmonella-infected murine Raw
264.7 macrophages, compared with mock infection control macrophages
(Fig. 1). Of the 18 target miRNAs,
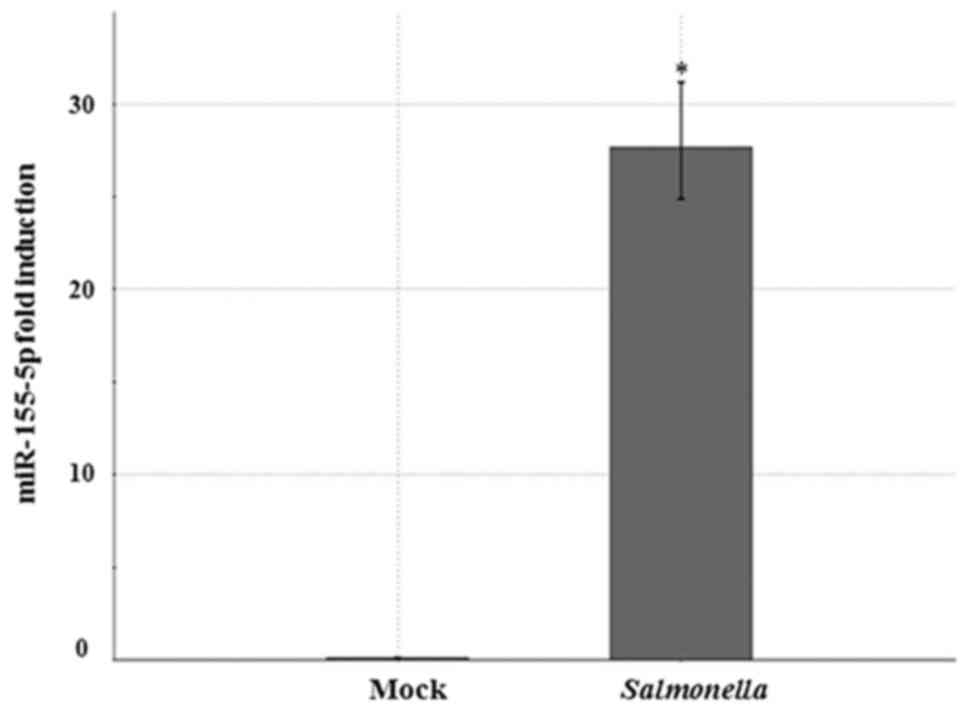
miR-155-5p had the highest expression ratio. When miR-155-5p
expression rate was determined using RT-qPCR assay in murine
macrophages infected with Salmonella for 24 h, miR-155-5p
levels increased 28-fold (Fig. 2),
showing that miR-155 is highly up-regulated upon Salmonella
infection.
Salmonella induced miR-155-mediated
cytotoxicity
Microscopic observations showed that
Salmonella infection for 48 h resulted in increased
macrophage death, when compared to mock infection control. Notably,
macrophage transfection study with synthetic miRNAs for 48 h
revealed that only miR-155 caused remarkable macrophage death,
similar to the result of Salmonella infection (data not
shown). To test whether miR-155 is involved in this
Salmonella cytotoxicity, macrophages were infected with
Salmonella or transfected with miRNAs, followed by
evaluation of their viability and death rates (%) at 48 h using NRU
and LDH-release assays, respectively. The results revealed that
cell viability decreased to 50% and cell death ratio increased to
30% at 48 h after Salmonella infection, compared to those of
mock infection (Fig. 3A and B).
Viability of macrophages transfected with miR-155 decreased up to
40% at 48 h post transfection, similar to that of
Salmonella-infected cells, while either anti-miR-155 or
nc-miR and anti-nc-miR as negative controls had a little or no
change in cell viability (Fig.
3A). Cell death ratios also showed results similar to those of
the viability analysis (Fig. 3B),
indicating that miR-155 could enhance death of macrophages.
Importantly, pre-transfection of anti-miR-155 into macrophages
prior to Salmonella infection relieved the Salmonella
cytotoxicity up to 20% (Fig. 4),
suggesting that miR-155 induction by Salmonella infection is
largely related to Salmonella toxicity on macrophages.
MiR-155 enhanced necrotic cell death
by targeting RIP1/3 activation
To test whether miR-155 induction by
Salmonella infection leads to macrophage death by
necroptosis, we examined the activation of RIP1 and RIP3 in
macrophages infected with Salmonella by Western blot
analysis. The activation of RIP1 and RIP3 were not observed at 6 h
after Salmonella infection (data not shown), consistent with
the delayed-death phenotype observed in cell viability and death
analysis (Fig. 3A and B). However,
upshifted forms representing phosphorylated RIP1 and RIP3 proteins
upon activation appeared at 18 h after Salmonella infection,
indicating necrotic cell death by Salmonella infection
(Fig. 5). Notably, the upshifts of
both RIP1 and RIP3 proteins similarly occurred at 18 h after
miR-155 transfection and these forms were not observed by the
transfection of anti-miR-155, nc-miR or anti-nc-miR, suggesting
that miR-155 up-regulation upon Salmonella infection causes
necrotic cell death of macrophages through RIP1 and RIP3
activation. Subsequently, we tested the effect of necrostatin-1
(Nec-1), a RIP1 specific inhibitor, on cell viability upon
Salmonella infection. As shown in Fig. 6, macrophages infected with
Salmonella or transfected with miR-155 showed approximately
40% reduction of cell viability. However, these reductions
attenuated up to 20% by the pre-treatment of macrophages with 10 µM
of Nec-1 for 24 h, suggesting that Nec-1 partly rescued the
macrophage death by either Salmonella infection or miR-155
transfection, and that RIP1 is involved in
Salmonella-induced cytotoxicity mediated by miR-155.
To further test whether this increase in cell death
by miR-155 up-regulation is caused by DNA damage, namely apoptosis
and necrosis, we scrutinized PARP-1 cleavage in macrophages after
Salmonella infection or miRNAs transfection by Western blot
analysis. In Raw 264.7 cells infected with Salmonella,
PARP-1 displayed an absence of the mature, uncleaved protein in 24
h (Fig. 7). This cleavage pattern
was detected only in miR-155 transfected cells, but not in
anti-miR-155, nc-miR, or anti-nc-miR transfected cells, showing
that miR-155 induction by Salmonella infection could enhance
PARP-1 cleavage.
Discussion
Salmonella induces macrophage death to
effectively avoid host innate immune responses as an essential
strategy for virulence (4,27). Firstly, a 454 deep sequencing
analysis revealed that host miRNA expression changed upon
Salmonella infection in RAW 264.7 macrophages and that
NF-kB-associated miRNAs, such as miR-155, miR-146a/b, and miR-21,
were shown to be strongly induced (14). Similar results were also observed
in human monocytes, suggesting that miRNAs play a first line host
defense against bacterial invasion (28,29).
However, these miRNAs did not respond to Salmonella
infection in epithelial HeLa cells, showing that certain miRNA
responses to bacterial infection could be cell-type specific
(14).
Among miRNAs, miR-155 is one of the best
characterized miRNAs. Dysregulation of miR-155 expression has been
reported in different types of cells. Firstly, miR-155 is highly
expressed in both activated B and T cells (30,31),
as well as in activated macrophages and monocytes (28). MiR-155 is also highly up-regulated
upon LPS stimulation of human primary dendritic cells (DCs) and
during DC maturation (32,33). A microarray analysis in bone
marrow-derived macrophages revealed that LPS significantly enhanced
miR-155 maturation from its precursors (34). Consistent with other results
(14,28), our microarray study also showed
that miR-155 was highly induced in RAW 264.7 cells upon
Salmonella infection. Besides miR-155 induction, we also
identified four immensely up-regulated miRNAs (miR-762, miR-2137,
miR-3547-5p and miR-6366). The possible roles of these miRNAs
induced by Salmonella infection are not known yet and remain
to be determined. We further validated that Salmonella
infection resulted in a 28-fold increase of miR-155 level by
RT-qPCR. From cell viability and cell death analysis, we
established that Salmonella infection or miR-155
transfection caused about 30% death of macrophages in 48 h.
Furthermore, the pretreatment of anti-miR-155 on macrophages before
Salmonella infection increased the cell viability up to 20%,
indicating that miR-155 may be a key regulator for causing
macrophage death by Salmonella.
Whether miR-155 is either enhancing or preventing
cell death is debatable, depending on the cell type. Evidences for
miR-155 anti-apoptotic activity were reported from several cell
types, including B lymphocytes (35). When macrophages are tested with
various stimuli including cisplatin, Helicobacter pylori
infection, and LPS, it can be observed that miR-155 enhances
macrophage resistance to apoptosis (22,36,37).
Targeting miR-155 in acute myelogenous leukemia cell HL-60 lines
reveals both cell growth inhibition and apoptosis induction
(38). When MG-63 osteosarcoma
cells are transfected with anti-miR-155, cell proliferation is
significantly reduced, demonstrating that miR-155 deficiency may
result in apoptotic cell death (39). However, several reports have
contrarily proven that miR-155 could lead to increased apoptosis
and cell cytotoxicity, as shown in our study. For example, the
overexpression of miR-155 in DCs results in p27kip1 protein
increase and apoptosis in DCs (33). Inhibition of miR-155, which is
highly up-regulated in the myocardium of LPS-treated mice,
decreases apoptosis in sepsis-induced cardiomyopathy through
targeting Pea15a (40).
PARP-1 is known to play two contradictory roles
during apoptotic cell death. Its stimulation leads to poly
(ADP-ribose) synthesis, whereas caspase activation causes PARP-1
cleavage and inactivation. In apoptotic cells, caspase-3-mediated
proteolysis causes DNA fragmentation and nuclear condensation, and
PARP-1 cleavage by caspases saves cellular energy levels. Thus,
D'Amours et al (41)
reported a support model in which the inactivation of PARP-1 by
caspase cleavage facilitates apoptosis by preventing DNA
repair-induced survival and by blocking energy depletion-induced
necrosis. However, caspase-3 is not activated during
Salmonella infection, and PARP-1 remains in its active,
uncleaved state, proposing that an unusual caspase-1-dependent
mechanism of necrosis is a cause for Salmonella-infected
macrophage death (5). Since
inhibition of PARP-1 fails to protect Jurkat cells from
necroptosis, activation of PARP-1 may not be involved in
necroptosis (42). Nevertheless,
the results showing that Nec-1, a RIP1 specific inhibitor, blocks
translocation of apoptosis inducing factor from the mitochondria to
the nucleus and inhibits activation of PARP-1 (43,44)
suggest that PARP-1 induced necrosis may act downstream of
necroptosis (2). Moreover, PARP-1
could be a direct or indirect substrate for RIP kinase1 (RIPK1)-
and/or RIPK3-mediated kinase cascade (45), because PARP-1 activity may be
regulated by phosphorylation (46). Both RIPK1 and RIPK3 are required
for initiation of necroptosis, but it can also occur in the absence
of RIPK1 (47,48), suggesting that RIPK3 drives
necroptosis whereas RIPK1 is involved in specific cellular
contexts.
Here, we found that upshifts of both RIP1 and RIP3
were observed in RAW264.7 macrophages by either Salmonella
infection or miR-155 transfection, and that pre-treatment of Nec-1
also similarly enhanced the cell viability up to 20% in both
Salmonella-infected and miR-155-transfected macrophages,
suggesting that activation of RIP1 and RIP3 by miR-155 may induce
macrophage death by necroptosis. During a previous study on HT-29
cells, a human colorectal adenocarcinoma cell line, RIP3 activation
following the induction of necroptosis requires the activity of
HSP90 and CDC37 cochaperone complex, and this activation induces
upshift of both RIP1 and RIP3 by phosphorylation (49). In addition to RIP1/3 activation by
miR-155, we also found that PARP-1 displayed an absence of the
mature, uncleaved protein at 24 h in both
Salmonella-infected and miR-155-transfected cells. This
atypical size shift and disappearance of PARP-1 protein was
previously reported in L929T cells undergoing TNF-induced
necroptosis, and this phenomenon was not due to a general
necroptotic destruction of cellular proteins by caspases (50,51).
Other reports also showed that the disappearance of PARP-1 signal
is an indicator of PARP-1 activation rather than PARP-1
destruction. The disappearance of PARP-1 signal during TNF-induced
necroptosis was due to heavy poly ADP-ribosylation, rendering
PARP-1 inaccessible for detection by PARP-1 antibodies (3).
Taken together, our results revealed that miR-155
up-regulation in macrophages by Salmonella infection causes
macrophage death and it may be mediated by both RIP1/3-related
necroptosis and PARP-1-mediated necrosis. Differing from our
results, however, overexpressing miR-155 in CMPCs revealed that
miR-155 attenuated necroptosis by about 40% via targeting RIP1,
independent of the activation of Akt pro-survival pathway (20). Hereby, we propose that miR-155 is a
potential alternative either for inducing cell death or for
improving cell survival by targeting the RIP family. Additionally,
it has been observed that it acts with cell-type specificity.
Acknowledgements
Not applicable.
Funding
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
20171D1A1B04031530).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHL conceived and designed the experiments. YTR, GHJ
and SAJ performed the experiments. YTR, EHL, JDS and JHL analyzed
the data. YTR wrote the paper. EHL, JDS and JHL revised the
manuscript critically for important intellectual content. All of
the authors have read and approved the paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CMPCs
|
cardiomyocyte progenitor cells
|
|
DCs
|
dendritic cells
|
|
IFN
|
interferon
|
|
LDH
|
lactate dehydrogenase
|
|
LPS
|
lipopolysaccharide
|
|
miRNAs
|
microRNAs
|
|
MOI
|
multiplicity of infection
|
|
Nec-1
|
necrostatin-1
|
|
NRU
|
neutral red uptake
|
|
PARP-1
|
poly (adenosine diphosphate-ribose)
polymerase-1
|
|
RIP
|
receptor interacting protein
|
|
RIPK
|
RIP kinase
|
|
TNF
|
tumor-necrosis factor
|
References
|
1
|
Majno G and Joris I: Apoptosis, oncosis,
and necrosis. An overview of cell death. Am J Pathol. 146:3–15.
1995.PubMed/NCBI
|
|
2
|
Wu W, Liu P and Li J: Necroptosis: An
emerging form of programmed cell death. Crit Rev Oncol Hematol.
82:249–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sosna J, Voigt S, Mathieu S, Lange A, Thon
L, Davarnia P, Herdegen T, Linkermann A, Rittger A, Chan FK, et al:
TNF-induced necroptosis and PARP-1-mediated necrosis represent
distinct routes to programmed necrotic cell death. Cell Mol Life
Sci. 71:331–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen LM, Kaniga K and Galán JE: Salmonella
spp. are cytotoxic for cultured macrophages. Mol Microbiol.
21:1101–1115. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brennan MA and Cookson BT: Salmonella
induces macrophage death by caspase-1-dependent necrosis. Mol
Microbiol. 38:31–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watson PR, Gautier AV, Paulin SM, Bland
AP, Jones PW and Wallis TS: Salmonella enterica serovars
Typhimurium and Dublin can lyse macrophages by a mechanism distinct
from apoptosis. Infect Immun. 68:3744–3747. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robinson N, McComb S, Mulligan R, Dudani
R, Krishnan L and Sad S: Type I interferon induces necroptosis in
macrophages during infection with Salmonella enterica serovar
Typhimurium. Nat Immunol. 13:954–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang S and Qin X: Critical role of type I
interferon-induced macrophage necroptosis during infection with
Salmonella enterica serovar Typhimurium. Cell Mol Immunol.
10:99–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borralho PM, Kren BT, Castro RE, da Silva
IB, Steer CJ and Rodrigues CM: MicroRNA-143 reduces viability and
increases sensitivity to 5-fluorouracil in HCT116 human colorectal
cancer cells. FEBS J. 276:6689–6700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ostenfeld MS, Bramsen JB, Lamy P,
Villadsen SB, Fristrup N, Sørensen KD, Ulhøi B, Borre M, Kjems J,
Dyrskjøt L and Orntoft TF: miR-145 induces caspase-dependent
and-independent cell death in urothelial cancer cell lines with
targeting of an expression signature present in Ta bladder tumors.
Oncogene. 29:1073–1084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Connell RM, Taganov KD, Boldin MP, Cheng
G and Baltimore D: MicroRNA-155 is induced during the macrophage
inflammatory response. Proc Natl Acad Sci USA. 104:1604–1609. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schulte LN, Eulalio A, Mollenkopf HJ,
Reinhardt R and Vogel J: Analysis of the host microRNA response to
Salmonella uncovers the control of major cytokines by the let-7
family. EMBO J. 30:1977–1989. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharbati S, Sharbati J, Hoeke L, Bohmer M
and Einspanier R: Quantification and accurate normalisation of
small RNAs through new custom RT-qPCR arrays demonstrates
Salmonella-induced microRNAs in human monocytes. BMC Genomics.
13:232012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Metzler M, Wilda M, Busch K, Viehmann S
and Borkhardt A: High expression of precursor microRNA-155/BIC RNA
in children with Burkitt lymphoma. Genes Chromosomes Cancer.
39:167–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Ma T, Huang C, Hu T and Li J: The
pivotal role of microRNA-155 in the control of cancer. J Cell
Physiol. 229:545–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Faraoni I, Antonetti FR, Cardone J and
Bonmassar E: miR-155 gene: A typical multifunctional microRNA.
Biochim Biophys Acta. 1792:497–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elton TS, Selemon H, Elton SM and
Parinandi NL: Regulation of the MIR155 host gene in physiological
and pathological processes. Gene. 532:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, van Mil A, Vrijsen K, Zhao J, Gao
L, Metz CH, Goumans MJ, Doevendans PA and Sluijter JP: MicroRNA-155
prevents necrotic cell death in human cardiomyocyte progenitor
cells via targeting RIP1. J Cell Mol Med. 15:1474–1482. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holler N, Zaru R, Micheau O, Thome M,
Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp
J: Fas triggers an alternative, caspase-8-independent cell death
pathway using the kinase RIP as effector molecule. Nat Immunol.
1:489–495. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Santis R, Liepelt A, Mossanen JC, Dueck
A, Simons N, Mohs A, Trautwein C, Meister G, Marx G,
Ostareck-Lederer A and Ostareck DH: miR-155 targets Caspase-3 mRNA
in activated macrophages. RNA Biol. 13:43–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Zhang X, Zhang C, Zhai F, Li Y and
Huang Z: MicroRNA-155 targets MAP3K10 and regulates osteosarcoma
cell growth. Pathol Res Pract. 213:389–393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JH, Jung SA, Kwon YA, Chung JL and Kim
US: Expression of microRNAs in fibroblast of pterygium. Int J
Ophthalmol. 9:967–972. 2016.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borenfreund E and Puerner JA: Toxicity
determined in vitro by morphological alterations and neutral red
absorption. Toxicol Lett. 24:119–124. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lindgren SW, Stojiljkovic I and Heffron F:
Macrophage killing is an essential virulence mechanism of
Salmonella typhimurium. Proc Natl Acad Sci USA. 93:4197–4201. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schulte LN, Westermann AJ and Vogel J:
Differential activation and functional specialization of miR-146
and miR-155 in innate immune sensing. Nucleic Acids Res.
41:542–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haasch D, Chen YW, Reilly RM, Chiou XG,
Koterski S, Smith ML, Kroeger P, McWeeny K, Halbert DN, Mollison
KW, et al: T cell activation induces a noncoding RNA transcript
sensitive to inhibition by immunosuppressant drugs and encoded by
the proto-oncogene, BIC. Cell Immunol. 217:78–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eis PS, Tam W, Sun L, Chadburn A, Li Z,
Gomez MF, Lund E and Dahlberg JE: Accumulation of miR-155 and BIC
RNA in human B cell lymphomas. Proc Natl Acad Sci USA.
102:3627–3632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ceppi M, Pereira PM, Dunand-Sauthier I,
Barras E, Reith W, Santos MA and Pierre P: MicroRNA-155 modulates
the interleukin-1 signaling pathway in activated human
monocyte-derived dendritic cells. Proc Natl Acad Sci USA.
106:2735–2740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu C, Huang X, Zhang X, Roensch K, Cao Q,
Nakayama KI, Blazar BR, Zeng Y and Zhou X: miR-221 and miR-155
regulate human dendritic cell development, apoptosis and IL-12
production through targeting of p27kip1, KPC1 and SOCS-1. Blood.
117:4293–4303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruggiero T, Trabucchi M, De Santa F, Zupo
S, Harfe BD, McManus MT, Rosenfeld MG, Briata P and Gherzi R: LPS
induces KH-type splicing regulatory protein-dependent processing of
microRNA-155 precursors in macrophages. FASEB J. 23:2898–2908.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Linnstaedt SD, Gottwein E, Skalsky RL,
Luftig MA and Cullen BR: Virally induced cellular microRNA miR-155
plays a key role in B-cell immortalization by Epstein-Barr virus. J
Virol. 84:11670–11678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sheridan C, Brumatti G, Elgendy M, Brunet
M and Martin SJ: An ERK-dependent pathway to Noxa expression
regulates apoptosis by platinum-based chemotherapeutic drugs.
Oncogene. 29:6428–6441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koch M, Mollenkopf HJ, Klemm U and Meyer
TF: Induction of microRNA-155 is TLR- and type IV secretion
system-dependent in macrophages and inhibits DNA-damage induced
apoptosis. Proc Natl Acad Sci USA. 109:E1153–E1162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang H, Dong Z, Liu JF, Chuang W, Gao LZ
and Ren YG: Targeting miR-155 suppresses proliferation and induces
apoptosis of HL-60 cells by targeting Slug/PUMA signal. Histol
Histopathol. 32:899–907. 2017.PubMed/NCBI
|
|
39
|
Wang C, Zhang C, Liu LAX, Chen B, Li Y and
Du J: Macrophage-derived mir-155-containing exosomes suppress
fibroblast proliferation and promote fibroblast inflammation during
cardiac injury. Mol Ther. 25:192–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Bei Y, Huang P, Zhou Q, Shi J, Sun
Q, Zhong J, Li X, Kong X and Xiao J: Inhibition of miR-155 protects
against LPS-induced cardiac dysfunction and apoptosis in mice. Mol
Ther Nucleic Acids. 5:e3742016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
D'Amours D, Sallmann FR, Dixit VM and
Poirier GG: Gain-of-function of poly(ADP-ribose) polymerase-1 upon
cleavage by apoptotic proteases: Implications for apoptosis. J Cell
Sci. 114:3771–3778. 2001.PubMed/NCBI
|
|
42
|
Degterev A, Huang Z, Boyce M, Li Y, Jagtap
P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA and Yuan J:
Chemical inhibitor of nonapoptotic cell death with therapeutic
potential for ischemic brain injury. Nat Chem Biol. 1:112–119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu X, Chua CC, Kong J, Kostrzewa RM,
Kumaraguru U, Hamdy RC and Chua BH: Necrostatin-1 protects against
glutamate-induced glutathione depletion and caspase-independent
cell death in HT-22 cells. J Neurochem. 103:2004–2014. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu X, Chua CC, Zhang M, Geng D, Liu CF,
Hamdy RC and Chua BH: The role of PARP activation in
glutamate-induced necroptosis in HT-22 cells. Brain Res.
1343:206–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jouan-Lanhouet S, Arshad MI,
Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van
Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D,
Vandenabeele P, et al: TRAIL induces necroptosis involving
RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ.
19:2003–2014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gagné JP, Moreel X, Gagné P, Labelle Y,
Droit A, Chevalier-Paré M, Bourassa S, McDonald D, Hendzel MJ,
Prigent C and Poirier GG: Proteomic investigation of
phosphorylation sites in poly(ADP-ribose) polymerase-1 and
poly(ADP-ribose) glycohydrolase. J Proteome Res. 8:1014–1029. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ,
Lin SC, Dong MQ and Han J: RIP3, an energy metabolism regulator
that switches TNF-induced cell death from apoptosis to necrosis.
Science. 325:332–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Upton JW, Kaiser WJ and Mocarski ES: Virus
inhibition of RIP3-dependent necrosis. Cell Host Microbe.
7:302–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li D, Xu T, Cao Y, Wang H, Li L, Chen S,
Wang X and Shen Z: A cytosolic heat shock protein 90 and
cochaperone CDC37 complex is required for RIP3 activation during
necroptosis. Proc Natl Acad Sci USA. 112:5017–5022. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Strelow A, Bernardo K, Adam-Klages S,
Linke T, Sandhoff K, Krönke M and Adam D: Overexpression of acid
ceramidase protects from tumor necrosis factor-induced cell death.
J Exp Med. 192:601–612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Thon L, Mathieu S, Kabelitz D and Adam D:
The murine TRAIL receptor signals caspase-independent cell death
through ceramide. Exp Cell Res. 312:3808–3821. 2006. View Article : Google Scholar : PubMed/NCBI
|