Introduction
Gastric cancer is one of the most common
malignancies in the world with poor prognosis (1,2).
Despite the reduced incidence in recent decades, gastric cancer
remains the third leading cause of global cancer-associated
mortality, and has the highest incidence in East Asia (3,4). The
early diagnosis of gastric cancer requires improvement, as it
frequently only identified in the advanced stage (5). Therefore, the prognosis of patients
with gastric cancer is very poor (6–8). The
primary reason for failure in the treatment of gastric cancer is
cell infiltration and metastasis (9).
MicroRNAs (miRNAs/miRs) are evolutionarily conserved
non-coding RNA of ~19–25 nucleotides, which function by regulating
one or more mRNA to regulate gene expression for translation
inhibition or cleavage (10,11).
miR serve a key role in cell proliferation, cell death and organ
development (12,13). In addition, previous studies
demonstrated that miRs are key regulators in the development and
progression of gastric cancer (14,15).
However, the expression status and biological function of specific
miRs in gastric cancer require further examination. miR-145 was
first reported to be consistently decreased in precancerous
colorectal lesions (16). Other
previous studies observed that miR-145 as a tumor-suppressive miRNA
regulates cell growth in tumor cells by targeting epidermal growth
factor receptor, Myc proto-oncogene protein (c-Myc) and POU domain,
class 5, transcription factor 1 (17–20).
In addition, miR-145 has been studied in gastric cancer (21). Lei et al (22) reported that miR-145 inhibits
gastric cancer cell migration and metastasis by inhibiting MYO6
expression. The results of the research of Qiu et al
(23) indicated that miR-145
suppressed the proliferation, migration, invasion and cell cycle
progression of gastric cancer cells through decreasing Sp1
expression. Gao et al (24)
suggested that miR-145 suppresses gastric cancer metastasis by
inhibiting N-cadherin protein translation. However, the exact
function and underlying molecular mechanism of miR-145 in gastric
cancer remains largely unclear and requires further
examination.
In the present study, the expression of miR-145 was
significantly decreased in gastric cancer cells. Further, miR-145
overexpression was able to inhibit the proliferation of gastric
cancer cells and induce apoptosis. In addition, it was observed
that miR-145 mimics inhibited gastric cancer cell invasion and
migration by regulating the phosphoinositide 3-kinase
(PI3K)/protein kinase B (AKT) signaling pathway. The present
results demonstrated that miR-145 functions as an anti-tumor gene
in gastric cancer cell, and is a potential therapeutic target.
Materials and methods
Cell culture and transfection
Gastric cancer cell line SGC-7901 and normal gastric
epithelial cells GES-1 were obtained from the Cell Bank of Chinese
Academy of Sciences (Shanghai, China). All cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin/streptomycin. Cells were incubated in a humidified
incubator at 37°C and 5% CO2.
The miR-145 mimics (5′-GTCCAGTTTTCCCAGGAATCCCT-3′)
(50 nM), miR-145 inhibitor (5′-AGGGATTCCTCCCAAAACTGGAC-3′) (100 nM)
and the negative control vector (5′-GUAGGAGUAGUGAAAGGCC-3′) (NC; 50
nM; Shanghai GenePharma Co., Ltd., Shanghai, China) were
transfected into SGC-7901 cells using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After transfection for 48–72 h, cells were
collected for further assays. Cells in the blank group (BL) were
untreated cells.
Cell proliferation
The proliferation of SGC-7901 cells transfected with
miR-145 mimics, miR-145 inhibitor or NC were examined using MTT
colorimetric assays. After transfection for 48 h, SGC-7901 cells
(1×105) were seeded in 96-well plates in triplicate. MTT
(20 µl; 5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
added to each well at 48 h after transfection. Following incubation
for 4 h, the MTT medium was removed and 150 µl dimethyl sulfoxide
was added. After shaking for 15 min at room temperature, the
optical density at the 490 nm of each sample was determined with
scanning multi-well spectrophotometer.
Flow cytometry analysis
SGC-7901 cells were transfected with miR-145 mimics,
miR-145 inhibitor or NC, and 48 h after transfection, the cells
were collected and washed with PBS. To detect the cell cycle, the
cells were fixed in 70% methanol at −20°C overnight. Subsequently,
the cells were washed with PBS twice and stained with propidium
iodide (PI). Finally, flow cytometry was used to detect cell
cycle.
To detect cellular apoptosis, cells were stained
with an Annexin V/PI (apoptosis detection kit; BD Biosciences,
Franklin Lakes, NJ, USA) according to the manufacturer's protocol.
After incubating with Annexin V/PI for 15 min in the dark, cellular
apoptosis (Q1: Dead cells; Q2: Late apoptosis; Q3: Normal cells;
Q4: Early apoptosis) was detected using a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). Data were analyzed by using
WinMDI version 2.5 (Purdue University Cytometry Laboratories;
http://www.cyto.purdue.edu/flowcyt/software/Catalog.htm).
Cell invasion assay
The effect of miR-145 on SGC-7901 cell invasive
capability was detected using a 24-well Transwell plate (8 mm pore
size; Corning Incorporated, Corning, NY, USA). Chamber inserts were
coated with 200 mg/ml Matrigel and dried overnight under sterile
conditions. SGC-7901 cells were transfected with miR-145 mimic,
miR-145 inhibitor or NC, and 48 h after transfection, the cells
(1×104) in RPMI-1640 medium were added to the top
chamber, and RPMI-1640 medium supplemented with 20% FBS was added
to the lower chamber. Following incubation for 48 h at 37°C, the
top chambers were wiped with cotton wool to remove the noninvasive
cells and subsequently fixed in 100% methanol at room temperature
for 10 min. Following staining in hematoxylin-eosin solution at
room temperature for 20 min, the invading cells on the underside of
the membrane were counted at five random fields under a light
microscope (Olympus Corporation, Tokyo, Japan).
Cell migration assay
The scratch wound healing assay was used to detect
the effect of miR-145 on SGC-7901 cell metastasis. SGC-7901 cells
were transfected with miR-145 mimics, miR-145 inhibitor or NC, and
cells (1×104 cells/well) were subsequently seeded
homogeneously on 6-well plates. When the cells formed a monolayer,
a sterile plastic 200 µl pipette tip was used to scratch the cells.
The floating cells were washed with RPMI-1640 medium. At 0, 24 and
48 h after scratch wound formation, images were obtained using an
inverted microscope at a magnification of ×200 and measured using
Image-Pro Plus software version 7.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Western blot analysis
SGC-7901 cells, which were transfected with miR-145
mimics, miR-145 inhibitor or NC for 48 h, were collected and the
total proteins were extracted using 40 mM Tris-HCl (pH 7.4)
containing 150 mM NaCl and 1% (v/v) Triton X-100, supplemented with
protease inhibitors. Protein concentration was determined using the
bicinchoninic acid protein assay. Equal amounts of protein (30 µg
per lane) were resolved on 10% SDS-PAGE, and subsequently
transferred to a polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA). Following blocking with 5% skimmed milk in
Tris buffered saline with 0.1% Tween-20 at room temperature for 1.5
h, samples were probed with antibodies against c-Myc (1:1,000; cat.
no. 5605; Cell Signaling Technology, Inc., Danvers, MA, USA),
phosphorylated (p-)AKT (1:1,000; cat. no. 4060; Cell Signaling
Technology, Inc.), PI3K (1:1,000; cat. no. 4249; Cell Signaling
Technology, Inc.), P21 (1:1,000; cat. no. 2947; Cell Signaling
Technology, Inc.), matrix metalloproteinase (MMP) 2 (1:1,000; cat.
no. 40994; Cell Signaling Technology, Inc.), MMP9 (1:1,000; cat.
no. 13667; Cell Signaling Technology, Inc.) and GAPDH (1:1,000;
cat. no. 8884; Cell Signaling Technology, Inc.). Following washing
with 1X PBST (10 min/wash) for three times, blots were incubated
with horseradish peroxidase-conjugated secondary antibody (1:2,000;
cat. no. 7074; Cell Signaling Technology, Inc.) at room temperature
for 2 h. Immunoreactive bands were visualized using SignalFire™
Plus ECL Reagent (cat no. 12630; Cell Signaling Technology, Inc.).
The protein expression levels of the stripes were normalized based
on the gray value of GAPDH. Image J version 2.0 software (Bio-Rad
Laboratories Inc, USA) was used to quantify the intensity of the
bands.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using RNAiso Plus
(Takara Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. RT was conducted to synthesize cDNA with
the ThermoScript RT-PCR system (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. qPCR
was performed to analyze the synthesized cDNA by using the
SYBR® Premix Ex Taq™ II kit (Takara Bio, Inc., Otsu,
Japan). Primer sequences were listed as following: U6 forward:
5′GCTTCGGCAGCACATATACTAAAAT3′ and reverse:
5′CGCTTCACGAATTTGCGTGTCAT3′; miR-145 forward:
5′-GTCCAGTTTTCCCAGGAATCCCT-3′ and reverse:
5′-GCTGTCAACGATACGCTACCTA-3′. The conditions of qPCR used for
amplification were as follows: 95°C for 5 min, 40 cycles at 95°C
for 30 sec, 55°C for 30 sec, 72°C for 30 sec, and 72°C for 10 min.
U6 served as an internal control. The relative gene expression was
assessed by using the 2−ΔΔCq method (25). The experiment was repeated at least
three times.
Statistical analysis
SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used to analyze the data. Dare are presented as the mean ±
standard deviation of experiments performed in triplicate. Data
were analyzed by one-way analysis of variance followed by Tukey's
post-hoc test, or the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-145 inhibits the proliferation of
SGC-7901 cells
The RT-qPCR results demonstrated that the expression
of miR-145 was significantly decreased in the gastric cancer cell
line SGC-7901 compared with normal gastric epithelial GES-1 cells
(Fig. 1A; P<0.001). To expand
the limited understanding of the role served by miR-145 in gastric
cancer cells, the effects of miR-145 gain and loss-of-function on
SGC-7901 cells were examined. For this, miR-145 mimics, miR-145
inhibitor or NC were transfected into SGC-7901 cells, and RT-qPCR
was performed to detect the transfection efficiency. As presented
in Fig. 1B, miR-145 mimics
significantly increased the expression level of miR-145 in SGC-7901
cells and the expression level of miR-145 was significantly
decreased in the miR-145 inhibitor-transfected SGC-7901 cells
(P<0.001). After transfection for 48 h, cell morphology was
imaged (Fig. 2A), and cells were
plated into 96-well plates to measure the absorbance. The MTT
results demonstrated that transfection with miR-145 mimics
significantly suppressed SGC-7901 cell proliferation compared with
the NC (Fig. 2B; P<0.001).
miR-145 mimics promotes SGC-7901 cell
apoptosis
SGC-7901 cell apoptosis was detected 48 h after
transfection with miR-145 mimics, miR-145 inhibitor or NC (Fig. 3A). Flow cytometry analysis revealed
that early apoptosis and late apoptosis were upregulated in miR-145
mimics transfected SGC-7901 cells compared with the NC (Fig. 3B). These data demonstrated that
overexpression of miR-145 induced gastric cancer cellular
apoptosis.
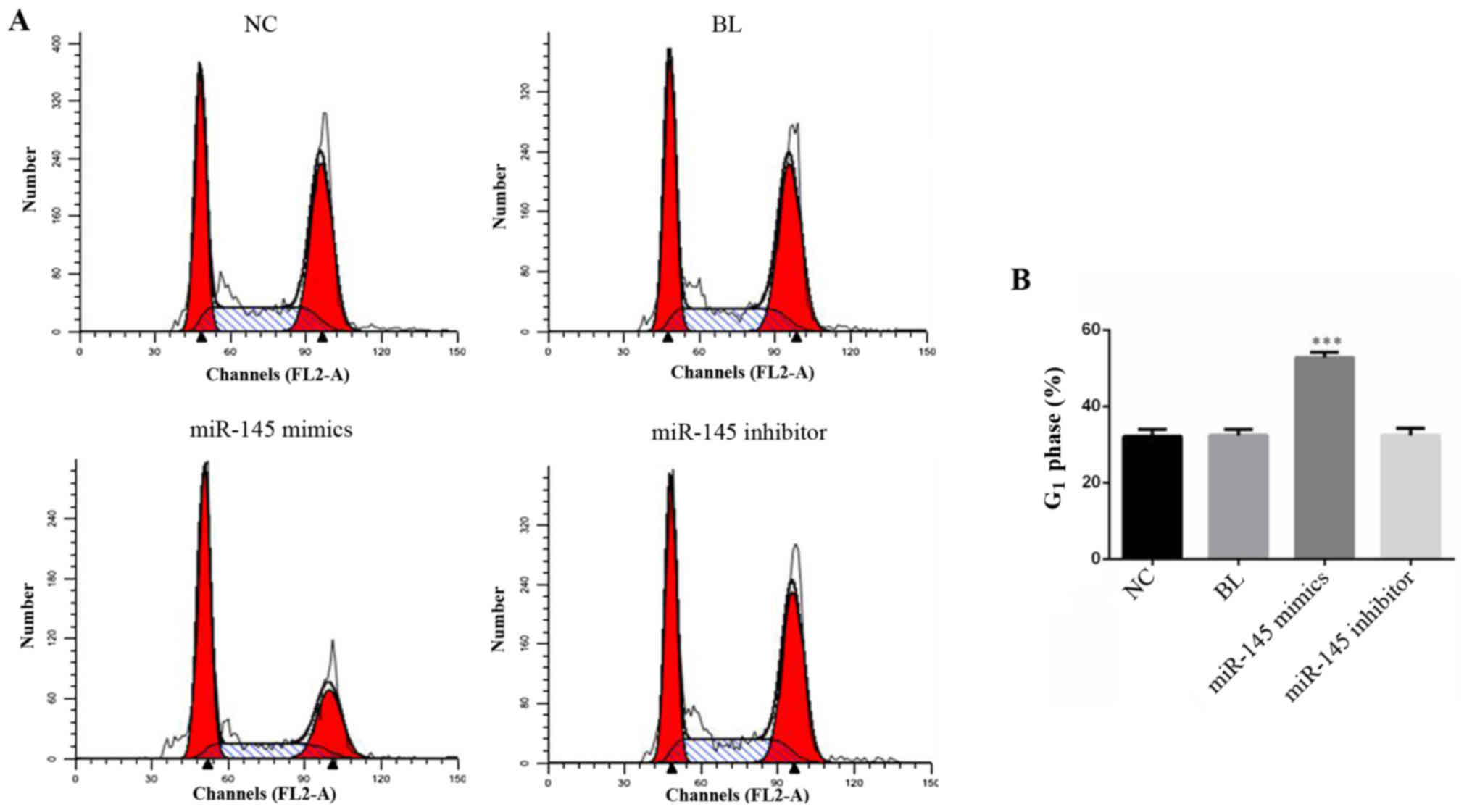
miR-145 mimics induces cell-cycle
arrest in SGC-7901 cells
Cell cycle analysis was used to examine the possible
mechanism by which miR-145 inhibits proliferation. SGC-7901 cells,
which were transfected with miR-145 mimics, miR-145 inhibitor or NC
for 48 h, were detected by cell cycle analysis (Fig. 4A). The results suggested that
overexpression of miR-145 significantly increased the percentage of
cells in the G1 phase (Fig.
4B; P<0.001).
Overexpression of miR-145 inhibits the
invasion and migration of gastric cancer cells
To investigate the metastatic effect of miR-145 on
SGC-7901 cells, migration and invasion assays were performed. The
mimics of miR-145 and NC were transfected into SGC-7901 cells,
which were identified to express relatively low levels of miR-145.
The results of the Matrigel Transwell assay demonstrated that
numbers of adhesive cells in the miR-145 mimics group was
significantly decreased compared with the NC group (Fig. 5A and B; P<0.05). To examine
whether cell migration ability was similarly suppressed by miR-145
overexpression, a scratch wound healing assay was performed. The
results revealed that overexpression of miR-145 suppressed the
wound healing rate of SGC-7901 cells compared with the NC group
(Fig. 5C and D). Collectively,
these data suggested that miR-145, as a regulatory molecule, is
involved in gastric cancer cell migration and invasion.
miR-145 inhibits SGC-7901 cell growth
by regulating the PI3K/AKT signaling pathway
To further investigate the molecular mechanisms
regulated by miR-145, western blotting was performed to detect
c-Myc, PI3K, p-AKT, MMP2 and MMP9 in SGC-7901 cells transfected
with the miR-145 mimics, miR-145 inhibitor or NC. The data
demonstrated that overexpression of miR-145 decreased protein
expression levels of c-Myc, PI3K, p-AKT, MMP2 and MMP9 (Fig. 6). Western blot analysis
demonstrated that the expression of p21, a cell cycle regulatory
protein, was significantly increased, suggesting that miR-145 may
block the cell cycle by regulating p21 expression. These data
revealed the potential molecular mechanism of miR-145 inhibiting
gastric cancer progression involves these proteins.
 | Figure 6.miR-145 inhibits SGC-7901 cell growth
by regulating the PI3K/AKT signaling pathway. miR-145
overexpression significantly decreased the expression of PI3K,
p-AKT, c-Myc, MMP2 and MMP9 while increased the expression of p21.
***P<0.001 vs. NC group. miRNA, micro RNA; NC, negative control;
BL, blank; PI3K, phosphoinositide 3-kinase; p-AKT, phosphorylated
protein kinase B; c-Myc, Myc proto-oncogene protein; MMP, matrix
metalloproteinase. |
Discussion
In previous years, research has revealed the
occurrence and development of gastric cancer. Gastric cancer
remains one of the most common malignancies and causes
approximately one million novel cases each year in the world
(26). miRNA has been reported to
express abnormalities or mutations in various tumors. Previous
studies demonstrated that miR-145 is commonly downregulated in
various types of cancer (27–30),
thus in the present study, the role of miR-145 in gastric cancer
was evaluated.
The miR-145 expression was examined in gastric
cancer SGC-7901 cells and normal gastric epithelial cells GES-1.
Consistent with a previous study, the present study identified that
the expression of miR-145 significantly decreased in gastric cancer
cells (22). Furthermore, the role
of miR-145 in gastric cancer was investigated. Transfection with
miR-145 mimics suppressed SGC-7901 cell growth, induced cellular
apoptosis and blocked cell cycle in the G1 phase.
Overexpression of miR-145 was able to inhibit SGC-7901 cell
invasion and migration. Collectively, miR-145 as a tumor suppressor
may inhibit gastric cancer progression. Although the present
results are consistent with previous studies (22–24),
there were differences. The present study systematically studied
the effect of miR-145 on biological behavior, including
proliferation, apoptosis, migration, invasion and cell cycle
distribution of gastric cancer cells for the first time, to the
best of the authors' knowledge. Different from other previous
studies, the effect of miR-145 downregulation on gastric cancer
cells was additionally investigated. Furthermore, notably, a novel
molecular mechanism of the influence of miR-145 on the biological
behavior of gastric cancer cells was identified in the present
study.
c-Myc, an oncogene, is involved in cell
proliferation, transformation and cell death regulation (31). Increased c-Myc protein expression
levels strongly contribute to the apoptotic stimuli of epithelial
cells, including DNA damage (32).
Therefore, the downregulation of c-Myc is necessary for cell cycle
arrest (33). In the present
study, c-Myc expression was significantly decreased in miR-145
overexpressed SGC-7901 cells. In addition, overexpression of
miR-145 was able to inhibit the SGC-7901 cell proliferation and
block cell cycle in G1 phase, which were consistent with
a previous study (34). c-Myc may
be a potential target of miR-145.
The PI3K/AKT signaling pathway may regulate a
variety of biological functions. Activated AKT may induce a
downstream phosphorylation cascade, regulation of cell growth and
survival, proliferation and apoptosis, angiogenesis, cell migration
and cell cycle and other cell activity and biological effects
(35,36). In the present study, it was
observed that overexpression of miR-145 in SGC-7901 cells decreased
the protein expression level of PI3K and p-AKT. The present data
suggested that miR-145 suppressed gastric cancer cell growth by
regulating the PI3K/AKT signaling pathway.
The primary reason for the failure of gastric cancer
treatment is cell infiltration and metastasis. The present study
demonstrated that gastric cancer cell invasion and migration was
suppressed by miR-145. In the MMP family, MMP2 and MMP9 are the
most important enzymes to degrade type IV collagen and serve an
important role in tumor vascularization, tumor cell invasion and
metastasis formation (37).
Therefore, the expression level of MMP2 and MMP9 were detected in
the present study, and it was observed that MMP2 and MMP9 protein
were decreased in miR-145 mimics transfected SGC-7901 cells. These
data suggested that miR-145 inhibited gastric cancer cell invasion
and metastasis by regulating MMP2 and MMP9 expression.
In conclusion, the present study demonstrated that
miR-145 is downregulated in gastric cancer cells. Furthermore, it
was identified that miR-145 inhibited SGC-7901 cell proliferation
and induced cellular apoptosis through repression of the
pro-oncogene c-Myc. In addition, miR-145 suppressed gastric cancer
cell invasion and metastasis by regulating MMP2 and MMP9
expression. Therefore, restoration of miR-145 may be utilized as a
potential novel therapeutic strategy in gastric cancer in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
National Clinical Research Base of Traditional Chinese Medicine in
Jiangsu, China (grant no. JD201511).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and ZS assessed and analyzed the data, and
contributed to the paper preparation. SY contributed to data
analysis. FG collaborated to design the study. All authors
collaborated to interpret the results and develop the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu AM, Huang L, Liu W, Gao S, Han WX and
Wei ZJ: Neoadjuvant chemotherapy followed by surgery versus surgery
alone for gastric carcinoma: Systematic review and meta-analysis of
randomized controlled trials. PLoS One. 9:e869412014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN, 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Memon MA, Subramanya MS, Khan S, Hossain
MB, Osland E and Memon B: Meta-analysis of D1 versus D2 gastrectomy
for gastric adenocarcinoma. Ann Surg. 253:900–911. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu AM, Huang L, Zhu L and Wei ZJ:
Significance of peripheral neutrophil-lymphocyte ratio among
gastric cancer patients and construction of a treatment-predictive
model: A study based on 1131 cases. Am J Cancer Res. 4:189–195.
2014.PubMed/NCBI
|
|
8
|
Huang L, Xu A, Li T, Han W, Wu S and Wang
Y: Detection of perioperative cancer antigen 72-4 in gastric juice
pre-and post-distal gastrectomy and its significances. Med Oncol.
30:6512013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghosn M, Tabchi S, Kourie HR and Tehfe M:
Metastatic gastric cancer treatment: Second line and beyond. World
J Gastroenterol. 22:3069–3077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2010. View Article : Google Scholar
|
|
14
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Michael MZ, O'Connor SM, van Holst
Pellekaan NG, Young GP and James RJ: Reduced accumulation of
specific microRNAs in colorectal neoplasia. Mol Cancer Res.
1:882–891. 2003.PubMed/NCBI
|
|
17
|
Hu J, Guo H, Li H, Liu Y, Liu J, Chen L,
Zhang J and Zhang N: MiR-145 regulates epithelial to mesenchymal
transition of breast cancer cells by targeting Oct4. PLoS One.
7:e459652012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Xia J, Wang N and Zong H: miR-145
inhibits proliferation and invasion of esophageal squamous cell
carcinoma in part by targeting c-Myc. Onkologie. 36:754–758.
2013.PubMed/NCBI
|
|
19
|
Cho WC, Chow AS and Au JS: MiR-145
inhibits cell proliferation of human lung adenocarcinoma by
targeting EGFR and NUDT1. RNA Biol. 8:125–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sachdeva M and Mo YY: MicroRNA-145
suppresses cell invasion and metastasis by directly targeting mucin
1. Cancer Res. 70:378–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui SY, Wang R and Chen LB: MicroRNA-145:
A potent tumour suppressor that regulates multiple cellular
pathways. J Cell Mol Med. 18:1913–1926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei C, Du F, Sun L, Li T, Li T, Min Y, Nie
A, Wang X, Geng L, Lu Y, et al: miR-143 and miR-145 inhibit gastric
cancer cell migration and metastasis by suppressing MYO6. Cell
Death Dis. 8:e31012017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang
Z, Zhu W, Shu Y and Liu P: miR-145, miR-133A and miR-133b inhibit
proliferation, migration, invasion and cell cycle progression via
targeting transcription factor Sp1 in gastric cancer. FEBS Lett.
588:1168–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao P, Xing AY, Zhou GY, Zhang TG, Zhang
JP, Gao C, Li H and Shi DB: The molecular mechanism of microRNA-145
to suppress invasion-metastasis cascade in gastric cancer.
Oncogene. 32:491–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guimarães RM and Muzi CD: Trend of
mortality rates for gastric cancer in Brazil and regions in the
period of 30 years (1980–2009). Arq Gastroenterol. 49:184–188.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo X, Burwinkel B, Tao S and Brenner H:
MicroRNA signatures: Novel biomarker for colorectal cancer? Cancer
Epidemiol Biomarkers Prev. 20:1272–1286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Sempere LF, Galimberti F,
Freemantle SJ, Black C, Dragnev KH, Ma Y, Fiering S, Memoli V, Li
H, et al: Uncovering growth-suppressive MicroRNAs in lung cancer.
Clin Cancer Res. 15:1177–1183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hermeking H and Eick D: Mediation of
c-Myc-induced apoptosis by p53. Science. 265:2091–2093. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maclean KH, Keller UB, Rodriguez-Galindo
C, Nilsson JA and Cleveland JL: c-Myc augments gamma
irradiation-induced apoptosis by suppressing Bcl-XL. Mol Cell Biol.
23:7256–7270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cannell IG, Kong YW, Johnston SJ, Chen ML,
Collins HM, Dobbyn HC, Elia A, Kress TR, Dickens M, Clemens MJ, et
al: p38 MAPK/MK2-mediated induction of miR-34c following DNA damage
prevents Myc-dependent DNA replication. Proc Natl Acad Sci USA.
107:5375–5380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao Y, Qu Y, Dang S, Yao B and Ji M:
MiR-145 inhibits oral squamous cell carcinoma (OSCC) cell growth by
targeting c-Myc and Cdk6. Cancer Cell Int. 13:512013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 (Suppl
2):S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aksamitiene E, Kiyatkin A and Kholodenko
BN: Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt
pathways: A fine balance. Biochem Soc Trans. 40:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|