Introduction
Lung cancer is a type of cancer that causes the
highest number of cancer-associated mortalities in men and women
globally, accounting for 26% of all cancer mortalities in men and
25% in women in 2018 (1). The
numbers of novel cases and mortalities due to lung cancer were
estimated to be 234,030 and 154,050, respectively, in the United
States in 2018 (1). In China,
there were ~700,000 novel cases of lung cancer and ~600,000 lung
cancer-associated mortalities in 2015 (2). Among patients with lung cancer,
>50% are diagnosed with metastatic disease and the five-year
survival rate is <5% (3).
Although the development of novel targeted therapies based on the
predictive biomarkers of lung cancer have markedly improved the
clinical outcome, it remains poor considering the extensive
invasion, recurrence and the metastasis of non-small cell lung
cancer (NSCLC) (4). Accumulating
evidence suggests that although patients with identical histology,
differentiation, location and stage of diagnosis receive similar
therapy, the survival of these patients is heterogeneous. This
indicates that the current methods of tumor classification and
staging are insufficient for selecting the most appropriate
treatment (5). Therefore, the
development of novel biomarkers that accurately predict patients at
greatest risk of metastasis is essential in order to select the
appropriate treatment strategy in order to increase the survival
rate.
In NSCLC, circulating tumor cells (CTCs) have been
used as a prognostic, predictive and pharmacodynamic biomarker
(6,7). The characterization of CTCs may
identify the mechanism of metastasis (8). In general, tumor dissemination
includes a number of steps, including: The generation of invasive
subpopulations of tumor cells, dissolution of the stroma, transport
into the peripheral circulation or lymphatic system, establishment
at a secondary lesion at a novel site and angiogenesis (9). CTCs may be discharged into the blood
circulation at an earlier stage compared with the metastasis of
cancer and may be detected in the early stage of cancer development
(10). In these invasive tumor
cells, the epithelial-mesenchymal transition (EMT) is a key process
in metastasis for the progression of cancer, including in NSCLC
(4) and is considered to be an
initiating event for distant dissemination (5,11).
Previous studies have also revealed that EMT is associated with
chemotherapeutic resistance (12,13).
Epithelial markers including epithelial cell adhesion molecule
(EpCAM) and cytokeratins (CKs) have been demonstrated to be
downregulated and mesenchymal markers including vimentin and twist
family BHLH transcription factor 1 (TWIST1) have been demonstrated
to be upregulated (14–16). Therefore, CTCs in the initial
stages of dissemination are assumed to express the EMT phenotype.
Detection of the EMT changes of CTCs may aid the identification of
the aggressive behavior of NSCLC.
The Canpatrol™ CTC assay classifies CTCs into
epithelial, bi-phenotypic (epithelial and mesenchymal) and
mesenchymal subtypes by using a cocktail of epithelial (EpCAM and
CK8/18/19) and mesenchymal (vimentin and TWIST1) markers (14,17).
In the present study, the EMT phenotypes of CTCs isolated from the
peripheral blood samples of a cohort of patients with NSCLC were
characterized using the Canpatrol™ CTC technique.
Patients and methods
Patient enrollment and blood
collection
The present study was ethically approved by the
Ethics Committee of the First Affiliated Hospital of Zhejiang
University (Hangzhou, China). A total of 85 NSCLC patients and 25
benign patients were enrolled in the study between December 2016
and June 2018 and written informed consent was obtained from the
patients prior to their enrollment. The patients who were
pathologically diagnosed with NSCLC without any history of other
types of malignant cancer were recruited into the NSCLC cohort, and
the patients who were clinically diagnosed with benign lung disease
were in the benign cohort. Patients aged ≤18 years old were
excluded. In NSCLC cohort, there were 53 males and 32 females. The
median age of the patients was 63 years old with the range between
32 and 83. All the NSCLC patients were classified into groups with
and without distant metastasis according to the 8th edition of the
American Joint Committee on Cancer tumor-node-metastasis staging
system for lung cancer (18).
Peripheral blood samples (5 ml) from 85 patients with NSCLC were
collected in EDTA tubes by venipuncture and stored at 4°C until
cell isolation, which was performed within 4 h. The blood samples
were collected prior to surgery, radiation therapy and chemotherapy
from patients with NSCLC of different stages. Peripheral blood
samples were also collected from 25 patients with benign diseases,
of which 2 patients presented with granuloma, 2 were pseudotumor, 4
were pulmonary nodule and 17 were tuberculosis.
Isolation and classification of
CTCs
CTCs were isolated and classified using a Canpatrol™
CTC assay (SurExam, Guangzhou, China; http://www.surexam.com/) as previously described
(14). Briefly, erythrocyte lysis
buffer, provided in the assay kit, was added to the peripheral
blood samples within 4 h of venipuncture and incubated for 30 min
at room temperature. The blood samples were then filtered using an
8-µm diameter pore-calibrated membrane (EMD Millipore, Billerica,
MA, USA) in order to collect the CTCs. The enriched CTCs were
subjected to RNA in situ hybridization with a combination of
epithelial (EpCAM and CK8/18/19) and mesenchymal (vimentin and
TWIST1) markers (provided in the assay kit). The CTCs were then
stained with a concentration of 4′,6-diamidino-2-phenylindole
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 100 ng/ml for 5
min at room temperature and analyzed with an automated imaging
fluorescent microscope (Carl Zeiss AG, Oberkochen, Germany). The
CTCs of each patient were classified based on the identification of
the markers. The Canpatrol™ CTC assay protocol is described in a
previously published study (17).
Briefly, CTCs were classified into three subpopulations according
to epithelial and mesenchymal markers that they expressed,
including epithelial CTCs (E+ CTCs), biophenotypic
epithelial/mesenchymal CTCs (E+/M+ CTCs), and
mesenchymal CTCs (M+ CTCs). E+ CTCs refer to
the cells that predominantly express EpCAM and/or CK8/18/19 but do
not express CD45; M+ CTCs mainly express vimentin and/or
TWIST markers but not CD45; and E+/M+ CTCs
express epithelial and mesenchymal markers but not CD45.
Statistical analysis
The Mann-Whitney U test was used to compare the EMT
phenotype of CTCs from patients of different subgroups for
non-normally distributed data. Comparisons of the rate between each
subgroup were tested using χ2 and Fisher's exact tests.
Receiver operating characteristic (ROC) curves and the area under
the curve (AUC) were determined in order to assess the predictive
and diagnostic power of different types of CTC subset. The optimal
cut-off value of every subset was selected using the Youden index
(19), which represents the
threshold value when the following is maximal: Sensitivity +
specificity-1. The statistical analyses were two-sided and
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was conducted using SPSS software,
version 22.0 (IBM Corp., Armonk, NY, USA). The measured data of
CTCs was presented as mean ± standard deviation. The age of
patients was presented as median (range).
Results
Patient characteristics
In the 85 NSCLC patients, 61.2% had a history of
smoking. The type of NSCLC was predominantly adenocarcinoma (67.1%;
Table I). In total, 18.8% of
patients were diagnosed with stage I disease, 15.3% were stage II,
17.6% were stage III and 48.2% were stage IV. The assessment of
distant metastasis was determined according to the
Tumor-Node-Metastasis staging system based on clinical analysis,
radiography and histology. Among the 85 patients with NSCLC, 41
patients exhibited distant metastasis (M1) and 44 patients
presented no distant metastasis (M0). Peripheral blood samples were
also collected from 17 men and 8 women (age range, 41–70 years)
with benign diseases including interstitial pneumonia and benign
small pulmonary nodules.
 | Table I.Characterization of CTCs in patients
with NSCLC. |
Table I.
Characterization of CTCs in patients
with NSCLC.
| Parameters | Total patients
(n=110) | Benign (n=25) | NSCLC (n=85) | P-value | M0 patient | M1 patient | P-value |
|---|
| Age (years), median
(range) | 63
(32–83) | 63
(41–70) | 63
(32–83) | >0.05 | 63
(47–83) | 63
(32–83) | >0.05 |
| Male % (n) | 63.6 (70/110) | 68.0 (17/25) | 62.4 (53/85) | >0.05 | 63.6 (28/44) | 61.0 (25/41) | >0.05 |
| Smoking history %
(n) | 60.9 (67/110) | 60.0 (15/25) | 61.2 (52/85) | >0.05 | 61.4 (27/44) | 60.9 (25/41) | >0.05 |
| Adenocarcinoma %
(n) | – | – | 67.1 (57/85) | >0.05 | 56.8 (25/44) | 53.7 (22/41) | >0.05 |
| Total CTCs |
|
|
|
|
|
|
|
| ≥1
cell/5 ml blood % (n) | 81.1 (77/95) | 60.0 (15/25) | 85.9 (73/85) | <0.05 | 81.8 (36/44) | 90.2 (37/41) | >0.05 |
|
Range | 0-57 | 0-2 | 0-57 |
| 0-17 | 0-57 |
|
|
Median | 4 | 1 | 5 |
| 4 | 7 |
|
| Mean ±
SD | 6.47±7.80 | 0.84±0.80 | 7.16±7.90 | <0.001 | 5.66±5.16 | 8.78±9.97 | >0.05 |
| E+
CTCs |
|
|
|
|
|
|
|
| ≥1
cell/5 ml blood % (n) | 64.2 (61/95) | 60.0 (15/25) | 67.1 (57/85) | >0.05 | 59.1 (26/44) | 75.6 (31/41) | >0.05 |
|
Range | 0-12 | 0-2 | 0-12 |
| 0-12 | 0-9 |
|
|
Median | 2 | 1 | 2 |
| 2 | 2 |
|
| Mean ±
SD | 2.27±2.61 | 0.84±0.80 | 2.47±2.67 | <0.05 | 2.5±3.03 | 2.43±2.26 | >0.05 |
|
E+/M+ CTCs |
|
|
|
|
|
|
|
| ≥1
cell/5 ml blood % (n) | 67.4 (64/95) | 0 (0/25) | 75.3 (64/85) | <0.05 | 72.7 (32/44) | 73.2 (30/41) | >0.05 |
|
Range | 0-45 | 0 | 0-45 |
| 0-10 | 0-45 |
|
|
Median | 2 | 0 | 2 |
| 2 | 2 |
|
| Mean ±
SD | 3.16±5.75 | 0.00±0.00 | 3.53±5.97 | <0.001 | 2.63±2.69 | 4.48±8.08 | >0.05 |
| M+
CTCs |
|
|
|
|
|
|
|
| ≥1
cell/5 ml blood % (n) | 40.0 (38/95) | 0 (0/25) | 44.7 (38/85) | <0.05 | 25.0 (11/44) | 65.8 (27/41) | <0.001 |
|
Range | 0-10 | 0 | 0-10 |
| 0-5 | 0-10 |
|
|
Median | 0 | 0 | 0 |
| 0 | 1 |
|
| Mean ±
SD | 1.04±1.76 | 0.00±0.00 | 1.16±1.83 | <0.001 | 0.52±1.15 | 1.85±2.15 | <0.001 |
EMT phenotypes of total CTCs from
patients with NSCLC and benign pulmonary diseases
The EMT phenotypes of CTCs were analyzed in blood
samples isolated from 85 (77.3%) patients with NSCLC and 25 (22.7%)
patients with benign pulmonary diseases. Among the 110 patients
recruited, 88 (80.0%) patients were characterized as CTC positive
with EMT markers (≥1 cell/5 ml blood) using a Canpatrol™ CTC assay
(Fig. 1A). Representative images
of the different phenotypic CTCs are shown in Fig. 1B.
The total number of CTCs in patients with benign
pulmonary diseases (mean, 0.84±0.80; median, 1.00) was lower
compared with that of patients with NSCLC (M0: mean, 5.66±5.16 and
median, 4.00; M1: mean, 8.78±9.97 and median, 7.00). The difference
in the total number of CTCs between patients with benign pulmonary
diseases and NSCLC was statistically significant (P<0.001),
while that between M0 and M1 was not (Fig. 1A).
The CTC-positive rate in patients with benign
pulmonary diseases, NSCLC, non-distant metastatic NSCLC (M0), and
distant metastatic NSCLC (M1) was 60.0 (15/25), 85.9 (73/85), 81.8
(36/44) and 90.2% (37/41), respectively (Fig. 2; Table
I). The difference in the CTC-positive rate between patients
with benign pulmonary diseases and NSCLC was statistically
significant (P<0.05), while that between M0 and M1 was not
(Fig. 2; Table I).
Phenotypic characterization of CTCs
from patients of different subgroups
According to the EMT markers, the CTCs from patients
with benign pulmonary and NSCLC were classified into three
subpopulations: Epithelial (E+), bi-phenotypic
(E+/M+) and mesenchymal (M+).
The mean numbers of CTCs of these three
subpopulations (E+, E+/M+ and
M+ CTCs) in patients with benign pulmonary diseases were
0.84±0.80, 0.00±0.00 and 0.00±0.00, respectively. The mean numbers
of CTCs of each subpopulation were demonstrated to be statistically
higher in patients with NSCLC compared with patients with benign
diseases (2.47±2.67, 3.53±5.97 and 1.16±1.83, respectively;
P<0.05; Fig. 1A; Table I).
The rate of E+ CTCs in patients with
benign pulmonary diseases was lower compared with that in patients
with NSCLC [60.0 (15/25) and 67.1% (57/85), respectively], but the
difference was not significant. The E+/M+ and
M+ CTC-positive rates were significantly higher in
patients with NSCLC [75.3 (64/85) and 44.7% (38/85), respectively]
compared with patients with benign pulmonary diseases [0.0 (0/25)
and 0.0% (0/25), respectively] (P<0.05; Fig. 2; Table
I).
Comparing between patients with distant and
non-distant metastasis, no significant difference was observed with
regards to the numbers of E+,
E+/M+ and total CTCs, respectively
(P>0.05). The number of M+ CTCs was significantly
higher in the M1 subgroup compared to the M0 subgroup (M1: mean,
1.85±2.15; M0: mean, 0.52±1.15; P<0.001; Fig. 1A). The rate of M+
CTC-positive patients was significantly higher in the M1 subgroup
compared to the M0 subgroup [M1: 65.9% (27/41); M0: 25.0% (11/44);
P<0.001; Fig. 2; Table I].
ROC curve and the optimal cut-off
value
ROC curve analyses were performed to evaluate the
diagnostic and predictive potential of the CTC populations.
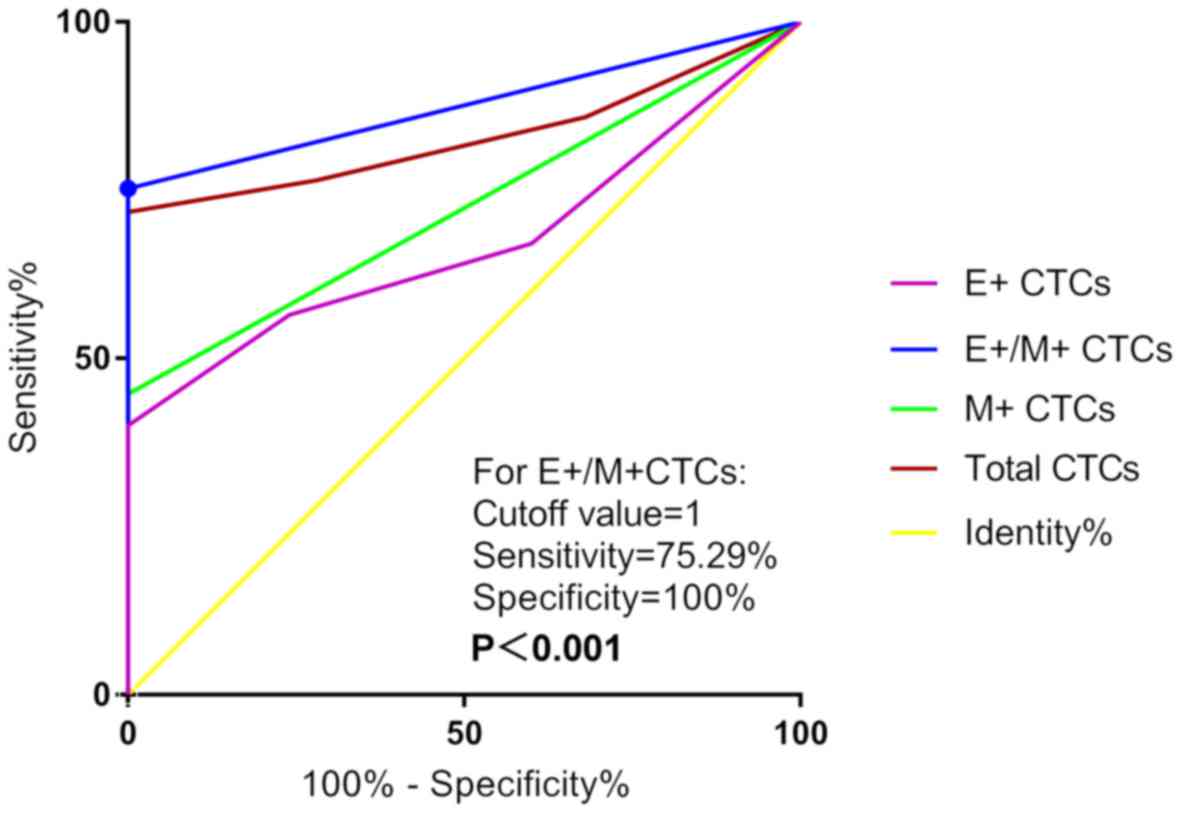
E+/M+ CTCs displayed the highest AUC [0.876;
95% confidence interval (CI), 0.814–0.939; P<0.001] compared
with E+ CTCs (0.672; 95% CI, 0.573–0.772), M+
CTCs (0.724; 95% CI, 0.629–0.818) and total CTCs (0.830; 95% CI,
0.756–0.903) in discriminating patients with NSCLC and benign
pulmonary diseases, with P<0.001 (Fig. 3). The optimal cut-off value of
E+/M+ CTCs was 1, which indicated that
patients with NSCLC exhibited E+/M+ CTCs and
≥1 cell/5 ml blood. The sensitivity and specificity of this value
were 75.29 and 100.00%, respectively.
ROC curve and AUC analyses were conducted in order
to distinguish NSCLC patients with distant metastasis from those
with non-distant metastasis. M+ CTCs exhibited the
highest AUC value of 0.723 (95% CI, 0.612–0.833; P<0.001;
Fig. 4) compared with
E+ CTCs (0.541; 95% CI, 0.417–0.665),
E+/M+ CTCs (0.543; 95% CI, 0.410–0.658) and
total CTCs (0.604; 95% CI, 0.484–0.725). The optimal cut-off value
of M+ CTCs was 1, which indicated that patients with
NSCLC exhibit M+ CTCs and ≥1 cell/5 ml blood. The
sensitivity and specificity of this value were 65.85 and 75.00%,
respectively (Fig. 4).
Phenotypic characterization of CTCs in
the cohort
The distribution of the three CTC subpopulations in
patients with benign and NSCLC varied substantially (Fig. 5A). Among the patients with NSCLC,
31.8% carried all three phenotypic CTCs, 37.7% carried two types of
CTCs (25.9% with E+ and E+/M+
CTCs; 7.1% with E+/M+ and M+ CTCs;
4.7% with E+ and M+ CTCs) and 14.1% were CTC
negative. Of the patients with benign disease, 40.0% were CTC
negative and 60% exhibited E+ CTCs. The patients with
NSCLC with or without distant metastasis are presented in Fig. 5B. Among the NSCLC patients with
distant metastasis, the majority were positive for all three
subpopulations (46.3%). Among the patients without distant
metastasis, the majority exhibited E+ and
E+/M+ CTCs (36.4%; Fig. 5B).
Discussion
The important function served by the EMT phenotype
has been demonstrated in lung cancer progression and drug
resistance (20,21). A previous study confirmed the
capability of mesenchymal cells to escape from the primary tumor
and reach distant sites (12). The
clinical use of CTCs focusing on enumeration has been reported and
validated by using the peripheral blood of patients with metastatic
breast, prostate and colorectal cancer types (22,23).
However, there are few studies involving direct association
analysis between EMT phenotypic CTCs and distant metastasis.
Furthermore, there is a lack of a standard for the determination of
CTCs in a clinical setting. A recent study has demonstrated that
CTCs are able to express EMT-like phenotypes (24). Detection of the mesenchymal or
hybrid EMT phenotype may be more informative for patients with
aggressive disease subtypes or poor outcomes (25–29).
Therefore, the application of novel technology to detect, capture
and analyze the epithelial or mesenchymal subpopulations of CTCs is
promising (30).
In the present study, the CTCs in a cohort of
patients with NSCLC were characterized using a clinically feasible
CTC assay with four epithelial markers, EpCAM and CK8/18/19, and
two mesenchymal markers, vimentin and TWIST1, and the distribution
and clinical value of CTCs with an EMT phenotype were studied.
Vimentin is a highly conserved intermediate filament protein
normally expressed in cells of mesenchymal origin, and its high
expression is associated with a poor prognosis (31) and the occurrence of metastases
(32) in patients with NSCLC.
TWIST is a highly conserved basic helix-loop-helix transcription
factor regulator of embryogenesis which is silent in most healthy
adult tissues, and its overexpression in patients with NSCLC is
involved in metastatic potential and poor survival (33,34).
Given the importance of vimentin and TWIST in the progression and
prognosis of lung cancer, it is reasonable to select them as the
marker for M type CTCs in lung cancer. For the selection and
validation of CTCs with epithelial and mesenchymal phenotypes, the
use of members of CK, EpCAM, vimentin and TWIST1 families has
become the standard method in patients with cancer (35). When epithelial cells start the
procedure of EMT and progress into mesenchymal cells, the capacity
for the invasiveness and metastasis of lung cancer is increased.
Thus, the expression of epithelial cell markers including EpCAM and
members of CKs are downregulated and that of mesenchymal cell
markers is upregulated (14–16,35).
Milano et al (36) designed
a study to detect the EMT of CTCs in patients with metastatic NSCLC
and analyzed the expression of epithelial markers (carcinoembryonic
antigen-CK19), EMT-associated genes (vimentin) and EMT
transcription factors (zinc finger protein SNAI2, zinc finger
E-box-binding homeobox 1–2 and TWIST1-2) using a reverse
transcription-polymerase chain reaction assay in order to calculate
the cell numbers. However, in the present study, RNA in situ
hybridization with a combination of epithelial (EpCAM and
CK8/18/19) and mesenchymal (vimentin and TWIST1) markers were used
to detect the epithelial and mesenchymal phenotype of CTCs, and an
automated imaging fluorescent microscope was used to directly count
the cells with different phenotypes. Furthermore, in the study by
Milano et al (36), only 10
metastatic patients with NSCLC and 10 healthy volunteers were
recruited to test the feasibility of CTC testing in the clinic.
This previous study revealed that 3 of 10 samples were positive for
CTCs at the baseline and the positive percentage of the patients
increased subsequent to treatment with the platinum-based
chemotherapy. During the dynamic testing, the expression of the
mesenchymal phenotype appeared to be associated with a more
unfavorable prognostic factor. However, the clinical significance
of the EMT phenotypic CTC needs to be further validated considering
the limited patient number in the study by Milano et al
(36). In the present study, 110
patients (85 patients with NSCLC from different clinical stages and
25 patients with benign diseases) were recruited. The ROC curves
revealed that E+/M+ CTCs display the highest
AUC (0.876; 95% CI, 0.805–0.948; P<0.001) in discriminating
patients with NSCLC and benign pulmonary diseases. More
importantly, it was revealed that the EMT expression of CTCs in
patients is significantly associated with distant metastasis.
In the analysis of CTCs from the peripheral blood
samples of patients in the present study, the number and incidence
of total CTCs in patients with NSCLC and benign pulmonary diseases
were compared. The distribution of the three distinct CTC
subpopulations, namely E+, E+/M+
and M+ CTCs, were examined. The incidence and total
number of CTCs were higher in patients with NSCLC compared with
patients with benign pulmonary diseases. Furthermore, ROC curve
analysis identified 1 unit of E+/M+ CTCs as
the optimal cut-off threshold with a sensitivity of 75.29% and
specificity of 100% in distinguishing NSCLC from benign pulmonary
diseases. These results are consistent with those of previous
studies in patients with breast cancer (14) and in cancer cell lines (35,37–39).
Previous studies have described that the most notable challenge of
CTC detection is the extremely low rate of CTCs, so the prognostic
cut-off values were lowered (40–43).
In a previous study by Chen et al (44), the optimal cut-off value in
distinguishing patients with NSCLC from controls (healthy donors
and benign diseases) was 8.93 units from the total number of CTCs
with a sensitivity of 74.7% and specificity of 86.6%. The
inconsistency may be explained by the following reasons. First, the
latter study detected the CTCs by folate receptor instead of EMT
markers. Second, the number of CTCs was determined according to the
quantity of folate receptor using a quantitative polymerase chain
reaction assay, while in the present study, intact cells were
captured using a pore-calibrated membrane and then characterized
using the EMT markers. In addition, the EMT phenotype has also been
observed and used as a marker for prognosis in other types of
cancer, including breast cancer (45,46),
colorectal cancer (47) and
hepatocellular carcinoma (48).
Tumor dissemination usually occurs in a number of
steps, including the generation of invasive cancer cells,
dissolution of the stroma, intravasation into the peripheral
circulation or lymphatic system, extravasation to the secondary
lesions and angiogenesis at distal sites (9). This dynamic process has been
hypothesized to transform cells with an epithelial phenotype into a
mesenchymal type, and then result in the loss of adhesion between
cells and the acquisition of enhanced motility, the recruitment of
vasculature and resistance to apoptotic signals (30). In this process, the cellular
cytoskeleton has been reported to be remodeled; the expression
levels of CKs, which compose the epithelial cell cytoskeleton,
decrease and those of vimentin, which compose the mesenchymal cell
cytoskeleton, increase (30,49,50).
Based on several studies in human cancer cell lines and mouse
models, the aberrant activation of the EMT phenotype has been
implicated in the progression of metastasis (15,49,51).
In order to extend the EMT analysis to CTC
subpopulations in metastasis, the distribution of E+,
E+/M+ and M+ CTCs in patients with
NSCLC with distant metastasis (M1) or without (M0) were detected
and analyzed in the present study. Compared with patients with M0,
the incidence and total number of E+ CTCs were higher in
patients with M1, but this difference was not significant. The
differences in the total cell numbers and incidence of
E+/M+ CTCs were also not significant.
However, the incidence and number of M+ CTCs were
significantly higher in patients with M1 compared with patients
with M0 (P<0.001). The results are consistent with the
aforementioned theory of the EMT phenotype, suggesting an
association between mesenchymal cells and cancer metastasis. In
addition, a previous study reported that CTCs express the
epithelial phenotype predominantly in the early stage of EMT, the
epithelial and mesenchymal phenotypes in the intermediate stage and
the mesenchymal phenotype in the last stage (14). The expression of EMT phenotypes in
CTCs may function as a marker for the metastatic cascade, tumor
dissemination and tumor progression (52). In the present study, the level of
M+ CTCs was substantially higher in the patients with
distant metastasis, compared with the patients without distant
metastasis. Furthermore, the results of the ROC curve and AUC
analyses additionally demonstrate the predictive value of
M+ CTCs. The optimal cut-off threshold in the diagnosis
for NSCLC with distant metastasis compared with no distant
metastasis was 1 unit with a sensitivity of 65.85% and specificity
of 75.00%, indicating that M+ CTCs have an immense
potential as a predictive factor for distant metastasis and tumor
progression (14).
On the other hand, a number of studies have proposed
a reversible EMT metastasis model, namely MErT, in which tumor
cells activate EMT to invade and then undergo a reversion progress
to form epithelial metastases once they arrive at distant sites
(11,15,30,53).
Mesenchymal cells cannot easily be distinguished and the epithelial
phenotype may be detected in the majority of metastatic lesions
(12,54). This dynamic progression may explain
the proportions of all types of subpopulation presented in Fig. 5, of which 70.1% of M1 patients
carried either E+/M+ CTCs only or
E+/M+ CTCs accompanied with other phenotypic
CTCs, and may be the reason why the numbers of E+ and
M+ CTCs were lower compared with that of the
E+/M+ CTCs.
In summary, three CTC subpopulations (E+,
E+/M+ and M+ CTCs) were detected
and analyzed in blood samples from 110 patients in the present
study using Canpatrol™ CTC assays. The results demonstrate the
predictive value of E+/M+ CTCs in
distinguishing patients with NSCLC from those with benign diseases,
in addition to that of M+ CTCs in distinguishing
patients with distant metastasis from those with non-distant
metastasis. Although the role of EMT in metastatic progress remains
controversial in different types of cancer, the results of the
present study indicate the predictive value of the EMT phenotype of
CTCs in peripheral blood, and it is a potential predictive
biomarker for distant metastasis in patients with NSCLC.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Projects
of Medical and Health Technology in Zhejiang Province (grant no.
N20090540), the Project of Zhejiang Provincial Administration of
Traditional Chinese Medicine (grant no. 2016ZB069) and the Project
of Clinical Scientific Research Fund of Zhejiang Medical
Association (grant no. 2017ZXC-A13).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JiaZ and SZ contributed equally to the study concept
and design. XZ and LW contributed equally to the clinical and
experimental studies and writing of the article. JL collected the
clinical data and JinZ performed the statistical analysis.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Zhejiang University (Hangzhou,
Zhejiang, China). Written informed consent was obtained from the
patients prior to enrollment.
Patient consent for publication
The patients provided written informed consent for
the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
CTCs
|
circulating tumor cells
|
|
NSCLC
|
non-small cell lung cancer
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curve
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pécuchet N, Zonta E, Didelot A, Combe P,
Thibault C, Gibault L, Lours C, Rozenholc Y, Taly V, Laurent-Puig
P, et al: Base-position error rate analysis of next-generation
sequencing applied to circulating tumor DNA in non-small cell lung
cancer: A prospective study. PLoS Med. 13:e10021992016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi ZM, Wang L, Shen H, Jiang CF, Ge X, Li
DM, Wen YY, Sun HR, Pan MH, Li W, et al: Downregulation of miR-218
contributes to epithelial-mesenchymal transition and tumor
metastasis in lung cancer by targeting Slug/ZEB2 signaling.
Oncogene. 36:2577–2588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reka AK, Chen G, Jones RC, Amunugama R,
Kim S, Karnovsky A, Standiford TJ, Beer DG, Omenn GS and Keshamouni
VG: Epithelial-mesenchymal transition-associated secretory
phenotype predicts survival in lung cancer patients.
Carcinogenesis. 35:1292–1300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frick MA, Kao GD, Aguarin L, Chinniah C,
Swisher-McClure S, Berman AT, Levin WP, Cengel KA, DeCesaris C,
Hahn SM, et al: Circulating tumor cell assessment in presumed early
stage non-small cell lung cancer patients treated with stereotactic
body radiation therapy: A prospective pilot study. Int J Radiat
Oncol Biol Phys. 102:536–542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morbelli S, Alama A, Ferrarazzo G, Coco S,
Genova C, Rijavec E, Bongioanni F, Biello F, Dal Bello MG, Barletta
G, et al: Circulating tumor DNA reflects tumor metabolism rather
than tumor burden in chemotherapy-naive patients with advanced
non-small cell lung cancer: 18F-FDG PET/CT study. J Nucl
Med. 58:1764–1769. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krebs MG, Sloane R, Priest L, Lancashire
L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G,
et al: Evaluation and prognostic significance of circulating tumor
cells in patients with non-small-cell lung cancer. J Clin Oncol.
29:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanssen A, Loges S, Pantel K and Wikman H:
Detection of circulating tumor cells in non-small cell lung cancer.
Front Oncol. 5:2072015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao D, Dai C and Peng S: Mechanism of the
mesenchymal-epithelial transition and its relationship with
metastatic tumor formation. Mol Cancer Res. 9:1608–1620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Wu T, Peng X, Liu J, Liu F, Wu S,
Liu S, Dong Y, Xie S and Ma S: Mesenchymal phenotype of circulating
tumor cells is associated with distant metastasis in breast cancer
patients. Cancer Manag Res. 9:691–700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. 8. Cham
Switzerland: Springer; 2017
|
|
18
|
Wu S, Liu S, Liu Z, Huang J, Pu X, Li J,
Yang D, Deng H, Yang N and Xu J: Classification of circulating
tumor cells by epithelial-mesenchymal transition markers. PLoS One.
10:e01239762015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fluss R, Faraggi D and Reiser B:
Estimation of the youden index and its associated cutoff point.
Biom J. 47:458–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kraljevic Pavelic S, Sedic M, Bosnjak H,
Spaventi S and Pavelic K: Metastasis: New perspectives on an old
problem. Mol Cancer. 10:222011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin Y, Li F, Zheng C, Wang Y, Fang Z, Guo
C, Wang X, Liu H, Deng L, Li C, et al: NEDD9 promotes lung cancer
metastasis through epithelial-mesenchymal transition. Int J Cancer.
134:2294–2304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oakman C, Pestrin M, Bessi S, Galardi F
and Di Leo A: Significance of micrometastases: Circulating tumor
cells and disseminated tumor cells in early breast cancer. Cancers
(Basel). 2:1221–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonnomet A, Brysse A, Tachsidis A, Waltham
M, Thompson EW, Polette M and Gilles C: Epithelial-to-mesenchymal
transitions and circulating tumor cells. J Mammary Gland Biol
Neoplasia. 15:261–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nauseef JT and Henry MD:
Epithelial-to-mesenchymal transition in prostate cancer: Paradigm
or puzzle? Nat Rev Urol. 8:428–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Busch EL, Keku TO, Richardson DB, Cohen
SM, Eberhard DA, Avery CL and Sandler RS: Evaluating markers of
epithelial-mesenchymal transition to identify cancer patients at
risk for metastatic disease. Clin Exp Metastasis. 33:53–62. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan TZ, Miow QH, Miki Y, Noda T, Mori S,
Huang RY and Thiery JP: Epithelial-mesenchymal transition spectrum
quantification and its efficacy in deciphering survival and drug
responses of cancer patients. EMBO Mol Med. 6:1279–1293. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raimondi C, Nicolazzo C and Gradilone A:
Circulating tumor cells isolation: The ‘post-EpCAM era’. Chin J
Cancer Res. 27:461–470. 2015.PubMed/NCBI
|
|
29
|
Ferreira MM, Ramani VC and Jeffrey SS:
Circulating tumor cell technologies. Mol Oncol. 10:374–394. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lowes LE and Allan AL: Circulating tumor
cells and implications of the epithelial-to-mesenchymal transition.
Adv Clin Chem. 83:121–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Saad S, Al-Shibli K, Donnem T, Persson
M, Bremnes RM and Busund LT: The prognostic impact of NF-kappaB
p105, vimentin, E-cadherin and Par6 expression in epithelial and
stromal compartment in non-small-cell lung cancer. Br J Cancer.
99:1476–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dauphin M, Barbe C, Lemaire S,
Nawrocki-Raby B, Lagonotte E, Delepine G, Birembaut P, Gilles C and
Polette M: Vimentin expression predicts the occurrence of
metastases in non small cell lung carcinomas. Lung Cancer.
81:117–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pallier K, Cessot A, Côté JF, Just PA,
Cazes A, Fabre E, Danel C, Riquet M, Devouassoux-Shisheboran M,
Ansieau S, et al: TWIST1 a new determinant of epithelial to
mesenchymal transition in EGFR mutated lung adenocarcinoma. PLoS
One. 7:e299542012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hui L, Zhang S, Dong X, Tian D, Cui Z and
Qiu X: Prognostic significance of twist and N-cadherin expression
in NSCLC. PLoS One. 8:e621712013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mirza S, Jain N and Rawal R: Evidence for
circulating cancer stem-like cells and epithelial-mesenchymal
transition phenotype in the pleurospheres derived from lung
adenocarcinoma using liquid biopsy. Tumour Biol.
39:10104283176959152017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Milano A, Mazzetta F, Valente S, Ranieri
D, Leone L, Botticelli A, Onesti CE, Lauro S, Raffa S, Torrisi MR
and Marchetti P: Molecular detection of EMT markers in circulating
tumor cells from metastatic non-small cell lung cancer patients:
Potential role in clinical practice. Anal Cell Pathol (Amst).
2018:35068742018.PubMed/NCBI
|
|
37
|
Houthuijzen JM, Daenen LG, Roodhart JM and
Voest EE: The role of mesenchymal stem cells in anti-cancer drug
resistance and tumour progression. Br J Cancer. 106:1901–1906.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kitamura T, Qian BZ and Pollard JW: Immune
cell promotion of metastasis. Nat Rev Immunol. 15:73–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tripathi SC, Peters HL, Taguchi A,
Katayama H, Wang H, Momin A, Jolly MK, Celiktas M,
Rodriguez-Canales J, Liu H, et al: Immunoproteasome deficiency is a
feature of non-small cell lung cancer with a mesenchymal phenotype
and is associated with a poor outcome. Proc Natl Acad Sci USA.
113:E1555–E1564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lowes LE, Lock M, Rodrigues G, D'Souza D,
Bauman G, Ahmad B, Venkatesan V, Allan AL and Sexton T: The
significance of circulating tumor cells in prostate cancer patients
undergoing adjuvant or salvage radiation therapy. Prostate Cancer
Prostatic Dis. 18:358–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lucci A, Hall CS, Lodhi AK, Bhattacharyya
A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM and Krishnamurthy S:
Circulating tumour cells in non-metastatic breast cancer: A
prospective study. Lancet Oncol. 13:688–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lowes LE, Lock M, Rodrigues G, D'Souza D,
Bauman G, Ahmad B, Venkatesan V, Allan AL and Sexton T: Circulating
tumour cells in prostate cancer patients receiving salvage
radiotherapy. Clin Transl Oncol. 14:150–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meyer CP, Pantel K, Tennstedt P, Stroelin
P, Schlomm T, Heinzer H, Riethdorf S and Steuber T: Limited
prognostic value of preoperative circulating tumor cells for early
biochemical recurrence in patients with localized prostate cancer.
Urol Oncol. 34:235.e11–e16. 2016. View Article : Google Scholar
|
|
44
|
Chen X, Zhou F, Li X, et al: Folate
Receptor-Positive Circulating Tumor Cell Detected by LT-PCR-Based
Method as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer. J
Thorac Oncol. 10:1163–1171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Franken B, de Groot MR, Mastboom WJ,
Vermes I, van der Palen J, Tibbe AG and Terstappen LW: Circulating
tumor cells, disease recurrence and survival in newly diagnosed
breast cancer. Breast Cancer Res. 14:R1332012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kasimir-Bauer S, Hoffmann O, Wallwiener D,
Kimmig R and Fehm T: Expression of stem cell and
epithelial-mesenchymal transition markers in primary breast cancer
patients with circulating tumor cells. Breast Cancer Res.
14:R152012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yokobori T, Iinuma H, Shimamura T, Imoto
S, Sugimachi K, Ishii H, Iwatsuki M, Ota D, Ohkuma M, Iwaya T, et
al: Plastin3 is a novel marker for circulating tumor cells
undergoing the epithelial-mesenchymal transition and is associated
with colorectal cancer prognosis. Cancer Res. 73:2059–2069. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun YF, Guo W, Xu Y, Shi YH, Gong ZJ, Ji
Y, Du M, Zhang X, Hu B, Huang A, et al: Circulating tumor cells
from different vascular sites exhibit spatial heterogeneity in
epithelial and mesenchymal composition and distinct clinical
significance in hepatocellular carcinoma. Clin Cancer Res.
24:547–559. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Willipinski-Stapelfeldt B, Riethdorf S,
Assmann V, Woelfle U, Rau T, Sauter G, Heukeshoven J and Pantel K:
Changes in cytoskeletal protein composition indicative of an
epithelial-mesenchymal transition in human micrometastatic and
primary breast carcinoma cells. Clin Cancer Res. 11:8006–8014.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim MY, Oskarsson T, Acharyya S, Nguyen
DX, Zhang XH, Norton L and Massagué J: Tumor self-seeding by
circulating cancer cells. Cell. 139:1315–1326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chao Y, Wu Q, Shepard C and Wells A:
Hepatocyte induced re-expression of E-cadherin in breast and
prostate cancer cells increases chemoresistance. Clin Exp
Metastasis. 29:39–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gao D, Joshi N, Choi H, Ryu S, Hahn M,
Catena R, Sadik H, Argani P, Wagner P, Vahdat LT, et al: Myeloid
progenitor cells in the premetastatic lung promote metastases by
inducing mesenchymal to epithelial transition. Cancer Res.
72:1384–1394. 2012. View Article : Google Scholar : PubMed/NCBI
|