Introduction
More people are now paying serious attention to
personal and non-visible health problems. For many modern women,
the menopausal syndrome seriously affects their quality of life. In
addition, diseases such as breast cancer, endometrial cancer and
osteoporosis, among others, occur frequently; therefore, there is
an urgent need to identify methods that prevent and/or control
their occurrence. In China, traditional Chinese medicine (TCM) is
often used to prevent and treat these diseases. Phytoestrogens
(PEs), which are derived from plant ingredients, can be activated
and combined with human estrogen receptors, as the structures of
PEs are similar to that of endogenous estrogen in mammals and
humans; therefore, using PEs to mimic the role of estrogen may
facilitate the prevention and control of specific diseases. In
addition, the use of estrogen for hormone therapy has extensive
side effects that cannot be ignored, rendering the cost-benefit of
this approach debatable. Therefore, it is imperative to investigate
whether certain TCM compounds that contain PEs may be used as
hormone replacement therapy (1,2), and
to characterize their efficiency and toxicity profiles relative to
those of standard hormone therapy. It is necessary to develop and
fully characterize these alternative derivative drugs, in order to
generate the most effective compositions for use in subsequent
clinical applications (3–6).
The complexity of TCM arises from its multi-system,
multi-target, multi-channel pharmacodynamics and treatment
characteristics. Some previous studies have focused on the effects
of estrogen and its mechanism of action (7,8).
Notably, the actual effect of TCM depends on the correlation
between its chemical constituents and its components with
pharmacological activity (8). In
addition, clarifying the overall efficacy and function of TCM is
central to ensuring drug safety and quality control.
In recent years, the spectrum-effect relationship
model has been used to investigate the correlation between the
chromatographic fingerprint of a TCM drug and its pharmacodynamic
effects. Firstly, chromatogram characteristic absorption peaks are
obtained and recorded via liquid chromatography (LC). Secondly,
these are combined with pharmacological indices to evaluate
efficacy. Finally, mass spectrometry (MS) is used to qualitatively
characterize the ingredients and reveal the active substances. The
relationship between the chemical constituents and pharmacological
activity of a TCM drug is used to assess whether its efficacy and
material basis is consistent with its functional characteristics.
This relationship is then used to identify the active components in
a TCM drug, to formulate control standards and to reflect the
internal quality of the drug. The spectrum-effect relationship has
been applied in various areas, including assessing
pharmaceutical/chemical material basis, identifying active
ingredient combinations, and in technology optimization (9–11).
In addition, it is used for the optimization of novel formulae, the
tracking and separation of target ingredients, and the development
of novel drugs (12–15).
Cuscuta chinensis Lam. is a type of edible
TCM. It was initially recorded in Shen Nong's Herbal, and was
listed as a top-grade herb (16).
From its extract, various chemical constituents have been separated
and identified, including flavonoids, glycosides, trace elements,
amino acids, coumarins, alkaloids, tannic acids and triterpene
acids (17,18). Cuscuta chinensis Lam. is
commonly used to support the normal functions of the liver and
kidneys, strengthen muscles and bones, and exert anti-osteoporotic
and antioxidant activities (19–21).
Based on preliminary experiments, this extract has been confirmed
to possess significant estrogenic activity (16). The chemical spectra of Cuscuta
chinensis Lam. samples obtained from various regions have been
recorded using high-performance LC (HPLC) (22,23).
In the present study, the estrogenic activity of Cuscuta
chinensis Lam. was assessed using a uterus growth test and an
MTT assay. The comprehensive results of bivariate, principal
component and Gray relational analyses indicated several active
compounds of Cuscuta chinensis Lam. that may be further
investigated in future studies. This study provided a theoretical
basis for the material efficacy of Cuscuta chinensis Lam.,
and laid the foundation for its further development and clinical
application.
Materials and methods
Instrumentation, animals and
reagents
Agilent 1290 HPLC system, Agilent 6530 Series
Quadrupole Time-of-Flight (Q-TOF) LC/MS system (Agilent
Technologies, Inc., Santa Clara, CA, USA) and Chemical HPLC-3D
workstations (Agilent Technologies, Inc.) were used for analytical
chemistry and data processing. Milli-Q ultrapure water (Merck KGaA,
Darmstadt, Germany) was used for preparation of samples and
standards. Additional instruments included the electronic
analytical balance AR1140 (Ohaus Corporation, Parsippany, NJ, USA),
680 Microplate Reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and Allegra 64R High-Speed Centrifuge (Beckman Coulter, Inc.,
Brea, CA, USA).
Immature female Kunming mice (~21 days old, weaned,
n=220), weighing 12±2 g, were purchased from Changchun National
Biological Industry Base Laboratory Animal Center [SCXK-(China)
2003–0004; Changchun, China]. The mice were divided into 22 groups
(n=10/group) and housed in a temperature-regulated room (22±2°C)
with humidity 60±10% and unrestricted access to food and water.
Cuscuta chinensis Lam. was purchased from San
Keshu Chinese Herbal Medicine Market (Harbin, China) and was
identified by Prof. Zhang Delian (Harbin University of Commerce,
Harbin, China). The samples of C. chinensis had been
collected from 20 diverse habitats, namely Shuangyashan (site
2012011501; Zongyi Medicine Station, Harbin, China), Qingdao (site
2012011502; Anguoyutai Medicine Station, Harbin, China), Dezhou
(site 2012011503; Jingtai Medicine Station, Harbin, China), Hulin
(site 2012011504; Longfukang Medicine Station, Harbin, China),
Liaoning (site 2012011505; Guangyuan Medicine Station, Harbin,
China), Shanxi (site 2012011506; Beiguo Medicine Station, Harbin,
China), Shanghai (site 2012011507; Wanbei Medicine Station, Harbin,
China), Jiangsu (site 2012011508; Renhetang Medicine Station,
Harbin, China), Anhui (site 2012011509; Chaoyang Medicine Station,
Harbin, China), Zhejiang (site 2012011510; Zhonglian Medicine
Station, Harbin, China), Nanning (site 2012011511; Qiancao Medicine
Station, Harbin, China), Shaanxi (site 2012011512; Dushitang
Medicine Station, Harbin, China), Guangdong (site 2012011513;
Ruixintang Medicine Station, Harbin, China), Beihai (site
2012011514; Hongtai Medicine Station, Harbin, China), Henan (site
2012011515; Yixintang Medicine Station, Harbin, China), Hebei
(2012011516, Xinhe Medicine Station, Harbin, China), Alashan (site
2012011517; Taida Medicine Station, Harbin, China), Neimeng (site
2012011518; Longyuan Medicine Station, Harbin, China), Sichuan
(site 2012011519; Tianran Medicine Station, Harbin, China) and
Ningxia (site 2012011520; Anjiang Medicine Station, Harbin, China).
Diethylstilbestrol was purchased from Hefei Jiulian Pharmaceutical
Co., Ltd. (Hefei, China). RPMI 1640 and RPMI 1640 without phenol
red were purchased from HyClone; GE Healthcare (Logan, UT, USA).
MTT and dimethyl sulfoxide (DMSO) were purchased from
Sigma-Aldrich; Merck KGaA. The MCF7 human breast cancer cell line
was provided by the Research Center of the Life and Environmental
Sciences, Harbin University of Commerce. The standards used were
chlorogenic acid, hyperin, quercitin, kaempferol and quercetin,
which were obtained from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China). The purity
of each standard was ≥98%. Acetonitrile, methanol and formic acid
were of MS-grade and water was ultrapure. Other reagents were of
analytical grade and were commercially available.
Preparation of the sample
solution
A solution of the Cuscuta chinensis Lam. (1.0
g/ml) was prepared in distilled water for oral administration. The
positive control solution consisted of 20 µg/ml diethylstilboestrol
in distilled water. The solution (0.1 g/ml) of Cuscuta
chinensis Lam. was prepared in 80% methanol. Chlorogenic acid,
hyperin, quercitin, kaempferol and quercetin (2.0 mg each) were
dissolved in 10 ml 80% methanol solution. The sample and standard
solutions were filtered using a 0.45-µm filter prior to
analysis.
LC-MS conditions
A Waters Symmetry Shield RP18 (4.6×250 mm, 5 µm;
Waters Corporation, Milford, MA, USA) was used to analyze the
samples. The mobile phase consisted of methanol (A) and 0.2% (v/v)
formic acid aqueous solution (B), and was pumped at a flow rate of
0.5 ml/min. The injection volume of each sample was 10 µl. The
gradient elution program was as follows: Mobile phase A was
initiated at 21% and increased linearly to 29% at 30 min. Solution
A was subsequently increased to 36% (30–50 min), 38% (50–65 min),
44% (65–90 min) and 65% (90–125 min). The mobile phase A was then
decreased to 21% for the last 5 min of the run (125–130 min). The
column temperature was maintained at 30°C. The chromatograms were
monitored at 328 nm. The atomization gas pressure was 0.25 MPa, and
the capillary voltage was 8 kV. The flow rate of the dry gas was
set to 8.0 l/min with the temperature at 30°C. The temperature of
the sheath gas was set at 400°C, and its flow rate was 34.0 l/min.
The collision energy was set to 10–30 eV. The mass spectra were
recorded within the scan range between 100 and 1,700 Da, and were
in negative ion scanning mode.
Fingerprint evaluation
LC fingerprints of the samples collected from 10
different batches of Cuscuta chinensis Lam. were
established, and matched automatically using the Similarity
Evaluation System for Chromatographic Fingerprint of TCM (version
2012; China Pharmacopoeia Committee, Beijing, China). Furthermore,
the reference chromatogram map was generated using the median
method. In addition, cluster analysis was applied to evaluate the
quality of the samples from 20 diverse habitats.
Principal component analysis
(PCA)
PCA is a widely used multivariate statistical
method. In the present study, characteristic peaks from the LC
chromatograms were further screened by PCA, with the aim of
identifying active compounds based on spectrum-effect
relationship.
Uterus growth test
The mice were divided into 22 groups (n=10/group).
The sample solutions that were prepared from Cuscuta
chinensis Lam. were administered twice daily (8 h were between
administrations in the morning and evening) by oral gavage at a
dose of 24 g/kg body weight for 4 days. In our previous study, four
different doses (3, 6, 12 and 24 g/kg) were administered, in order
to evaluate the efficacy of the drug; finally, 24 g/kg was
determined to be the effective dose (24). The blank and positive control
groups comprised equal volumes of distilled water and
diethylstilbestrol solution, respectively, which were also
administered twice daily for 4 days. On day 5, drug-containing
blood samples were obtained from the orbit of the anesthetized
mice. The samples were centrifuged at 3,802 × g for 10 min at 4°C,
and the supernatants (drug-containing serum samples) were
collected. The drug-containing serum samples were inactivated by
heating at 56°C in a water-bath for 30 min, and were filtered
through a 0.22-µm filter prior to the MTT assay. The uteri of the
mice were immediately excised and weighed following
anaesthetization and decapitation, in order to calculate uterine
weight indices. Animal experimentation was initiated following 5
days of acclimation. The mice were fasted overnight with free
access to water prior to administration of the test solutions. The
present study was conducted in strict accordance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (25). All experimental procedures were
reviewed and approved by the Animal Ethical Committee of the Harbin
University of Commerce. All potentially stressful procedures were
performed under sodium pentobarbital anesthesia. Pentobarbital
sodium was prepared to 1% of the concentration, with an i.p.
injection dose of 40 mg/kg. If the anesthetic effect was not
appropriate, 10 mg/kg was supplemented after 20 min.
MTT assay
MCF7 cells were co-cultured in RPMI 1640 without
phenol red containing 5% charcoal-dextran-treated fetal bovine
serum (HyClone; GE Healthcare Life Sciences) for 4 days in an
incubator containing 5% CO2 at 37°C. The cells were
seeded in 96-well plates at a density of 2,000 cells/well. An
adherent culture was achieved and after 72 h of culture, the cells
were co-cultured in RPMI 1640 medium containing 10% serum samples
from Cuscuta chinensis Lam.-treated mice. The experiments
were conducted in 6 replicates. Subsequently, 20 µl MTT solution (5
mg/ml in PBS) was added to each well, and the cells were incubated
for 4 h at 37°C. After the removal of medium, 150 µl DMSO was
added, in order to dissolve the formazan crystals, and the plates
were agitated in the dark for 15 min at room temperature. The
absorbance was measured at 570 nm using a microplate reader. The
proliferation rate was calculated as the average absorbance value
of the drug-containing serum group divided by the average
absorbance value of the blank control group (cells cultured in RPMI
1640 without serum).
Statistical analysis
Paired-sample t-test (two-tailed) and one-way
analysis of variance followed by Tukey's test were used to identify
statistically significant differences among values in the blank
control and experimental groups. P<0.05 was considered to
indicate a statistically significant difference. The results of the
aforementioned analytical methods were consistent.
Pearson's correlation coefficient was used to
calculate the relative peak areas, which corresponded to estrogenic
activity of the samples. Correlation coefficients >0.3 were
considered significant (P<0.05), whereas correlation
coefficients >0.5 were considered highly significantly
(P<0.01).
PCA was used to evaluate characteristic peak areas
in the chromatograms of the samples from 20 diverse habitats.
Bivariate analysis and Grey relational analysis were used to assess
the correlation of peak areas and their estrogenic activity.
Paired-sample t-test (two-tailed), ANOVA followed by Tukey's test
and bivariate analysis were conducted using SPSS software (SPSS for
Windows 21.0; IBM Corporation, Armonk, NY, USA). Grey relational
analysis was calculated using DPS version 8.0 (Fiberhome
Telecommunication Technologies Co., Ltd., Wuhan, China). P<0.05
was considered to indicate a statistically significant
difference.
Results and Discussion
Cluster analysis and similarity
assessment
The relative standard deviation of the retention
time and peak area of characteristic peaks were 0.77 and 0.62% for
precision, 0.52 and 0.14% for reproducibility, and 0.95 and 0.85%
for stability, respectively. These results indicated that the
established LC fingerprinting method was feasible. The similarities
of the chromatograms from 10 different batches of Cuscuta
chinensis Lam. from the same habitat were all >0.9. Perhaps
be due to the limitation of the instrument hardware, or since the
content cannot reach the detection limit, the chromatographic peaks
for all samples may not fully represent the characteristic
constituents of Cuscuta chinensis Lam. as peaks could
possibly been lost. Therefore, the sample matching number of peaks
analyzed was adjusted from 26 to 22, in order to obtain a more
accurate chromatogram map. A total of 26 peaks were obtained, and
the sum of their areas was >0.8 compared with those of the
overall peak area in the sample (the sum of the areas is the sum of
26 characteristic peak areas. The overall peak area is the area of
all peaks in the chromatogram). Therefore, it was concluded that
these chromatographic peaks could effectively reflect the main
active constituents of Cuscuta chinensis Lam. The results of
cluster analysis are shown in Fig.
1. A total of 20 diverse habitats were divided into three
categories as follows: A: S12, Shaanxi; S13, Guangdong; S6, Shanxi;
S9, Anhui; S7, Shanghai; S19, Sichuan; S11, Nanning; S1,
Shuangyashan; S2, Qingdao; and S5, Liaoning; B: S18, Neimeng; S4,
Hulin; S20, Ningxia; S3, Dezhou; S14, Beihai; S17, Alashan; S10,
Zhejiang; S16, Hebei; and S8, Jiangsu; and C: S15, Henan.
Identification of chemical
constituents
Active ingredients were orally administered in the
form of mixtures rather than monomers, because TCM exerts its
pharmacological effects based on the synergy between components.
Conversely, individually separated single compounds may not exert
therapeutic effects (7,8). Previous studies have been conducted
to identify the chemical compounds of Cuscuta chinensis Lam.
(22,23). The correlation between inherent
constituents and their activities indicated that they could be
identified based on the combination of LC with the corresponding
indices of pharmacological activity. Q-TOF-MS is widely used for
constituent identification of TCM, since it can provide the exact
molecular mass. By analyzing the primary and secondary MS data, 26
compounds were identified. The present study aimed to reveal
compounds that have estrogenic activity among the known components
of Cuscuta chinensis Lam.; therefore, nuclear magnetic
resonance spectroscopy was not required to validate their identity.
Based on the results of MS, mixtures of active ingredients were
prepared and their estrogenic activity was validated, in order to
determine the bioactive components with estrogenic activity of
Cuscuta chinensis Lam. In addition, PCA was used to assess
the size of contribution for these compounds with regards to their
estrogenic activity. A contribution of the activity is indicated by
an eigen value >1 and a cumulative contribution rate of
variables >0.8. The higher the absolute value of the eigen load,
the higher the influence of the compound on estrogenic indices.
The chromatograms of Cuscuta chinensis Lam.
and the standard samples were compared in order to identify
individual compounds under the same LC-MS conditions. As shown in
Table I, 26 constituents were
identified. Peak 1 indicated a predominant deprotonated ion with a
m/z ratio of 695
(C30H32O19), which was identical
to the elemental composition of
quercetin-3-O-(2‘-O-α-rhamnosy-6’-O-malony)-β-D-glucoside. This was
supported by the loss of a glucose moiety that yielded a fragment
ion with a m/z ratio of 533, further supporting the initial
prediction. Peak 2 indicated a predominant deprotonated ion with a
m/z ratio of 725
(C32H38O19), which was identical
to the elemental composition of
kaempferol-3-O-β-D-aplosyl-(1→2)-[-α-L-rhamnosy-(1→6)]-β-D-glucoside,
and was supported by the loss of a glucose moiety and a rhamnopyl
moiety, forming a fragment ion with a m/z ratio of 359. Peak
5 demonstrated a predominant deprotonated ion with a m/z
ratio of 487 (C22H16O13), which is
identical to the elemental composition of
6-O-(E)-P-coumaroyl)-β-D-fructofuranosyl-(2→1)-α-D-glucopyranoside.
The loss of a coumaryl moiety formed a fragment ion with a
m/z ratio of 341, and was followed by a subsequent loss of
an additional OH moiety to form a fragment ion with a m/z
ratio of 325, which was in agreement with the aforementioned data.
Peak 11 indicated a predominant deprotonated ion with a m/z
ratio of 499 (C22H28O13), which
was identical to the elemental composition of
4-caffeoyl-5-coumaroylquinic acid. The loss of a COOH moiety formed
a fragment ion with a m/z ratio of 453. Peak 25 indicated a
predominant deprotonated ion with a ratio m/z of 411
(C29H48O), which was identical to the
elemental composition of stigmasterol. The loss of a CH3
moiety, forming a fragment ion with a ratio m/z of 397,
further supported the inference. The retention time and MS/MS data,
as well as the identification of 26 compounds are listed in
Table I. The structures of 22
compounds are shown in Fig. 2.
 | Table I.Identification of the chemical
composition of the peaks. |
Table I.
Identification of the chemical
composition of the peaks.
| Author, year | Peak |
tR (min)a | Negative mode (m/z)
[M-H]-(m/z)b
MS2 (m/z)c | Formula | Predicted
compound | (Refs.) |
|---|
|
| 1 | 10.76 | 695.2134/533.1871
[M-H-Glc]− |
C30H32O19 |
Quercetin-3-O-(2‘-O-α-rhamnosy-6’-O-malony)-β-D-glucoside |
|
|
| 2 | 13.46 | 725.2170/359.0983
[M-H-C14H22O11]− |
C32H38O19 |
Kaempferol-3-O-β-D-aplosyl-(1→2)-[-α-L-rhamnosy-(1→6)]-β-D-glucoside |
|
|
| 5 | 29.78 | 487.1678/341.0885
[M-H-C6H10O4]− |
C22H16O13 |
6-O-(E)-P-coumaroyl)-β-D-fructofuranosyl-(2→1)-α-D-glucopyranoside |
|
|
| 6 | 32.53 | 431.1977/325.0935
[M-H-C4H10O3]− |
C21H20O10 |
Kaempferol-7-rhamnosy |
|
|
| 9 | 41.06 | 461.1102/182.9910
[M-H-C15O6]− |
C21H18O12 |
Kaempferol-3-β-D-glucuronide |
|
|
| 10 | 43.66 | 269.1399/137.0266
[M-H-C8H4O2]− |
C15H10O5 | Apigenin |
|
|
| 11 | 47.17 | 499.1805/452.9227
[M-H-COOH]− |
C22H28O13 |
4-caffeoyl-5-coumaroylquinic acid |
|
|
| 12 | 52.81 | 417.1203/195.0520
[M-H-C11H10O5]− |
C20H18O10 |
Kaempferol-3-arabofuranoside |
|
|
| 13 | 57.84 | 595.1400/149.0461
[M-H-C21H18O11]− |
C26H28O16 |
Quercetin-3-O-β-D-apiofuranosyl-(1→2)-β-D-galactoside |
|
|
| 14 | 65.46 | 515.1417/211.0466
[M-H-C18H8O5]− |
C25H24O12 | Dicaffeoylquinic
acid |
|
|
| 15 | 68.37 | 463.0982/353.1245
[M-H-C5H2O3]− |
C21H20O12 | Hyperin |
|
|
| 16 | 86.82 | 447.1073/895.1957
[2M-H]− |
C21H20O11 | Quercitin |
|
|
| 17 | 88.44 | 477.1070/353.0888
[M-H-C5O4]− |
C21H18O13 | Querciturone |
|
|
| 18 | 95.97 | 316.9482/631.1687
[2M-H]− |
C16H12O7 | Isorhamnetin |
|
|
| 19 | 99.79 | 661.1808/353.0884
[M-H-C18H12O5]− |
C34H30O14 |
Coumaroyl-dicaffeoylquinic acid |
|
|
| 20 | 101.16 | 353.0896/191.0560
[M-H-Glc]− |
C16H18O9 | Chlorogenic
acid |
|
|
| 21 | 104.67 | 609.1262/384.9358
[M-H-C12H16O4]− |
C27H30O16 | Kaempferol-3,
7-O-diglucuronide |
|
| Yang et al
(2011) | 22 | 106.65 | 301.0361 |
C15H10O7 | Quercetin | (19) |
| Liao et al
(2014) |
|
|
|
|
| (20) |
|
| 23 | 107.87 | 599.2110/447.2425
[M-H-C6H4O4]− |
C28H24O15 |
Quercltrin-2″-gallate |
|
|
| 24 | 110.77 | 593.1337/301.0356
[M-H-2rha]− |
C27H30O15 | Quercetin-3,
7-α-L-dirhamnoside |
|
|
| 25 | 117.17 | 411.2005/397.2047
[M-H-CH2]− |
C29H48O | Stigmasterol |
|
|
| 26 | 119.15 | 285.0642/571.0921
[2M-H]− |
C15H10O6 | Kaempferol |
|
PCA
As shown in Table
II, the eigen values of the first 19 principal components were
>1, and the cumulative contribution rate of the first six
principal components was 85.447%. Therefore, the first six
principal components were evaluated further. Table II indicates their eigen values and
factor loadings. The first principal component exhibited higher
load based on the eigen values of the eight peaks (>0.8). These
peaks were the following: 2, 5, 8, 9, 10, 11, 13 and 20. The second
principal component revealed only peak 22 had a load value >0.8.
Consequently, kaempferol-3-O-β-D-aplosyl-(1→2)-
[-α-L-rhamnosy-(1→6)]-β- D-glucoside, 6-O-(E)-P- coumaroyl)
-β-D-fructofuranosyl-(2→1)- α-D-glucopyranoside,
kaempfe-rol-3-β-D-glucuronide, apigenin,
4-caffeoyl-5-coumaroylquinic acid,
quercetin-3-O-β-D-apiofuranosyl-(1→2)- β-D-galactoside, chlorogenic
acid, quercetin, and one unknown compound, corresponding to a total
of nine peaks were selected as representative variables, and their
estrogenic activity was analyzed (Fig.
3).
 | Table II.Eigen values and corresponding
percentages of the variables (top 19 principal components). |
Table II.
Eigen values and corresponding
percentages of the variables (top 19 principal components).
|
| Initial eigen
values | Extraction sums of
squared loadings |
|---|
|
|
|
|
|---|
| Component | Total | Variance (%) | Cumulative (%) | Total | Variance (%) | Cumulative (%) |
|---|
| 1 | 10.935 | 39.053 | 39.053 | 10.935 | 39.053 | 39.053 |
| 2 | 5.546 | 19.806 | 58.859 | 5.546 | 19.806 | 58.859 |
| 3 | 2.971 | 10.612 | 69.471 | 2.971 | 10.612 | 69.471 |
| 4 | 1.833 | 6.545 | 76.016 | 1.833 | 6.545 | 76.016 |
| 5 | 1.476 | 5.271 | 81.287 | 1.476 | 5.271 | 81.287 |
| 6 | 1.165 | 4.159 | 85.447 | 1.165 | 4.159 | 85.447 |
| 7 | 0.896 | 3.199 | 88.646 |
|
|
|
| 8 | 0.815 | 2.911 | 91.557 |
|
|
|
| 9 | 0.694 | 2.479 | 94.036 |
|
|
|
| 10 | 0.422 | 1.506 | 95.541 |
|
|
|
| 11 | 0.338 | 1.207 | 96.748 |
|
|
|
| 12 | 0.285 | 1.019 | 97.768 |
|
|
|
| 13 | 0.231 | 0.825 | 98.593 |
|
|
|
| 14 | 0.166 | 0.592 | 99.185 |
|
|
|
| 15 | 0.106 | 0.380 | 99.565 |
|
|
|
| 16 | 0.073 | 0.262 | 99.827 |
|
|
|
| 17 | 0.027 | 0.096 | 99.923 |
|
|
|
| 18 | 0.017 | 0.060 | 99.984 |
|
|
|
| 19 | 0.005 | 0.016 | 100.000 |
|
|
|
Determination of estrogenic
activity
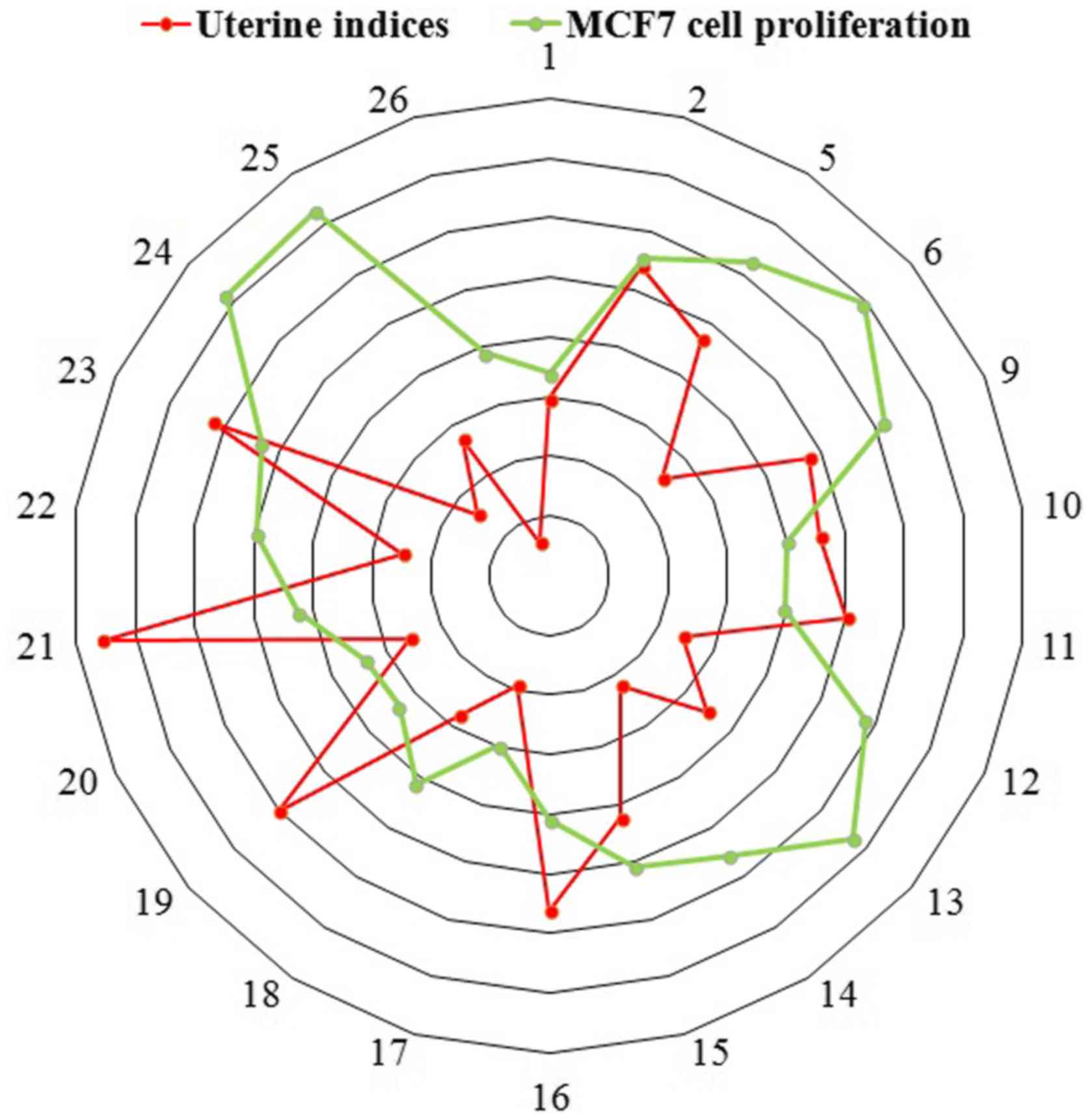
The ratio of uterine wet weight to body weight of
each mouse was calculated (Fig.
4). Animals exposed to Cuscuta chinensis Lam. from 15
habitats exhibited significant estrogenic activities by increasing
uterine weight compared with mice in the blank control group. In
addition the proliferation rate of MCF7 cells treated with serum
from Cuscuta chinensis Lam.-treated mice was determined.
Cells treated with sera from mice that were administered Cuscuta
chinensis Lam. from 15 habitats exhibited significantly
increased growth compared with cells devoid of treatment (Fig. 4).
Bivariate analysis
The present study revealed that the uterine weights
of immature female Kunming mice were increased and the
proliferation of MCF7 human breast cancer cells was promoted by
some of the Cuscuta chinensis Lam. components. These
findings confirmed the estrogenic activity of Cuscuta
chinensis Lam. The uterine indices and MCF7 cell proliferation
rates of these constituents are shown in Fig. 5. Pearson's correlation coefficient
was used to calculate the relative peak areas, which corresponded
to estrogenic activity of the samples. Correlation coefficients
>0.3 were considered significant (P<0.05), whereas
correlation coefficients >0.5 were considered highly
significantly (P<0.01). Based on these results, Cuscuta
chinensis Lam. was revealed to possess estrogenic activity. A
total of nine compounds were significantly correlated with the
increase in the uterine weight (P<0.05), whereas 11 compounds
were significantly correlated with MCF7 cellular proliferation
(P<0.05). The compounds that were significantly correlated with
uterine weight and cell proliferation were
quercetin-3-O-(2‘-O-α-rhamnosy-6’-O-malony)-β-D-glucoside,
kaempferol-3-O-β-D-aplosyl-(1→2)-[-α-L-rhamnosy-(1→6)]-β-D-glucoside,
6-O-(E)-P-coumaroyl)-
β-D-fructofuranosyl-(2→1)-α-D-glucopyranoside, kaempferol-
7-rhamnosy, kaempferol-3-β-D-glucuronide, apigenin,
kaempferol-3-arabofuranoside, dicaffeoylquinic acid, hyperin,
quercitin, isorhamnetin, quercetin, quercltrin-2″-gallate,
quercetin-3, 7-α-L-dirhamnoside and stigmasterol (Fig. 5). These 15 compounds exerted
estrogenic activities.
Gray relational analysis method
The relative correlation of the characteristic peaks
of the 26 compounds with uterine indices is shown in Fig. 6, and the relative correlation of
characteristic peaks of the 26 compounds with cell proliferation
rates are shown in Fig. 7. The
five compounds that exhibited the highest correlation with uterine
indices were quercitin, apigenin, stigmasterol,
4-caffeoyl-5-coumaroylquinic acid and
kaempferol-3-O-β-D-aplosyl-(1→2)-[-α-L-rhamnosy-(1→6)]-
β-D-glucoside (Fig. 6, in
descending order); whereas the five compounds that exhibited the
highest correlation with MCF7 cell proliferation were quercitin,
stigmasterol, kaempferol-3-arabofuranoside,
kaempferol-3-O-β-D-aplosyl-(1→2)-[-α-L-rhamnosy-(1→6)]-β-D-glucoside
and kaempferol-7-rhamnosy (Fig. 7,
in descending order). The relative correlations of quercitin,
apigenin, stigmasterol, 4-caffeoyl-5-coumaroylquinic acid,
kaempferol-3-O-β-D-aplosyl-(1→2)-[-α-L-rhamnosy-(1→6)]-β-D-glucoside,
kaempferol-3-arabofuranoside, and kaempferol-7-rhamnosy to uterine
weight and cell proliferation were >0.5.
Conclusions
LC-Q-TOF-MS technology was used to identify
estrogenic active constituents of Cuscuta chinensis Lam. The
present study illustrated that quercetin-3-O-(2‘-O-α-rhamnosy-
6’-O-malony)-β-D-glucoside,
kaempferol-3-O-β-D-aplosyl-(1→2)-[-α-L-rhamnosy-(1→6)]-β-D-glucoside,6-O-(E)-P-coumaroyl)-β-D-fructofuranosyl-(2→1)-
α-D-glucopyranoside, kaempferol-7-rhamnosy,
kaempferol-3-β-D-glucuronide, apigenin,
4-caffeoyl-5-coumaroylquinic acid, kaempferol-3-arabofuranoside,
quercetin-3-O-β-D-apiofuranosyl-(1→2)-β-D-galactoside,
dicaffeoylquinic acid, hyperin, quercitin, isorhamnetin,
chlorogenic acid, quercetin, quercltrin-2′-gallate, quercetin-3,
7-α-L-dirhamnoside, stigmasterol, and one unknown compound, were
the main active constituents of Cuscuta chinensis Lam., as
analyzed by gray relational analysis and bivariate analysis.
However, these findings require further studies in order to explore
the in vivo effects and mechanism of action of the
constituents of this extract.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81073015), the
Nature Scientific Foundation of Heilongjiang Province (grant no.
ZD2017014) and the Young Innovative Talent Training Plan of the
College in the Heilongjiang Province (grant no.
UNPYSCT-2017209).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DX was responsible for sorting and processing data
and writing the manuscript. LW carried out the overall design of
the experiment. HY established the fingerprints. SH determined
estrogenic activity. SM analyzed the correlation between chemical
components and estrogenic activity. JB identified the active
components. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were reviewed and
approved by the Animal Ethical Committee of the Harbin University
of Commerce.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TCM
|
traditional Chinese medicine
|
|
HPLC/Q- TOF-MS
|
high-performance liquid
chromatography/quadrupole time-of-flight mass spectrometry
|
|
BA
|
bivariate analysis
|
|
PCA
|
principal component analysis
|
|
DMSO
|
dimethyl sulfoxide
|
References
|
1
|
An BH, Jeong H, Zhou W, Liu X, Kim S, Jang
CY, Kim HS, Sohn J, Park HJ, Sung NH, et al: Evaluation of the
biological activity of opuntia ficus indica as a tissue- and
estrogen receptor subtype-selective modulator. Phytother Res.
30:971–980. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
van de Schans MG, Vincken JP, de Waard P,
Hamers AR, Bovee TF and Gruppen H: Glyceollins and
dehydroglyceollins isolated from soybean act as SERMs and ER
subtype-selective phytoestrogens. J Steroid Biochem Mol Biol.
156:53–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Xia Q, Liu X, Liu W, Huang W, Mei
X, Luo J, Shan M, Lin R, Zou D and Ma Z: Phytochemistry,
pharmacology, quality control and future research of Forsythia
suspensa (Thunb.) Vahl: A review. J Ethnopharmacol. 210:318–339.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shu Y, Liu Z, Zhao S, Song Z, He D, Wang
M, Zeng H, Lu C, Lu A and Liu Y: Integrated and global
pseudotargeted metabolomics strategy applied to screening for
quality control markers of Citrus TCMs. Anal Bioanal Chem.
409:4849–4865. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang B, Wang Y, Shan L, Zou J, Wu Y, Yang
F and Zhang Y, Li Y and Zhang Y: A novel and practical
chromatographic ‘Fingerprint-ROC-SVM’ strategy applied to quality
analysis of traditional chinese medicine injections: Using KuDieZi
injection as a case study. Molecules. 22:E12372017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuo L, Peng J, Zhao Y, Li D, Xie X, Tong
L and Yu Z: Screening bioactive quality control markers of
QiShenYiQi dripping pills based on the relationship between the
ultra-high performance liquid chromatography fingerprint and
vascular protective activity. J Sep Sci. 40:4076–4084. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spagnuolo P, Rasini E, Luini A, Legnaro M,
Luzzani M, Casareto E, Carreri M, Paracchini S, Marino F and
Cosentino M: Isoflavone content and estrogenic activity of
different batches of red clover (Trifolium pratense L.) extracts:
An in vitro study in MCF-7 cells. Fitoterapia. 94:62–69. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang DD, Liang J, Yang WZ, Hou JJ, Yang M,
Da J, Wang Y, Jiang BH, Liu X, Wu WY and Guo DA:
HPLC/qTOF-MS-oriented characteristic components data set and
chemometric analysis for the holistic quality control of complex
TCM preparations: Niuhuang Shangqing pill as an example. J Pharm
Biomed Anal. 89:130–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hernaez R and Thrift AP: High negative
predictive value, low prevalence, and spectrum effect: Caution in
the interpretation. Clin Gastroenterol Hepatol. 15:1355–1358. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Sun X, Liu B, Zhang L, Fan Z and Ji
Y: Screening and identification of hepatotoxic component in Evodia
rutaecarpa based on spectrum-effect relationship and UPLC-Q-TOFMS.
Biomed Chromatogr. 30:1975–1983. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng Q, Zhao Y, Wang J, Liu T, Zhang B,
Gong M, Li J, Liu H, Han B, Zhang Y, et al: Spectrum-effect
relationships between UPLC fingerprints and bioactivities of crude
secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its
three processed products on mitochondrial growth coupled with
canonical correlation analysis. J Ethnopharmacol. 153:615–623.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi Z, Liu Z, Liu C, Wu M, Su H, Ma X,
Zang Y, Wang J, Zhao Y and Xiao X: Spectrum-effect relationships
between chemical fingerprints and antibacterial effects of
lonicerae japonicae flos and lonicerae flos base on UPLC and
microcalorimetry. Front Pharmacol. 7:122016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Yu H, Wu H, Pan Y, Wang K, Liu L,
Jin Y and Zhang C: Tracing novel hemostatic compounds from heating
products of total flavonoids in Flos Sophorae byspectrum-effect
relationships and column chromatography. J Sep Sci. 38:1691–1699.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Wang XL, Wu L, Li H, Qin KM, Cai H,
Pei K, Liu T and Cai BC: Investigation on the spectrum-effect
relationships of Da-Huang-Fu-Zi-Tang in rats by UHPLC-ESI-Q-TOF-MS
method. J Ethnopharmacol. 154:606–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie RF, Zhou X, Shi ZN, Li YM and Li ZC:
Study on spectrum-effect relationship of rhizoma Rhei, cortex
Magnoliae Officinalis, fructus Aurantii immaturus and their
formula. J Chromatogr Sci. 51:524–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding JX, Li WL, Bai J and Ji YB: Studies
on spectrum-effect correlation of mimic estrogenic activity of
Cuscuta chinensis. Chin Pharm J. 337–340. 2013.(In Chinese).
|
|
17
|
Sun SL, Guo L, Ren YC, Wang B, Li RH, Qi
YS, Yu H, Chang ND, Li MH and Peng HS: Anti-apoptosis effect of
polysaccharide isolated from the seeds of Cuscuta chinensis Lam on
cardiomyocytes in aging rats. Mol Biol Rep. 41:6117–6124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang J, Li X, Geng J, Han L, Tang J, Jin Y
and Zhang Y: Determination of hyperin in seed of Cuscuta chinensis
Lam. by enhanced chemiluminescence of CdTe quantum dots on
calcein/K3Fe(CN)(6) system. Food Chem. 134:2383–2388. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang L, Chen Q, Wang F and Zhang G:
Antiosteoporotic compounds from seeds of Cuscuta chinensis. J
Ethnopharmacol. 135:553–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao JC, Chang WT, Lee MS, Chiu YJ, Chao
WK, Lin YC, Lin MK and Peng WH: Antinociceptive and
anti-inflammatory activities of Cuscuta chinensis seeds in mice. Am
J Chin Med. 42:223–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S, Xu X, Xu H, Xu S, Lin Q, Jia Z,
Han T, Zhang H, Zhang Y, Liu H, et al: Purification,
characterization and biological effect of reversing the kidney-yang
deficiency of polysaccharides from semen cuscutae. Carbohydr Polym.
175:249–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou D, Wang J, Zhang B, Xie S, Wang Q, Xu
K and Lin R: Analysis of chemical constituents in Wuzi-Yanzong-Wan
by UPLC-ESI-LTQ-Orbitrap-MS. Molecules. 20:21373–21404. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen ML, Miao L, Cao J, Ip SP and Che CT:
Quantitative analysis of biologically active ingredients of five
seeds combo by liquid chromatography-quadrupole time-of-flight mass
spectrometry for quality control of commercial herbal product. J
Sep Sci. 35:1612–1618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding JX: Correlation of researches on the
fingerprints and estrogenic activity of Cuscuta chinensis. Master's
degree thesis of Harbin University of Commerce. 18–19. 2013.(In
Chinese).
|
|
25
|
Chatkupt TT, Libal NL, Mader SL, Murphy SJ
and Saunders KE: Effect of continuous trio breeding compared with
continuous pair breeding in ‘Shoebox’ caging on measures of
reproductive performance in Estrogen receptor knockout mice. J Am
Assoc Lab Anim Sci. 2018. View Article : Google Scholar :
|