Introduction
Skin aging is caused by various factors such as
hormonal imbalance, certain metabolic pathway disorders and,
especially, exposure to ultraviolet (UV) irradiation (1). Photoaging mainly results from chronic
solar UV irradiation (2), and it
is characterized clinically by telangiectasias, dyschromia,
roughness, laxity and wrinkle formation (3–5).
Ultraviolet radiation B (UVB) (280–320 nm) is the principal reason
for skin photoaging because it can cross the whole epidermis layer
and penetrate the dermis (inner layer) of human skin (6), induce keratinocyte apoptosis
(7) and reactive oxygen species
(ROS)-mediated inflammation (8),
and increase the expression and activity of matrix
metalloproteinases (MMPs) via mitogen-activated protein kinases
(MAPK) signaling pathway (9,10).
Overexpression (OE) of MMP-1 (a collagenase) could induce the
degradation of extracellular matrix (ECM) by initiating the
degradation of type I collagen (COL-I) and type III collagen
(COL-III), the major structural protein of ECM, which is secreted
by fibroblasts (11,12).
Klotho is a soluble protein and transmembrane that
shows glucosidase activity, it serves as a novel biomarker involved
in aging (13). Klotho is related
to insulin sensitivity, mineralization, cell renewing, reparative
processes and electrolytic balance (14). Klotho mutant mice develop a
phenotype characterized by accelerated skin atrophy, aging,
osteoporosis, lung emphysema, vascular calcification and delayed
wound healing (13).
Co-culture methods are widely used in tissue
engineering to drive tissue formation with the direct or indirect
interaction of multiple cell types (15). Co-culture can effectively
recapitulate the relationships among cell types within processes
and native tissue that are inefficient when relying solely on
soluble factors and scaffolds. On the one hand, when acting as the
target cells, stem cells differentiate and eventually synthesize
the ECM or metabolites that confer function to a tissue in
co-culture systems (15). On the
other hand, as assisting cells, stem cells can also promote
homeostasis of engineered tissues. For instance, stem cells promote
the tissue repair directly (16),
and they have the ability to inhibit cell apoptosis, locally
suppress the immune system and promote cell differentiation and
proliferation indirectly (17,18).
Thus, in this study, we evaluated the protective effects of klotho
overexpressed adipose-derived stem cells (ADSCs) on UVB-induced
photoaging in co-cultured human fibroblasts cell line human skin
fibroblasts (HSF2) in vitro, and relative phosphorylated P38
MAPK (p-P38) signaling were investigated during this process.
Materials and methods
Chemicals and reagents
FITC mouse anti-human CD90, CD44, CD45 and CD11b
antibodies were purchased from Becton-Dickinson (BD Biosciences,
San Jose, CA, USA). Dulbecco's modified Eagle's medium (DMEM) was
purchased from Hyclone (GE Healthcare, Logan, UT, USA). Fetal
bovine serum (FBS) was obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). TRIzol reagents were purchased
from Invitrogen (Thermo Fisher Scientific, Inc.). ELISA kits of
human COL-I, COL-III, MMP1 and MMP3 were purchased from Cell
Signaling Technology Inc. (Danvers, MA, USA). Primary antibodies of
klotho (1:800; polyclonal, cat. no. ab203576), COL-I (1:2,000;
polyclonal, cat. no. ab34710), COL-III (1:5,000; polyclonal, cat.
no. ab7778), MMP1 (1:2,000; polyclonal, cat. no. ab38929), MMP3
(1:500; polyclonal, cat. no. ab53015), P38 (1:1,000; polyclonal,
cat. no. ab27986) and p-P38 (1:2,000; polyclonal, cat. no. ab47363)
were purchased from Abcam (Cambridge, MA, USA). Primary antibody of
GAPDH (1:1,000; monoclonal, cat. no. 5174) was purchased from Cell
Signaling Technology, Inc. Horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (1:1,000; polyclonal, cat. no. A0208) was
purchased from Beyotime Institute of Biotechnology (Shanghai,
China).
Cells
HSF2 (cat. no. bio-51608; Beijing Baiou Bowei
Biotechnology Co., Ltd., Beijing, China) and 293 (cat. no.
bio-72947; Beijing Baiou Bowei Biotechnology Co., Ltd.). Eight
human subcutaneous adipose tissue samples of healthy normal weight
males (aged, 20–28 years) were collected during physical
examination after informed consent was completed and signed by the
donors at Shanghai East Hospital Affiliated to Tongji University
(Shanghai, China) in June, 2017. One of the samples was used in the
present study. ADSCs were isolated from human subcutaneous adipose
tissue as previously described (19). This study was approved by the
Ethics Committee of Shanghai East Hospital Affiliated to Tongji
University. Researchers were blinded to the experimental
groups.
The 3rd generation of ADSCs (1×104) was
incubated for 10 h at 4°C with FITC mouse anti-human CD90, CD44,
CD45 and CD11b antibodies, respectively. Secondary antibodies
labeled with FITC were added and incubated for 1 h at 4°C. The
expression of stromal markers (CD11b-FITC, CD44-FITC, CD45-FITC,
CD90-FITC) on the cellular membrane was analyzed by flow cytometry
(cat. no. E670006-0300, Shanghai, China). The multipotentiality of
ADSCs (adipogenic, chondrogenic and osteogenic) were tested. The
3rd generation of ADSCs (approximately 80% covered) was seeded in a
6-well culture plate with the adipogenic, chondrogenic and
osteogenic differentiation medium (Cyagen Biosciences Inc.,
Guangzhou, China) for 2 weeks (at 37°C with 5% CO2),
respectively, according to the manufacturer's protocol. Finally,
these ADSCs were stained by Oil Red O, Safranin O and alkaline
phosphatase staining kits according to the protocol, and then
detected and distinguished by optical microscope (Olympus,
Corporation; Tokyo, Japan).
Co-culture system
In the present study, HSF2 cells and ADSCs were
co-cultured indirectly in a Transwell co-culture plate (0.4 µm
polyester film). The HSF2 cells (1×105) were seeded in
the upper layer and ADSCs (1×105) were seeded in the
lower layer. The cells were cultured at 37°C (DMEM with 10% FBS and
5% CO2). Three biological replicates were prepared for
each sample and the experiment was repeated 3 times.
UVB irradiation
The HSF2 cells were collected and washed three times
with sterile PBS and evenly distributed in PBS in uncovered petri
dishes. UVB irradiation was performed using the UVB lamps (LEITUO
illumination, Shenzhen, China) in a biosafety cabinet at room
temperature (25°C) and the UV radiation intensity was validated by
the UV photometric detector (Deshengxing Technology Co., Ltd.,
Shenzhen, China). The irradiating distance was set at 15 cm. The
UVB irradiation doses were set at 0 (dark treatment), 10, 20 and 40
mJ/cm2 for different groups respectively. After each UVB
irradiation, the cells were washed by PBS then cultured at 37°C
(DMEM with 10% FBS and 5% CO2). The irradiation lasted
60 min/day for 3 days. Cells in each group were harvested at 0, 24,
48 and 72 h in radiation experiment.
Construction and identification of
lentivirus vectors
The klotho (Homo sapiens klotho; GenBank;
AB005142.1) OE/empty plasmid lentivirus vectors were constructed by
JRDun Biotech (Shanghai, China). Recombinant lentivirus was
generated using AdEasy technology as previously described with
minor modifications (20).
Recombinant lentivirus was produced and amplified in packaging 293
cells.
Viral supernatants were diluted in culture medium to
give the desired concentration and added to logarithmic phase
monolayer ADSCs. After being cultured for 48 h, the function of
vectors in ADSCs was identified. Relative mRNA expression and
protein level of klotho in ADSCs were tested by quantitative PCR,
and western blot analysis, respectively.
Effect of klotho OE on the
proliferation of ADSCs
ADSCs were divided into 3 groups: ADSCs group (NC),
ADSCs + empty plasmid control group (EPC) and ADSCs + klotho OE
group (OE). ADSCs in EPC and OE were transduced with klotho OE and
empty plasmid lentivirus respectively. Cells in each group were
harvested at 0, 24, 48 and 72 h after incubation. The proliferation
of ADSCs at each time-point was tested by CCK-8 assays kit
according to a previous report (21).
Effect of UVB irradiation on HSF2
UVB irradiation was carried out using a UV lighter
(LEITUO illumination, Shenzhen, China). Immediately after the
irradiation, the PBS was aspirated and replaced with complete
medium. UVB irradiation doses were set at 0 (dark treatment), 10,
20 and 40 mJ/cm2 for further experiment. The irradiation
lasted 60 min/day for 3 days. Cells in each group were harvested at
0, 24, 48 and 72 h in the radiation experiment. The proliferation
of HSF2 at each time-point was tested by CCK-8 assays kit according
to a previous report (21). The
relative expression of MMP1, MMP3, COL-I and COL-III were measured
by ELISA kits according to the protocol, and the protein level of
P38 and p-P38 was tested by western blot analysis at the end of the
experiment.
Effect of klotho overexpressing ADSCs
on co-cultured HSF2 under UVB irradiation
ADSCs were divided into 3 groups: ADSCs, ADSCs +
empty plasmid control group and ADSCs + klotho OE group. In ADSCs +
empty plasmid control group and ADSCs + klotho OE group, ADSCs were
transduced with klotho OE and empty plasmid lentivirus,
respectively. Cells in each group were incubated at normal
conditions for 48 h, and these cells were used in the co-culture
system.
The co-culture cells were divided into 3 groups:
HSF2 + ADSCs (NC), HSF2 + ADSCs + empty plasmid control group (EPC)
and HSF2 + ADSCs + klotho OE group (OE). The co-culture system was
achieved according to a previous report (22). Briefly, ADSCs and HSF2 were mixed
and incubated with complete DMEM containing 10% FBS with 100 U/ml
penicillin/streptomycin in a humidified atmosphere at 37°C with 5%
CO2. UVB irradiation dose was set at 20
mJ/cm2. The irradiation lasted 60 min/day for 3 days.
HSF2 cells were separated and collected at the end of experiment.
Relative mRNA expression of MMP1 and MMP3 were measured by
quantitative PCR. Protein level of MMP1, MMP3, COL- I, COL-III, P38
and p-P38 were tested by western blot analysis.
RT-qPCR
The level of the mRNA expression was determined
using qPCR. The total RNA (2 µg of each sample) was extracted and
reverse transcribed to cDNA by the reverse transcription kits
(Thermo Fisher Scientific, Inc.). The obtained cDNA was used as
template for RT-qPCR analysis on quantitative real-time PCR machine
(ABI-7300) with SYBR-Green reagents (Thermo Fisher Scientific,
Inc.).
Primers (Table I)
for the qPCR were designed by Primer 5.0 and synthesized by JRDun
Biotech. Relative mRNA expression was evaluated by
2−ΔΔCq relative quantitative analysis in each sample
against GAPDH gene expression. ΔΔCq= (Cq, target gene in the
treated group - Cq, reference gene in the treated group) - (Cq,
target gene in the control group - Cq, reference gene in the
control group) (23).
 | Table I.Primers used in qPCR assays. |
Table I.
Primers used in qPCR assays.
| Genes | Sequence
(5′-3′) | Description |
|---|
| MMP1 |
ATTCTACTGATATCGGGGCTTTGA | F |
| MMP1 |
ATGTCCTTGGGGTATCCGTGTAG | R |
| MMP3 |
TATGGATCCCCCCCTGACTCCCCTGAG | F |
| MMP3 |
ATGGAATTCAGGTTCAAGCTTCCTGAGG | R |
| Klotho |
CACGGCAAGGGTGCGTCCAT | F |
| Klotho |
TCGCGCCCACGAGATGGAGA | R |
| GAPDH |
CTCATGACCACAGTCCATGC | F |
| GAPDH |
TTCAGCTCTGGGATGACCTT | R |
Western blot analysis
The protein levels of MMP1, MMP3, COL-I, COL-III,
P38 and p-P38 were tested by western blot analysis. Total protein
was extracted with Cell Protein Extraction Reagent (cat. no. 89802,
Thermo Fisher Scientific, Inc.). Total protein was determined using
BCA Protein Assay kit (cat. no. 23227, Thermo Fisher Scientific,
Inc.). Protein (35 µg) was added per lane and separated by 10%
SDS-PAGE and then transferred onto PVDF membranes as previously
described (24). The membranes
were blocked with 5% fat-free dry milk at 25°C for 1 h then
incubated with the respective primary antibody (MMP1, MMP3, COL-I,
COL-III, P38, p-P38 and GAPDH) overnight at 4°C, followed by a
secondary antibody [horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG] for 2 h at 4°C. Finally, the protein bands were
detected by an ECL-detecting kit (Beyotime Institute of
Biotechnology). Each blot was normalized to its corresponding
internal control-GAPDH value. Band intensities were quantifed by
densitometry using Image-Pro plus Software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The data are expressed as mean ± standard deviation.
Statistical analyses of data were performed by one-way analysis of
variance (ANOVA) and Tukey's honest significant difference (HSD)
post hoc test. Data analysis was carried out using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of ADSCs
The flow cytometry results indicated that CD44- and
CD90-positive cells were 97.7% and 93.9%, while CD11b (1.1%) and
CD45 (1.0%) were negative on the 3rd generation ADSCs (Fig. 1A). The results suggested that the
majority of these cells are ADSCs after three generation
subculture. Oil Red O staining of the ADSCs after 2 weeks of
culture demonstrated numerous intracellular lipid droplets.
Safranin O and alkaline phosphatase staining was positive (Fig. 1B). Thus, our experiment
successfully isolated ADSCs, the cell surface marks were consistent
with the characteristics of ADSCs. The study successfully induced
ADSCs to adipogenic, chondrogenic and osteogenic cells. The
isolated ADSCs have the ability of multi-potential differentiation
and it could be used in the co-culture system.
Identification of lentivirus
vectors
Viral supernatants were diluted in culture medium to
give the desired concentration and added to logarithmic phase
monolayer cell cultures for 48 h. Then the function of vectors in
ADSC cells was identified. The mRNA expression as well as protein
level of klotho was tested. As shown in Fig. 2, the mRNA expression as well as
protein level of klotho was significantly upregulated for klotho OE
in ADSCs.
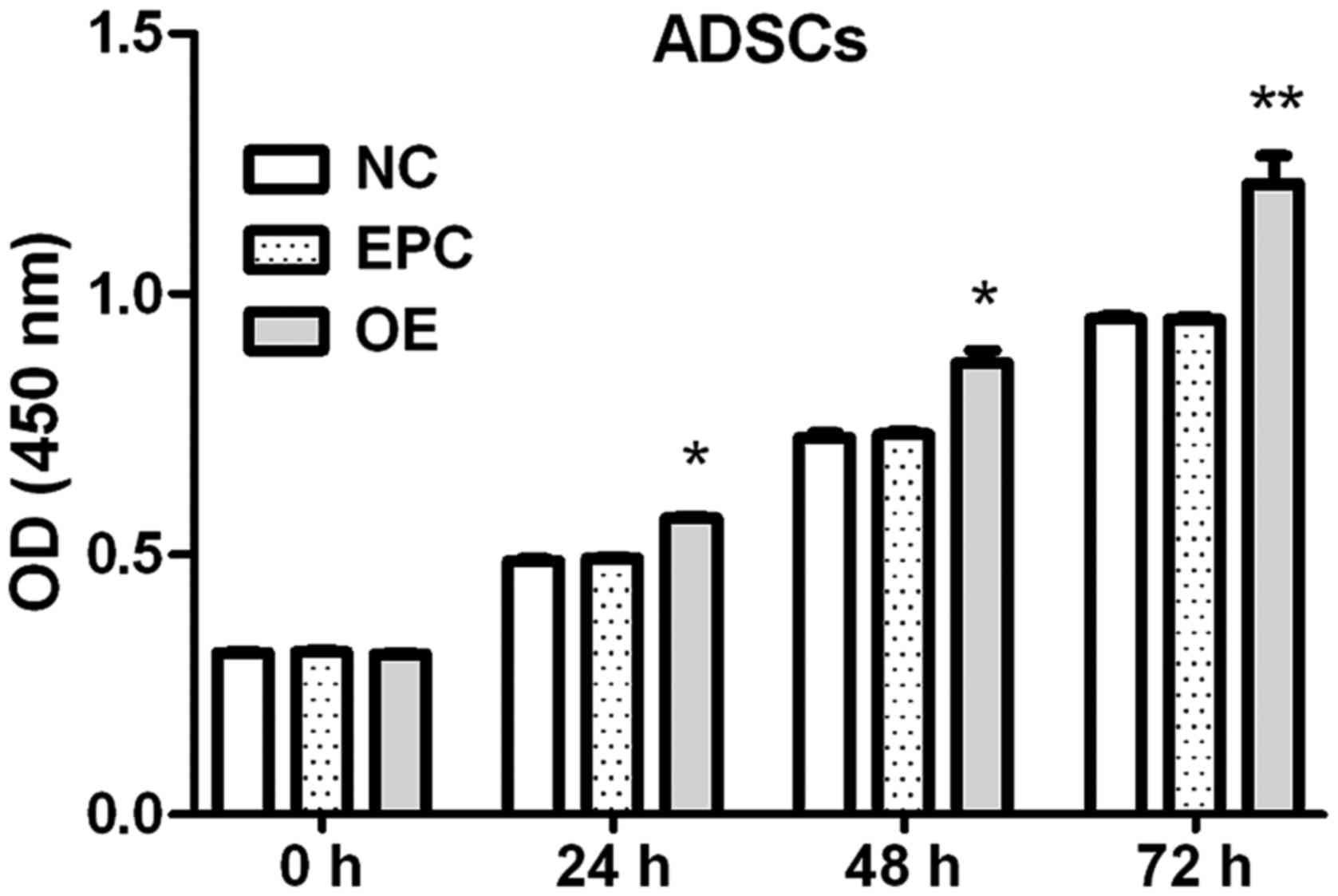
Klotho OE promotes the proliferation
of ADSCs
Effects of klotho OE on the proliferation of ADSCs
at each time-point were measured by CCK-8 assay.
The proliferation of ADSCs was increased by klotho
OE (8.2% at 24 h, 14.5% at 48 h and 25.8% at 72 h compared to EPC
group) (Fig. 3).
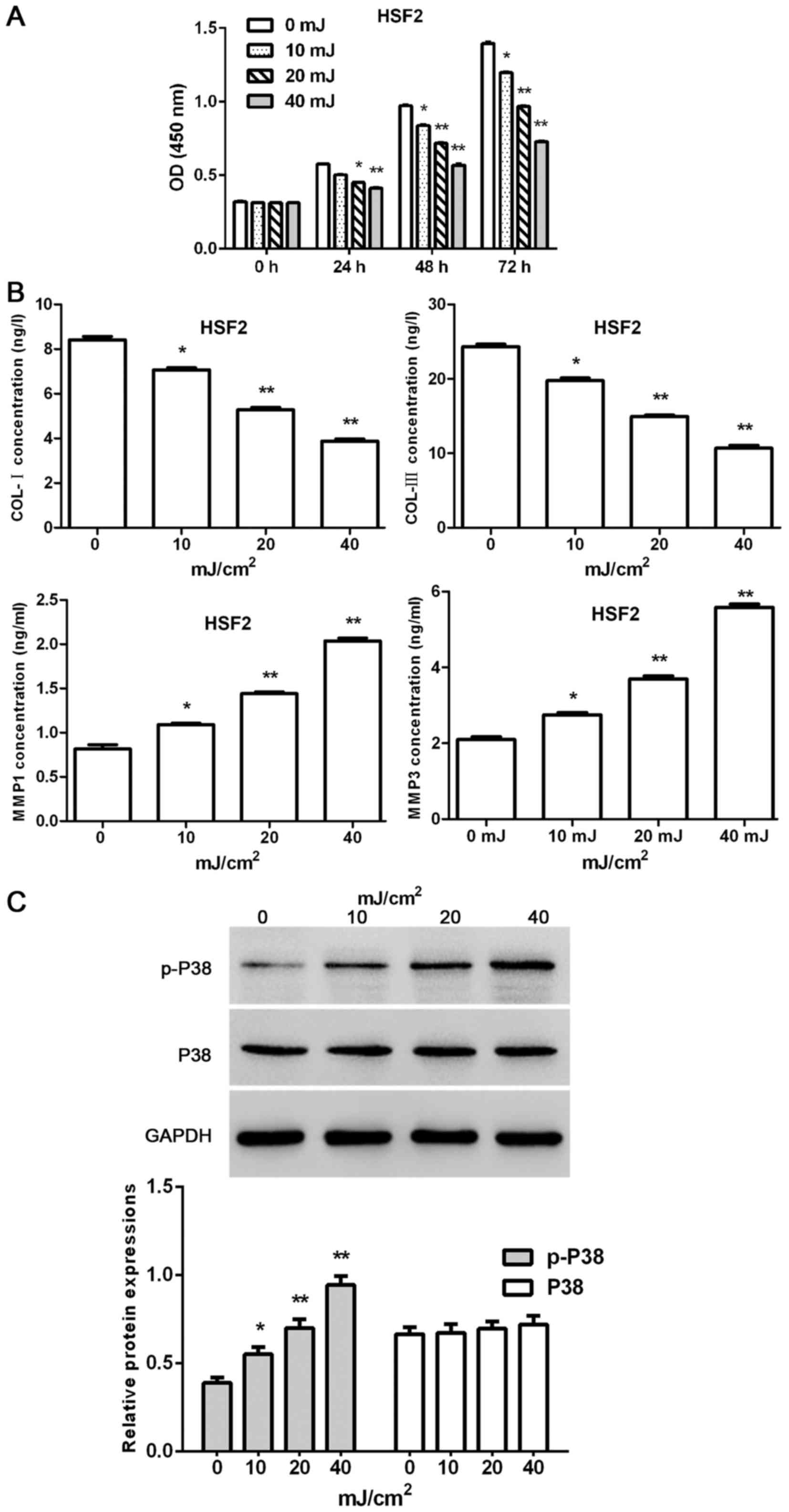
Effect of UVB irradiation on HSF2
The proliferation of HSF2 at 0, 24, 48 and 72 h was
tested by CCK-8 assays kit after various doses of UVB irradiation.
Moreover, concentration of MMP1, MMP3, COL-I and COL-III were
measured by ELISA kits according to the protocol, and the protein
level of P38 and p-P38 were tested by western blot analysis at the
end of the experiment.
The present results indicated that the proliferation
of HSF2 cells was time- and dose-dependently decreased by UVB
irradiation (except UVB 10 mJ/cm2 at the time-point of
24 h; 7.3, 13.5 and 19.9% for UVB 10 mJ/cm2 at 24, 48
and 72 h; 12.6, 25.5 and 42.8% for UVB 20 mJ/cm2 24, 48
and 72 h; 16.3, 40.5 and 66.7% for UVB 10 mJ/cm2 24, 48
and 72 h, respectively) (Fig. 4A).
Furthermore, UVB irradiation dose-dependently reduced the
concentrations of COL-I and COL-III, and increase the
concentrations of MMP1 and MMP3 in HSF2 cells (Fig. 4B). Furthermore, the protein level
of p-P38 in HSF2 cells was dose-dependently upregulated by UVB
irradiation. By contrast, the protein level of P38 showed no
changes when exposed to UVB irradiation (Fig. 4C).
Effect of klotho overexpressed ADSCs
on co-cultured HSF2 under UVB irradiation
HSF2 were separated from the co-culture system at
the end of experiment. Relative mRNA expression of MMP1 and MMP3 as
well as protein level of COL-I, COL-III, MMP1, MMP3, p-P38 and P38
in each group were tested.
As shown in Fig.
5A, the mRNA expression of MMP1 and MMP3 as well as the protein
level of MMP1, MMP3 and p-P38 in HSF2 cells were downregulated by
klotho-overexpressed ADSCs in the co-culture system exposed to UVB
irradiation. By contrast, the protein level of COL-I and COL-III
were upregulated, and the protein level of P38 had no obvious
change (Fig. 5B).
 | Figure 5.Effect of klotho overexpressed ADSCs
on co-cultured HSF2 under UVB irradiation. (A) Relative mRNA
expressions of MMP1 and MMP3 in HSF2 cells. (B) Proteins level of
COL-I, COL-III, MMP1, MMP3, p-P38 and P38 in HSF2 cells. NC, HSF2
cells separated from HSF2 + ADSCs group; EPC, HSF2 cells separated
from HSF2 + ADSCs + empty plasmid control group; OE, HSF2 cells
separated from HSF2 + ADSCs + klotho OE group. *P<0.05,
**P<0.01 compared with EPC (n=3). OE, overexpression; ADSCs,
adipose-derived stem cells; HSF2, human skin fibroblasts; EPC,
empty plasmid control group. |
Discussion
UV rays from the sun are a common environmental
factor affecting humans. Chronic UV irradiation could lead to skin
cancer (25,26), accelerated aging of the skin
(27,28), cataract (29,30)
and immunosuppression (31,32).
Photoaging skin is characterized clinically by coarseness, laxity
and wrinkles, and this is closely associated with disorganization
of collagen fibers and reduction in collagen content (33,34).
On the other hand, UVB irradiation-induced ROS mediate the
phosphorylation of protein kinases through MAPK signaling pathway
(9). ROS can cause upregulation of
the expression and activity of MMPs, which is associated with
collagen degradation in photoaged skin (35,36).
The present results show that the proliferation and the collagen
content of HSF2 were decreased by UVB irradiation. By contrast, the
protein level of MMP1, MMP3 and p-P38 in HSF2 were upregulated.
Klotho is an aging suppressor gene, and its OE in
mice extends the life span of the animals in vivo (37). Furthermore, klotho regulates
cellular stress responses to oxidative stress by inhibiting
insulin/IGF-1 signaling pathway (38), enhancing FoxO forkhead
transcription factor FoxO3-mediated manganese superoxide dismutase
expression by negatively regulating PI3K/AKT pathway (39) and suppressing Nox2 by cAMP/PKA
pathway (40). In addition, klotho
expression was inhibited by activating NF-κB signaling in melanoma
cells (41). Stem cells are the
future in tissue regeneration and engineering. In the cell
co-culture system, stem cells provide a target cell source with
multipotent differentiation capacity, as well as assisting cells
that promote tissue metabolism, homeostasis, repair and growth.
Their incorporation into co-culture systems seems to play an
important role in the formation of complex tissues or organs
(15). In the present study, ADSC
cells act as assisting cells, and the protective effect was
achieved indirectly. The positive effects come from the soluble
klotho and various growth factors secreted by klotho overexpressed
ADSC cells.
In conclusion, OE of klotho in ADSCs ameliorate
UVB-induced photoaging in co-cultured HSF2 in vitro, and
these antiaging effects were potentially achieved by increasing the
collagen content and decreasing the protein level of MMP-1, MMP-3
and p-P38. Future longitudinal research is required to explore the
function of ADSCs in skin repair and functional reconstruction
in vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FF was involved in drafting the manuscript and cell
culture. YuL helped with UVB irradiation. YiL constructed the
vector. LS and JY were responsible for CCK-8 assays. ZL contributed
to ELISA. All authors read and approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shanghai East Hospital Affiliated to Tongji University (Shanghai,
China). Signed informed consents were obtained from the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no conflicts of
interest in this study.
References
|
1
|
Huang CY, Lin YT, Kuo HC, Chiou WF and Lee
MH: Compounds isolated from Eriobotrya deflexa leaves
protect against ultraviolet radiation B-induced photoaging in human
fibroblasts. J Photochem Photobiol B. 175:244–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grether-Beck S, Wlaschek M, Krutmann J and
Scharffetter-Kochanek K: Photodamage and photoaging - prevention
and treatment. J Dtsch Dermatol Ges. 3(s2): S19–S25. 2005.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rokhsar CK, Lee S and Fitzpatrick RE:
Review of photorejuvenation: Devices, cosmeceuticals, or both?
Dermatol Surg. 31:1166–1178; discussion 1178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldman MP, Weiss RA and Weiss MA: Intense
pulsed light as a nonablative approach to photoaging. Dermatol
Surg. 31:1179–1187; discussion 1187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munavalli GS, Weiss RA and Halder RM:
Photoaging and nonablative photorejuvenation in ethnic skin.
Dermatol Surg. 31:1250–1260; discussion 1261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nichols JA and Katiyar SK: Skin
photoprotection by natural polyphenols: Anti-inflammatory,
antioxidant and DNA repair mechanisms. Arch Dermatol Res.
302:71–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lei X, Liu B, Han W, Ming M and He YY:
UVB-Induced p21 degradation promotes apoptosis of human
keratinocytes. Photochem Photobiol Sci. 9:1640–1648. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duncan FJ, Martin JR, Wulff BC, Stoner GD,
Tober KL, Oberyszyn TM, Kusewitt DF and Van Buskirk AM: Topical
treatment with black raspberry extract reduces cutaneous
UVB-induced carcinogenesis and inflammation. Cancer Prev Res
(Phila). 2:665–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharma SD, Meeran SM and Katiyar SK:
Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative
stress and activation of mitogen-activated protein kinases and
nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol
Cancer Ther. 6:995–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin XJ, Kim EJ, Oh IK, Kim YK, Park CH and
Chung JH: Prevention of UV-induced skin damages by
11,14,17-eicosatrienoic acid in hairless mice in vivo. J Korean Med
Sci. 25:930–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YL, Lee MH, Chang HJ, Huang PY, Huang
IJ, Cheng KT and Leu SJ: Taiwanese native plants inhibit matrix
metalloproteinase-9 activity after ultraviolet B irradiation.
Molecules. 14:1062–1071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kähäri VM and Saarialho-Kere U: Matrix
metalloproteinases in skin. Exp Dermatol. 6:199–213. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lanske B and Razzaque MS: Premature aging
in klotho mutant mice: Cause or consequence? Ageing Res Rev.
6:73–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Talotta R, Bongiovanni S, Letizia T,
Rigamonti F, Ditto MC, Atzeni F, Salaffi F, Batticciotto A, Gerardi
MC, Antivalle M, et al: Measurement of serum klotho in systemic
sclerosis. Dis Markers. 2017:95459302017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paschos NK, Brown WE, Eswaramoorthy R, Hu
JC and Athanasiou KA: Advances in tissue engineering through stem
cell-based co-culture. J Tissue Eng Regen Med. 9:488–503. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orlic D, Kajstura J, Chimenti S, Limana F,
Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A and Anversa
P: Mobilized bone marrow cells repair the infarcted heart,
improving function and survival. Proc Natl Acad Sci USA.
98:10344–10349. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scadden DT: The stem-cell niche as an
entity of action. Nature. 441:1075–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo J, Tang M, Huang J, He BC, Gao JL,
Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, et al: TGFbeta/BMP type I
receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic
signaling in mesenchymal stem cells. J Biol Chem. 285:29588–29598.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen T, Shen J, Zheng QQ, Li QS, Zhao HL,
Cui L and Hong CY: Cell viability and extracellular matrix
synthesis in a co-culture system of corneal stromal cells and
adipose-derived mesenchymal stem cells. Int J Ophthalmol.
10:670–678. 2017.PubMed/NCBI
|
|
22
|
Xu X, Wang HY, Zhang Y, Liu Y, Li YQ, Tao
K, Wu CT, Jin JD and Liu XY: Adipose-derived stem cells cooperate
with fractional carbon dioxide laser in antagonizing photoaging: A
potential role of Wnt and β-catenin signaling. Cell Biosci.
4:242014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-DeltaDeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blumer MJ, Longato S and Fritsch H:
Structure, formation and role of cartilage canals in the developing
bone. Ann Anat. 190:305–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elwood JM and Jopson J: Melanoma and sun
exposure: An overview of published studies. Int J Cancer.
73:198–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ley RD and Reeve VE: Chemoprevention of
ultraviolet radiation-induced skin cancer. Environ Health Perspect.
105 Suppl 4:981–984. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
MacKie RM: Long-term health risk to the
skin of ultraviolet radiation. Prog Biophys Mol Biol. 92:92–96.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harrison GI and Young AR: Ultraviolet
radiation-induced erythema in human skin. Methods. 28:14–19. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sliney DH: Exposure geometry and spectral
environment determine photobiological effects on the human eye.
Photochem Photobiol. 81:483–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suh MH, Kwon JW, Wee WR, Han YK, Kim JH
and Lee JH: Protective effect of ascorbic Acid against corneal
damage by ultraviolet B irradiation: A pilot study. Cornea.
27:916–922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vink AA, Yarosh DB and Kripke ML:
Chromophore for UV-induced immunosuppression: DNA. Photochem
Photobiol. 63:383–386. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Norval M: Effects of solar radiation on
the human immune system. J Photochem Photobiol B. 63:28–40. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsieh HY, Lee WC, Senadi GC, Hu WP, Liang
JJ, Tsai TR, Chou YW, Kuo KK, Chen CY and Wang JJ: Discovery,
synthetic methodology, and biological evaluation for antiphotoaging
activity of bicyclic[1,2,3]triazoles: In vitro and in vivo studies.
J Med Chem. 56:5422–5435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wlaschek M, Tantcheva-Poór I, Naderi L, Ma
W, Schneider LA, Razi-Wolf Z, Schüller J and Scharffetter-Kochanek
K: Solar UV irradiation and dermal photoaging. J Photochem
Photobiol B. 63:41–51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung YR, Kim DH, Kim SR, An HJ, Lee EK,
Tanaka T, Kim ND, Yokozawa T, Park JN and Chung HY: Anti-wrinkle
effect of magnesium lithospermate B from Salvia miltiorrhiza
BUNGE: Inhibition of MMPs via NF-kB signaling. PLoS One.
9:e1026892014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Staniforth V, Huang WC, Aravindaram K and
Yang NS: Ferulic acid, a phenolic phytochemical, inhibits
UVB-induced matrix metalloproteinases in mouse skin via
posttranslational mechanisms. J Nutr Biochem. 23:443–451. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dërmaku-Sopjani M, Kolgeci S, Abazi S and
Sopjani M: Significance of the anti-aging protein Klotho. Mol Membr
Biol. 30:369–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sopjani M, Rinnerthaler M, Kruja J and
Dermaku-Sopjani M: Intracellular signaling of the aging suppressor
protein Klotho. Curr Mol Med. 15:27–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lim SW, Jin L, Luo K, Jin J, Shin YJ, Hong
SY and Yang CW: Klotho enhances FoxO3-mediated manganese superoxide
dismutase expression by negatively regulating PI3K/AKT pathway
during tacrolimus-induced oxidative stress. Cell Death Dis.
8:e29722017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Kuro-o M and Sun Z: Klotho gene
delivery suppresses Nox2 expression and attenuates oxidative stress
in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging
Cell. 11:410–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie B, Cao K, Li J, Chen J, Tang J, Chen
X, Xia K, Zhou X, Cheng Y, Zhou J, et al: Hmgb1 inhibits Klotho
expression and malignant phenotype in melanoma cells by activating
NF-κB. Oncotarget. 7:80765–80782. 2016. View Article : Google Scholar : PubMed/NCBI
|