Introduction
Helicobacter pylori (H. pylori) is a
spiral-shaped gram-negative bacterium that can be selectively
planted in the human stomach, resulting in various gastric
diseases, including gastritis, gastric adenocarcinoma, peptic ulcer
disease and gastric mucosa-associated lymphoma (1,2).
H. Pylori promotes the production of various types of
cytokines in gastric mucosa, which leads to chronic inflammation
and epithelial cell damage.
Previous studies indicated that excessive production
of interleukin (IL)-17A was associated with a variety of
inflammatory diseases, including experimental autoimmune
encephalomyelitis (3), extrinsic
allergic alveolitis (4),
inflammatory bowel diseases (5),
collagen-induced arthritis (6) and
psoriasis (7). IL-17A belongs to
the IL-17 family, which are produced by cluster of differentiation
(CD)4+ Th17 cells and other subsets of immune cells
(8). IL-17A induces the production
of cytokines and chemokines, including IL-8 and growth-regulated
oncogene α (GRO-α) in epithelial cells and macrophages, and
exhibits a pro-inflammatory effects (9). In addition, upregulation of IL-17A is
associated with H. pylori infection in the gastric mucosa
(10,11). IL-17A also stimulates gastric
mononuclear and epithelial cells to produce IL-8, suggesting that
IL-17A serves an important role in H. pylori-induced
inflammation (10,12); however, the factors that
participate in IL-17A-mediated inflammatory responses in H.
pylori-associated gastritis remain unknown.
MicroRNAs (miRNAs/miRs) serve biological functions
in the immune system (13–15). Aberrant expression of miRNAs, such
as miR-146a, is associated with numerous inflammatory disorders
(16), and is enhanced by the
stimulation with inflammatory cytokines (17). For example, miR-146a was
upregulated by IL-1β and Toll-like receptors (TLRs), such as TLR2
(18,19). Previous studies have indicated that
miR-146a was upregulated following H. pylori infection in a
nuclear factor-κB (NF-κB)-dependent manner (20–22).
In addition, miR-146a overexpression regulates inflammation
responses by inhibiting its target genes, including tumor necrosis
factor (TNF) receptor-associated factor 6 (TRAF6) and IL-1
receptor-associated kinase 1 (IRAK1) (23,24).
TRAF6 and IRAK1 function as signal transducers in the NF-κB pathway
that activates iκB kinase in response to proinflammatory cytokines
(23). NF-κB is an essential
signaling factor involved in the progression of H. pylori
infection (25). It has been
reported that miR-146a could inhibit TRAF6- and IRAK1-mediated
signaling by acting as a negative regulator in the inflammatory
state (26); however, whether
miR-146a suppresses inflammatory responses by stimulating IL-17A
during H. pylori infection remains unknown.
In the present study, the expression of miR-146a was
increased by IL-17A stimulation in gastric epithelial cells;
whether the induction of miR-146a affects the activities of IL-17A
induced inflammatory responses in H. pylori-associated
gastritis was investigated. In addition, the correlation of IL-17A
and miR-146a expression in H. pylori-infected gastric
tissues was evaluated.
Materials and methods
Cell culture and H. pylori strain
A human gastric cancer cell line, SGC-7901 was
purchased from the cell bank of Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences (Shanghai, China). The cells
were routinely cultured in RPMI-1640 medium purchased from Gibco
supplemented with 10% FBS (both Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a humidified atmosphere of 5%
CO2. H. pylori strain 26695 was obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in brain-heart infusion agar (BHI; Becton, Dickinson and Company,
Franklin Lakes, NJ, USA) plates with 10% rabbit blood (cat. no.
HQ50073; Shanghai Bangsheng Biotechnology Co., Ltd., Shanghai,
China) at 37°C under the microaerophilic conditions of 5%
O2, 10% CO2 and 85% N2.
Infection of SGC-7901 cells with H.
pylori 26695
H. pylori was collected from the culture
plates using 2 ml sterile PBS followed by centrifugation at 2500 ×
g for 5 min at room temperature, and resuspended in RPMI-1640
medium. The concentration of H. pylori 26695 was determined
at 600 nm using the Agilent 8453E UV-visible Spectroscopy System
(Agilent Technologies, Inc., Santa Clara, CA, USA): Optical density
(600 nm) = 1×109 colony forming unit/ml. SGC-7901 cells
were infected with H. pylori 26695 for 6 h at 37°C, with the
multiplicity of infection 100.
Cytokine measurements by ELISA
An ELISA was employed to evaluate the inflammatory
response of SGC-7901 cells subjected to IL-17A stimulation at
serial concentrations (0, 0.01, 0.1, 1 or 10 ng/ml) over 24 h at
37°C, or treated with 10 ng/ml IL-17A at different time points (0,
6, 12, 18 or 24 h) at 37°C. Cell culture supernatants were
collected by centrifugation at 1,000 × g for 20 min at room
temperature, and the expression levels of IL-17A (cat. no. D1700),
GRO-α (cat. no. DGR00B) and IL-8 (cat. no. D8000C) in the
supernatant were determined using DuoSet ELISA Development System
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer's protocols. Absorbance at 450 nm was detected using a
microplate reader.
Analysis of miR-146a and mRNA by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from tissues or cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. For RT-qPCR
analysis of miR-146a, the total RNA isolated from cells was reverse
transcribed to cDNA using a TaqMan MicroRNA Reverse Transcription
Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) The
reactions were incubated at 16°C for 30 min, followed by 42°C for
30 min, then 85°C for 5 min before being held at 4°C. The
expression levels of miR-146a were evaluated using TaqMan miRNA
assays (Ambion; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. The reactions were performed using a
Bio-Rad IQ5 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with
the following program: 95°C for 2 min, 40 cycles of 95°C for 15 sec
and 60°C for 30 sec. U6 was used as internal reference. The
upstream and downstream primers of miR-146a and U6 were synthesized
by Sangon Biotech Co., Ltd. (Shanghai, China) and the sequences
were as follows: miR-146a forward, 5′-GTGCAGGGTCCGAGGT-3′ and
reverse, 5′-CGGCGGTGAGAACTGAATTCC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Relative expression of miR-146a was analyzed using
2−∆∆Cq method (27).
The results were repeated at least three times.
The expression levels of IL-17A were determined
using a PrimeScript RT-PCR kit (Takara Bio, Inc., Otsu, Japan). The
expression was normalized using β-actin. The primer sequences are
as follows: IL-17A forward, 5′-AGGAATCACAATCCCACGAA-3′ and reverse,
5′-ACTTTGCCTCCCAGATCACA-3′; β-actin forward,
5′-TTCCTTCCTGGGCATGGAGTCC-3′ and reverse,
5′-TGGCGTACAGGTCTTTGCGG-3′.
Oligonucleotide transfection
All oligonucleotides were obtained from Shanghai
GenePharma Co., Ltd. (Shanghai, China) and transfected into
SGC-7901 cells using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Briefly, SGC-7901 cells were
seeded in 6-well plates at a concentration of 2.0×105
cells/well in RPMI-1640 supplemented with 10% FBS. After
subculturing at 37°C for 24 h under 5% CO2, the medium
was replaced with fresh serum-free medium purchased from Gibco
(Opti-MEM® I Reduced Serum Medium; Thermo Fisher
Scientific, Inc.), and SGC-7901 cells were transfected with
miR-146a mimics (50 nM; 5′-UGAGAACUGAAUUCCAUGGGUU-3′;
5′-CCCAUGGAAUUCAGUUCUCAUU-3′), scrambled miR control (50 nM;
forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′), IRAK1 small interfering (si)RNA (100
nM; forward, 5′-GGUUUCGUCACCCAAACAUtt-3′ and reverse,
5′-AUGUUUGGGUGACGAAACCtg-3′) or TRAF6 siRNA (100 nM; forward,
5′-GGUUGUUUGCACAAGAUGGtt-3′ and reverse,
5′-CCAUCUUGUGCAAACAACCtt-3′). The mock treatment group was
transfected with transfection reagent only. FAM-labeled negative
control siRNA (100 nM) purchased from Ambion (cat. no. AM4620;
Thermo Fisher Scientific, Inc.) was transfected into SGC-7901 cells
to evaluate the transfection efficiency; >80% of the cells were
successfully transfected (data not shown). After transfection for
24 h, the cells were treated with 10 ng/ml IL-17A (cat. no. 200-17;
Peprotech, Inc., Rocky Hill, NJ, USA) for 24 h.
Western blotting
Western blot analysis was performed as previously
described (28). The blot was
probed with primary antibodies at 4°C overnight: Anti-IRAK1
(1:1,000; cat. no. 4362) and anti-TRAF6 (1:1,000; cat. no. 4743),
were used to determine the expression levels of IRAK1 and TRAF6,
respectively; Anti-β-actin (1:1,000; cat. no. 5125; all Cell
Signaling Technology, Inc., Danvers, MA, USA) was used as an
internal control. Next, membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies, goat-anti-mouse
(1:5,000; cat. no. 115-035-003) or goat-anti-rabbit (1:5,000; cat.
no. 111-035-003; both Jackson ImmunoResearch Laboratories., Inc.,
West Grove, PA, USA) at 37°C for 2 h. Protein bands were visualized
using SuperSignal West Dura Duration substrate reagent (cat. no.
34080; Thermo Fisher Scientific, Inc.). Blots were quantified using
Image J software (version 1.48; National Institutes of Health,
Bethesda, MD, USA).
Evaluation of NF-κB activity
For NF-κB activity analysis, SCG-7901 cells were
co-transfected with 0.8 mg of the reported luciferase vector
pNF-κB-TA-Luc (cat. no. 631904; Clontech Laboratories, Inc.,
Mountainview, CA, USA), 0.04 mg of Renilla control vector
(pRL-TK; cat. no. 2241; Promega Corporation, Madison, WI, USA) and
miR-control/miR-146a mimics by using Lipofectamine 2000 according
to the manufacturer's protocols. After 24 h, the cells were
stimulated with or without 10 ng/ml IL-17A (cat. no. 200-17;
Peprotech, Inc.) for 12 h at 37°C. Luciferase activity was analyzed
using the Dual-Luciferase Reporter Assay System (Promega
Corporation). Firefly luciferase activity was normalized to that of
Renilla luciferase.
Patient samples
In total, 40 samples from were collected from
patients with gastric mucosa (18 women and 22 men, 25–60-years-old)
who underwent endoscopy due to dyspeptic symptoms at Southwest
Hospital (Chongqing, China) between January 2013 and December 2014,
including 20 patients with H. pylori–induced chronic
gastritis and 20 healthy donors. None of the patients had been
administered nonsteroidal anti-inflammatory drugs, antibiotics or
proton pump inhibitor drugs. Identification of H. pylori
infection in gastric mucosa was performed via H. pylori
culture, C13-urea breath tests and histological analysis. The
present study was approved by the Ethics Committee of the
Institutional Review Board at The Third Military Medical University
(Chongqing, China), and written informed consent was obtained from
all patients.
Statistical analyses
Data were presented as the mean ± standard deviation
of at least three independent experiments. Data were analyzed using
a Student's t-test or analysis of variance followed by a Dunnett's
post-hoc test. The association between the expression levels of
miR-146a and IL-17A was analyzed using Pearson's correlation. All
statistical analyses were performed using GraphPad Prism software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
IL-17A enhances the production of
GRO-α and IL-8 in SGC-7901 cells
Previous studies reported that H. pylori
infection may stimulate the secretion of IL-17A (10,11,29),
which was also confirmed in human gastric cancer cell line SGC-7901
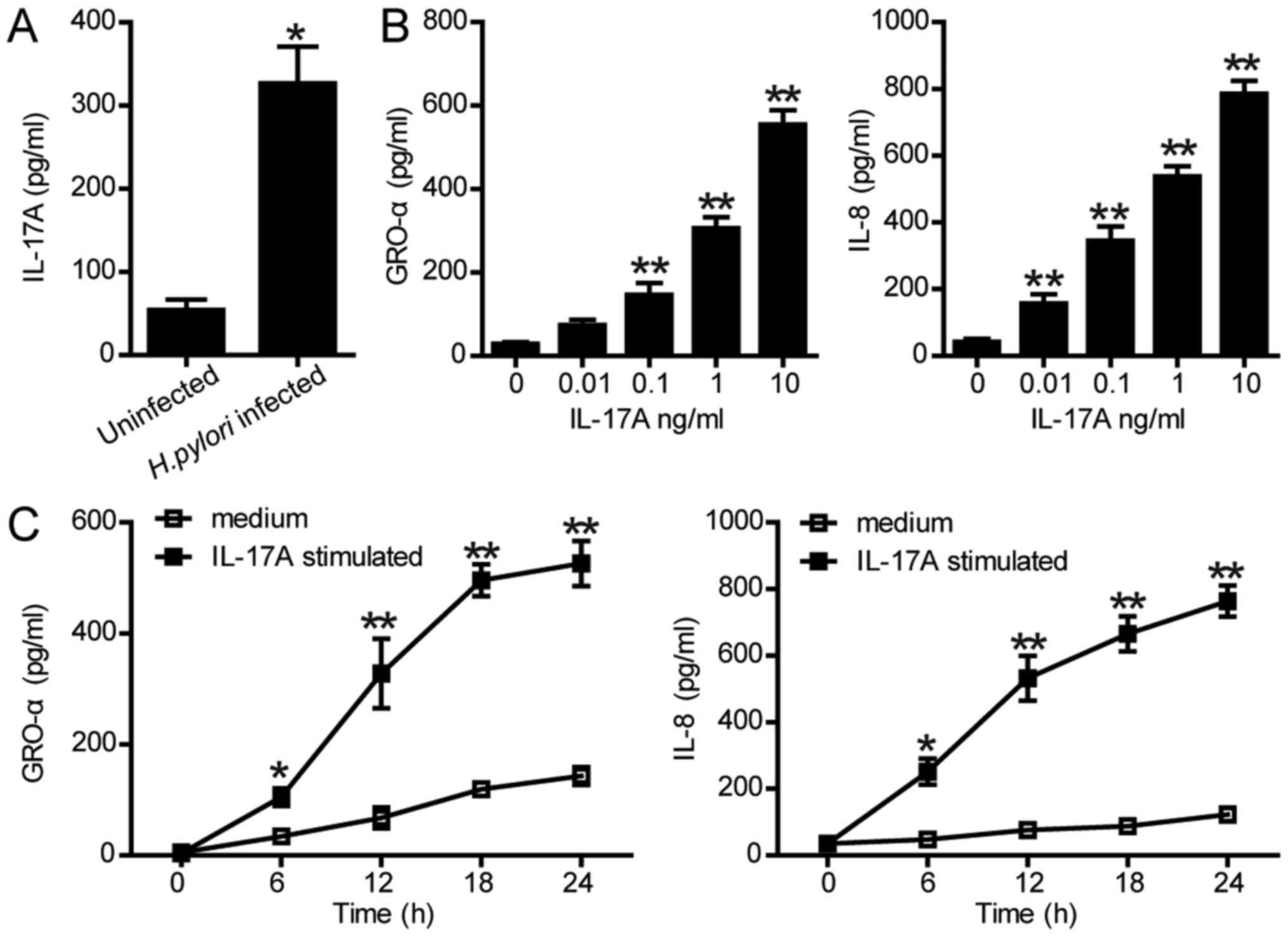
in the present study. As presented in Fig. 1A, H. pylori infection
significantly increased the protein levels of IL-17A in SGC-7901
cells compared with the control. Furthermore, to investigate the
roles of IL-17A in inflammation, the expression levels of GRO-α and
IL-8 were examined by ELISA. As presented in Fig. 1B, production of GRO-α and IL-8 were
significantly increased in SGC-7901 cells following treatment with
0.01–10 ng/ml IL-17A over 24 h compared with the control. The
expression levels of GRO-α and IL-8 in SGC-7901 cells were also
significantly increased in a time-dependent manner (Fig. 1C). These data indicated that H.
pylori-associated production of IL-17A could upregulate GRO-α
and IL-8 in gastric epithelial cells, suggesting a potential role
of IL-17A in H. pylori-induced inflammation.
miR-146a is upregulated in SGC-7901
cells upon IL-17A stimulation
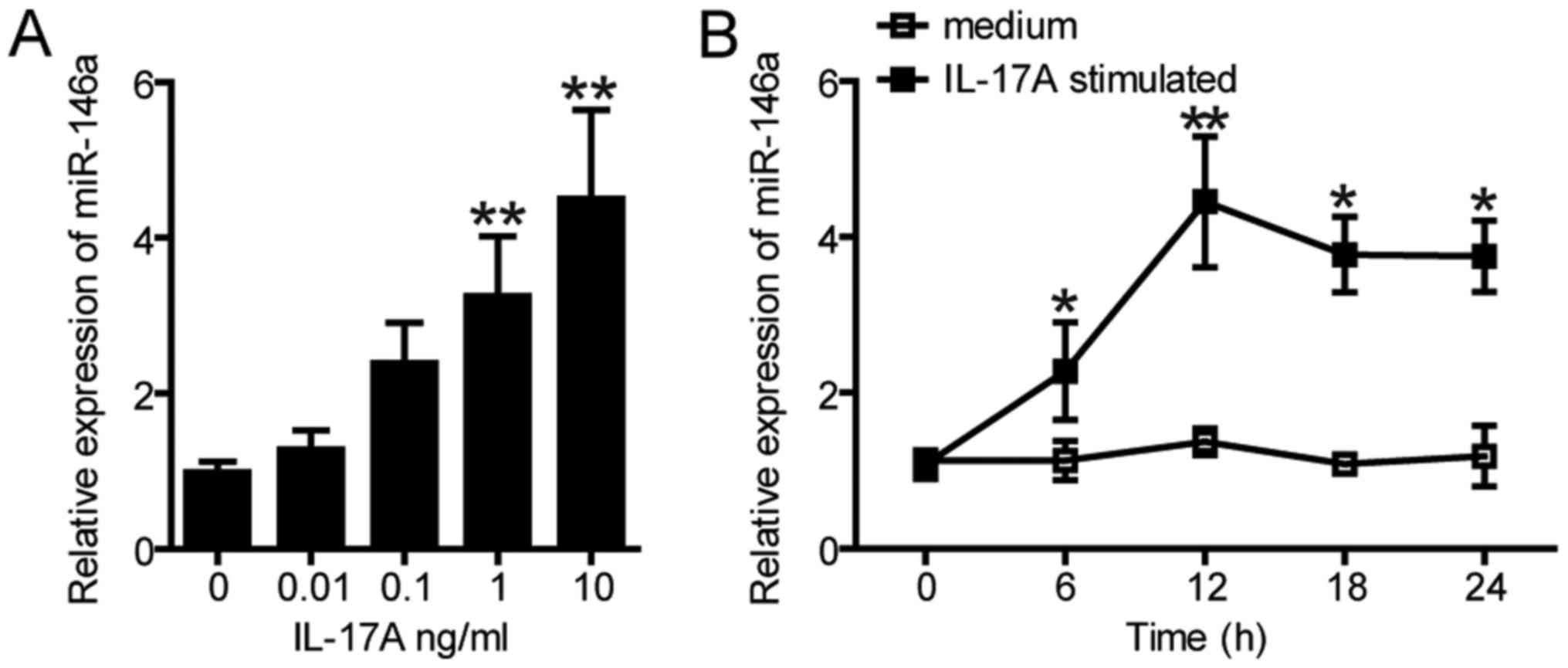
Previous studies have revealed that the expression
levels of miR-146a was significantly increased in gastric
epithelial cells following the stimulation by H. pylori
(20,30). Whether H. pylori-induced
IL-17A can enhance the expression of miR-146a in SGC-7901 cells was
further investigated in the present study. As presented in Fig. 2A, the expression levels of miR-146a
were significantly increased in SGC-7901 cells treated with 1 and
10 ng/ml IL-17A over 12 h compared with the control; but no
significant difference was observed in SGC-7901 cells treated with
0.01 or 0.1 ng/ml IL-17A. In addition, the expression of miR-146a
was significantly upregulated from 6–24 h following treatment with
10 ng/ml IL-17A compared with the control; expression peaked at 12
h and reduced thereafter (Fig.
2B). These results suggested that miR-146a expression is
increased in gastric epithelial cells upon the stimulation with
IL-17A.
miR-146a downregulates IRAK1 and
TRAF6, which are involved in IL-17A-mediated inflammation in
vitro
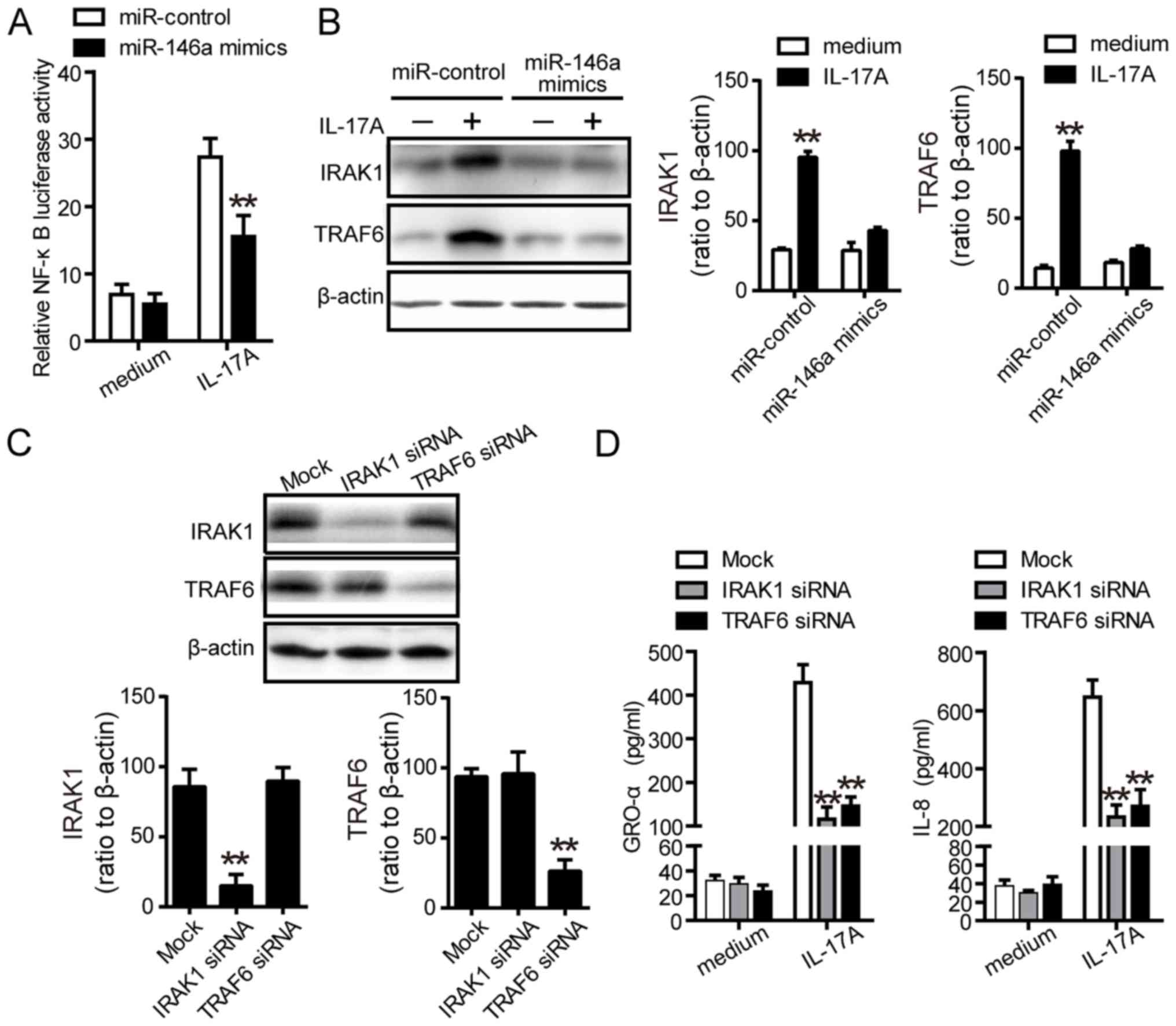
A previous study indicated that IL-17A induced the
production of proinflammatory cytokines via the NF-κB signaling
pathway (31). Thus, whether
miR-146a regulated proinflammatory cytokines in a NF-κB-dependent
manner was investigated. As presented in Fig. 3A, luciferase report assays revealed
that miR-146a mimics significantly inhibited the activity of NF-κB
compared with the control. Furthermore, IRAK1 and TRAF6 have been
reported as two potential targets of miR-146a, and are involved in
the activation of NF-κB (30). In
the present study, miR-146a significantly reduced the protein
expression of IRAK1 and TRAF6 upon stimulation with IL-17A
(Fig. 3B). To further determine
the biological functions of IRAK1 and TRAF6 on IL-17A-stimulated
inflammatory responses, specific siRNAs were used to inhibit the
expression of IRAK1 and TRAF6 (Fig.
3C). Western blot analysis revealed that downregulation of
IRAK1 and TRAF6 significantly decreased the protein levels of GRO-α
and IL-8 compared with the control (Fig. 3D), which suggested that
IL-17A-induced expression of miR-146a may suppress the expression
of IRAK1 and TRAF6, attenuating NF-κB activity and affecting
inflammation.
Overexpression of miR-146a suppresses
IL-17A-induced inflammatory responses in SGC-7901 cells
Previous studies revealed that miR-146a participated
in the negative feedback regulation of inflammation during H.
pylori infection (20–22,30);
thus the effects of miR-146a on IL-17A-induced inflammatory
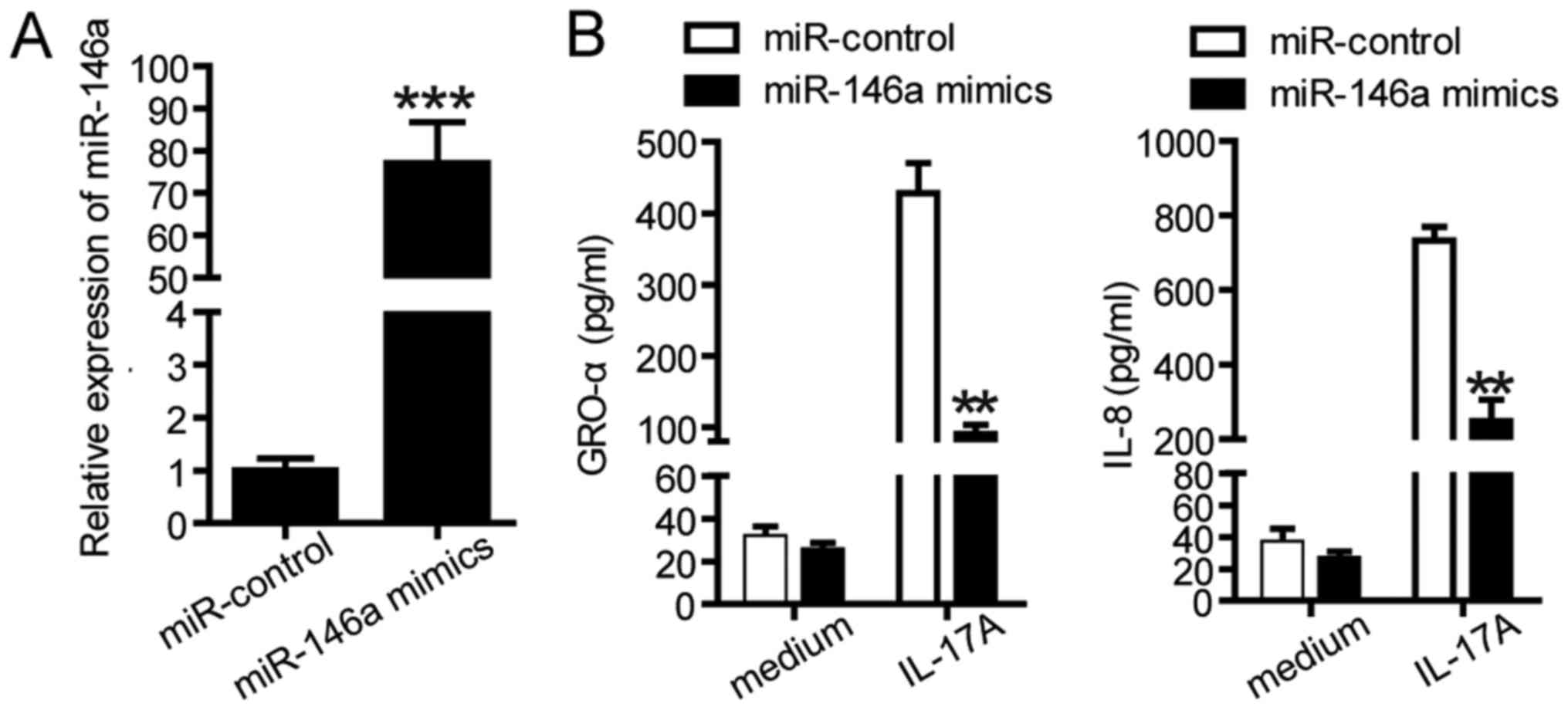
responses in SGC-7901 cells were investigated. The expression
levels of miR-146a in transfected SGC-7901 cells were determined
using RT-qPCR (Fig. 4A). As
presented in Fig. 4B, SGC-7901
cells were transfected with miR control or miR-146a mimics followed
by treatment with IL-17A. As presented in Fig. 4B, miR-146a mimics significantly
decreased the protein levels of GRO-α and IL-8 compare with the
control. In summary, these data suggested that miR-146a serves a
potential role in IL-17A-induced inflammatory responses.
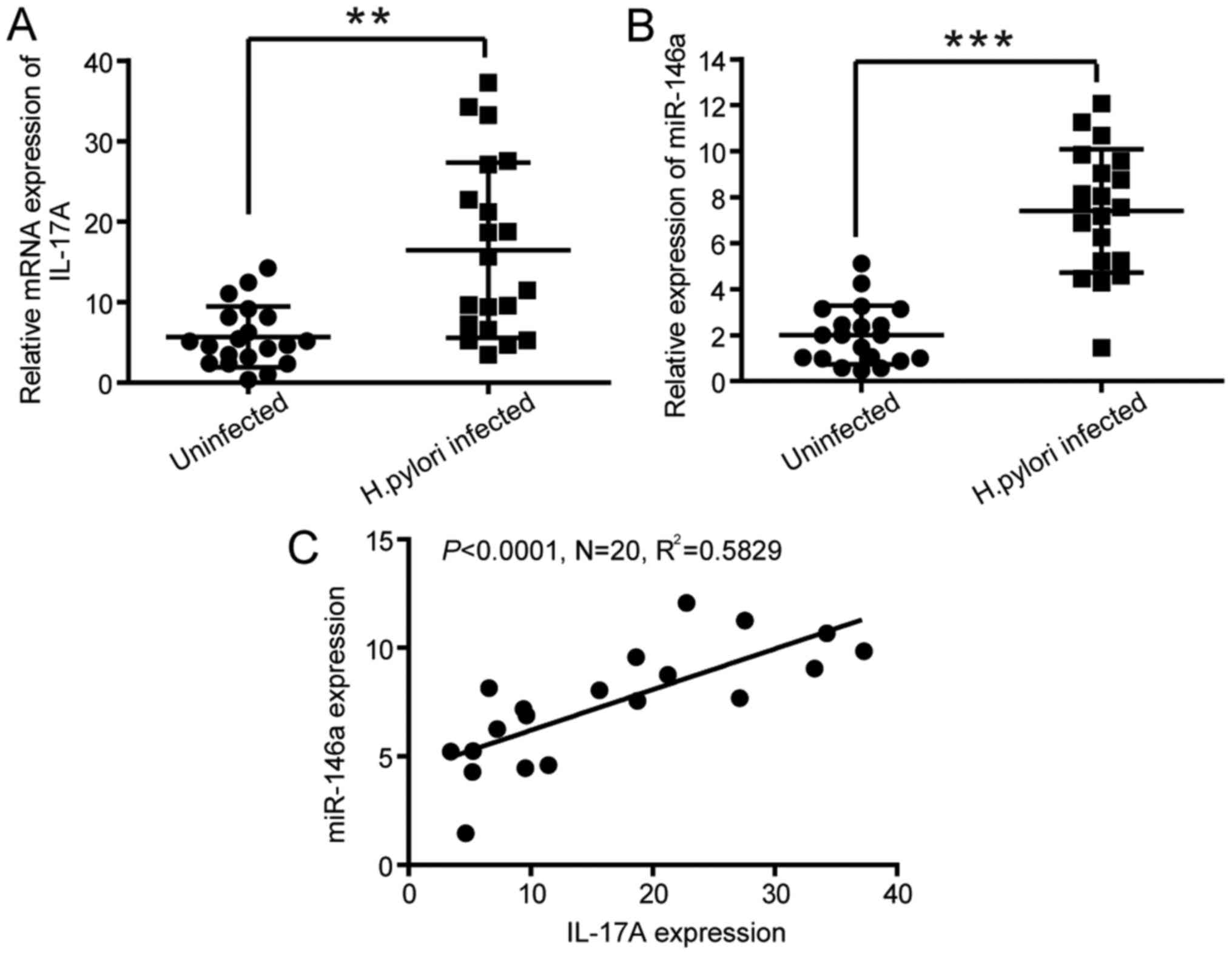
Correlation between the expression of
IL-17A and miR-146a in human gastric mucosa infected with H.
pylori
To determine whether the expression levels of IL-17A
were increased in H. pylori-infected gastric mucosa in
vivo, the mRNA expression of IL-17A in gastric mucosa tissues
from patients with H. pylori infection was determined. The
mRNA levels of miR-146a and IL-17A were evaluated in gastric mucosa
tissues from 20 patients with H. pylori-induced chronic
gastritis and 20 healthy donors. The results of RT-qPCR revealed
that the expression levels of IL-17A and miR-146a (Fig. 5A and B) were upregulated in H.
pylori-infected gastric mucosa tissues compared with the normal
tissues. Furthermore, Pearson's correlation analysis indicated a
positive correlation between the mRNA levels of miR-146a and IL-17A
(R2=0.5829, P<0.0001; Fig. 5C), suggesting that miR-146a is
correlated with the production of IL-17A in H.
pylori-infected human gastric mucosa.
Discussion
In the present study, the results revealed that: i)
H. pylori infection in gastric mucosa stimulated the
production of IL-17A, resulting in the synthesis of GRO-α and IL-8;
ii) IL-17A induced the expression of miR-146a in gastric epithelial
cells; iii) overexpression of miR-146a suppressed IL-17A-induced
inflammatory responses in SGC-7901 cells; iv) miR-146a
downregulated the expression of IRAK1 and TRAF6, which were
involved in IL-17A-mediated inflammation in vitro; and vi)
the expression levels of miR-146a were correlated with the
production of IL-17A in H. pylori-infected human gastric
mucosa. In summary, these findings suggested that IL-17A-induced
miR-146a may function as a negative regulator on inflammation
response in gastric mucosa during the infection of H.
pylori.
The persistent infection of H. pylori may
cause severe diseases including peptic ulcers and gastric carcinoma
(32). Previous studies have
reported that H. pylori infection can stimulate the
production of IL-17A in the stomachs of human and mice (11,33).
In addition, the results of the present study indicated that the
mRNA levels of IL-17A were increased in H. pylori-infected
patients compared with the normal controls. IL-17A may be
responsible for mucosal damage in H. pylori-induced
gastritis (34,35); these findings together with the
present study have demonstrated that IL-17A served a role in the
regulation of GRO-α and IL-8, which are the proinflammatory
cytokines associated H. pylori infection. It has been
reported that IL-17−/− mice exhibited suppressed
inflammatory responses and reduced H. pylori colonization in
gastric mucosa (36), suggesting
that IL-17A serves an important roles in inflammatory responses and
host defense during the infection of H. pylori.
In the last decade, numerous studies focused on the
role of miRNAs in immune responses. A previous study suggested that
miRNAs contributed to the pathogenesis of immune disorders involved
in Th17-mediated responses (37).
A recent study indicated that CD4+ T cells transduced
with miR-301a increased the levels of IL-17A and TNF-α in
inflammatory bowel disease (38).
In addition, miR-146a may regulate the production of IL-17 in the
peripheral blood mononuclear cell and synovium in patients with
rheumatoid arthritis (39). Liu
et al (40) reported that
IL-17 affected the expression of miRNA in brain astrocytes, such as
miR-873, which regulates inflammatory cytokines and chemokines
in vitro and in vivo, affecting the development of
experimental autoimmune encephalomyelitis. The present study
revealed that miR-146a expression was significantly elevated in
gastric epithelial cells treated with IL-17A, and miR-146a
overexpression could downregulate the expression of inflammatory
cytokines, including GRO-α and IL-8. Additionally, miR-146a and
IL-17A were upregulated in human gastric mucosa infected with H.
pylori, suggesting that miR-146a may be an essential regulator
in IL-17A-mediated inflammatory responses during the infection of
H. pylori.
IL-17A can activate the NF-κB signaling pathway
(41,42). In the present study, IL-17A
activated the NF-κB signaling during the infection of H.
pylori. Liu et al (40)
revealed that miR-873 directly targeted A20 and promoted the
activation of NF-κB, consequently stimulating the production of
inflammatory cytokines and chemokines in astrocytes. As NF-κB
activation is critical in response to infection, downregulation of
this signaling is also essential to prevent excess inflammation,
tissue damage and autoimmunity (43). miR-146a is a NF-κB-dependent gene
but also can downregulate several signaling mediators involved in
inflammatory responses, such as TRAF6 and IRAK1, which are located
at the upstream of NF-κB and function in a negative feedback loop
in regulating of NF-κB activity (44,45);
this prevents the constitutive activation of NF-κB during
inflammatory response (46). The
results of the present study demonstrated that miR-146a regulated
IL-17A-induced proinflammatory cytokines by affecting the
expression of IRAK1 and TRAF6 during the infection of H.
pylori. IRAK1 and TRAF6 were identified as the direct targets
of miR-146a, which downregulated the mRNA levels of IRAK1 and TRAF6
upon stimulation with IL-17A. In addition, silencing of IRAK1 or
TRAF6 resulted in suppressed IL-17A-stimulated inflammatory
responses in vitro. These findings indicated that miR-146a
may be involved in IL-17A-induced pathogenesis of H. pylori
infection by regulating IRAK1 and TRAF6.
In summary, miR-146a may have suppressed the
inflammatory responses upon IL-17A stimulation during infection
with H. pylori in an NF-κB-dependent manner. These findings
provide insight into a potential novel regulatory mechanism in the
inflammation associated with H. pylori infection. miR-146a
may function as a inflammatory suppressor gene in H.
pylori-associated gastritis, and may serve as a biomarker or
therapeutic target in gastritis therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. NSFC, 81501721) and
Presidential Foundation of General Hospital of Ji'nan Military
Region (grant no. 2017MS06).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NL conducted the study, collected the data, analyzed
the data and wrote the manuscript. JW, WY, KD and FY collected the
data and contributed to the introduction. BS and BT performed the
experiments and reviewed/edited the manuscript. YZ, TW and BQ
designed the study, contributed to the discussion, and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Institutional Review Board at The Third Military
Medical University, and written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
H. pylori
|
Helicobacter pylori
|
|
IL-17A
|
interleukin-17A
|
|
IL-8
|
interleukin-8
|
|
GRO-α
|
growth-regulated oncogene α
|
|
IRAK1
|
interleukin-1 receptor-associated
kinase 1
|
|
TRAF6
|
tumor necrosis factor
receptor-associated factor 6
|
References
|
1
|
Marshall BJ and Warren JR: Unidentified
curved bacilli in the stomach of patients with gastritis and peptic
ulceration. Lancet. 1:1311–1315. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atherton JC and Blaser MJ: Coadaptation of
Helicobacter pylori and humans: Ancient history, modern
implications. J Clin Invest. 119:2475–2487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Komiyama Y, Nakae S, Matsuki T, Nambu A,
Ishigame H, Kakuta S, Sudo K and Iwakura Y: IL-17 plays an
important role in the development of experimental autoimmune
encephalomyelitis. J Immunol. 177:566–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simonian PL, Roark CL, Wehrmann F, Lanham
AM, Born WK, O'Brien RL and Fontenot AP: IL-17A-expressing T cells
are essential for bacterial clearance in a murine model of
hypersensitivity pneumonitis. J Immunol. 182:6540–6549. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Troncone E, Marafini I, Pallone F and
Monteleone G: Th17 cytokines in inflammatory bowel diseases:
Discerning the good from the bad. Int Rev Immunol. 32:526–533.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakae S, Nambu A, Sudo K and Iwakura Y:
Suppression of immune induction of collagen-induced arthritis in
IL-17-deficient mice. J Immunol. 171:6173–6177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khmaladze I, Kelkka T, Guerard S, Wing K,
Pizzolla A, Saxena A, Lundqvist K, Holmdahl M, Nandakumar KS and
Holmdahl R: Mannan induces ROS-regulated, IL-17A-dependent
psoriasis arthritis-like disease in mice. Proc Natl Acad Sci USA.
111:E3669–E3678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cua DJ and Tato CM: Innate IL-17-producing
cells: The sentinels of the immune system. Nat Rev Immunol.
10:479–489. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung PF, Wong CK and Lam CW: Molecular
mechanisms of cytokine and chemokine release from eosinophils
activated by IL-17A, IL-17F, and IL-23: Implication for Th17
lymphocytes-mediated allergic inflammation. J Immunol.
180:5625–5635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luzza F, Parrello T, Monteleone G, Sebkova
L, Romano M, Zarrilli R, Imeneo M and Pallone F: Up-regulation of
IL-17 is associated with bioactive IL-8 expression in
Helicobacter pylori-infected human gastric mucosa. J
Immunol. 165:5332–5337. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizuno T, Ando T, Nobata K, Tsuzuki T,
Maeda O, Watanabe O, Minami M, Ina K, Kusugami K, Peek RM and Goto
H: Interleukin-17 levels in Helicobacter pylori-infected
gastric mucosa and pathologic sequelae of colonization. World J
Gastroenterol. 11:6305–6311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sebkova L, Pellicanò A, Monteleone G,
Grazioli B, Guarnieri G, Imeneo M, Pallone F and Luzza F:
Extracellular signal-regulated protein kinase mediates interleukin
17 (IL-17)-induced IL-8 secretion in Helicobacter
pylori-infected human gastric epithelial cells. Infect Immun.
72:5019–5026. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du C, Liu C, Kang J, Zhao G, Ye Z, Huang
S, Li Z, Wu Z and Pei G: MicroRNA miR-326 regulates TH-17
differentiation and is associated with the pathogenesis of multiple
sclerosis. Nat Immunol. 10:1252–1259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu LF, Boldin MP, Chaudhry A, Lin LL,
Taganov KD, Hanada T, Yoshimura A, Baltimore D and Rudensky AY:
Function of miR-146a in controlling Treg cell-mediated regulation
of Th1 responses. Cell. 142:914–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stittrich AB, Haftmann C, Sgouroudis E,
Kühl AA, Hegazy AN, Panse I, Riedel R, Flossdorf M, Dong J,
Fuhrmann F, et al: The microRNA miR-182 is induced by IL-2 and
promotes clonal expansion of activated helper T lymphocytes. Nat
Immunol. 11:1057–1062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan EK, Ceribelli A and Satoh M:
MicroRNA-146a in autoimmunity and innate immune responses. Ann
Rheum Dis. 72 Suppl 2:ii90–ii95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao JL, Rao DS, Boldin MP, Taganov KD,
O'Connell RM and Baltimore D: NF-kappaB dysregulation in
microRNA-146a-deficient mice drives the development of myeloid
malignancies. Proc Natl Acad Sci USA. 108:9184–9189. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nahid MA, Satoh M and Chan EK: Interleukin
1β-responsive MicroRNA-146a is critical for the cytokine-induced
tolerance and cross-tolerance to toll-like receptor ligands. J
Innate Immun. 7:428–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li N, Xu X, Xiao B, Zhu ED, Li BS, Liu Z,
Tang B, Zou QM, Liang HP and Mao XH: H. pylori related
proinflammatory cytokines contribute to the induction of miR-146a
in human gastric epithelial cells. Mol Biol Rep. 39:4655–4661.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao B, Zhu ED, Li N, Lu DS, Li W, Li BS,
Zhao YL, Mao XH, Guo G, Yu PW and Zou QM: Increased miR-146a in
gastric cancer directly targets SMAD4 and is involved in modulating
cell proliferation and apoptosis. Oncol Rep. 27:559–566.
2012.PubMed/NCBI
|
|
22
|
Liu Z, Wang D, Hu Y, Zhou G, Zhu C, Yu Q,
Chi Y, Cao Y, Jia C and Zou Q: MicroRNA-146a negatively regulates
PTGS2 expression induced by Helicobacter pylori in human
gastric epithelial cells. J Gastroenterol. 48:86–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nahid MA, Pauley KM, Satoh M and Chan EK:
miR-146a is critical for endotoxin-induced tolerance: IMPLICATION
IN INNATE IMMUNITY. J Biol Chem. 284:34590–34599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim DJ, Park KS, Kim JH, Yang SH, Yoon JY,
Han BG, Kim HS, Lee SJ, Jang JY, Kim KH, et al: Helicobacter
pylori proinflammatory protein up-regulates NF-kappaB as a
cell-translocating Ser/Thr kinase. Proc Natl Acad Sci USA.
107:21418–21423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou J, Wang P, Lin L, Liu X, Ma F, An H,
Wang Z and Cao X: MicroRNA-146a feedback inhibits RIG-I-dependent
Type I IFN production in macrophages by targeting TRAF6, IRAK1, and
IRAK2. J Immunol. 183:2150–2158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang B, Li N, Gu J, Zhuang Y, Li Q, Wang
HG, Fang Y, Yu B, Zhang JY, Xie QH, et al: Compromised autophagy by
MIR30B benefits the intracellular survival of Helicobacter
pylori. Autophagy. 8:1045–1057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimang'a A, Revathi G, Kariuki S, Sayed S,
Devani S, Vivienne M, Kuester D, Mönkemüller K, Malfertheiner P and
Wex T: IL-17A and IL-17F gene expression is strongly induced in the
mucosa of H. pylori-infected subjects from Kenya and Germany. Scand
J Immunol. 72:522–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Xiao B, Tang B, Li B, Li N, Zhu E,
Guo G, Gu J, Zhuang Y, Liu X, et al: Up-regulated microRNA-146a
negatively modulate Helicobacter pylori-induced inflammatory
response in human gastric epithelial cells. Microbes Infect.
12:854–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Liao MY, Gao XL, Zhong Q, Tang TT,
Yu X, Liao YH and Cheng X: IL-17A induces pro-inflammatory
cytokines production in macrophages via MAPKinases, NF-κB and AP-1.
Cell Physiol Biochem. 32:1265–1274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ernst PB and Gold BD: The disease spectrum
of Helicobacter pylori: The immunopathogenesis of
gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol.
54:615–640. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caruso R, Fina D, Paoluzi OA, Del Vecchio
Blanco G, Stolfi C, Rizzo A, Caprioli F, Sarra M, Andrei F, Fantini
MC, et al: IL-23-mediated regulation of IL-17 production in
Helicobacter pylori-infected gastric mucosa. Eur J Immunol.
38:470–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Numasaki M, Watanabe M, Suzuki T,
Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze
MT, Kolls JK and Sasaki H: IL-17 enhances the net angiogenic
activity and in vivo growth of human non-small cell lung cancer in
SCID mice through promoting CXCR-2-dependent angiogenesis. J
Immunol. 175:6177–6189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vykhovanets EV, Maclennan GT, Vykhovanets
OV and Gupta S: IL-17 Expression by macrophages is associated with
proliferative inflammatory atrophy lesions in prostate cancer
patients. Int J Clin Exp Pathol. 4:552–565. 2011.PubMed/NCBI
|
|
36
|
Shiomi S, Toriie A, Imamura S, Konishi H,
Mitsufuji S, Iwakura Y, Yamaoka Y, Ota H, Yamamoto T, Imanishi J
and Kita M: IL-17 is involved in Helicobacter pylori-induced
gastric inflammatory responses in a mouse model. Helicobacter.
13:518–524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krebs CF, Kapffer S, Paust HJ, Schmidt T,
Bennstein SB, Peters A, Stege G, Brix SR, Meyer-Schwesinger C,
Müller RU, et al: MicroRNA-155 drives TH17 immune response and
tissue injury in experimental crescentic GN. J Am Soc Nephrol.
24:1955–1965. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He C, Shi Y, Wu R, Sun M, Fang L, Wu W,
Liu C, Tang M, Li Z, Wang P, et al: miR-301a promotes intestinal
mucosal inflammation through induction of IL-17A and TNF-α in IBD.
Gut. 65:1938–1950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Niimoto T, Nakasa T, Ishikawa M, Okuhara
A, Izumi B, Deie M, Suzuki O, Adachi N and Ochi M: MicroRNA-146a
expresses in interleukin-17 producing T cells in rheumatoid
arthritis patients. BMC Musculoskelet Disord. 11:2092010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu X, He F, Pang R, Zhao D, Qiu W, Shan
K, Zhang J, Lu Y, Li Y and Wang Y: Interleukin-17 (IL-17)-induced
microRNA 873 (miR-873) contributes to the pathogenesis of
experimental autoimmune encephalomyelitis by targeting A20
ubiquitin-editing enzyme. J Biol Chem. 289:28971–28986. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Doreau A, Belot A, Bastid J, Riche B,
Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C,
Trolliet P, et al: Interleukin 17 acts in synergy with B
cell-activating factor to influence B cell biology and the
pathophysiology of systemic lupus erythematosus. Nat Immunol.
10:778–785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shalom-Barak T, Quach J and Lotz M:
Interleukin-17-induced gene expression in articular chondrocytes is
associated with activation of mitogen-activated protein kinases and
NF-kappaB. J Biol Chem. 273:27467–27473. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruland J: Return to homeostasis:
Downregulation of NF-κB responses. Nat Immunol. 12:709–714. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Selvamani SP, Mishra R and Singh SK:
Chikungunya virus exploits miR-146a to regulate NF-κB pathway in
human synovial fibroblasts. PLoS One. 9:e1036242014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He X, Zheng Y, Liu S, Shi S, Liu Y, He Y,
Zhang C and Zhou X: MiR-146a protects small intestine against
ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB
pathway. J Cell Physiol. 233:2476–2488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sundaravinayagam D, Kim HR, Wu T, Kim HH,
Lee HS, Jun S, Cha JH, Kee Y, You HJ and Lee JH: miR146a-mediated
targeting of FANCM during inflammation compromises genome
integrity. Oncotarget. 7:45976–45994. 2016. View Article : Google Scholar : PubMed/NCBI
|