Introduction
Retinal neovascularization (RNV) may be a result of
numerous types of proliferative ischemic retinopathy, including
retinopathy of prematurity (ROP), proliferative diabetic
retinopathy (DR) and retinal vein occlusion (RVO). Although
anti-vascular endothelial growth factor (VEGF) drugs, including
ranibizumab and aflibercept, have exhibited potential as a
treatment for RNV, an improved understanding of the mechanism
underlying RNV is required in order to develop novel strategies for
the prevention of neovascular retinal diseases.
Oxidative stress may be both a cause and a
consequence of numerous types of vascular complications (1). The mammalian retina is an organ with
high metabolic demand and reactive oxygen species (ROS) are
generated through increased consumption of oxygen (2). NADPH oxidase (NOX) appears to be the
only enzymatic source of ROS in the retina that is clearly involved
in pathological neovascularization (3). Excessive levels of ROS cause
endothelial dysfunction, which results in the loss of vascular
cells and ischemia, and in turn triggers blood vessel growth
(4).
VEGF is a factor in the direct stimulation of
endothelial cell proliferation and tube formation in
ischemia-induced RNV (5).
Accumulating evidence supports the idea that ROS respond to VEGF
and hypoxia-inducible factor-1α (HIF-1α) (6,7).
Furthermore, NOX appears to be an important effector in redox
signaling for the activation and signaling of HIF-1α and VEGF
(8).
Oxidative stress induces inflammation. Increased
levels of ROS influence various physiological and pathological
processes, including inflammation. An important mechanism of
inflammation is the induction of inflammasomes, which activate
caspase-1 and process inflammatory cytokines (9). This may result in pyroptosis, a form
of inflammatory programmed cell death induced by inflammatory
caspases (10). Pyroptosis
releases the cytoplasmic contents from dying host cells, thereby
providing potent signals to initiate an inflammatory cascade
(11).
Increased generation of ROS in cells may induce the
process of autophagy through transcriptional and
post-transcriptional regulation (12). In general, ROS may be considered to
be inducers of autophagy (13).
Abnormal inflammation disrupts cellular homeostasis (14). Exposure to a highly inflammatory
environment causes severe damage to tissues. Damaged cellular
components may be digested by autophagy, a process of ‘self-eating’
that is induced by oxidative stress and hypoxia in order to
maintain homeostasis (15).
In the present study, the role of oxidative stress,
autophagy and pyroptosis in retinal angiogenesis was investigated
in a mouse model of OIR. The results suggested that oxidative
stress is potentially implicated in the development of retinal
vasculature through upregulation of pyroptosis and downregulation
of autophagy.
Materials and methods
Animals and oxygen-induced retinopathy
(OIR) model (16)
Pregnant female C57BL/6 mice at 8–10 weeks of age
and 20–25 g post-mating were purchased from Jackson Laboratory (Bar
Harbor, ME, USA). They were randomly divided into two groups: A
normoxia group [wild-type (WT) group; n=32] and an oxygen-exposed
group (ROP group; n=30). Postnatal day 7 (P7) mice and their
mothers were placed in conditions of hyperemia (75±5% oxygen) for 5
days, and were subsequently removed and placed in air (21% oxygen)
for an additional 5 days. The incubator temperature was maintained
at 21±2°C with 45–60% humidity, on a 12/12 h day/night cycle with
food and water ad libitum. Oxygen levels were checked using
a CY-12C portable oxygen measuring instrument (AIPU Instruments,
Ltd., Hangzhou, China). The mice in the normoxia group were
maintained in normal room air from birth until P17. Following
fundus photography, the mice were sacrificed by an overdose of
pentobarbital (100 mg/kg; intra-peritoneal injection;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The eyes were
removed and prepared for further histological and molecular
analysis. All investigations were approved by the Animal Care and
Institutional Ethics Committee of Dalian Medical University (no.
LCKY2016-31) and conformed to the US National Institutes of Health
(Bethesda, MD, USA) Guide for the Care and Use of Laboratory
Animals and the ARRIVE guidelines (17).
Fundus photography
Mydriatic eye drops (5% tropicamide) were given to
dilate the pupil in order to obtain a better view of the ocular
fundus of wild-type and OIR mice on P17, following general
anesthesia. Once the pupil was dilated, a fundus camera (OPTO-RIS;
Optoprobe Science, Ltd., Richmond, BC, Canada) was used to view the
inner surfaces of the eyes in a dark room. The appearance of the
fundus was documented.
Hematoxylin and eosin (H&E)
staining
The eyes were fixed with 40 g/l paraformaldehyde in
PBS overnight at 4°C and subsequently embedded in paraffin.
Sections (5-µm-thick) of the whole eye were stained with H&E at
room temperature, according to a standard method. The nuclei of new
vessels extending from the retina to the vitreous were counted in
six sections at magnification ×400 in each group using an Olympus
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Acquired images were processed using Image J version 1.48 (National
Institutes of Health).
Dihydroethidium (DHE) staining
The ROS levels were measured in situ with the
fluorescent probe DHE. Fresh frozen eye sections (−26°C; 6-µm
thickness) were incubated with DHE (1 µM in PBS) for 30 min at
37°C. Digital images were taken of 10 random fields from each
sample using an Olympus fluorescence microscope at magnification
×400 (Olympus Corporation), and the positive areas were analyzed
using Image J analysis software.
Western blotting
Pups from all groups were sacrificed on P17. Retinas
from three mice mixed for one sample, total proteins were extracted
using radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China). Equal amounts of protein (40 µg) by
bicinchoninic acid protein assay were loaded onto 12% SDS-PAGE
gels. Following electrophoresis and wet transfer to a
polyvinylidene difluoride (PVDF) membrane, the membrane was blocked
in 5% skim milk in TBS with Tween-20 (TBST) at room temperature for
1 h. Immunostaining was conducted using antibodies against VEGF-A
(cat. no. ab52917; 1:500), caspase-1 (cat. no. ab1872; 1:1,000),
pro-caspase-1 (cat. no. ab179515; 1:1,000), interleukin (IL)-1β
(cat. no. ab2105; 1:500), pro-IL-1β (cat. no. ab2105; 1:500),
microtubule associated protein 1 light chain 3α (LC3; cat. no.
ab48394; 1:500) (Abcam, Cambridge, MA, USA), HIF-1α (cat. no.
36169; 1:1,000), autophagy protein (Atg)5 (cat. no. 12994;
1:1,000), Atg7 (cat. no. 8558; 1:1,000), Atg12 (cat. no. 4180;
1:1,000), Beclin1 (cat. no. 3495; 1:1,000), p62 (cat. no. 23214;
1:1,000), NOD-like receptor family pyrin domain-containing 3
(NLRP3; cat. no. 15101; 1:1,000) and GAPDH (cat. no. 5174; 1:1,000;
Cell Signaling Technology, Inc, Danvers, MA, USA) at 4°C overnight.
The blots were subsequently incubated with horseradish
peroxidase-conjugated secondary antibody (cat. no. A0208; 1:1,000;
Beyotime Institute of Biotechnology) at room temperature for 2 h.
All blots were developed using a chemiluminescence system (Fluor
Chem M System; ProteinSimple, San Jose, CA, USA) and signal
intensities were analyzed with a Gel-pro 4.5 Analyzer (Media
Cybernetics, Inc., Rockville, MD, USA). After measuring the
intensity of each band by densitometry using the image processing
software Image J, the relative intensities were calculated by
normalizing to GAPDH from the corresponding sample.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from retinas using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
The first strand cDNA was synthesized from 1–2 µg total RNA via
oligo (dT)-primed RT (priming for 5 min at 25°C; RT for 20 min at
46°C; RT inactivation for 1 min at 95°C; iScriptcDNA synthesis kit;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primers were
designed using Primer-BLAST, based on the published GenBank
sequence (https://blast.ncbi.nlm.nih.gov/Blast.cgi; http://www.ncbi.nlm.nih.gov/genbank/).
All primer pairs are listed in Table
I. RT-qPCR with SYBR (Takara Bio, Inc., Osaka, Japan) was
performed with an ABI 7500 Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and quantified by
2−ΔΔCq method (18).
 | Table I.Sequences of polymerase chain
reaction primers. |
Table I.
Sequences of polymerase chain
reaction primers.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| NOX1 |
CAGTTATTCATATCATTGCACACCTATTT |
CAGAAGCGAGAGATCCATCCA |
| NOX4 |
GCACGCTGTTGATTTTTATGG |
GCGAGGCAGGAGAGTCAGTA |
| GAPDH |
GTGTTTCCTCGTCCCGTAGA |
AATCTCCACTTTGCCACTGC |
Immunofluorescence staining
Eyes were fixed with 40 g/l paraformaldehyde in PBS
overnight at 4°C and infiltrated with 25% sucrose. The cryosections
were blocked with 1% bovine serum albumin (cat. no. HZB0148;
Sigma-Aldrich; Merck KGaA) at room temperature for 0.5 h. Following
incubation with anti-LC3 antibody (cat. no. ab48394; 1:500; Abcam)
at 4°C overnight, sections were incubated with anti-rabbit IgG
(cat. no. A0453; Alexa Fluor 555; 1:100; Beyotime Institute of
Biotechnology) at 4°C for 1 h. DAPI (1:10,000; Sigma-Aldrich; Merck
KGaA) was used to label the nucleus at a concentration of 5 µg/ml
at room temperature for 5 min. Fluorescence images were acquired
using a confocal Olympus microscope at magnification, ×400 (Olympus
Corporation).
Transmission electron microscopy
Eyes were isolated and fixed in 2.5% glutaraldehyde
at 4°C overnight and 1% osmium tetroxide at room temperature
successively, followed by dehydration in ethanol. Following
incubation in acetone for 20 min, the eyes were treated with 50% (1
h), 75% (3 h) and 100% (overnight) epoxy resin and heated at 70°C
overnight. The embedded eyes were sliced to ultrathin sections (70
nm) using an MT-5000 Sorvall microtome (Sorvall; Thermo Fisher
Scientific, Inc.). Sections were stained with 3% uranyl acetate and
3% lead citrate for 15 min at room temperature and were visualized
with a transmission electron microscope system.
Statistical analysis
All experiments were performed at least three times,
and the results of one representative experiment are presented as
the mean ± standard deviation. For comparisons between two groups,
the independent sample t-test (normally distributed) or
Mann-Whitney test (non-normally distributed) was used. P<0.05
was considered to indicate a statistically significant difference.
Analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY,
USA).
Results
RNV is clearly generated in OIR
mice
To investigate whether the mouse model of OIR was
successfully established, a fundus camera was used to capture
images of the retina. The fundus images revealed that neovascular
tufts were successfully generated in the OIR mice. Large numbers of
pale neovascular vessels had grown into the vitreous cavity in the
OIR mice (Fig. 1A) and the number
of neovascular nuclei was significantly greater compared with that
in the WT mice (Fig. 1B and
C).
 | Figure 1.RNV is generated in OIR mice. (A)
Fundus photography facilitated visualization of the inner surfaces
of the eyes of the WT and OIR mice. The representative images
indicate the large number of pale neovascular vessels that had
grown into the vitreous cavity in the OIR mice, compared with the
WT mice. (B) Representative H&E staining of retinal sections. A
large number of the neovascular nuclei were present beyond the
internal limiting membrane in the OIR mice, while there were only a
few neovascular nuclei in the WT mice. (C) The number of outgrowth
endothelial cells in the retinas was calculated using Image-Pro
Plus software. The number of neovascular nuclei in the OIR mice was
significantly greater compared with that of the control mice. Data
are presented as the mean ± standard deviation of the mean (n=6
mice per group). ***P<0.001 vs. WT mice. GCL, ganglion cell
layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL,
outer plexiform layer; ONL, outer nuclear layer; RPE, retinal
pigment epithelium. H&E, hematoxylin and eosin; OIR,
oxygen-induced retinopathy; WT, wild-type. |
Angiogenesis is activated in the
retina of OIR mice
The effect on retinal angiogenesis in OIR mice was
determined. When OIR had been achieved, the protein expression
levels of VEGF-A and those of its upstream regulatory molecule
HIF-1α were significantly increased in the retinas of the OIR mice
compared with the WT control (Fig.
2). These results indicated that angiogenesis was activated,
via the HIF-1α/VEGF-A signaling pathway, in this model.
Retinal oxidative stress is increased
in OIR mice
ROS is one of the principal triggers of blood vessel
growth. To assess the alteration in the levels of ROS in the OIR
mice in the present study, retinal sections were assessed using DHE
staining. The retinas from the OIR mice exhibited a significant
increase in the intensity of DHE staining (Fig. 3A and B). A major source of ROS in
endothelial cells is NOX. In particular, the isoforms NOX1 and NOX4
are known to be involved in the generation of retinal ROS (19,20).
The mRNA expression levels of NOX1 and NOX4 were determined in the
present study using RT-qPCR analysis. As with the levels of ROS,
the mRNA expression levels of NOX1 and NOX4 were significantly
increased in the retinas from the OIR mice compared with the WT
control mice (Fig. 3C and D).
 | Figure 3.Retinal oxidative stress is increased
in OIR mice. (A) The reactive oxygen species levels in the retinas
of the WT and OIR mice were detected using the DHE fluorescent
probe and fluorescent images of the retinas were visualized with a
fluorescence microscope. Scale bar, 50 µm. (B) Quantification of
DHE intensity in the retinas (n=6 mice per group). The retinas were
harvested for RNA extraction and qPCR detection. (C) NOX1 and (D)
NOX4 were upregulated at the mRNA level in the retinas from the OIR
mice compared with the WT mice. The relative mRNA expression levels
were normalized to GAPDH (n=6 mice per group). Data are presented
as the mean ± standard deviation of the mean. *P<0.05,
**P<0.01 vs. WT mice. IPL, inner plexiform layer; INL, inner
nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear
layer; RPE, retinal pigment epithelium; OIR, oxygen-induced
retinopathy; WT, wild-type; VEGF, vascular endothelial growth
factor; DHE, dihydroethidium. |
Pyroptosis is activated in the retinas
of OIR mice
In order to elucidate the involvement of
inflammasomes in OIR, the expression of NLRP3 was analyzed using
western blotting. There was a significant increase in the levels of
NLRP3 expression in the OIR mice compared with the WT control mice
(Fig. 4). As oxidative stress
activates inflammasome-caspase-1-IL-1β signaling, whether caspase-1
is activated in the hypoxia situation of the retinas was
investigated. The protein levels of caspase-1 and pro-caspase-1,
which were detected using western blotting, were significantly
increased in the OIR mice compared with the WT control mice
(Fig. 4). Furthermore, the
pro-inflammatory cytokines IL-1β and pro-IL-1β also exhibited an
increase at the protein level (Fig.
4).
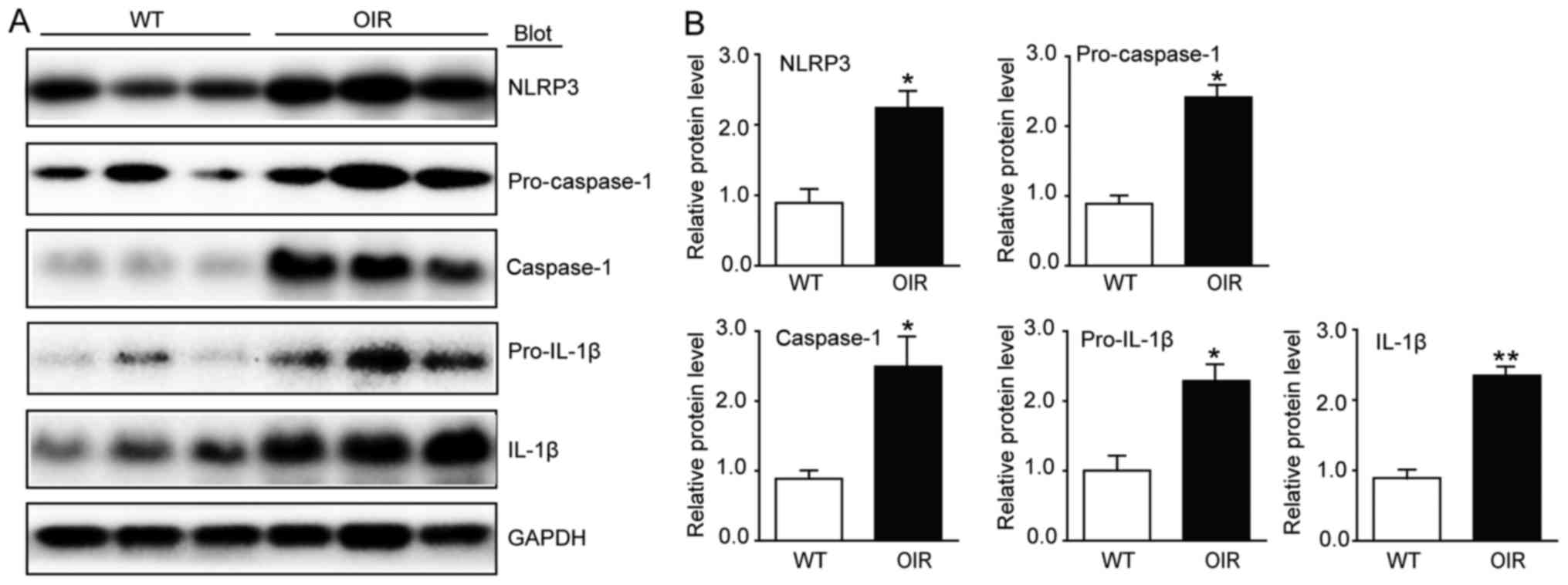 | Figure 4.Inflammasome activity is increased in
the retinas of OIR mice. (A) Immunoblot analysis of the protein
expression levels of NLRP3, caspase-1, IL-1β, pro-caspase-1 and
pro-IL-1β in the retinas. (B) Quantification revealed an increase
in the expression levels of NLRP3, caspase-1, IL-1β, pro-caspase-1
and pro-IL-1β in the retinas of the OIR mice compared with the WT
mice. The relative protein expression levels were normalized to
GAPDH (n=6 mice per group). Data are presented as the mean ±
standard deviation of the mean. *P<0.05, **P<0.01 vs. WT
mice. IL, interleukin; NLRP3, NOD-like receptor family pyrin
domain-containing 3; OIR, oxygen-induced retinopathy; WT,
wild-type. |
Autophagic flux is reduced in the
retinas of OIR mice
In recent years, autophagy has been identified as a
biological process associated with inflammation in different
cellular contexts, as it targets inflammasome components for
degradation (21). On that basis,
it was hypothesized that autophagy may be involved in OIR. As
lipidation of LC3 is a useful marker of autophagy, LC3 was analyzed
using immunofluorescence and western blotting. The expression
levels of Atg5, Atg7, Atg12 and Beclin1 were also determined using
western blotting. The immunostaining identified that the
distribution of LC3 was reduced in the OIR mice compared with the
WT control mice (Fig. 5A).
Furthermore, transmission electron micrographs of the retinas
demonstrated that there were fewer autophagosomes in the OIR mice
compared with the WT control mice (Fig. 5B). There was a significant
reduction in the expression levels of LC3II/I, Atg5, Atg7, Atg12
and Beclin1 in the retinas from the OIR mice compared with the WT
control mice (Fig. 5C and D).
However, a reduction in LC3II/I, which is expressed in
autophagosomes, does not always confirm autophagic flux (22). Thus, the protein levels of p62, a
selective substrate of autophagy, were also analyzed. The results
demonstrated that there was an increase in the p62 protein levels
in the OIR mice compared with the WT control mice (Fig. 5C and D), indicating that autophagic
flux was blocked in the retinas of the OIR mice.
 | Figure 5.Autophagic flux is reduced in the
retinas of OIR mice. (A) Immunostaining of the LC3 (red) in the
retinal sections (left) and quantification of fluorescence
intensity (right; n=6 mice per group). Nuclei were counterstained
with DAPI (blue). Scale bar, 50 µm. (B) Transmission electron
micrographs and quantification of the autophagosomes in retinas.
Scale bar, 500 nm. (C) Immunoblot analysis of the protein
expression levels of Beclin1, Atg5, Atg7, Atg12, LC3 and p62 in the
retinas. (D) Quantification of the protein bands (n=6 mice per
group). GAPDH as an internal control. Data are the mean ± standard
deviation of the mean. *P<0.05 vs. WT mice. OIR, oxygen-induced
retinopathy; GCL, ganglion cell layer; IPL, inner plexiform layer;
INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer
nuclear layer; RPE, retinal pigment epithelium; Atg, autophagy
protein; LC3, microtubule associated protein 1 light chain 3α; WT,
wild-type. |
Discussion
Aberrant RNV is a principal cause of visual
impairment and blindness in the context of neovascular ocular
diseases, including ROP, DR and RVO. Among them, ROP is a major
cause of childhood blindness worldwide (23). In certain countries, particularly
those with middle and low incomes, the incidence of ROP is >40%
due to the increasing survival rate of premature infants and
limited fundoscopic follow-up evaluation (24). Although anti-VEGF and laser
ablation therapies may be successful, the long-term efficacy
remains uncertain. A clear understanding of the mechanisms
underlying RNV generation is required in order to develop novel
therapeutic alternatives.
RNV is stimulated by one or more angiogenic factors
that are released by the retina under ischemic or hypoxic
conditions, including VEGF, HIFs and cytokines (25). VEGF is an important pathogenic
factor that stimulates endothelial cell proliferation and tube
formation and mediates ischemia-induced RNV (5). The expression of retinal VEGF is
increased under stress conditions in order to generate
neovascularization (26).
Therefore, understanding of the regulation of VEGF may aid
investigations regarding the role of transcriptional regulators in
RNV. In the present study, increased expression of VEGF-A and
HIF-1α in the OIR mice suggested that the angiogenesis in OIR mice
is generated via the HIF-1α/VEGF-A signaling pathway.
Oxidative stress occurs when the generation of
pro-oxidants or ROS exceeds the capacity of endogenous
antioxidants. ROS, including the superoxide anion
(O2−), hydroxyl radical (OH), hydrogen
peroxide (H2O2) and singlet oxygen
(1O2), are primarily generated by mitochondria and NOX.
The NOX family members serve a vital role in multiple cellular
biological processes, including signal transduction, migration and
proliferation (27). Furthermore,
NOX appears to be the only enzymatic source of ROS in the retina
that is clearly involved in pathological neovascularization
(3). NOX1 and NOX4 are the major
isoforms of NOX that are highly expressed in retinal endothelial
cells of mice following stress (19,20).
In hypoxic environments, increased amounts of ROS are generated to
mediate the hypoxia-induced cellular response (28). In the present study, DHE staining,
NOX1 and NOX4 were detected in order to demonstrate that hypoxia
stimulates the accumulation of ROS in the retinas of the OIR
mice.
Increased generation of intracellular ROS may induce
programmed cell death, such as autophagy and pyroptosis, via
execution by lysosomal proteases or caspases (12). Pro-IL-1β and pro-caspase-1 are
stored in secretory lysosomes, where they await an
exocytosis-inducing stimulus; in the absence of such a stimulus,
these molecules may undergo lysosomal degradation (29). IL-1β activation and release require
the synthesis of pro-IL-1β and caspase-1 activation. Pyroptosis is
a pro-inflammatory type of programmed cell death that is triggered
by increased oxidative stress and the subsequent activation of
inflammasome-caspase-1-IL-1β signaling, resulting in tissue injury
(30). NOX-mediated oxidative
stress is an initial signal that induces inflammasome activation.
The inflammasome is a large supramolecular complex that is largely
composed of dimers of the adaptor protein apoptosis-associated
speck-like protein containing a CARD. In particular, the generation
of ROS via NOX causes thioredoxin-interacting protein to associate
with NLRP3, which facilitates inflammasome formation (31). Subsequently, caspase-1 is activated
by the inflammasome and promotes the cascade and release of the
highly pro-inflammatory cytokine IL-1β. Previous studies have
suggested that pyroptosis may be functionally linked with
autophagy. A recent study demonstrated that reactive oxygen species
induce NLPR3 inflammasomes mediated pyroptosis in the intestinal
cells (32). It has been
demonstrated that the inhibition of autophagy stimulates
pneumococcus-induced pyroptosis and protects microglial cells
against pyroptosis (33). The
present study revealed an increase in the protein expression levels
of NLRP3, caspase-1 and IL-1β in OIR mice, which indicated that
pyroptosis is activated during vascularization of the retina.
Autophagy, also termed ‘self-eating’, is a highly
sensitive cellular process that is induced in response to a wide
range of stresses, including starvation, hypoxia, cytotoxicity and
infection, in order to maintain homeostasis (15). Prolonged exposure to high levels of
ROS and inflammasomes represents a stressful environment. A number
of studies have suggested that ROS induce autophagy as upstream
modulators (34,35). In RPE cells under oxidative stress,
autophagy is increased and autophagic flux is reduced, which are
stimulated by acute and chronic stress, respectively (36). Oxidative stress produces a large
amount of damaged proteins, which may lead to overloading of the
autophagosomal system and result in a reduction in autophagic
activity (36,37). In the high-glucose conditions of
Müller cells, autophagic machinery is triggered, although
autophagic flux is compromised. The autophagic substrate p62
accumulates in the cytosol, signaling apoptosis and extensive VEGF
production (38). The level of
LC3II/I is an indicator of the initiation of autophagy. It has been
well demonstrated that autophagy depends on the activity of Atg5,
Atg7 and Atg12. Of all these indicators, Beclin1 is required for
Atg-7-dependent autophagy (22).
The results of the present study demonstrated that the autophagic
flux was weakened in the retinas of the OIR mice. As increased
protein aggregation may contribute to inflammasome activation and
tissue injury, reduced autophagy and increased inflammation may be
involved in angiogenesis (39,40).
ROS act as a ‘double-edged sword’ in the
vasculature. In wound healing, physiological angiogenesis is
induced by tissue hypoxia and ROS, resulting in the production of
VEGF (41,42). However, the supply of oxygen and
nutrients caused by pathological angiogenesis results in the
uncontrolled generation of new blood vessels, which leads to an
abnormal vascular pattern (43).
The present study revealed that RNV was generated via HIF-1α/VEGF-A
signaling. Hypoxia stimulated the accumulation of ROS, which was
mediated by NOX, and increased pyroptosis through activation of the
NLRP3-caspase-1-IL-1β pathway, while autophagic activity was
compromised in this process.
A recent study demonstrated that impaired autophagy
induces pro-inflammatory and pro-angiogenic proteins, which results
in angiogenesis (39). Autophagic
dysfunction and oxidative stress are implicated in retinitis
pigmentosa (44). Mild oxidative
stress triggers cell survival and repair mechanisms, including the
autophagic pathway. However, in the case of severe oxidative
stress, excessive levels of ROS for a prolonged period leads to
oxidative damage and, ultimately, cell death (45). The results of certain studies
suggest that pyroptosis may be functionally associated with
autophagy. Inhibition of autophagy stimulates pneumococcus-induced
pyroptosis and protects microglial cells against pyroptosis
(33). The present study
demonstrated, for the first time to the best of our knowledge, that
ROS and pyroptosis are activated and autophagy is compromised in
OIR mice, suggesting that severe oxidative stress induces
upregulation of pyroptosis and inhibition of autophagy. The
combination of oxidative stress, pyroptosis and impaired autophagy
may serve an important role in the pathophysiology of OIR. A
possible limitation of the present study was that only the
phenomena of oxidative stress, autophagy, and pyroptosis were
investigated; the mechanisms accounting for their association
remain unclear, and alterations in apoptosis were not detected.
Further investigation is warranted to illustrate these changes in
retinal neovascularization.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural
Foundation of Liaoning Province (grant nos. 2015E21SF005 and
20180550740).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW and L-YJ performed the research. L-YJ, SW and LL
analyzed the data. SW, L-YJ and J-ML conceived and designed the
research. J-ML wrote the manuscript.
Ethics approval and consent to
participate
All investigations were approved by the Animal Care
and Use Committee of Dalian Medical University and conformed to the
US National Institutes of Health Guide for the Care and Use of
Laboratory and the ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhatt L, Groeger G, McDermott K and Cotter
TG: Rod and cone photoreceptor cells produce ROS in response to
stress in a live retinal explant system. Mol Vis. 16:283–293.
2010.PubMed/NCBI
|
|
3
|
Al-Shabrawey M, Bartoli M, El-Remessy AB,
Platt DH, Matragoon S, Behzadian MA, Caldwell RW and Caldwell RB:
Inhibition of NAD(P)H oxidase activity blocks vascular endothelial
growth factor overexpression and neovascularization during ischemic
retinopathy. Am J Pathol. 167:599–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo C, Yang M, Jing L, Wang J, Yu Y, Li Y,
Duan J, Zhou X, Li Y and Sun Z: Amorphous silica nanoparticles
trigger vascular endothelial cell injury through apoptosis and
autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and
PI3K/Akt/mTOR signaling. Int J Nanomed. 11:5257–5276. 2016.
View Article : Google Scholar
|
|
5
|
Adamis AP and Shima DT: The role of
vascular endothelial growth factor in ocular health and disease.
Retina. 25:111–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zepeda AB, Pessoa A Jr, Castillo RL,
Figueroa CA, Pulgar VM and Farías JG: Cellular and molecular
mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell
Biochem Funct. 31:451–459. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saito S, Lin YC, Tsai MH, Lin CS, Murayama
Y, Sato R and Yokoyama KK: Emerging roles of hypoxia-inducible
factors and reactive oxygen species in cancer and pluripotent stem
cells. Kaohsiung J Med Sci. 31:279–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kietzmann T and Görlach A: Reactive oxygen
species in the control of hypoxia-inducible factor-mediated gene
expression. Semin Cell Dev Biol. 16:474–486. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Green DR, Galluzzi L and Kroemer G:
Mitochondria and the autophagy-inflammation-cell death axis in
organismal aging. Science. 333:1109–1112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vande Walle L and Lamkanfi M: Pyroptosis.
Curr Biol. 26:R568–R572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Tan J, Miao Y, Lei P and Zhang Q:
ROS and autophagy: Interactions and molecular regulatory
mechanisms. Cell Mol Neurobiol. 35:615–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levonen AL, Hill BG, Kansanen E, Zhang J
and Darley-Usmar VM: Redox regulation of antioxidants, autophagy,
and the response to stress: Implications for electrophile
therapeutics. Free Radic Biol Med. 71:196–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lapaquette P, Guzzo J, Bretillon L and
Bringer MA: Cellular and molecular connections between autophagy
and inflammation. Mediators Inflamm. 2015:3984832015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blasiak J, Petrovski G, Veréb Z, Facskó A
and Kaarniranta K: Oxidative stress, hypoxia, and autophagy in the
neovascular processes of age-related macular degeneration. Biomed
Res Int. 2014:7680262014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grossniklaus HE, Kang SJ and Berglin L:
Animal models of choroidal and retinal neovascularization. Prog
Retin Eye Res. 29:500–519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilkinson-Berka JL, Deliyanti D, Rana I,
Miller AG, Agrotis A, Armani R, Szyndralewiez C, Wingler K, Touyz
RM, Cooper ME, et al: NADPH oxidase, NOX1, mediates vascular injury
in ischemic retinopathy. Antioxid Redox Signal. 20:2726–2740. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Wang JJ, Yu Q, Chen K, Mahadev K and
Zhang SX: Inhibition of reactive oxygen species by Lovastatin
downregulates vascular endothelial growth factor expression and
ameliorates blood-retinal barrier breakdown in db/db mice: Role of
NADPH oxidase 4. Diabetes. 59:1528–1538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris J: Autophagy and cytokines.
Cytokine. 56:140–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zin A and Gole GA: Retinopathy of
prematurity-incidence today. Clin Perinatol. 40:185–200. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gilbert C, Fielder A, Gordillo L, Quinn G,
Semiglia R, Visintin P and Zin A: International NO-ROP Group:
Characteristics of infants with severe retinopathy of prematurity
in countries with low, moderate, and high levels of development:
Implications for screening programs. Pediatrics. 115:e518–e525.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cabral T, Mello LGM, Lima LH, Polido J,
Regatieri CV, Belfort R Jr and Mahajan VB: Retinal and choroidal
angiogenesis: A review of new targets. Int J Retina Vitreous.
3:312017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cervia D, Catalani E, Dal Monte M and
Casini G: Vascular endothelial growth factor in the ischemic retina
and its regulation by somatostatin. J Neurochem. 120:818–829. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu Q, He Y, Ji J, Yao Y, Shen W, Luo J,
Zhu W, Cao H, Geng Y, Xu J, et al: Hypoxia-inducible factor 1α
(HIF-1α) and reactive oxygen species (ROS) mediates
radiation-induced invasiveness through the SDF-1α/CXCR4 pathway in
non-small cell lung carcinoma cells. Oncotarget. 6:10893–10907.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Andrei C, Dazzi C, Lotti L, Torrisi MR,
Chimini G and Rubartelli A: The secretory route of the leaderless
protein interleukin 1beta involves exocytosis of
endolysosome-related vesicles. Mol Biol Cell. 10:1463–1475. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou R, Tardivel A, Thorens B, Choi I and
Tschopp J: Thioredoxin-interacting protein links oxidative stress
to inflammasome activation. Nat Immunol. 11:136–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu D, Han R, Deng S, Liu T, Zhang T, Xie H
and Xu Y: Protective effects of flagellin A N/C against
radiation-induced NLR pyrin domain containing 3
inflammasome-dependent pyroptosis in intestinal cells. Int J Radiat
Oncol Biol Phys. 101:107–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JY, Paton JC, Briles DE, Rhee DK and
Pyo S: Streptococcus pneumoniae induces pyroptosis through the
regulation of autophagy in murine microglia. Oncotarget.
6:44161–44178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szumiel I: Autophagy, reactive oxygen
species and the fate of mammalian cells. Free Radic Res.
45:253–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Filomeni G, Desideri E, Cardaci S, Rotilio
G and Ciriolo MR: Under the ROS…thiol network is the principal
suspect for autophagy commitment. Autophagy. 6:999–1005. 2014.
View Article : Google Scholar
|
|
36
|
Mitter SK, Song C, Qi X, Mao H, Rao H,
Akin D, Lewin A, Grant M, Dunn W Jr, Ding J, et al: Dysregulated
autophagy in the RPE is associated with increased susceptibility to
oxidative stress and AMD. Autophagy. 10:1989–2005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaarniranta K, Sinha D, Blasiak J,
Kauppinen A, Veréb Z, Salminen A, Boulton ME and Petrovski G:
Autophagy and heterophagy dysregulation leads to retinal pigment
epithelium dysfunction and development of age-related macular
degeneration. Autophagy. 9:973–984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lopes de Faria JM, Duarte DA, Montemurro
C, Papadimitriou A, Consonni SR and Lopes de Faria JB: Defective
autophagy in diabetic retinopathy. Invest Ophthalmol Vis Sci.
57:4356–4366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, Copland DA, Theodoropoulou S, Chiu
HA, Barba MD, Mak KW, Mack M, Nicholson LB and Dick AD: Impairing
autophagy in retinal pigment epithelium leads to inflammasome
activation and enhanced macrophage-mediated angiogenesis. Sci Rep.
6:206392016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Hanus J, Abu-Asab M, Shen D,
Ogilvy A, Ou J, Chu XK, Shi G, Li W, Wang S and Chan CC: NLRP3
upregulation in retinal pigment epithelium in age-related macular
degeneration. Int J Mol Sci. 17(pii): E732016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sen CK, Khanna S, Babior BM, Hunt TK,
Ellison EC and Roy S: Oxidant-induced vascular endothelial growth
factor expression in human keratinocytes and cutaneous wound
healing. J Biol Chem. 277:33284–33290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schreml S, Szeimies RM, Prantl L, Karrer
S, Landthaler M and Babilas P: Oxygen in acute and chronic wound
healing. Br J Dermatol. 163:257–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chung AS and Ferrara N: Developmental and
Pathological Angiogenesis. Annu Rev Cell Dev Biol. 27:563–584.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moreno ML, Mérida S, Bosch-Morell F,
Miranda M and Villar VM: Autophagy dysfunction and oxidative
stress, two related mechanisms implicated in retinitis pigmentosa.
Front Physiol. 9:10082018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sureshbabu A, Ryter SW and Choi ME:
Oxidative stress and autophagy: Crucial modulators of kidney
injury. Redox Biol. 4:208–214. 2015. View Article : Google Scholar : PubMed/NCBI
|



















