Introduction
Diabetic retinopathy (DR) is a severe microvascular
complication of diabetes. It is the main cause of loss of vision in
diabetic patients (1). Diabetic
patients are known to suffer from hyperglycemia due to
abnormalities in insulin signaling pathways; these abnormal levels
of sugar can cause various pathogenetic processes, resulting in the
following complications in diabetic patients: i) microvascular
defects of the retina and ii) dysfunction and degeneration of the
neuro-retina (2). The progression
of DR depends upon two major types of diabetic mellitus. Research
has shown that DR is the most common vision-threatening lesion in
patients with type I diabetes. Diabetic macular edema seems to be
more prevalent in patients with type II diabetes (3). Several studies have tried to
determine the causes and progression of DR in diabetic patients
(4–6); however, scientists have not yet been
successful in completely elucidating the mechanisms associated with
the pathogenesis of DR. Currently, diabetes mellitus cannot be
prevented or treated effectively in clinical practice.
In the field of molecular biology, scientists have
made major breakthroughs in understanding non-coding RNAs (ncRNAs).
The prognosis of diabetic patients has improved tremendously
because of various path-breaking studies conducted by researchers
in the field of molecular biology (7). Recent studies have reported that long
non-coding RNAs (lncRNAs) are involved in various biological
processes (7), and it was found
that lncRNAs are widely involved in signaling pathways, which
regulate numerous aspects of life processes (7). In the context of oncology, pan-cancer
molecular portraits of human tumors have been associated with
essential lncRNAs (8). The
activity of essential oncogenic pathways was altered with
dysregulation in the quantities of lncRNAs (8). lncRNAs impact post-translation
processes partly based on the competitive endogenous RNA (ceRNA)
mechanism, where lncRNA interacts with microRNA (miRNA) through
miRNA-binding sites (MREs) and hence regulate the expression of
certain genes (9). As a result,
ceRNA regulation is highly associated with several pathogenic
processes.
lncRNAs and miRNAs were previously demonstrated to
be associated with the pathogenesis of DR (10–12).
Previous findings highlight that the development of DR is
associated with various key molecules, such as miR-1273g-3p, miR-21
and miR-29a (13–15). Research has elucidated how miRNAs
play a pivotal role in the diagnosis of DR (16). For example, Yan et al found
that the expression of lncRNAs was aberrant in the early stages of
DR. In other words, DR may develop as follows. The aberrantly
expressed lncRNAs play pivotal roles in modulating target
molecules, and they regulate multiple pathogenetic pathways
(11). Presently, scientists have
reported that disease-related lncRNAs are associated with
function-related messenger RNAs (mRNAs) and miRNAs (17,18).
In many other diseases except DR, ceRNA mechanism have been
investigated to date; this has radically transformed the approach
used to understand the pathogenesis of several diseases (19), including cardiac hypertrophy
(20), rheumatoid arthritis
(21) and papillary renal cell
carcinoma (22). However, the role
played by the lncRNA-miRNA-mRNA network in the pathogenesis of DR
remains unclear. We have preliminary knowledge of the function and
physiological significance of ceRNAs.
Here, based on ceRNA theory, we attempted to explore
the crosstalk between lncRNA-miRNA-mRNA complexities. Key molecules
were identified by performing Gene Ontology (GO) analysis and
Weighted Gene Co-expression Network Analysis (WGCNA) (Fig. 1). In this study, ceRNA theory was
used for the first time in DR to compute a systematic profile of
the lncRNA-miRNA-mRNA network. Our results may be useful in
comprehending the role played by ceRNAs in the pathogenesis of
DR.
Materials and methods
Data availability
Using eight week-old C57BL/6 mice, a mouse model of
streptozotocin (STZ)-induced diabetes was constructed. Two months
after administering STZ injection to diabetic mice, total RNAs were
isolated from the retinas of the diabetic mice. The mice were
considered to be diabetic when their blood glucose levels exceeded
250 mg/dl. Using TRIzol reagent, wild-type mice were matched
according to age and sex. Agilent Mouse Gene Expression Microarrays
(Product Number G4852A; Agilent Technologies, Santa Clara, CA, USA)
were used to generate microarray data from mouse mRNA and lncRNA.
The raw data were provided by Yan et al, which has been
described in a previous study (11), and the ethics statement was
previously provided. To generate microarray data of miRNAs from
STZ-induced diabetic rats, we referred to a previous study and its
supplementary material (12).
Screening of differentially expressed
lncRNAs, miRNAs and mRNAs
As for the differential expression analysis, the
Benjamini-Hochberg method was applied to calculate the adjusted
P-value to minimize the false discovery rate (FDR) as was
previously described (12). |Fold
change|>2 and P<0.05 were set as cut-offs.
Prediction of target lncRNAs and mRNAs
of miRNAs
The target lncRNAs of miRNAs was predicted by
referring to Miranda (23). The
minimum free energy (MFE) was calculated under the following
condition: Max energy ≤-20 and score >160 were considered as
cut-offs. The target mRNAs of miRNAs was predicted on the basis of
miRTarBase (24), miRecords
(25) and starBase version 2.0
(26).
Construction of the ceRNA network
The lncRNA-miRNA-mRNA network was reconstructed with
ceRNA theory. The expression correlation of lncRNAs and mRNAs was
determined with Pearson correlation coefficient (PCC). The
mRNA-lncRNA pair was considered as the co-expressed one
(P<0.05). The ceRNA network was constructed by assembling all
the co-expressed competing triplets, and this network was
visualized with Cytoscape software v3.5.1 (https://cytoscape.org/).
Function enrichment analysis
While performing GO analysis, these databases were
used for annotation and visualization. Integration Discovery
(DAVID) and Cytoscape plug-in BinGO were used to complete the GO
analysis. To perform WGCNA analysis, we used R WGCNA package
(https://cran.r-project.org/package=WGCNA). The cut
height value was set to 0.992. We only considered modules in which
the distance between two consecutive modules was <0.1. All the
modules were then combined into a single module. The heat map was
drawn by using pheatmap R package (https://cran.r-project.org/web/packages/pheatmap/).
Results
Screening of lncRNAs, mRNAs and miRNAs
is associated specifically with DR
We compared expression profiles of lncRNAs, miRNAs
and mRNAs, which were obtained from the retinas of different
diabetic mice. To determine the key RNA molecules, differential
expression analysis was performed (2). In this study, 305 differentially
expressed lncRNAs and 17 differentially expressed miRNAs (diabetic
vs. non-diabetic ones) were observed. Among them, 89 lncRNAs and 14
miRNAs showed upregulated expression, whereas 214 lncRNAs and 3
miRNAs showed downregulated expression in the diabetic retina
samples compared with the controls (Fig. 2). The occurrence and development of
DR was regulated by the differentially expressed genes.
Construction of lncRNA-miRNA and
mRNA-miRNA co-expression network
lncRNAs can competitively interact with miRNAs, and
they may function as ceRNAs. With the help of miRanda, the
interactions between lncRNAs and miRNAs were predicted. The
interactions were predicted by referring to the minimal free energy
of miRNA-lncRNA duplexes (max energy ≤-20 and score >160). In
total, we predicted 246 pairs of lncRNA-miRNA; 17 miRNAs and 121
lncRNAs were involved in these 246 pairs (Fig. 3A). Using miRTarBase, miRecords and
starBase version 2.0, we identified the target mRNAs of miRNAs.
Thus, we identified 664 target mRNAs of 14 miRNAs (Fig. 3B).
Construction of the competitive
endogenous RNA network
Since lncRNAs were found to be specifically
associated with DR, we further investigated them with Cytoscape
software. Then, the results of the analyses were used to construct
the ceRNA network. Based on previous results of the mRNA-miRNA and
lncRNA-miRNA co-expression network, the interactions between the
lncRNA-miRNA-mRNA network were visualized (Fig. 4). In total, 802 nodes (121 lncRNA
nodes, 17 miRNA nodes, and 664 mRNA nodes) and 949 edges were found
in this network. The key miRNAs were as follows: miR-223-3p,
miR-34c-5p, and miR-200b-3p; these miRNAs were essential hubs in
the entire network. Moreover, these miRNAs potentially play an
important role in the pathogenesis of DR.
Functional evaluation of the diabetic
retinopathy-specific lncRNAs
We observed that mRNAs were connected to lncRNAs in
the network. To comprehend the functions of every lncRNA, the
functions of connected mRNAs were assessed. Thus, we deciphered how
gene products can be enriched in biological processes, cellular
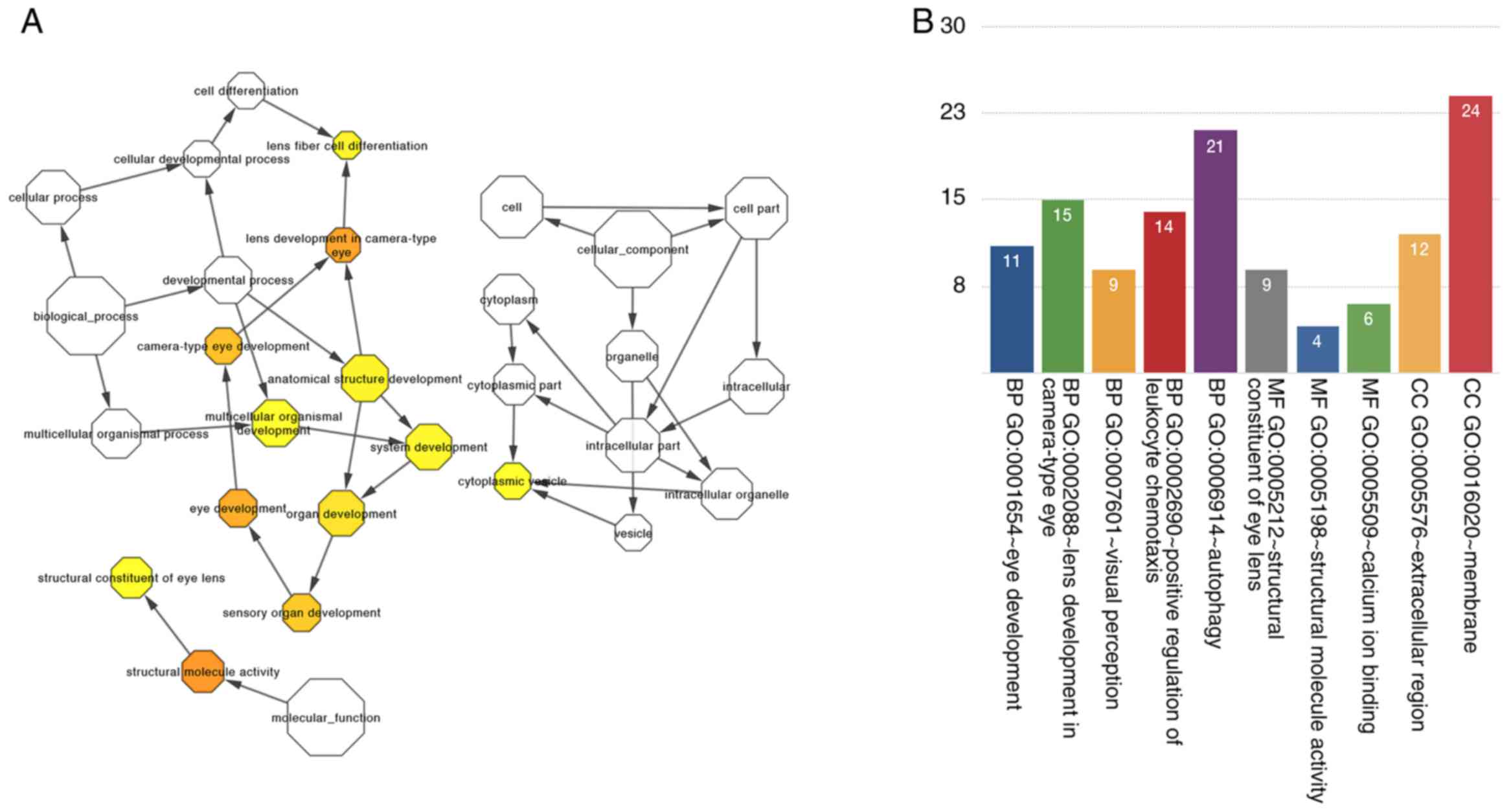
components, and molecular functions. Surprisingly, the results of
GO analysis indicate that enriched GO terms were associated with
the following events: the development of eyes and lens in
camera-type eye, visual perception, structural constituents of eye
lens, structural molecule activity and calcium-binding ions
(Fig. 5A). We visualized gene
function enrichment in the network by using BinGO (Fig. 5B).
Key lncRNA-miRNA-mRNA sub-network
based on the module analysis
To investigate the cross-talks between mRNAs and
lncRNAs, key lncRNAs were extracted; moreover, the correlation
between mRNAs and miRNAs was determined by WGCNA analysis. Thus, we
constructed a novel sub-network of lncRNA-miRNA-mRNA. We discovered
a module that was significantly related to DR (P<0.001,
R=0.980). In this module, 33 lncRNAs and 10 mRNAs were involved
(Fig. 6).
Discussion
In the past few decades, many studies have been
conducted to determine the molecular mechanisms underlying diabetic
retinopathy (DR). Protein-coding genes or miRNAs have been
previously identified. Yet, very few studies have determined the
function of lncRNAs which are associated with the pathogenesis of
DR. Scientists have not been successful in elucidating the
functions of the lncRNA-miRNA-mRNA network in DR to date. Here, we
report certain lncRNAs which can be used as miRNA sponges, where
these sponges are involved in the pathogenesis of DR. To the best
our knowledge, this is the first study to determine whether ceRNAs
play a pivotal role in the development of DR.
In the present study, we performed GO analysis to
determine the possible functions of the differentially expressed
lncRNAs. We found that the functions of lncRNAs were completely
related to the functions of the connected mRNAs; this notion was
used to determine gene product enrichment in biological processes,
cellular components and molecular functions. By performing GO
analysis of mRNAs, we found that differentially expressed genes
were involved in the following ophthalmic functions: Eye
development, lens development in camera-type eye, visual perception
and structural constituents of eye lens. This indicates that
disease-related lncRNAs play important roles in the pathogenesis of
DR. Furthermore, we also performed KEGG analysis; however, the
results indicated that disease-specific genes were not
significantly associated with certain pathways.
It has been established that WGCNA is a widely-used
bioinformatic method that describes correlation patterns among
genes of microarray samples. We performed WGCNA analysis for the
following purposes: i) to identify clusters (modules) of highly
correlated genes, ii) to summarize clusters (module) by using eigen
gene or an intramodular hub gene, iii) to relate modules with one
another and external sample traits using eigen gene network
methodology, and iv) to calculate module membership measures.
Although the analysis was performed with utmost diligence, we
obtained a paradoxical result: We found that only one module was
specifically related to DR. This module included 33 lncRNAs and 10
mRNAs. These 33 lncRNAs might serve as potential diagnostic
biomarkers and therapeutic targets for DR.
Importantly, previous studies have reported that
some key hub miRNAs, depicted in this study, play an important role
in the pathogenesis of different diseases. For example, Bozec et
al investigated the functions of miR-223-3p, reporting that
this molecule is associated with the angiogenesis of head and neck
squamous cell carcinoma (27).
This indicates that miR-223-3p plays a pivotal role in the
development of DR. In another study, miR-223-3p was found to be
involved in the pathogenesis and progression of diabetic kidney
disease (28). Another study
reported that miR-34c-5p is an inflammation-related miRNA, which is
associated with the vascular repair factor HGF and miR-574-3p. It
is upregulated in type 2 diabetes monocytes, implying that
anti-inflammatory cells play a pivotal role in adhesion, vascular
repair and invasion (29).
Moreover, miR-34c-5p is also a tumor suppressor (30), and it is associated with apoptosis
and differentiation of cancer cells. Furthermore, miR-200b-3p is
also a hub molecule in ceRNA network. It was found that miR-200b-3p
is associated with several types of cancers, dysregulating
monocyte/macrophage (31) and
differentiating epithelial-to-mesenchymal transition of glioma
cancer cells (32).
Our study has several limitations. A major concern
is the insufficient data and a relatively small sample size. Owing
to the limited sample size, the miRNA-lncRNA-mRNA network might be
restricted. Moreover, the type of diabetes was not reported by
study participants. Another major concern of this research study is
that computational results might be noises and false-positive
results; however, we set cut-offs to the most used values.
Moreover, we used the widely accepted methods of computation. For
example, the target mRNAs of miRNAs were predicted on the basis of
three widely used databases (miRTarBase, miRecords and starBase).
These results need further confirmation in terms of cell lines and
animal models.
In summary, we constructed a competitive endogenous
RNA network and identified several potential key molecules. We
described the important role played by non-coding RNAs in the
pathogenesis of DR. Our study highlighted specific lncRNAs and
miRNAs related to the pathogenesis of DR, which might be used as
novel diagnostic biomarkers and therapeutic targets for DR.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the
National Natural Science Foundation of China (81501559 and
61671255), the Jiangsu Overseas Research and Training Program for
University Prominent Young and Middle-aged Teachers and Presidents
2016, and the Graduate Research and Innovation Plan Project of
Nantong University (YKC15056, YKC16072), the Innovative Training
Program of Undergraduate Students of Nantong University (2018144)
and the National Key R&D Program of China (2018YFC1314902).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and HW designed the study, analyzed and
interpreted the data, and wrote the manuscript. KJ, AS, LW, LS, KJ
and JD conducted the data analysis and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klein R, Klein BE, Moss SE, Davis MD and
DeMets DL: The Wisconsin epidemiologic study of diabetic
retinopathy. II. Prevalence and risk of diabetic retinopathy when
age at diagnosis is less than 30 years. Arch Ophthalmol.
102:520–526. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding J and Wong TY: Current epidemiology
of diabetic retinopathy and diabetic macular edema. Curr Diab Rep.
12:346–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu H, Wu H, Shi L, Yuan X, Yin Y, Yuan M,
Zhou Y, Hu Q, Jiang K and Dong J: The association of haptoglobin
gene variants and retinopathy in type 2 diabetic patients: A
meta-analysis. J Diabetes Res. 2017:21950592017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu H, Geng X, Zhang X, Qiu M, Jiang K,
Tang L and Dong J: A self-adaptive distance regularized level set
evolution method for optical disk segmentation. Biomed Mater Eng.
24:3199–3206. 2014.PubMed/NCBI
|
|
6
|
Wu HQ, Wu H, Shi LL, Yu LY, Wang LY, Chen
YL, Geng JS, Shi J, Jiang K and Dong JC: The association between
retinal vasculature changes and stroke: A literature review and
Meta-analysis. Int J Ophthalmol. 10:109–114. 2017.PubMed/NCBI
|
|
7
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiu HS, Somvanshi S, Patel E, Chen TW,
Singh VP, Zorman B, Patil SL, Pan Y, Chatterjee SS, Sood AK, et al:
Cancer Genome Atlas Research Network: Pan-cancer analysis of lncRNA
regulation supports their targeting of cancer genes in each tumor
context. Cell Rep. 23:297–312.e12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Sun X, Icli B and Feinberg MW:
Emerging roles for MicroRNAs in diabetic microvascular disease:
Novel targets for therapy. Endocr Rev. 38:145–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan B, Tao ZF, Li XM, Zhang H, Yao J and
Jiang Q: Aberrant expression of long noncoding RNAs in early
diabetic retinopathy. Invest Ophthalmol Vis Sci. 55:941–951. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kovacs B, Lumayag S, Cowan C and Xu S:
MicroRNAs in early diabetic retinopathy in streptozotocin-induced
diabetic rats. Invest Ophthalmol Vis Sci. 52:4402–4409. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang LQ, Cui H, Wang L, Fang X and Su S:
Role of microRNA-29a in the development of diabetic retinopathy by
targeting AGT gene in a rat model. Exp Mol Pathol. 102:296–302.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Q, Qiu F, Zhou K, Matlock HG,
Takahashi Y, Rajala RV, Yang Y, Moran E and Ma JX: Pathogenic role
of microRNA-21 in diabetic retinopathy through downregulation of
PPARα. Diabetes. 66:1671–1682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye Z, Li ZH and He SZ: miRNA-1273g-3p
involvement in development of diabetic retinopathy by modulating
the autophagy-lysosome pathway. Med Sci Monit. 23:5744–5751. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gomaa AR, Elsayed ET and Moftah RF:
MicroRNA-200b expression in the vitreous humor of patients with
proliferative diabetic retinopathy. Ophthalmic Res. 58:168–175.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang C, Wu D, Gao L, Liu X, Jin Y, Wang D,
Wang T and Li X: Competing endogenous RNA networks in human cancer:
Hypothesis, validation, and perspectives. Oncotarget.
7:13479–13490. 2016.PubMed/NCBI
|
|
18
|
Wang Y, Hou J, He D, Sun M, Zhang P, Yu Y
and Chen Y: The emerging function and mechanism of ceRNAs in
cancer. Trends Genet. 32:211–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song C, Zhang J, Liu Y, Pan H, Qi HP, Cao
YG, Zhao JM, Li S, Guo J, Sun HL and Li CQ: Construction and
analysis of cardiac hypertrophy-associated lncRNA-mRNA network
based on competitive endogenous RNA reveal functional lncRNAs in
cardiac hypertrophy. Oncotarget. 7:10827–10840. 2016.PubMed/NCBI
|
|
21
|
Jiang H, Ma R, Zou S, Wang Y, Li Z and Li
W: Reconstruction and analysis of the lncRNA-miRNA-mRNA network
based on competitive endogenous RNA reveal functional lncRNAs in
rheumatoid arthritis. Mol BioSyst. 13:1182–1192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang C, Yuan N, Wu L, Wang X, Dai J, Song
P, Li F, Xu C and Zhao X: An integrated analysis for long noncoding
RNAs and microRNAs with the mediated competing endogenous RNA
network in papillary renal cell carcinoma. OncoTargets Therapy.
10:4037–4050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chou CH, Chang NW, Shrestha S, Hsu SD, Lin
YL, Lee WH, Yang CD, Hong HC, Wei TY, Tu SJ, et al: miRTarBase
2016: Updates to the experimentally validated miRNA-target
interactions database. Nucleic Acids Res. 44:D239–D247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao F, Zuo Z, Cai G, Kang S, Gao X and Li
T: miRecords: An integrated resource for microRNA-target
interactions. Nucleic Acids Res. 37:D105–D110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bozec A, Zangari J, Butori-Pepino M, Ilie
M, Lalvee S, Juhel T, Butori C, Brest P, Hofman P and
Vouret-Craviari V: MiR-223-3p inhibits angiogenesis and promotes
resistance to cetuximab in head and neck squamous cell carcinoma.
Oncotarget. 8:57174–57186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Li R, He J, Yang Q, Wu Y, Huang J
and Wu B: Co-expression analysis among microRNAs, long non-coding
RNAs, and messenger RNAs to understand the pathogenesis and
progression of diabetic kidney disease at the genetic level.
Methods. 124:46–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baldeón Rojas L, Weigelt K, de Wit H,
Ozcan B, van Oudenaren A, Sempértegui F, Sijbrands E, Grosse L, van
Zonneveld AJ, Drexhage HA and Leenen PJ: Study on
inflammation-related genes and microRNAs, with special emphasis on
the vascular repair factor HGF and miR-574-3p, in monocytes and
serum of patients with T2D. Diabetol Metab Syndr. 8:62016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li F, Chen H, Huang Y, Zhang Q, Xue J, Liu
Z and Zheng F: miR-34c plays a role of tumor suppressor in HEC1-B
cells by targeting E2F3 protein. Oncol Rep. 33:3069–3074. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu X, Wang QL, Li YF, Wang XD, Xu A and Li
Y: A novel miR-200b-3p/p38IP pair regulates monocyte/macrophage
differentiation. Cell Discov. 2:150432016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu J, Cui H, Zhu Z and Wang L:
MicroRNA-200b-3p suppresses epithelial-mesenchymal transition and
inhibits tumor growth of glioma through down-regulation of ERK5.
Biochem Biophys Res Commun. 478:1158–1164. 2016. View Article : Google Scholar : PubMed/NCBI
|