Introduction
Unresectable advanced pancreatic cancer (APC) is the
most lethal and the most aggressive human cancer (1). APC is predicted to increase from the
4th to the 2nd leading cause of mortality in the USA by 2020 due to
its lethal and malignant characteristics (2). Due to the limitations of diagnostic
techniques, the majority of patients and clinicians become aware of
the disease too late, as this cancer is frequently diagnosed in an
advanced stage (3,4). APC is characterized by a high
mortality rate worldwide, 90.8% in China (5), 78.5% in the USA (6) and 95.0% in Canada (7).
Gemcitabine (Gem) was more effective compared with
5-fluorouracil (5-FU) in patients with APC and improved the
survival rate; therefore, it was approved as a first-line regimen
by the US Food and Drug Administration (FDA) in 1996 (8). At present, the majority of
chemotherapy regimens are derived from Gem, which was used as the
control treatment in numerous previous studies (9–11).
Although a number of combination chemotherapy
regimens containing Gem (Gem+Xs) or monotherapies have become more
prevalent over the past decades (12), the improvement of the conditions of
the patients has been limited (12–15).
For example, the poor prognosis of APC leads to a low survival rate
(14,16,17),
which has remained relatively unaltered for ~5 decades (15). Nevertheless, the benefits and risks
of combination chemotherapy regimens remain unclear. Therefore,
first-line chemotherapy regimen data were pooled to comprehensively
evaluate the benefits and risks of these treatments.
Materials and methods
Study design
In order to assess the benefits and risks of various
chemotherapy regimens in distinct conditions, head-to-head
comparison clinical trials were selected. This network
meta-analysis followed the preferred reporting items of system
reviews and meta-analysis (PRISMA) statement (18) while integrating evidence from
direct and indirect treatment comparisons (19). The flow chart for study selection
is presented in Fig. 1.
Search strategy
The comprehensive search strategy was conducted
using the MEDLINE (www.pubmed.com), EMBASE (www.embase.com), Cochrane Central Register of
Controlled Trails (www.cochranelibrary.com) and ClinicalTrials.gov (https://ClinicalTrials.gov) databases with the
following search terms: (Advanced pancreatic cancer or pancreatic
cancer) AND (advanced pancreatic cancer or chemotherapy regimens).
The drug abbreviations and the combinations tested are listed in
Table I.
 | Table I.Abbreviation list of chemistry
regimens. |
Table I.
Abbreviation list of chemistry
regimens.
| Abbreviation | Chemistry
regimens |
|---|
| Gem | Gemcitabine |
| Gem+Axit |
Gemcitabine+axitinib |
| Gem+5-FU |
Gemcitabine+5-fluorouracil |
| Gem+Cap+Erl |
Gemcitabine+capecitabin+erlotinib |
| Gem+Cap |
Gemcitabine+capecitabine |
| Gem+Cet |
Gemcitabine+cetuximab |
| Gem+Cis |
Gemcitabine+cisplatin |
| Gem+Erl |
Gemcitabine+erlotinib |
| Gem+Erl+Bev |
Gemcitabine+erlotinib+bevacizumab |
| Gem+Eta |
Gemcitabine+etanercept |
| Gem+Exa |
Gemcitabine+exatecan |
| Gem+Iri |
Gemcitabine+irinotecan |
| Gem+Mar |
Gemcitabine+marismastat |
| Gem+Nab-p |
Gemcitabine+nab-paclitaxel |
| Gem+Pem |
Gemcitabine+pemetrexed |
| Gem+Radio |
Gemcitabine+radiotherapy |
| Gem+Sor |
Gemcitabine+sorafenib |
| Gem+Tip |
Gemcitabine+tipifarnib |
| Gem+Vis |
Gemcitabine+vismodegib |
|
Oxa+Iri+Leu+Flu+Inf |
Oxaliplatin+irinotecan+leucovorin+fluorouracil+infusion |
Study selection criteria and
outcomes
Experienced investigators independently selected the
studies and extracted the data, and any conflicts were resolved in
discussion. The study selection criteria were based on the National
Comprehensive Cancer Network 2017 criteria (20). The following inclusion criteria
were applied: (i) Parallel-group randomized controlled trials
(RCTs; phase II or III) with the Gem intervention set as the common
comparison treatment and including ≥2 arms; (ii) a minimum 6-month
follow-up period; (iii) patients ≥18 years old (i.e., adult
patients); (iv) diagnosis of unresectable APC; (v) application of
palliative treatments, including invasive radiation therapy,
chemotherapy or chemoradiation therapy, targeted therapies or
combination therapy with the respective placebo or control group;
(vi) either fixed-dose or flexible-dose RCTs with dose titration;
and (vii) the patient performance status reported as 0–2 scores in
the Eastern cooperative oncology group or 70–80% in Karnofsky
scales (21). Previous studies
that (i) included patients undergoing radical resection, (ii)
failed to report the number of patients, (iii) failed to report the
primary efficacy outcome [progression-free survival (PFS)], or (iv)
failed to report the data necessary to estimate the standard
deviation of the primary efficacy outcome were excluded.
Data extraction
The following data were extracted from each included
RCT: First author's name, published year, clinical phase, sample
size of each arm, age, treatment, dosage, route, duration, overall
survival (OS) in months, PFS in months, and 14 treatment-associated
categories of side effects associated with quality of life
(‘hepatotoxicity’, ‘haematological’, ‘mental/psychiatry’, ‘renal
toxicity’, ‘gastrointestinal’, ‘neuropathy’, ‘electrolytes
imbalance’, ‘pain’, ‘infection’, ‘skin’, ‘constitutional symptoms’,
‘cardiac/vascular’, ‘pulmonary’ and ‘other’). The details of the
outcomes of the included studies are presented in Table II.
 | Table II.General characteristics of the
included studies. |
Table II.
General characteristics of the
included studies.
| Author, year | Phase | N | Median age, years
(range) | Regimens | Dose | Route | Duration, median
(range) or mean ± SD | Overall survival,
months mean (95% CI) | Progression-free
survival, months mean (95% CI) | (Refs.) |
|---|
| Cunningham et
al, 2009 | 3 | 266 | 62 (26–83) | Gem | Gem (1,000
mg/m2/week) weekly | IV |
| 6.2 | 3.8 | (30) |
|
| 3 | 267 | 62 (37–82) | Gem+Cap | Gem (1,000
mg/m2/week) + Cap 1,660 mg/m2/day | IV and oral |
| 7.1 | 5.3 |
|
| Conroy et
al, 2005 | N/A | 171 | 61 (34–75) | Gem | Gem (1,000
mg/m2/week) | IV | 10 weeks | 6.8 (5.5–7.6) | 3.3 (2.2–3.6) | (32) |
|
| N/A | 171 | 61 (25–76) |
Oxa+Iri+Leu+Flu | Oxi (85
mg/m2) + Iri (180 mg/m2 + Leu (400
mg/m2) + Flu (400 mg/m2) | IV | 10 weeks | 11.1
(9.0–13.1) | 6.4 (5.5–7.2) |
|
| Berlin et
al, 2002 | 3 | 162 | 64.3 | Gem | Gem (1,000
mg/m2/week) | IV | 3 weeks of every
4 | 5.4 | 2.2 | (41) |
|
| 3 | 160 | 65.8 | Gem+5-FU | Gem (1,000
mg/m2/week) + 5-FU (600 mg/m2/week) | IV and bolus | 3 weeks of every
4 | 6.7 | 3.4 |
|
| Bramhall et
al, 2002 | N/A | 119 | 62 (37–85) | Gem | Gem (1,000
mg/m2/week) | IV | 10 weeks | 5.47 | 3.2 | (42) |
| N/A | 120 | 62 (32–83) | Gem+Mar | Gem (1,000
mg/m2/week) + Mar 10 mg b.i.d. | IV and bolus | 10 weeks | 5.52 | 3.08 |
|
|
| Rocha Lima et
al, 2004 | 3 | 180 | 60.2
(32.3–82.9) | Gem | Gem (1,000
mg/m2/week) (6.6–88) | IV | 12.9 weeks | 6.6
(0.03–22.8) | 3.0 (2.5–3.7) | (43) |
|
| 3 | 180 | 63.2
(38.7–81.2) | Gem+Iri | Gem (1,000
mg/m2/week) + Iri 100 mg/m2 | IV | 12.1 weeks
(3.0–83.9) | 6.3 (0.2–23.8) | 3.5 (2.8–4.2) |
|
| Van Cutsem et
al, 2004 | 3 | 347 | 62 (30–88) | Gem | Gem (1,000
mg/m2/week) | IV | 14 weeks | 6.07 | 3.63 | (44) |
| 3 | 341 | 61 (29–89) | Gem+Tip | Gem (1,000
mg/m2/week) + Tip 200 mg b.i.d. | IV and bolus | 12.1 weeks | 6.43 | 3.73 |
|
|
| Oettle et
al, 2005 | 3 | 273 | 63 (28–82) | Gem | Gem (1,250
mg/m2/week) | IV | 12 weeks | 6.2 (5.4–6.9) | 3.3 (2.5–3.6) | (45) |
|
| 3 | 273 | 63 (27–83) | Gem+Pem | Gem (1,000
mg/m2/week) + Pem 500 mg/m2 | IV | 12 weeks | 6.3 (5.4–6.9) | 3.9 (3.3–4.7) |
|
| Von Hoff et
al, 2005 | 3 | 430 | 63 | Gem | Gem (1,000
mg/m2/week) | IV | 3 months | 6.7 | 3.7 | (46) |
|
| 3 | 431 | 63 | Gem+Nab-p | N/A | IV | 4 months | 8.5 | 5.5 |
|
| Abou-Alfa et
al, 2006 | 3 | 174 | 62.3 (30–84) | Gem | Gem (1,000
mg/m2/week) | IV | 5.8±3.7 | 6.2 (5.2–7.5) | 3.8 (3–4.3) | (47) |
|
| 3 | 175 | 63 (36–85) | Gem+Exa | Gem (1,000
mg/m2/week) + Exa 2.0 mg/m2 | IV | months 6.4±4.2
months | 6.7 (5.4–7.9) | 3.7 (2.7–4.7) |
|
| Heinemann et
al, 2006 | 3 | 97 | 66 (43–85) | Gem | Gem (1,000
mg/m2/week) | IV | 4.1 months | 7.5 | 5.3 | (48) |
|
| 3 | 98 | 64 (37–82) | Gem+Cis | Gem (1,000
mg/m2/week) + Cis 50 mg/m2 | IV | 3.3 months | 6 | 3.1 |
|
| Stathopoulos et
al, 2006 | 3 | 70 | 64 (44–83) | Gem | Gem (900
mg/m2/week) | IV | 12 weeks | 6.5 | 2.9 | (49) |
|
| 3 | 60 | 64 (31–84) | Gem+Irl | Gem (900
mg/m2/week) + Iri 300 mg/m2 | IV | 9 weeks | 6.4 | 2.8 |
| Herrmann et
al, 2007 | 3 | 159 | N/A | Gem | Gem (1,000
mg/m2/week) | IV |
| 7.2 | 3.9 | (50) |
|
| 3 | 159 | N/A | Gem+Cap | Gem (1,000
mg/m2/week) + Cap 650 mg/m2 | IV and oral |
| 8.4 | 4.3 |
|
| Moore et al,
2007 | 3 | 284 | 64.0
(36.1–92.4) | Gem | Gem (1,000
mg/m2/week) | IV |
| 5.91 | 3.55 | (51) |
|
| 3 | 285 | 63.7
(37.9–84.4) | Gem+Erl | Gem (1,000
mg/m2/week) + Erl 100 mg/days | IV |
| 6.24 | 3.75 |
|
| Spano et al,
2008 | 2 | 34 | 61 (36–78) | Gem | Gem 1,000
mg/m2 on days 1,8 and 15 in 4-week cycles | IV | 4 (1–12)
cycles | 5.6 (3.9–8.8) | 3.7 (2.2–6.7) | (52) |
|
| 2 | 69 | 65 (44–81) | Gem+Axi | Gem 1,000
mg/m2 on day Axi 5 mg b.i.d. | PO | Axitinib 113
(7–481) days, |
|
|
|
|
|
|
|
|
|
|
| Gemcitabine 5
(1–18) cycles | 6.9 (5.3–10.1) | 4.2 (3.6–10.2) |
|
| Colucci et
al, 2010 | 3 | 199 | 63 (37–75) | Gem | Gem (1,000
mg/m2/week) | IV | 8 cycles | 8.3 | 3.9 | (40) |
|
| 3 | 201 | 63 (35–75) | Gem+Cis | Gem (1,000
mg/m2/week) + Cis 25 mg/m2 | IV | 7 cycles | 7.2 | 3.8 |
|
| Philip et
al, 2010 | 3 | 371 | 64.3 | Gem | Gem (1,000
mg/m2/week) | IV |
| 5.9 | 3 | (53) |
|
| 3 | 372 | 63.7 | Gem+Cet | Gem (1,000
mg/m2/week) + Cet 400 mg/m2 | IV |
| 6.3 | 3.4 |
|
| Kindler et
al, 2011 | 3 | 316 | 62 (35–89) | Gem | Gem (1,000
mg/m2/week) weekly | IV | 2.3 (0.03–11.0)
months | 8.3 (6.9–10.3) | 4.4 (3.7–5.2) | (54) |
|
| 3 | 314 | 61 (34–84) | Gem+Axi | Gem (1,000
mg/m2/week) + Axi 10 mg/day | IV and oral | 2.8 (0.03–11.0)
months | 8.5 (6.9–9.5) | 4.4 (4.0–5.6) |
|
| Loehrer et
al, 2011 | N/A | 37 | 67±8.7a | Gem | 1,000
mg/m2/week for weeks |
|
|
|
|
|
|
|
|
| 69 (49.7–83.7) |
| 1 to 6, followed by
1 week rest. Following rest, for 3 of 4 weeks | IV | 5.5 (2–8.3)
weeks | 9.2 (7.9–11.4) | 6.7 | (55) |
|
| N/A | 34 |
65.34±10.3a 66 (46.9–83.5) | Gem+Radio | 600
mg/m2/week | IV | 5.5 (2–8.3)
weeks | 11.1
(7.6–15.5) | 6 |
|
| Heinemann et
al, 2012 | N/A | 143 | 65 (32–78) | Gem | Gem (1,000
mg/m2/week) | IV | 5 cycles
(0–26) | 6.9 | 3.2 | (56) |
|
| N/A | 141 | 63 (38–75) | Gem+Cap+ Erl | Gem (1,000
mg/m2/week) + Cap (1,000 mg/m2 twice daily) +
Erl (150 mg daily) | IV | 5 cycles
(0–26) | 6.2 | 2.2 |
|
| Wu et al,
2013 | 1/2 | 8 | 59 (46–75) | Gem | Gem 1,000
mg/m2 weekly for 7 weeks with a one-week rest, followed
1,000 mg/m2 weekly for 3 weeks' with a one-week
rest | IV | 12.8 (8–22)
weeks | 8.1 (3.1–20.4) | 4.3 (2.2–8.1) | (57) |
|
| 1/2 | 30 | 59 (46–81) | Gem+Eta | Eta 25 mg twice
weekly | Subcutaneous | 12.2 (2–40)
weeks | 5.43
(1.5–16.9) | 2.23 (1.8–7.4) |
|
| Moehler et
al, 2014 | 2 | 48 | 64.5 (36–84) | Gem | Gem (1,000
mg/m2) was administered on days 1, 8, 15, 22, 29, 36 and
43 of the first cycle (8 weeks duration) and days 1, 8 and 15 of
all subsequent cycles (4 weeks duration) | IV | 4.2 (0.3–21.4)
months | 4.9 (3.5–7.7) | 4.9 (3.5–7.7) | (58) |
|
| 2 | 49 | 64.0 (44–83) | Gem+Sor | Sor 400 mg | IV and PO | 2.3 (0–19.1)
months | 3 (1.8–7.2) | 3 (1.8–7.2) |
|
| Catenacci et
al, 2015 | 1b/2 | 53 | 64 (39–84) | Gem | Gem 1,000 mg/m2 IV
over 30 min on days 1, 8 and 15 every 28 days | IV | 3 (0–14)
cycles | 6.1 (5.0–8.0) | 2.5 (1.9–3.8) | (59) |
|
| 1b/2 | 53 | 64 (49–82) | Gem+Vis | GDC-0449 150 mg
daily | | 4 (1–12)
cycles | 6.9 (5.8–8.0) | 4.0 (2.5–5.3) |
|
| Ramanathan et
al, 2016 | 3 | 430 | 63 (32–88) | Gem | Gem 1,000
mg/m2 weekly for 7 of 8 weeks (cycle 1); in subsequent
cycles, all patients were administered treatment on days 1, 8 and
15 every 4 weeks | IV |
| 6.7 (6.0–7.2) | 3.7 (3.6–4.0) | (60) |
|
| 3 | 431 | 62 (27–86) | Gem+Nab-p | Gem 1,000
mg/m2 on days 1, 8, 15, 29, 36 and 43 + Nab-P 1+++25
mg/m2 | IV |
| 8.5 (7.9–9.5) | 5.5 (4.5–5.9) |
|
Risk of bias assessment
To reduce the risk bias, the recommended approach of
Cochrane reviews was followed and the risk was assessed throughout
the process (22). The following
bias sources were independently assessed: Random sequence
generation, allocation concealment, blinding of investigators
and/or patients, blinding of outcome assessment and the degree of
data incompleteness. Each bias was scored as low, unclear or high,
as presented in Table III.
 | Table III.Risk assessment of the included
studies. |
Table III.
Risk assessment of the included
studies.
| Author, year | Random sequence
generation | Allocation
concealment | Blinding of
participants and personnel | Blinding of
assessment | Incomplete outcome
outcome data | Selective
reporting | Otherbias | Total scores | (Refs.) |
|---|
| Cunningham et
al, 2009 | L | L | L | L | H | U | L | 5 | (30) |
| Conroy et
al, 2005 | U | H | L | L | L | U | L | 4 | (32) |
| Berlin et
al, 2002 | L | L | L | L | L | L | L | 7 | (41) |
| Bramhall et
al, 2002 | L | L | L | L | L | L | L | 7 | (42) |
| Rocha Lima et
al, 2004 | L | L | H | L | L | L | L | 6 | (43) |
| Van Cutsem et
al, 2004 | L | L | L | L | L | U | L | 6 | (44) |
| Oettle et
al, 2005 | L | L | L | L | L | L | L | 7 | (45) |
| Von Hoff et
al, 2005 | L | L | L | L | L | L | L | 7 | (46) |
| Abou-Alfa et
al, 2006 | L | L | L | L | L | L | L | 7 | (47) |
| Heinemann et
al, 2006 | L | L | L | L | L | L | L | 7 | (48) |
| Stathopoulos et
al, 2006 | L | L | L | L | L | L | L | 7 | (49) |
| Herrmann et
al, 2007 | L | L | L | L | H | L | L | 6 | (50) |
| Moore et al,
2007 | L | L | L | L | H | L | L | 6 | (51) |
| Spano et al,
2008 | L | L | L | L | L | L | L | 7 | (52) |
| Colucci et
al, 2010 | L | L | L | L | L | L | L | 7 | (40) |
| Philip et
al, 2010 | L | L | L | L | H | L | L | 7 | (53) |
| Kindler et
al, 2011 | L | L | L | L | L | L | L | 7 | (54) |
| Loehrer et
al, 2011 | L | L | L | L | L | L | L | 7 | (55) |
| Heinemann et
al, 2012 | L | L | L | L | L | L | L | 7 | (56) |
| Wu et al,
2013 | L | L | L | L | L | L | L | 7 | (57) |
| Moehler et
al, 2014 | L | L | L | L | L | L | L | 7 | (58) |
| Catenacci et
al, 2015 | L | L | L | L | L | L | U | 6 | (59) |
| Ramanathan et
al, 2016 | L | L | L | L | H | L | L | 6 | (60) |
Statistical analysis
All statistical analyses were performed using the
network meta-analysis package in Stata (version 13.0; StataCorp LP,
College Station, TX, USA) (19).
For the endpoint outcomes, OS and PFS data were extracted from
references as medians and subsequently transformed into
standardized mean differences with 95% confidence intervals (CIs).
A network meta-analysis was conducted following the standard
workflow (19). The network map
presents the connection status of the studies; no loops and a
P-value >0.05 validated the consistency model to perform the
network meta-analysis. Heterogeneity was assessed with the
I2 metric. Heterogeneity was 0% for OS and 75.64% for
PFS. Therefore, the Mantel-Haenszel fixed-effects model was used
for OS, and the Mantel-Haenszel random-effects model was used for
PFS (21). All of the studies with
various treatments were included as drugs that were either directly
or indirectly associated with a common comparator (Gem only) for
further downstream ranking analysis. Subsequently, to rank the
effects of the treatments, the analysis of the surface under the
cumulative ranking (SUCRA) probabilities was performed under the
protocol of Stata (19), and the
results are presented as the percentage of the efficacy of each
intervention relative to a hypothetical ideal intervention
(23). A larger SUCRA score
indicated longer OS and PFS.
The data are presented in an ordinal data format
according to the 14 categories of side effects. Network
meta-analyses were separately conducted, and the data were
calculated as hazard ratios with 95% CIs. To examine and classify
the adverse effects that occurred among the different treatments, a
stack bar graph of each category was generated.
Results
Standard workflow via PRISMA
To ensure the general quality of the present study,
a PRISMA flowchart regarding the screening process of the study
used, is presented in Fig. 1. From
an initial set of 7,855 non-duplicated studies, a total of 23 RCTs
were included in this analysis. The drug abbreviations and the
combinations assessed are listed in Table I, and the general characteristics
of the included RCTs or studies are presented in Table II. The risk of bias assessment for
these included RCTs is depicted in Table III. The geometry evidence of the
OS network plot and its associated pooled forest plot are
summarized in Fig. 2. Furthermore,
the geometry evidence of the PFS network plot and its associated
pooled forest plot are summarized in Fig. 3. Using SUCRA, graphs of the rank of
the treatments associated with OS and PFS are listed in Fig. 4, and the 14 types of
treatment-associated toxicities are presented in Fig. 5.
 | Figure 2.Network and forest plot for overall
survival. (A) Geometry evidence of overall survival. (B) Overall
survival forest. Gem, gemcitabine; Axit, axitinib; 5-FU,
5-fluorouracil; Erl, erlotinib; Cap, capecitabine; Cet, cetuximab;
Cis, cisplatin; Bev, bevacizumab; Eta, etanercept; Exa, exatecan;
Iri, irinotecan; Mar, marismastat; Nab-p, nab-paclitaxel; Pem,
pemetrexed; Radio, radiotherapy; Sor, sorafenib; Tip, tipifarnib;
Vis, vismodegib. |
 | Figure 3.Network and forest plot for
progression-free survival. (A) Geometry evidence of
progression-free survival. (B) Progression-free survival forest.
Gem, gemcitabine; Axit, axitinib; 5-FU, 5-fluorouracil; Erl,
erlotinib; Cap, capecitabine; Cet, cetuximab; Cis, cisplatin; Bev,
bevacizumab; Eta, etanercept; Exa, exatecan; Iri, irinotecan; Mar,
marismastat; Nab-p, nab-paclitaxel; Pem, pemetrexed; Radio,
radiotherapy; Sor, sorafenib; Tip, tipifarnib; Vis, vismodegib. |
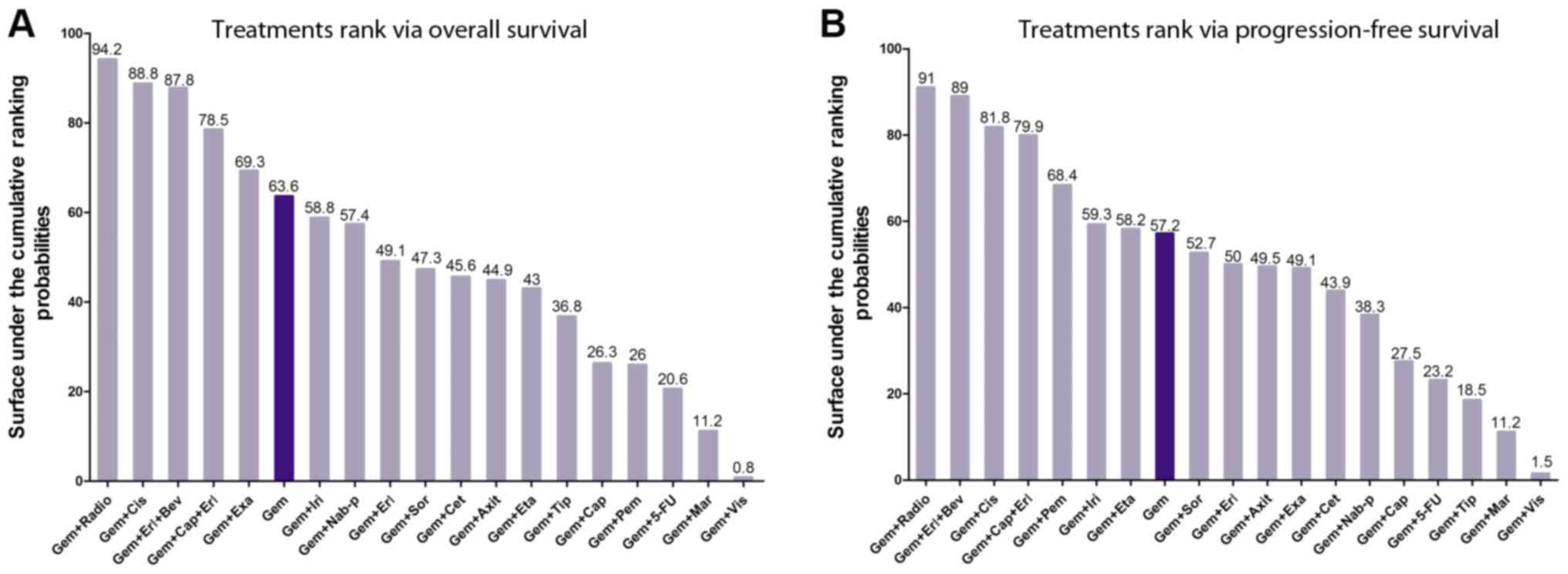 | Figure 4.Treatment ranking by overall survival
and progression-free survival. (A) Treatment ranking by overall
survival. (B) Treatment ranking by progression-free survival. Gem,
gemcitabine; Axit, axitinib; 5-FU, 5-fluorouracil; Erl, erlotinib;
Cap, capecitabine; Cet, cetuximab; Cis, cisplatin; Bev,
bevacizumab; Eta, etanercept; Exa, exatecan; Iri, irinotecan; Mar,
marismastat; Nab-p, nab-paclitaxel; Pem, pemetrexed; Radio,
radiotherapy; Sor, sorafenib; Tip, tipifarnib; Vis, vismodegib. |
 | Figure 5.Assessment of the occurrence rates of
14 dominant drug-associated toxicities among 19 chemotherapy
regimens. Gem, gemcitabine; Axit, axitinib; 5-FU, 5-fluorouracil;
Erl, erlotinib; Cap, capecitabine; Cet, cetuximab; Cis, cisplatin;
Bev, bevacizumab; Eta, etanercept; Exa, exatecan; Iri, irinotecan;
Mar, marismastat; Nab-p, nab-paclitaxel; Pem, pemetrexed; Radio,
radiotherapy; Sor, sorafenib; Tip, tipifarnib; Vis, vismodegib;
Oxa, oxaliplatin; Leu, leucovorin; Flu, fluorouracil. |
Network diagram (geometry and
forest)
Numerous combinations of various treatments were
analyzed. The network maps of OS and PFS demonstrated the geometry
of 18 chemotherapy regimens compared with a common treatment, Gem,
and no loops were identified (Figs.
2A and 3A). Furthermore, the
network forest plots indicated the effectiveness of the different
regimens compared with the pooled overall result (Figs. 2B and 3B).
Ranking treatments
Due to the variable conditions of APC, a critical
aspect to be considered by medical doctors is what chemotherapy
regimens are the most suitable and reasonable for the specific
conditions of their patients. Therefore, 19 chemotherapy regimens
were ranked according to their SUCRA probabilities based on OS and
PFS (Fig. 4).
Regarding OS (Fig.
4A), Gem ranked 6th with a SUCRA value of 63.6. The top five
combination regimens included Gem+radiotherapy (Radio),
Gem+cisplatin (Cis), Gem+erlotinib (Erl)+bevacizumab (Bev),
Gem+capecitabine (Cap)+Erl, and Gem+exatecan (Exa). The present
results suggested that radiotherapy was the most effective
treatment in extending the OS of patients, consistently with the
results observed for PFS (Fig.
4B). The SUCRA scores for Gem+irinotecan (Iri) to
Gem+tipifarnib presented a similar medium rank, and the scores for
Gem+Cap to Gem+vismodegib (Vis) presented a low rank.
For PFS (Fig. 4B),
Gem ranked 8th with a SUCRA value of 57.2. The top seven
combination regimens included Gem+Radio, Gem+Erl+Bev, Gem+Cis,
Gem+Cap+Erl, Gem+pemetrexed, Gem+Iri and Gem+etanercept. The SUCRA
scores from Gem+ sorafenib (Sor) to Gem+ nab-paclitaxel (Nab-p)
presented a medium rank, and Gem+Cap to Gem+Vis presented a low
rank.
Adverse events
In addition to survival, health-associated quality
of life issues are a central aspect for patients with APC.
Improving the health-associated quality of life by reducing
treatment-associated toxicities and the occurrence rate of adverse
events is important for patients. The majority of common
treatment-associated toxicities include ‘hepatotoxicity’,
‘haematological’, ‘mental/psychiatry’, ‘renal toxicity’,
‘gastrointestinal’, ‘neuropathy’, ‘electrolytes imbalance’, ‘pain’,
‘infection’, ‘skin’, ‘constitutional symptoms’, ‘cardiac/vascular’,
‘pulmonary’ and ‘other’. The majority of these toxicities seriously
affect the quality of life of the patient during the treatment
process.
Regarding the 19 chemotherapy regimens, each regimen
may cause various treatment-associated toxicities, and the
occurrence rate of each toxicity varied among treatments.
Nevertheless, the three treatment-associated toxicities with the
highest occurrence rates were ‘haematological’, ‘gastrointestinal’
and ‘constitutional symptoms’ for all regimens except Gem+Erl,
which presented the largest proportions of toxicities. Furthermore,
the remaining treatment-associated toxicities, including ‘skin’,
‘hepatotoxicity’, ‘infection’, ‘cardiac/vascular’, ‘neuropathy’,
and ‘mental/psychiatry’, were the most common adverse effects among
the majority of regimens, following ‘haematological’,
‘gastrointestinal’ and ‘constitutional symptoms’. ‘Renal toxicity’,
‘electrolytes imbalance’ and ‘pulmonary’ presented the lowest
occurrence rate among the regimens.
Treatment-associated toxicities always accompany the
therapeutic process. To achieve the best results from the
perspectives of the clinicians and the patients, patients must
consider a series of unavoidable treatment-associated toxicities,
leading to a complex selection process.
Discussion
Since Gem was approved as a first-line treatment for
APC by the FDA in 1996, a number of combination chemotherapy
regimens, including Gem+Xs, have emerged. Post-treatment long-term
survival remains poor and is a marked risk of the current
chemotherapy regimens (24). This
issue may be caused by a failure of local control and of diagnosing
localized APC in time (25).
Associated RCTs and studies published between 2002 and 2016,
covering a total of 14 years, were selected to assess the
advantages and disadvantages of each regimen compared with Gem
monotherapy.
Among the chemotherapy regimens, Gem+Radio presented
the principal improvement in extending OS and PFS. This finding
suggested that radiotherapy may block the progressive deterioration
associated with advanced cancer and is consistent with the previous
study of Youl et al (26),
which identified that a gross tumor volume <48 cm3
may be successfully targeted with radiotherapy. An additional three
regimens, Gem+Cis, Gem+Erl+Bev and Gem+Cap+Erl, were better
compared with Gem monotherapy in terms of OS and PFS. Regarding
other combination regimens, Gem+Iri, Gem+Sor, Gem+Erl,
Gem+Axitinib, Gem+Exa, Gem+Cetuximab, Gem+Nab-p and Gem+Cap
presented higher SUCRA probabilities compared with Gem+5-FU,
another double regimen. In contrast, Gem+marismastat and Gem+Vis
exhibited decreased SUCRA probabilities. Although numerous regimens
are available, side effects always accompany the therapeutic
effect. Therefore, selecting the regimen that may offer the longest
survival is a complex process that requires the clinician to
comprehensively assess the disease status of the patient in order
to identify a balance between the benefits and risks of the
treatment.
A previous study demonstrated that patients who
undergo 5-FU and 5-FU-based regimens following resection had
improved survival rates (27).
5-FU has been used as the principle chemoradiation and/or
chemotherapy regimens and was previously considered the principal
effective chemotherapeutic agent available in pancreatic cancer
treatment. In the 1990s, Gem was first demonstrated to be a safe
drug with low toxicity for APC treatment (28). Subsequent clinical trials
demonstrated the significant advantages of Gem for short-term and
long-term OS in the treatment of advanced and metastatic pancreatic
cancer (8,29,30);
these previous results demonstrated the effectiveness of Gem
therapy in pancreatic cancer.
In patients with optimal performance status,
concurrent chemotherapy, including FOLFIRINOX, albumin-bound
paclitaxel and 5-FU, in combination with Gem, or other Gem-based
combined chemotherapies, may provide increased survival benefits
(31,32). Although patients at similar
disease-stages receive the same chemical regimens, there may be
various outcomes due to individual variations.
Gem has been the standard for treating APC since
1996, providing a limited survival of 6 months due to the intrinsic
capacity of cancer cells and the surrounding microenvironment to
resist cytotoxicity (33,34). Combination therapies with Gem have
presented limited effectiveness in clinical trials, and the
identification of novel therapeutic strategies that may increase
median survival and PFS with reduced adverse effects, is required.
Certain treatments, including 5-FU monotherapy, presented
significantly decreased survival compared with Gem monotherapy and
have demonstrated the effectiveness of Gem as a first-line
chemotherapy for APC. Although certain trials examining combination
therapies demonstrated improved objective response rates, the
treatments failed to achieved improvement in all three of the most
common outcomes measured; Median survival, PFS and the objective
response rate (35–39). The present network meta-analysis of
patients with unresectable pancreatic cancer suggested that the
current median survival for patients treated with Gem was >6
months, and the objective response rate for Gem ranged between 4.4
and 17.3% (40). These results may
represent the standard to be compared with future results of
single-arm phase II trials involving Gem-based combination
regimens. The severity of the adverse effects among these regimens
was assessed by inconsistent scales, which may contribute to bias.
Future studies may consider a consistent scale among various
conditions in order to avoid possible biases.
In conclusion, numerous chemotherapy regimens are
used to extend the survival and reduce the treatment-associated
toxicities of patients with APC. The effect and
treatment-associated toxicities of a particular regimen requires
consideration to balance the benefits and risks for the patient.
The present study provides additional evidence for selecting
appropriate treatments according to the clinical situation.
Acknowledgements
The authors would like to thank Dr Kun Huang from
Chengdu TME Co., Ltd. (Chengdu, China) for advice on optimizing the
data search strategy and Professor Jinhui Tian from Lanzhou
University (Lanzhou, China) for the valuable discussion regarding
the implications of the results.
Funding
The present study was supported by the Science &
Technology Department of Sichuan Province Grants (Sichuan, China;
grant no. 2016JY0036).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JC, FX and SY conceived and designed the present
study. YX, LC, JY, XW, ZZ and NL performed the data extraction. JY
and FX ensured the quality of the data. FX and SY analyzed the
data. JC, FX and LC drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goral V: Pancreatic cancer: Pathogenesis
and diagnosis. Asian Pac J Cancer Prev. 16:5619–5624. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pancreatic Cancer Action Network: The
alarming rise of pancreatic cancer deaths in the United States. Why
we need to stem the tide today. Pancreatic Cancer Action Network;
Manhattan Beach, CA: 2012
|
|
3
|
Krechler T, Horejs J, Ulrych J, Zeman M,
Macásek J, Dusková J and Zák A: Current status of pancreatic cancer
diagnosis. Cas Lek Cesk. 150:587–593. 2011.(In Czech). PubMed/NCBI
|
|
4
|
Freelove R and Walling AD: Pancreatic
cancer: Diagnosis and management. Am Fam Physician. 73:485–492.
2006.PubMed/NCBI
|
|
5
|
Chen WQ, Liang D, Zhang SW, Zheng RS and
He YT: Pancreatic cancer incidence and mortality patterns in china,
2009. Asian Pac J Cancer Prev. 14:7321–7324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Schrag D, Brooks GA and Dominici
F: National trends in pancreatic cancer outcomes and pattern of
care among Medicare beneficiaries, 2000 through 2010. Cancer.
120:1050–1058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akhtar-Danesh GG, Finley C and
Akhtar-Danesh N: Long-term trends in the incidence and relative
survival of pancreatic cancer in Canada: A population-based study.
Pancreatology. 16:259–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbruzzese JL: New applications of
gemcitabine and future directions in the management of pancreatic
cancer. Cancer. 95 (Suppl 4):941–945. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macdonald JS: Clinical overview: Adjuvant
therapy of gastrointestinal cancer. Cancer Chemother Pharmacol. 54
Suppl 1:S4–S11. 2004.PubMed/NCBI
|
|
11
|
Okusaka T, Ishii H, Funakoshi A, Ueno H,
Furuse J and Sumii T: A phase I/II study of combination
chemotherapy with gemcitabine and 5-fluorouracil for advanced
pancreatic cancer. Jpn J Clin Oncol. 36:557–563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi S, Yao W, Xu J, Long J, Liu C and Yu
X: Combinational therapy: New hope for pancreatic cancer? Cancer
Lett. 317:127–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristensen A, Vagnildhaug OM, Grønberg BH,
Kaasa S, Laird B and Solheim TS: Does chemotherapy improve
health-related quality of life in advanced pancreatic cancer? A
systematic review. Crit Rev Oncol Hematol. 99:286–298. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gresham GK, Wells GA, Gill S, Cameron C
and Jonker DJ: Chemotherapy regimens for advanced pancreatic
cancer: A systematic review and network meta-analysis. BMC Cancer.
14:4712014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ansari D, Tingstedt B, Andersson B,
Holmquist F, Sturesson C, Williamsson C, Sasor A, Borg D, Bauden M
and Andersson R: Pancreatic cancer: Yesterday, today and tomorrow.
Future Oncol. 12:1929–1946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Birhanu G, Javar HA, Seyedjafari E and
Zandi-Karimi A: Nanotechnology for delivery of gemcitabine to treat
pancreatic cancer. Biomed Pharmacother. 88:635–643. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaimani A, Higgins JP, Mavridis D,
Spyridonos P and Salanti G: Graphical tools for network
meta-analysis in STATA. PLoS One. 8:e766542013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Biermann JS, Chow W, Reed DR, Lucas D,
Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, et
al: NCCN guidelines insights: Bone cancer, version 2.2017. J Natl
Compr Canc Netw. 15:155–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hernández-Quiles C, Bernabeu-Wittel M,
Pérez-Belmonte LM, Macías-Mir P, Camacho-González D, Massa B,
Maiz-Jiménez M and Ollero-Baturone M: PALIAR investigators:
Concordance of Barthel Index, ECOG-PS, and Palliative Performance
Scale in the assessment of functional status in patients with
advanced medical diseases. BMJ Support Palliat Care. 7:300–307.
2017.PubMed/NCBI
|
|
22
|
Lovato N, Lack L, Wright H and Kennaway
DJ: Evaluation of a brief treatment program of cognitive behavior
therapy for insomnia in older adults. Sleep. 37:117–126. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salanti G, Ades AE and Ioannidis JP:
Graphical methods and numerical summaries for presenting results
from multiple-treatment meta-analysis: An overview and tutorial. J
Clin Epidemiol. 64:163–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jutric Z and Melstrom LG: New treatment
options and management considerations in borderline resectable
pancreatic cancer. Oncology (Williston Park). 31:443–452.
2017.PubMed/NCBI
|
|
25
|
Sugarbaker PH: Strategies to improve local
control of resected pancreas adenocarcinoma. Surg Oncol. 26:63–70.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Youl M, Hashem S, Brade A, Cummings B,
Dawson LA, Gallinger S, Hedley D, Jiang H, Kim J, Krzyzanowska MK,
et al: Induction gemcitabine plus concurrent gemcitabine and
radiotherapy for locally advanced unresectable or resected
pancreatic cancer. Clin Oncol (R Coll Radiol). 26:203–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalser MH and Ellenberg SS: Pancreatic
cancer. Adjuvant combined radiation and chemotherapy following
curative resection. Arch Surg. 120:899–903. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aapro MS, Martin C and Hatty S:
Gemcitabine-a safety review. Anticancer Drugs. 9:191–201. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Regine WF, Winter KA, Abrams RA, Safran H,
Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm
ML, et al: Fluorouracil vs gemcitabine chemotherapy before and
after fluorouracil-based chemoradiation following resection of
pancreatic adenocarcinoma: A randomized controlled trial. JAMA.
299:1019–1026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cunningham D, Chau I, Stocken DD, Valle
JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J,
et al: Phase III randomized comparison of gemcitabine versus
gemcitabine plus capecitabine in patients with advanced pancreatic
cancer. J Clin Oncol. 27:5513–5518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vaccaro V, Sperduti I and Milella M:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 365:768–769; author reply 769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rhim AD, Oberstein PE, Thomas DH, Mirek
ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP,
Tattersall IW, et al: Stromal elements act to restrain, rather than
support, pancreatic ductal adenocarcinoma. Cancer Cell. 25:735–747.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fox RG, Lytle NK, Jaquish DV, Park FD, Ito
T, Bajaj J, Koechlein CS, Zimdahl B, Yano M, Kopp J, et al:
Image-based detection and targeting of therapy resistance in
pancreatic adenocarcinoma. Nature. 534:407–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Citterio C, Baccini M, Orlandi E, Di
Nunzio C and Cavanna L: Second-line chemotherapy for the treatment
of metastatic pancreatic cancer after first-line gemcitabine-based
chemotherapy: A network meta-analysis. Oncotarget. 9:29801–29809.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiorean EG, Von Hoff DD, Tabernero J,
El-Maraghi R, Wee Ma W, Reni M, Harris M, Whorf R, Liu H, Shiansong
Li J, et al: Second-line therapy after nab-paclitaxel plus
gemcitabine or after gemcitabine for patients with metastatic
pancreatic cancer. Br J Cancer. 115:e132016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oettle H, Riess H, Stieler JM, Heil G,
Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V, et
al: Second-line oxaliplatin, folinic acid, and fluorouracil versus
folinic acid and fluorouracil alone for gemcitabine-refractory
pancreatic cancer: Outcomes from the CONKO-003 trial. J Clin Oncol.
32:2423–2429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walker EJ and Ko AH: Beyond first-line
chemotherapy for advanced pancreatic cancer: An expanding array of
therapeutic options? World J Gastroenterol. 20:2224–2236. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schultheis B, Reuter D, Ebert MP, Siveke
J, Kerkhoff A, Berdel WE, Hofheinz R, Behringer DM, Schmidt WE,
Goker E, et al: Gemcitabine combined with the monoclonal antibody
nimotuzumab is an active first-line regimen in KRAS wildtype
patients with locally advanced or metastatic pancreatic cancer: A
multicenter, randomized phase IIb study. Ann Oncol. 28:2429–2435.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Colucci G, Giuliani F, Gebbia V, Biglietto
M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E and Lopez M:
Gemcitabine alone or with cisplatin for the treatment of patients
with locally advanced and/or metastatic pancreatic carcinoma: A
prospective, randomized phase III study of the Gruppo Oncologia
dell'Italia Meridionale. Cancer. 94:902–910. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Berlin JD, Catalano P, Thomas JP, Kugler
JW, Haller DG and Benson AB III: Phase III study of gemcitabine in
combination with fluorouracil versus gemcitabine alone in patients
with advanced pancreatic carcinoma: Eastern Cooperative Oncology
Group Trial E2297. J Clin Oncol. 20:3270–3275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bramhall SR, Schulz J, Nemunaitis J, Brown
PD, Baillet M and Buckels JA: A double-blind placebo-controlled,
randomised study comparing gemcitabine and marimastat with
gemcitabine and placebo as first line therapy in patients with
advanced pancreatic cancer. Br J Cancer. 87:161–167. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rocha Lima CM, Green MR, Rotche R, Miller
WH Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G and
Miller LL: Irinotecan plus gemcitabine results in no survival
advantage compared with gemcitabine monotherapy in patients with
locally advanced or metastatic pancreatic cancer despite increased
tumor response rate. J Clin Oncol. 22:3776–3783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Van Cutsem E, van de Velde H, Karasek P,
Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype
C, Neumann H, et al: Phase III trial of gemcitabine plus tipifarnib
compared with gemcitabine plus placebo in advanced pancreatic
cancer. J Clin Oncol. 22:1430–1438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oettle H, Richards D, Ramanathan RK, van
Laethem JL, Peeters M, Fuchs M, Zimmermann A, John W, Von Hoff D,
Arning M and Kindler HL: A phase III trial of pemetrexed plus
gemcitabine versus gemcitabine in patients with unresectable or
metastatic pancreatic cancer. Ann Oncol. 16:1639–1645. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Von Hoff DD, Ervin TJ, Arena FP, Gabriela
Chiorean E, Infante JR, Moore M, Seay TE, Tjulandin S, Ma WW, Saleh
M, et al: Randomized phase III study of weekly nab-paclitaxel plus
gemcitabine versus gemcitabine alone in patients with metastatic
adenocarcinoma of the pancreas (MPACT). J Clin Oncol. 31:LBA148.
2013. View Article : Google Scholar
|
|
47
|
Abou-Alfa GK, Letourneau R, Harker G,
Modiano M, Hurwitz H, Tchekmedyian NS, Feit K, Ackerman J, De Jager
RL, Eckhardt SG and O'Reilly EM: Randomized phase III study of
exatecan and gemcitabine compared with gemcitabine alone in
untreated advanced pancreatic cancer. J Clin Oncol. 24:4441–4447.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heinemann V, Quietzsch D, Gieseler F,
Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M,
Heinrich B, et al: Randomized phase III trial of gemcitabine plus
cisplatin compared with gemcitabine alone in advanced pancreatic
cancer. J Clin Oncol. 24:3946–3952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Stathopoulos GP, Syrigos K, Aravantinos G,
Polyzos A, Papakotoulas P, Fountzilas G, Potamianou A, Ziras N,
Boukovinas J, Varthalitis J, et al: A multicenter phase III trial
comparing irinotecan-gemcitabine (IG) with gemcitabine (G)
monotherapy as first-line treatment in patients with locally
advanced or metastatic pancreatic cancer. Br J Cancer. 95:587–592.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Herrmann R, Bodoky G, Ruhstaller T,
Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A,
Pestalozzi B, et al: Gemcitabine plus capecitabine compared with
gemcitabine alone in advanced pancreatic cancer: A randomized,
multicenter, phase III trial of the Swiss Group for Clinical Cancer
Research and the Central European Cooperative Oncology Group. J
Clin Oncol. 25:2212–2217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Spano JP, Chodkiewicz C, Maurel J, Wong R,
Wasan H, Barone C, Létourneau R, Bajetta E, Pithavala Y, Bycott P,
et al: Efficacy of gemcitabine plus axitinib compared with
gemcitabine alone in patients with advanced pancreatic cancer: An
open-label randomised phase II study. Lancet. 371:2101–2108. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Philip PA, Benedetti J, Corless CL, Wong
R, O'Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC,
Rivkin SE, et al: Phase III study comparing gemcitabine plus
cetuximab versus gemcitabine in patients with advanced pancreatic
adenocarcinoma: Southwest Oncology Group-directed intergroup trial
S0205. J Clin Oncol. 28:3605–3610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kindler HL, Ioka T, Richel DJ, Bennouna J,
Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S,
et al: Axitinib plus gemcitabine versus placebo plus gemcitabine in
patients with advanced pancreatic adenocarcinoma: A double-blind
randomised phase 3 study. Lancet Oncol. 12:256–262. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Loehrer PJ Sr, Feng Y, Cardenes H, Wagner
L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR
and Benson AB III: Gemcitabine alone versus gemcitabine plus
radiotherapy in patients with locally advanced pancreatic cancer:
An Eastern Cooperative Oncology Group trial. J Clin Oncol.
29:4105–4112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Heinemann V, Vehling-Kaiser U, Waldschmidt
D, Kettner E, Märten A, Winkelmann C, Klein S, Kojouharoff G,
Gauler TC, von Weikersthal LF, et al: Gemcitabine plus erlotinib
followed by capecitabine versus capecitabine plus erlotinib
followed by gemcitabine in advanced pancreatic cancer: Final
results of a randomised phase 3 trial of the ‘Arbeitsgemeinschaft
Internistische Onkologie’ (AIO-PK0104). Gut. 62:751–759. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu C, Fernandez SA, Criswell T, Chidiac
TA, Guttridge D, Villalona-Calero M and Bekaii-Saab TS: Disrupting
cytokine signaling in pancreatic cancer: A phase I/II study of
etanercept in combination with gemcitabine in patients with
advanced disease. Pancreas. 42:813–818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Moehler M, Maderer A, Schimanski C,
Kanzler S, Denzer U, Kolligs FT, Ebert MP, Distelrath A, Geissler
M, Trojan J, et al: Gemcitabine plus sorafenib versus gemcitabine
alone in advanced biliary tract cancer: A double-blind
placebo-controlled multicentre phase II AIO study with biomarker
and serum programme. Eur J Cancer. 50:3125–3135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Catenacci DV, Junttila MR, Karrison T,
Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M,
Rajdev L, et al: Randomized phase Ib/II study of gemcitabine plus
placebo or vismodegib, a hedgehog pathway inhibitor, in patients
with metastatic pancreatic cancer. J Clin Oncol. 33:4284–4292.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ramanathan RK, Goldstein D, Korn RL, Arena
F, Moore M, Siena S, Teixeira L, Tabernero J, Van Laethem JL, Liu
H, et al: Positron emission tomography response evaluation from a
randomized phase III trial of weekly nab-paclitaxel plus
gemcitabine versus gemcitabine alone for patients with metastatic
adenocarcinoma of the pancreas. Ann Oncol. 27:648–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|



















