Introduction
Refractory diseases, including osteonecrosis of the
femoral head, are a growing worldwide health problem (1), and the potential of tissue
engineering strategies for their treatment are currently being
investigated. The fundamental elements of tissue engineering
include scaffolds, signals and cells. Tissue engineering scaffolds
are cytocompatible biomaterials that cells adhere to and/or replace
with extracellular matrix (ECM) to produce native tissues (2). Scaffolds are usually classified on
the basis of their source and ability to degrade. Tricalcium
phosphate (TCP) is a synthetic, degradable inorganic material that
has good biocompatibility, bioactivity and biodegradability; it is
an ideal tissue repair material (3). TCP is commonly used at low
temperature β phase, and β-TCP is mainly composed of calcium and
phosphorus, similar to the inorganic composition of bone (4). However, it is a challenging material
owing to its hydrophobicity and the absence of active groups to
interact with cells of interest.
Mesenchymal stem cells (MSCs) are the most common
type of cell used in orthopedic tissue engineering. MSCs have the
potential to differentiate into osteoblasts, chondrocytes and
adipocytes (5). At present, bone
marrow-derived MSCs (BMSCs) are considered the gold standard for
use in tissue engineering (2).
However, pathological tissues often have poor innate regenerative
capacity, with few or no viable MSCs (2). In tissue engineering and regenerative
medicine, seed cells (for example, BMSCs) should be combined with
scaffolds to repair tissues of interest (6). Strategies for taking full advantage
of biomaterials and the efficient recruitment of MSCs are being
increasingly investigated (7). The
precise and efficient adhesion of MSCs to biomaterials requires
investigation.
A number of attempts to enhance the affinity between
cells and biomaterials have been conducted, and surface
modification of biomaterials is widely used. For example, the
heptapeptide sequence, LTHPRWP (L7), with affinity towards
synovium-derived mesenchymal stem cells (SMSCs) has been covalently
conjugated to polycaprolactone electrospun meshes and to human
decalcified bone scaffolds; this elevates the adhesion and
spreading of SMSCs on scaffolds (8). A chondrocyte-affinity peptide (CAP),
DWRVIIPPRPSA, was identified by phage display technology (9). Polyethylenimine has been covalently
modified with CAP to construct a non-viral vector for
cartilage-targeted therapy (9). A
previous study revealed the enhanced adhesion of human dermal
fibroblasts on anorganic bovine bone mineral modified by the
functional synthetic 15-residue peptide sequence GTPGPQGIAGQRQVV
(P-15), which is a potent cell-binding domain in the α1 chain of
type I collagen (10,11). The RGD peptide, derived from
fibronectin in ECM, was demonstrated to promote cell adhesion in
1984 (12); since then, a number
of materials modified with RGD have been used in academic study and
clinical therapies (13,14).
Affinity peptides towards BMSCs are also commonly
used. Recently, a novel peptide, DPIYALSWSGMA (DPI), with specific
affinity towards BMSCs, was identified through phage display
technology (15). In the present
study, the affinity of the DPI peptide towards BMSCs was further
verified. β-TCP was selected as the scaffold modified by DPI and
cellular behavior on the modified scaffold was studied.
Materials and methods
Cell culture
C57BL/6 mouse BMSCs (cat. no. MUBMX-01001) were
obtained from Cyagen Biosciences, Inc. (Santa Clara, CA, USA). The
cells were cultured in cell culture flasks in a humidified
atmosphere with 5% CO2 at 37°C (Fig. 1). Cells were cultured in low
glucose Dulbecco's modified Eagle's medium (cat. no. 01-051-1A;
Biological Industries, Kibbutz Beit-Haemek, Israel) with
L-glutamine containing 10% fetal bovine serum (cat. no. 10099141;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin; cat.
no. 15140122; Gibco; Thermo Fisher Scientific, Inc.). The medium
was replaced every 2–3 days. Cells were detached and passaged at
80–90% confluency. BMSCs were used at passage 4–5 for further
experiments.
Peptide synthesis
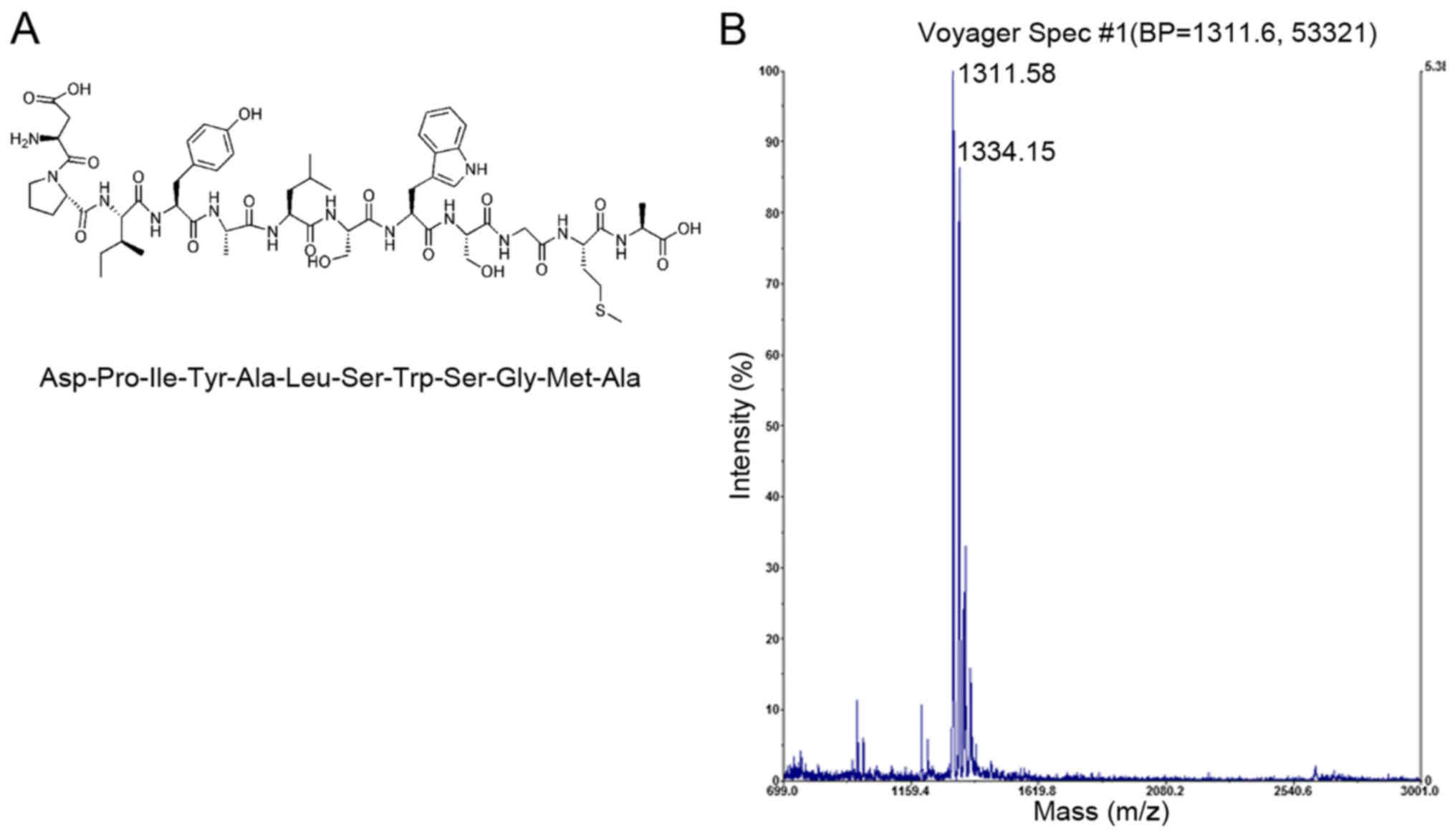
The DPI peptide sequence with high affinity towards
BMSCs (Fig. 2A) was previously
discovered using phage display (15). The linear dodecapeptide was derived
from the Ph.D.-12 phage display library (cat. no. E8110S; New
England Biolabs, Inc., Ipswich, MA, USA). The molecular weight of
the synthesized DPI peptide was confirmed to be 1,311.58 Da by mass
spectrometry (JBI Scientific, LLC, Huntsville, TX, USA) using
matrix-assisted laser desorption/ionization-time of flight method
with positive mode (Fig. 2B). The
synthesized DPI peptide was dissolved in 50% acetonitrile (ACN)
with 0.05% trifluoroacetic acid (TFA) to obtain a concentration of
100 µg/ml, and subsequently mixed with α-cyano-4-hydroxycinnamic
acid solution (10 mg/ml in 50% ACN, 0.05% TFA) at a 1:1 ratio. A
total of 1 µl of the mixture was loaded on target plate and air
dried. The instrument settings were: 20,000 V accelerating voltage,
95% grid voltage, acquisition mass range between 600 and 2,000 Da,
and extraction delay time was 200 nsec. A peptide sequence of the
same chain length as DPI, but scrambled (LSPSAGAYIDWM; LSP), was
used as the negative control. A peptide comprising three amino
acids (RGD) was used as the positive control. All peptides were
synthesized by solid-phase peptide synthesis using
9-fluorenylmethoxycarbonyl chemistry (Scilight-Peptide, Inc.;
Scilight Biotechnology, LLC Beijing, China). An extra aminohexanoic
acid was linked at the amino-terminus of all peptides to facilitate
fluorescein-5-isothiocyanate (FITC) labeling. The FITC-labeled
peptides, FITC-DPI, FITC-LSP and FITC-RGD, were stored at −20°C. A
concentration of 1 mg/ml was obtained by dissolving the peptides in
PBS (cat. no. 02-024-1A; Biological Industries) before use.
Peptide-affinity assay by flow
cytometry
The C57BL/6 mouse BMSCs were washed twice with PBS
and dissociated with 0.25% trypsin-EDTA (cat. no. 25200-056; Gibco;
Thermo Fisher Scientific, Inc.). The cell suspension was
centrifuged at 250 × g for 5 min at room temperature to collect
cell sedimentation. The cells were incubated with 100 µM
FITC-labeled peptides for 1 h at 37°C to allow cell binding and
internalization. The mouse BMSC affinity properties of the peptides
were analyzed quantitatively using flow cytometry at a wavelength
of 488 nm and FlowJo v7.6.1 (Tree Star, Inc., Ashland, OR, USA)
software. All procedures were repeated at least three times.
Peptide-affinity assay by fluorescence
cytochemistry
C57BL/6 mouse BMSCs were cultured in 24-well dishes
until 70–90% confluence was achieved. The cells were subsequently
incubated with 100 µM FITC-labeled peptides for 1 h at 37°C and
with rhodamine-labeled phalloidin (cat. no. CA1610; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) for 30
min at room temperature for cytoskeletal staining. The nuclei were
counterstained with DAPI (cat. no. C0065; Beijing Solarbio Science
& Technology Co., Ltd.). The cells were examined in the 24-well
dishes using a fluorescence microscope. All procedures were
repeated at least three times.
Synthesis of DPI-modified β-TCP
(β-TCP-DPI)
The synthesis of β-TCP-DPI was conducted following a
previously described procedure (10,16).
Functional β-TCP scaffolds were constructed using DPI peptides that
were adsorbed onto β-TCP through an adsorption/freeze-drying
strategy. Briefly, disk-shaped β-TCP (diameter, 6 mm; height, 2 mm;
Shanghai Bio-lu Biomaterials Co., Ltd, Shanghai, China) was
incubated for 24 h at room temperature in peptide solution
containing 100 µg/ml DPI in PBS in a ratio of 1.0 g β-TCP to 2.0 ml
solution with gentle agitation to ensure to equilibrate the peptide
over all exposed surfaces of the microporous β-TCP. Unadsorbed
peptide was removed from the scaffolds by washing five times in PBS
with gentle shaking over a 24 h period. The β-TCP-DPI composites
were dried in vacuo for 1 h and stored at −20°C in
moisture-proof containers. FITC-DPI peptide-modified β-TCP was also
synthesized and observed using ImageXpress Micro Confocal
(Molecular Devices, LLC, Sunnyvale, CA, USA). The β-TCP and
β-TCP-DPI composites were sterilized under ultraviolet (UV) light
for cell culture experiments.
Behavior of cells on β-TCP-DPI in
vitro
C57BL/6 mouse BMSCs were seeded at passage 4–5 onto
pure β-TCP and β-TCP-DPI scaffolds to investigate cell adhesion and
proliferation, as described below. All scaffolds were sterilized
through UV light exposure on a clean bench before use.
Cell adhesion assay
Cell Counting Kit-8 (CCK-8; cat. no., 96992;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to evaluate
the number of cells adhered onto the scaffolds (17). The cells were dissociated with
0.25% trypsin-EDTA. The cell suspension was centrifuged at 250 × g
for 5 min at room temperature and the sediment was collected. Cells
were resuspended at a specified concentration (6×104
cells/ml) and an appropriate volume (200 µl) in serum-free DMEM.
The cell suspension was added to 96-well plates containing β-TCP
and β-TCP-DPI and incubated for 3 h in a humidified atmosphere with
5% CO2 at 37°C; the scaffolds were removed to new wells
and washed thrice with PBS. Subsequently, 100 µl fresh medium and
10 µl CCK-8 reagent were added and the BMSCs that adhered to the
scaffolds were incubated for another 4 h at 37°C. The absorbance
was measured at 450 nm using an automated microplate reader. All
procedures were repeated at least three times.
Cell proliferation assay
CCK-8 assay was used to measure proliferation of
cells on β-TCP-DPI and unmodified β-TCP scaffolds. C57BL/6 mouse
BMSCs were seeded onto the unmodified β-TCP and β-TCP-DPI
scaffolds. Briefly, a total of 30 µl cell suspension containing
2×103 cells was slowly and carefully pipetted onto the
center-top surface of each β-TCP and β-TCP-DPI disk. The cell
suspension was not allowed to contact the sides of the wells to
ensure that all cells adhered to the scaffolds. Plates were gently
placed into an incubator. After 3-h incubation, an extra volume
(170 µl) fresh medium was added to each well gently and slowly
along the edge of the well; wells were not rinsed to avoid washing
out cells that did not firmly adhere to the scaffolds. After 3 days
of incubation, the scaffolds were washed thrice with PBS and
incubated with CCK-8 solution at 37°C for 3 h. Absorbance was
measured at 450 nm with an automated microplate reader. All
procedures were repeated at least three times.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Student's t-test was performed to compare two groups and one-way
analysis of variance followed by Dunnett's test was performed for
comparison of multiple groups. SPSS v24.0 (IBM Corp., Armonk, NY,
USA) software was used for data analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
DPI has a highly specific affinity
towards mouse BMSCs
The mouse BMSC affinity peptide DPI, the negative
control peptide LSP and the positive control peptide RGD were used
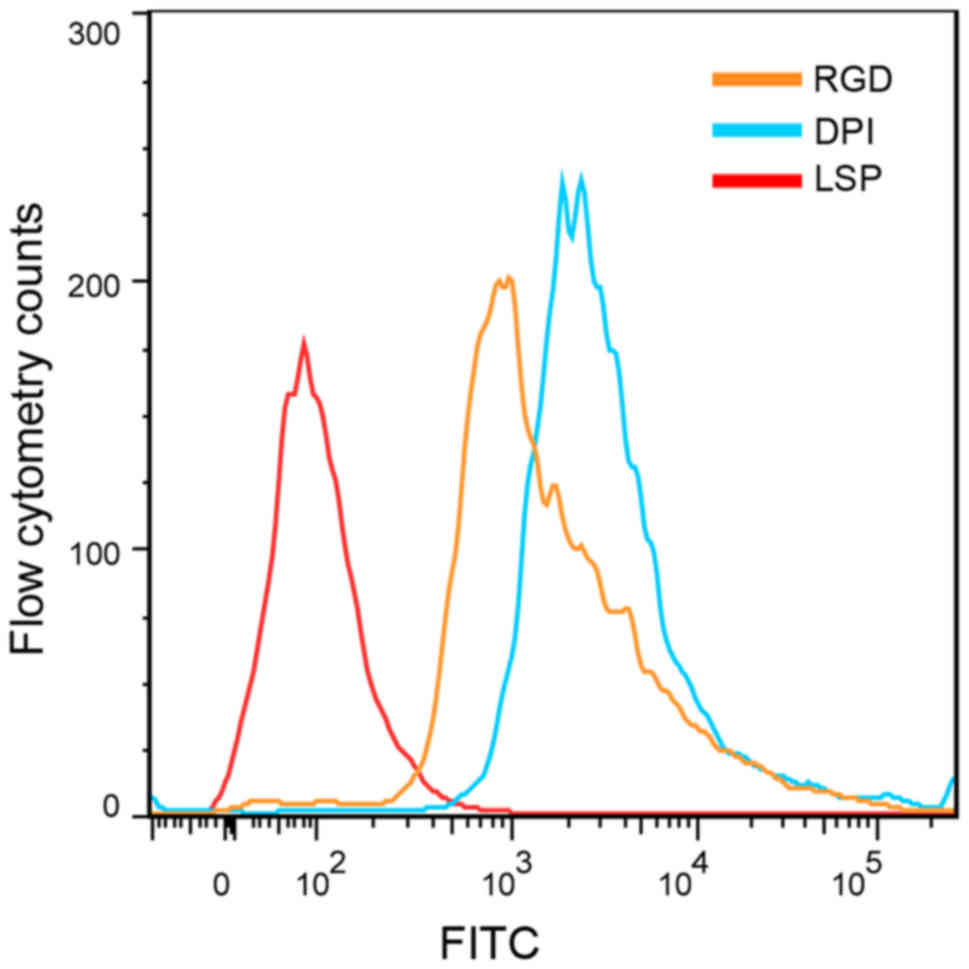
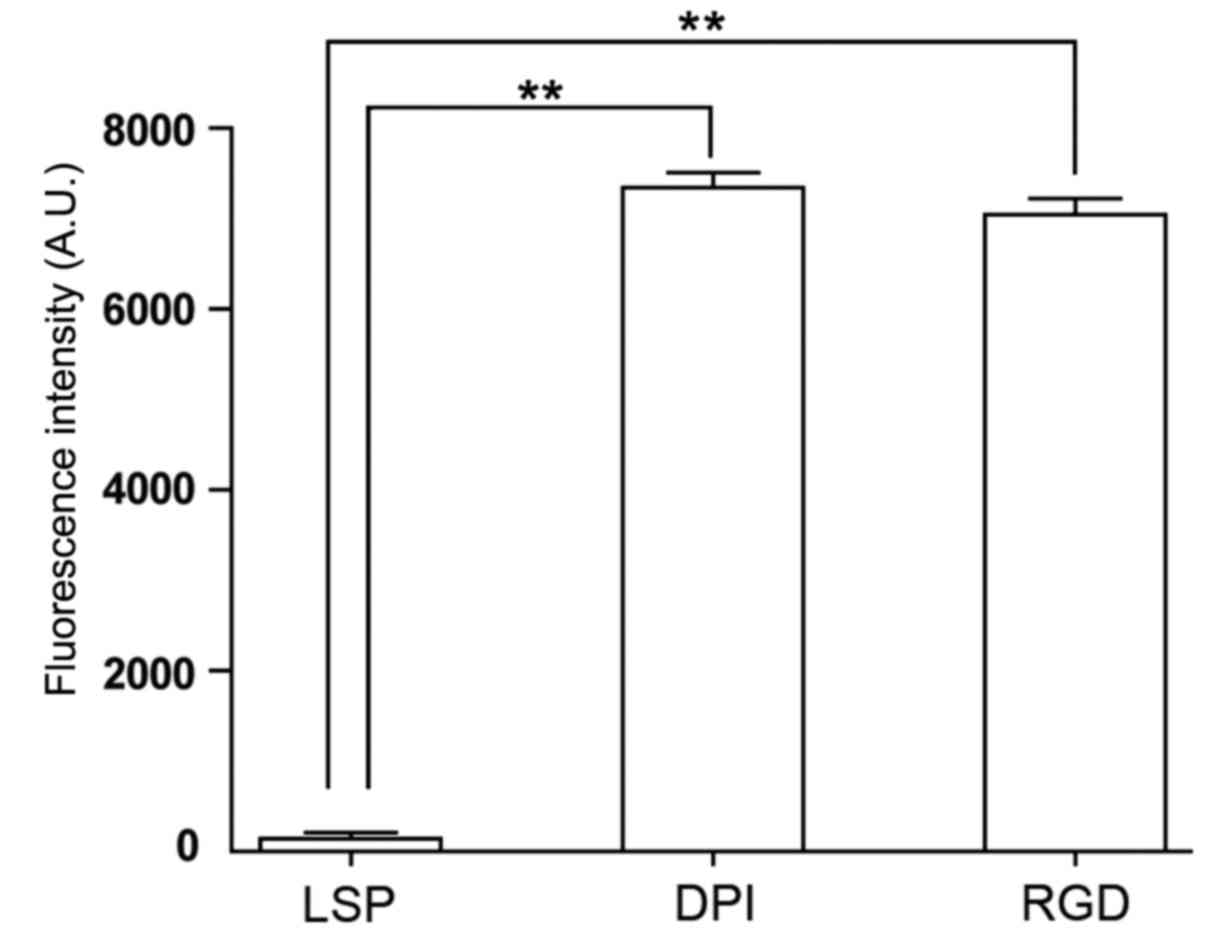
in this study. Mouse BMSCs were incubated for 1 h with FITC-labeled
DPI, LSP or RGD, and analyzed by flow cytometry. The average
fluorescence intensity was 7,343.5±167.6 for BMSCs incubated with
FITC-DPI, 7,042.0±179.6 for BMSCs incubated with FITC-RGD and
132.0±80.6 for BMSCs incubated with FITC-LSP. The average
fluorescence intensities for BMSCs incubated with FITC-DPI and
FITC-RGD were significantly higher compared with BMSCs incubated
with FITC-LSP (n=3; P<0.01; Figs.
3 and 4). The average
fluorescence intensity of cells incubated with FITC-DPI was
55.6-fold higher than that of FITC-LSP. The cells were also
observed under a fluorescence microscope. Strong fluorescent
signals were observed in the cells incubated with FITC-DPI and
FITC-RGD, whereas weak fluorescent signals were observed of the
cells incubated with FITC-LSP (Fig.
5). These results suggested that the DPI peptide may have a
high affinity for mouse BMSCs.
Successful synthesis of functional
β-TCP scaffolds
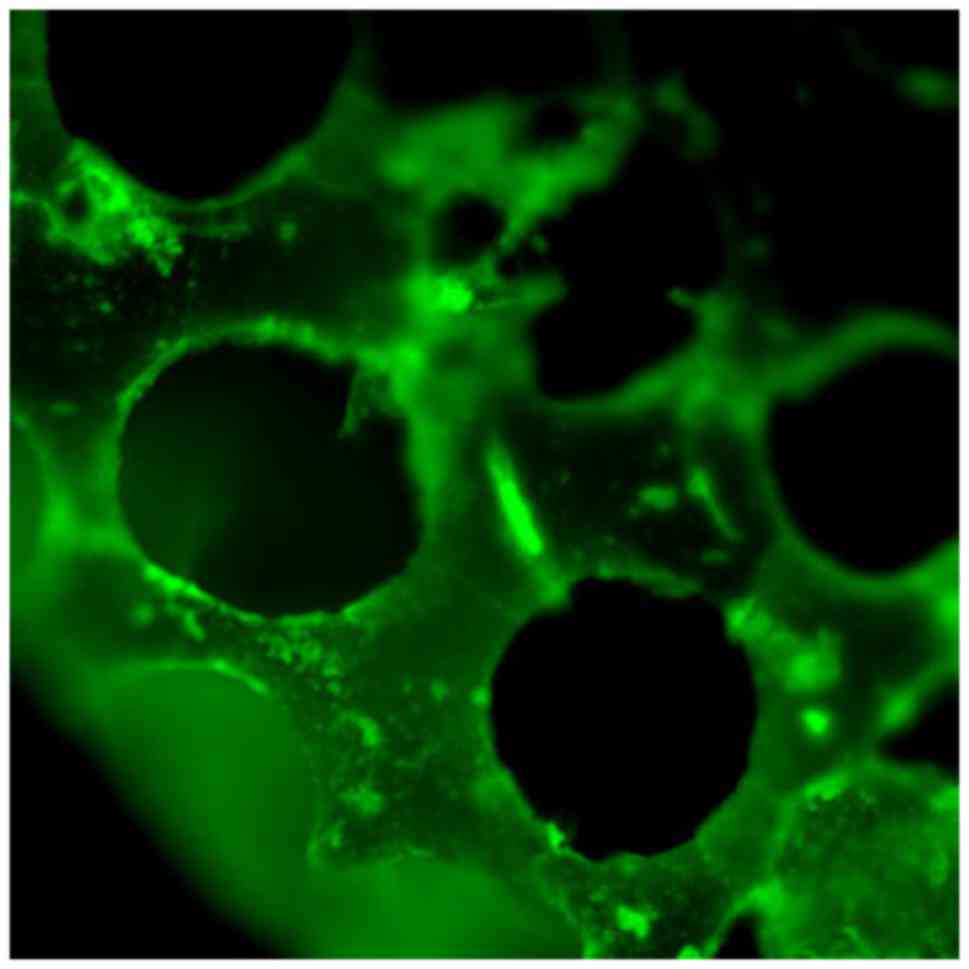
FITC-DPI was adsorbed onto the β-TCP scaffolds for
surface modification through an adsorption/freeze-drying strategy.
Following adsorption, the β-TCP-DPI scaffolds exhibited homogeneous
green fluorescence (Fig. 6). This
result indicated successful adsorption of DPI onto the surface of
β-TCP scaffolds and the successful construction of β-TCP-DPI
composite materials.
Adhesion and proliferation of BMSCs
onto the functional β-TCP-DPI scaffolds is enhanced compared with
pure β-TCP scaffolds
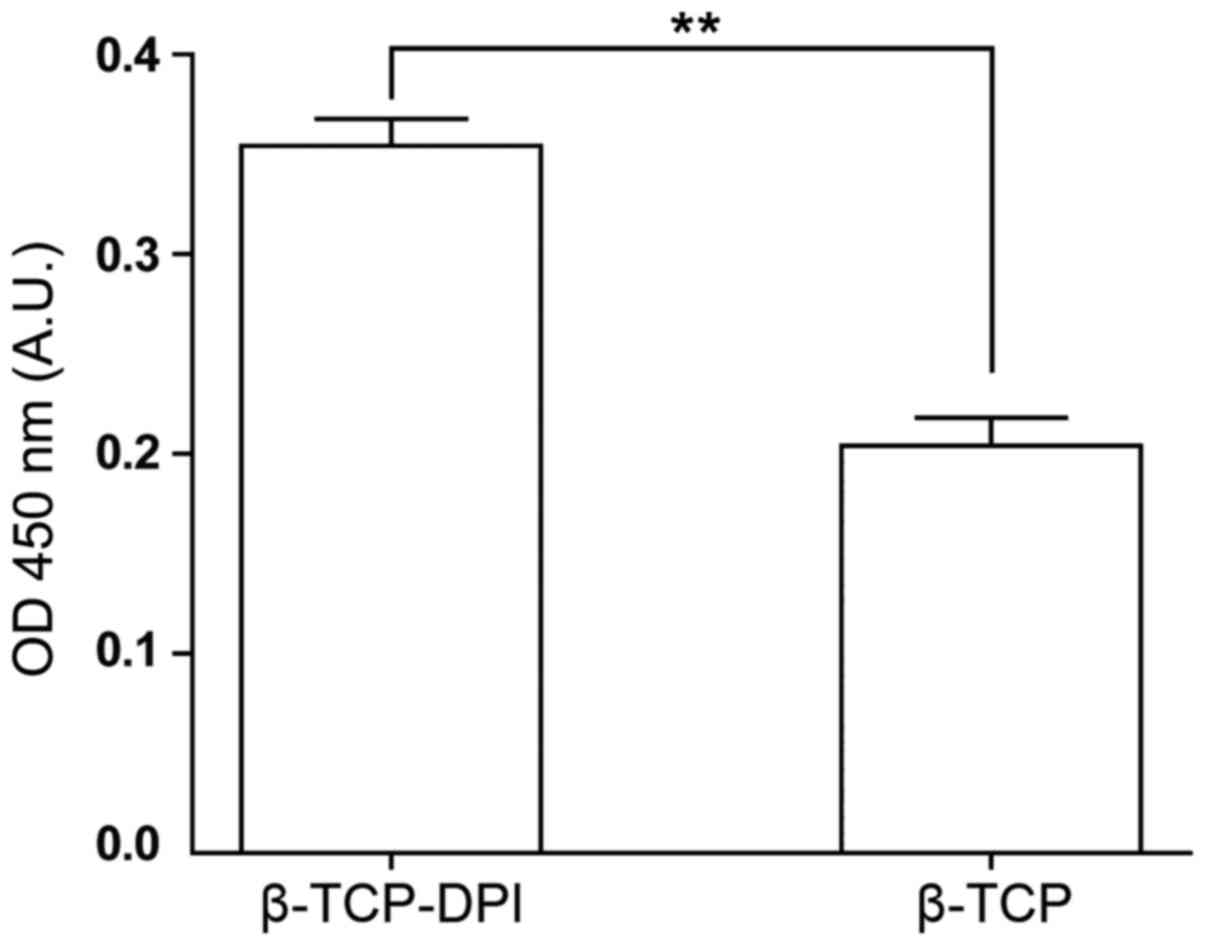
In the cell adhesion assay, the number of adherent
cells on the β-TCP-DPI composites was significantly higher compared
with those on the pure β-TCP scaffolds, according to optical
density (OD) measurements (n=3; P<0.01; Fig. 7). This result indicated that the
β-TCP-DPI composites are effective for mouse BMSC adhesion.
A CCK-8 assay was used to evaluate cell
proliferation on pure β-TCP and β-TCP-DPI scaffolds. Following 3
days of incubation, the OD value of β-TCP-DPI scaffolds was
significantly higher compared with pure β-TCP scaffolds, which
indicated that β-TCP-DPI may enhance BMSC proliferation compared
with unmodified β-TCP (n=3; P<0.01; Fig. 8).
Discussion
Tissue engineering uses concepts of biology and
engineering to develop functional substitutes for damaged tissue
(18), and MSCs are a common type
of seed cell used for tissue engineering. MSCs in human bone marrow
have been estimated to comprise 0.001–0.01% of the total nucleated
cells, and this number declines with age (19). Furthermore, MSCs are rare or absent
in pathological tissues such as the necrotic femoral head (2). A number of studies have proposed that
host progenitor cells build new bone following recruitment to the
site of repair (20,21). A method of efficient recruitment
and utilization of the limited number of MSCs remains to be
established. The strategy of recruiting BMSCs using affinity
peptides is commonly adopted (14,22).
Phage display provides a novel method for searching of highly
specific affinity peptides towards BMSCs. In this way, a number of
peptide sequences with highly specific affinity towards BMSCs have
been identified and used to modify biomaterials to improve their
surface properties and functions. For example, the peptide sequence
EPLQLKM (E7) is widely used to enhance the interaction between
BMSCs and various scaffolds (6,23–26).
In the present study, the DPI peptide, with affinity towards BMSCs,
was also reported to be discovered through phage display (15). Flow cytometry and fluorescence
cytochemistry were used to confirm the high affinity of DPI towards
BMSCs. It was also demonstrated that the β-TCP-DPI scaffolds
enhanced the adhesion and proliferation of BMSCs compared with pure
β-TCP scaffolds.
β-TCP is commonly used in absorbable bioceramics
(27). In tissue engineering,
β-TCP is an ideal biomaterial for the repair of osteonecrosis or
bone defects (2,28). β-TCP has been extensively studied
and applied as a bone repair and bone tissue engineering scaffold
material (27). It is
non-cytotoxic and has excellent biocompatibility and
osteo-conductivity (3,4). Stable associations between peptides
and materials are essential for the construction of functional
scaffolds (13). Methods of
connecting peptide molecules to materials include covalent
attachment, blending, co-polymerization, chemical and physical
treatment (13). As an inorganic
material, β-TCP has no innate biological stimulatory activity and
lacks functional groups. Biomolecules are, therefore, difficult to
conjugate to β-TCP (29). Amino
acids, peptides and proteins can be adsorbed to inorganic materials
(30–32). The mechanism of adsorption involves
the formation of complexes between the carboxyl group and surface
Ca2+, between free amino or guanidine groups and the
phosphate group (10). Thus, an
adsorption/freeze-drying strategy was used in the present study to
modify β-TCP scaffolds with DPI (10,16,33).
In the process of synthesizing functional β-TCP, unadsorbed peptide
should be rinsed sufficiently; otherwise non-adsorbed peptide
molecules may dissolve in the cell culture medium and inhibit cell
adhesion (12).
The present study aimed to investigate the ability
of C57BL/6 mouse BMSCs to adhere and proliferate on β-TCP-DPI
scaffolds. In the cell adhesion assay, the CCK-8 assay was used to
detect the number of adherent cells on the scaffolds. It was
hypothesized that BMSCs would be recruited onto the β-TCP-DPI
scaffolds more strongly and rapidly compared with the pure β-TCP
scaffolds. Cell adhesion to substrates is time-dependent, and
experimental adhesion time should be carefully considered (13). It usually assessed 1–4 h after cell
seeding, and in the present study, 3 h was selected as the time
point of verification. The results indicated that the adhesion of
BMSCs to the functional β-TCP scaffolds was enhanced compared with
the pure β-TCP scaffolds.
Numerous biomolecules have been reported to promote
the proliferation of BMSCs (6,34).
Therefore, it was considered necessary to study the effect of the
functional β-TCP scaffolds on proliferation. In cell adhesion
assay, cell suspension was added into the wells and cells adhered
to the scaffolds freely. In cell proliferation assay, cells were
seeded onto the scaffolds restrictedly; cell suspension was
carefully placed onto the center-top surface of the scaffolds to
ensure that all cells adhered to the scaffolds. Therefore, the
initial number of cells growing on scaffolds was fixed to
accurately investigate the changes of cell proliferation. The
result indicated that DPI-modified β-TCP scaffolds promoted the
proliferation of BMSCs.
Additional studies are required to investigate the
underlying mechanism of the affinity peptide towards BMSCs. The
repair effect of the functional β-TCP-DPI scaffolds should also be
further studied and evaluated in vivo.
In the present study, functional β-TCP scaffolds
were successfully synthesized by adsorption of the BMSC affinity
peptide, DPI, onto the surface of β-TCP using an
adsorption/freeze-drying strategy. In vitro experiments
demonstrated that the adhesion and proliferation of BMSCs on the
functional β-TCP scaffolds was enhanced. The functional scaffold
may be used as a potent biomaterial for MSC-based tissue
engineering therapy.
Acknowledgements
The authors would like to thank Dr Nianping Zhang,
Dr Tiantong Sun and Dr Li Qiao for revising the manuscript.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81271966).
Availability of data and materials
The data sets generated and analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
GW, ZM and SS designed the experiments. GW performed
the experiments. GW, HX, YL and CW analyzed the data. GW wrote the
manuscript. GW revised the manuscript. All authors reviewed the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Mont MA, Cherian JJ, Sierra RJ, Jones LC
and Lieberman JR: Nontraumatic osteonecrosis of the femoral head:
Where do we stand today? A ten-year update. J Bone Joint Surg Am.
97:1604–1627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tatara AM and Mikos AG: Tissue engineering
in orthopaedics. J Bone Joint Surg Am. 98:1132–1139. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ke D, Dernell W, Bandyopadhyay A and Bose
S: Doped tricalcium phosphate scaffolds by thermal decomposition of
naphthalene: Mechanical properties and in vivo osteogenesis in a
rabbit femur model. J Biomed Mater Res B Appl Biomater.
103:1549–1559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
LeGeros RZ: Properties of osteoconductive
biomaterials: Calcium phosphates. Clin Orthop Relat Res. 81–98.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Q, Xing D, Ma L and Gao C: Synthesis of
E7 peptide-modified biodegradable polyester with the improving
affinity to mesenchymal stem cells. Mater Sci Eng C Mater Biol
Appl. 73:562–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herrmann M, Verrier S and Alini M:
Strategies to stimulate mobilization and homing of endogenous stem
and progenitor cells for bone tissue repair. Front Bioeng
Biotechnol. 3:792015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao ZX, Zhang X, Pi YB, Yin L, Li L, Chen
H, Zhou C and Ao Y: Surface modification on polycaprolactone
electrospun mesh and human decalcified bone scaffold with
synovium-derived mesenchymal stem cells-affinity peptide for tissue
engineering. J Biomed Mater Res A. 103:318–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pi Y, Zhang X, Shi J, Zhu J, Chen W, Zhang
C, Gao W, Zhou C and Ao Y: Targeted delivery of non-viral vectors
to cartilage in vivo using a chondrocyte-homing peptide identified
by phage display. Biomaterials. 32:6324–6332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian JJ and Bhatnagar RS: Enhanced cell
attachment to anorganic bone mineral in the presence of a synthetic
peptide related to collagen. J Biomed Mater Res. 31:545–554. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhatnagar RS, Qian JJ and Gough CA: The
role in cell binding of a beta-bend within the triple helical
region in collagen alpha 1 (I) chain: Structural and biological
evidence for conformational tautomerism on fiber surface. J Biomol
Struct Dyn. 14:547–560. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pierschbacher MD and Ruoslahti E: Cell
attachment activity of fibronectin can be duplicated by small
synthetic fragments of the molecule. Nature. 309:30–33. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hersel U, Dahmen C and Kessler H: RGD
modified polymers: Biomaterials for stimulated cell adhesion and
beyond. Biomaterials. 24:4385–4415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H and Hollister S: Comparison of
bone marrow stromal cell behaviors on poly (caprolactone) with or
without surface modification: Studies on cell adhesion, survival
and proliferation. J Biomater Sci Polym Ed. 20:1975–1993. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramaraju H, Miller SJ and Kohn DH:
Dual-functioning peptides discovered by phage display increase the
magnitude and specificity of BMSC attachment to mineralized
biomaterials. Biomaterials. 134:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhatnagar RS, Qian JJ, Wedrychowska A,
Sadeghi M, Wu YM and Smith N: Design of biomimetic habitats for
tissue engineering with P-15, a synthetic peptide analogue of
collagen. Tissue Eng. 5:53–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang HX, Zhang XP, Xiao GY, Hou Y, Cheng
L, Si M, Wang SS, Li YH and Nie L: In vitro and in vivo evaluation
of calcium phosphate composite scaffolds containing BMP-VEGF loaded
PLGA microspheres for the treatment of avascular necrosis of the
femoral head. Mater Sci Eng C Mater Biol Appl. 60:298–307. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maijenburg MW, van der Schoot CE and
Voemans C: Mesenchymal stromal cell migration: Possibilities to
improve cellular therapy. Stem Cells Dev. 21:19–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caplan AI: New era of cell-based
orthopedic therapies. Tissue Eng Part B Rev. 15:195–200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caplan AI: Why are MSCs therapeutic? New
data: New insight. J Pathol. 217:318–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Lin CY and Hollister SJ: The
interaction between bone marrow stromal cells and RGD-modified
three-dimensional porous polycaprolactone scaffolds. Biomaterials.
30:4063–4069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shao Z, Zhang X, Pi Y, Wang X, Jia Z, Zhu
J, Dai L, Chen W, Yin L, Chen H, et al: Polycaprolactone
electrospun mesh conjugated with an MSC affinity peptide for MSC
homing in vivo. Biomaterials. 33:3375–3387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng Q, Man Z, Dai L, Huang H, Zhang X, Hu
X, Shao Z, Zhu J, Zhang J, Fu X, et al: A composite scaffold of MSC
affinity peptide-modified demineralized bone matrix particles and
chitosan hydrogel for cartilage regeneration. Sci Rep. 5:178022015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang H, Zhang X, Hu X, Shao Z, Zhu J, Dai
L, Man Z, Yuan L, Chen H, Zhou C and Ao Y: A functional biphasic
biomaterial homing mesenchymal stem cells for in vivo cartilage
regeneration. Biomaterials. 35:9608–9619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Man Z, Yin L, Shao Z, Zhang X, Hu X, Zhu
J, Dai L, Huang H, Yuan L, Zhou C, et al: The effects of
co-delivery of BMSC-affinity peptide and rhTGF-β1 from coaxial
electrospun scaffolds on chondrogenic differentiation.
Biomaterials. 35:5250–5260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu B and Lun DX: Current application of
β-tricalcium phosphate composites in orthopaedics. Orthop Surg.
4:139–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rh Owen G, Dard M and Larjava H:
Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as
regenerative material for the repair of complex bone defects. J
Biomed Mater Res B Appl Biomater. 106:2493–2512. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alvarez LM, Rivera JJ, Stockdale L, Saini
S, Lee RT and Griffith LG: Tethering of epidermal growth factor
(EGF) to beta tricalcium phosphate (βTCP) via fusion to a high
affinity, multimeric βTCP-binding peptide: Effects on human
multipotent stromal cells/connective tissue progenitors. PLoS One.
10:e01296002015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moreno EC, Kresak M and Hay DI: Adsorption
of molecules of biological interest onto hydroxyapatite. Calcif
Tissue Int. 36:48–59. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hay DI and Moreno EC: Differential
adsorption and chemical affinities of proteins for apatitic
surfaces. J Dent Res. 58:930–942. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gorbunoff MJ and Timasheff SN: The
interaction of proteins with hydroxyapatite. III. Mechanism. Anal
Biochem. 136:440–445. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang XB, Bhatnagar RS, Li S and Oreffo RO:
Biomimetic collagen scaffolds for human bone cell growth and
differentiation. Tissue Eng. 10:1148–1159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Parrish B, Breitenkamp RB and Emrick T:
PEG- and peptide-grafted aliphatic polyesters by click chemistry. J
Am Chem Soc. 127:7404–7410. 2005. View Article : Google Scholar : PubMed/NCBI
|