Introduction
Liver fibrosis is a reversible, wound healing
process which derives from excessive accumulation of the
extracellular matrix (ECM) (1). A
previous epidemiological and animal study indicated that the
consistent fibrosis eventually leads to cirrhosis resulting in
hepatocellular carcinoma (HCC) and liver failure (2). The occurrence of liver fibrosis is
associated with hepatic stellate cell (HSC) activation, ECM
synthesis and degradation, abnormal expression of inflammatory
factors and fiber-associated factors and liver cells apoptosis
(3–5). Previous studies demonstrated that the
activation of HSCs and their conversion to a myofibroblasts-like
phenotype is responsible for the deposition of excessive ECM in the
fibrotic liver (6,7). Therefore, it is of paramount
importance to develop anti-fibrotic therapies.
MicroRNAs (miRNAs/miRs) are highly conserved,
non-coding, small RNAs (21–23 nt) that directly regulate gene
expression at the post-transcriptional level by binding the
3′-untranslated regions (3′-UTRs) of specific mRNA (8). They serve important regulatory roles
in cell proliferation, apoptosis and development of human diseases
(9,10). Dysregulated miRNAs are associated
with various human diseases. As an example, miR-146a was
demonstrated to inhibit the proliferation of HSCs by regulating the
tumor growth factor-β/Smad4 signaling pathway (11), and miR-150 was demonstrated to
inhibit the activation of HSCs (12,13).
Previous studies identified that miRNAs may be an important
diagnostic and therapeutic target of liver fibrosis by regulating
the activation of HSCs (14,15).
Additionally, it was identified that astaxanthin
(AST), a xanthophyll carotenoid, was able to inhibit the activation
of HSCs in the progression of liver fibrosis (16,17).
It is separated from other molecules of the carotene subclass,
which contains 13 conjugated double polyunsaturated bonds
responsible for the unique chemical properties of AST (18). Due to biological implications,
anti-oxidant and anti-inflammatory effects of AST allow it to
protect against oxidative stress-associated and inflammatory
disease (17). In addition, AST
may have potential effects on various diseases, including cancer,
obesity, hypertriglyceridemia, hypercholesterolemia,
cardiovascular, gastrointestinal, liver, neurodegenerative,
ophthalmologic, bone, reproductive system and skin diseases
(19,20). However, the anti-fibrotic mechanism
of AST is not fully understood. Furthermore, the miRNAs in HSCs
involved in the anti-fibrotic mechanism of AST remain unknown.
Previous studies demonstrated that miR-29 family members are
downregulated in mouse models of liver fibrosis and in human
fibrotic livers, and its downregulation is negatively correlated
with the activation of HSCs (10,21).
However, at present, the role of miR-29b in AST regulation of HSCs
has not been reported, to the best of the authors' knowledge. In
the present study, in order to identify the role of AST in
anti-fibrotic effect and its effect on promoting HSCs apoptosis and
inhibiting HSCs proliferation, the role of miR-29b and specific
apoptosis-associated genes in the AST-treated HSCs was
investigated.
Materials and methods
Cell culture and treatment with
AST
The human HSC line LX-2 was provided by the Central
Laboratory of Central South University Xiangya Cell Library (Hunan,
China). Cells were cultured in low-glucose Dulbecco's modified
Eagle's medium (HyClone; GE Heathcare Life Sciences, Logan, UT,
USA) containing 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and
100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a
37°C cell culture incubator providing 5% CO2 and 55%
humidity.
AST standard (purity ≥98%) was purchased from Dr.
Ehrenstorfer GmbH (Augsburg, Germany). Prior to use, AST was
completely dissolved in dimethyl sulfoxide (DMSO; Beijing Solarbio
Science and Technology Co., Ltd., Beijing, China) to obtain AST
stock solution (8 mM) and stored at −20°C. A filter membrane (0.45
µm) was used to get rid of bacterium. Prior to cell treatment, AST
stock was incubated at 37°C for 30 min and dissolved in the cell
culture medium to obtain preferred concentrations. The final FBS
concentration in AST containing medium was 10%, and subsequently an
equal amount of FBS and DMSO were added to controls. LX-2 cells
were treated with AST at various concentrations (10, 20 and 40 µM)
or DMSO as a control in 37°C for 24 or 48 h. Cells were collected
to prepare total RNA and protein for subsequent experiments.
Transfection
LX-2 cells were seeded in 6-well plates and
subsequently serum-starved overnight when cells reached 30–50%
confluency. The miR-29b mimics (5′-UAGCACCAUUUGAAAUCAGUGUU-3′;
antisense, 5′-AUCGUGGUAAACUUUAGUCACAA-3′), miR-29b inhibitors
(5′-UAGCACCAUUUGAAAUCAGUGUU-3′) were synthesized by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). Subsequent to being starved
overnight, LX-2 cells were transfected with either miR-29b mimics
(miR-29b; 100 nmol/l) or mimic negative control (Con miR; 100
nmol/l) or miR-29b inhibitors (anti-miR-29b; 100 nmol/l) or
inhibitor negative control (Con Inh; 100 nmol/l) at 37°C for 48 h
using riboFECT™ CP reagent (Guangzhou RiboBio Co., Ltd.)
according to the manufacturer's protocol. When treated with AST
(DMSO, 10, 20 and 40 µM) for 24 and 48 h, miR-29b was upregulated
in a dose-dependent manner, particularly at 40 µM. Therefore, 40 µM
was selected for subsequent experiments. Subsequent to replacing
the cell culture medium, LX-2 cells were immediately treated with
AST (40 µM) or the vehicle (DMSO) in 37°C for 48 h. Cells were
collected to prepare total RNA and protein.
MTT analysis
Cell proliferation was detected by MTT assay, and
LX-2 cells were seeded in 96-well plates (Costar; Corning, Inc.,
Corning, NY, USA) in medium containing 10% FBS at ~2,000
cells/well. LX-2 cells were only treated with AST (ranging between
5 and 80 µM) in 37°C for 12, 24, 48 and 72 h. On the other hand,
LX-2 cells were transfected with miR-29b mimics or mimic negative
control or inhibitors or inhibitor negative control in 37°C for 48
h and subsequently AST (40 µM) or the vehicle (DMSO) were added to
the refreshed medium for 48 h. To assess cell viability, 20 µl MTT
solution (5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was added into each well for 4 h at 37°C. Following removal of
culture medium, 150 µl DMSO was added to each well. After 10 min,
absorbance (A) at a wavelength of 450 nm (A450) was
detected by a multifunctional microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The proliferation inhibition
rate was calculated from the following model: Proliferation
inhibition rate =[1-(A experimental group-A blank
group)/(A control group-A blank
group)]x100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells of each group
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The RNA quality and
quantity were determined with a Nanodrop spectrophotometer
(Nanodrop 2000c; Thermo Fisher Scientific, Inc., Wilmington, DE,
USA), and the RNA purity was determined by gel electrophoresis. RNA
(2 µg) was reverse-transcribed by Transcriptor First Strand cDNA
Synthesis kit (Roche Diagnostics, Bazel, Switzerland). RT-qPCR was
performed to determine the original number of specific transcripts
associated with fibrotic markers using FastStart Universal SYBR
Green Master (Roche Diagnostics). The miRNAs expression was
normalized to U6 as a housekeeping gene and the mRNA expression was
normalized against β-actin. RT-qPCR was conducted using the
PikoReal™ Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientifc, Inc.) with the following reaction conditions: Initial
denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C
for 15 sec, annealing at 58°C for 60 sec and extension at 65°C for
30 sec; followed by melting curve analysis. Each test was performed
in triplicate and the 2−ΔΔCq method (22) was used to calculate the expression
of miRNAs and mRNA in LX-2 cells. The primers sequences used in the
present study are listed in Table
I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer | Sequence
(5′-3′) |
|---|
| hsa-miR-29b | RT |
CCTGTTGTCTCCAGCCACAAAAGAGCACAATATTTCAGGAGACAACAGGAACACTG |
|
| Forward |
CGGGCTAGCACCATTTGAAAT |
|
| Reverse |
CAGCCACAAAAGAGCACAAT |
| U6 | RT |
AAAATATGGAACGCTTCACG |
|
| Forward |
CGCTTCGGCAGCACATATACTAAAATTGGAAC |
|
| Reverse |
GCTTCACGAATTTGCGTGTCATCCTGC |
| α-SMA | Forward |
GGCTCTGGGCTCTGTAAGG |
|
| Reverse |
CTCTTGCTCTGGGCTTCATC |
| Col1a1 | Forward |
CCCGGGTTTCAGAGACAACTTC |
|
| Reverse |
TCCACATGCTTTATTCCAGCAATC |
| Bcl-2 | Forward |
GTGCCTGCTTTTAGGAGACCGA |
|
| Reverse |
GAGACCACACTGCCCTGTTGATC |
| Bax | Forward |
TTTGCTTCAGGGTTTCATCCA |
|
| Reverse |
GAGACACTCGCTCAGCTTCTTG |
| Caspase-3 | Forward |
GTAGAAGTCTAACTGGAAAACCCAA |
|
| Reverse |
CATGTCATCATCAACACCACTGTCT |
| β-actin | Forward |
TCCTCCCTGGAGAAGAGCTA |
|
| Reverse |
TCAGGAGGAGCAATGATCTTG |
Western blot analysis
LX-2 cells were lysed by radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) to obtain total protein, and a bicinchoninic protein assay
kit (CWBIO Corporation, Allston, MA, USA) was used to detect the
protein concentration. Total protein samples (30–50 µg) were
electrophoresed on 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). After the
proteins were fully transferred, the membranes were blocked with 5%
bovine serum albumin (w/v) in Tween-20/Tris-buffered saline (TBST)
at room temperature for 2 h, followed by 2 h incubation at room
temperature with antibodies against B cell lymphoma (Bcl)-2
(1:1,000; cat. no. ab32124; Abcam, Cambridge, UK), α-SMA (1:3,000;
cat. no. ab32575; Abcam), collagen α-1(I) chain (Col1a1; 1:5,000;
cat. no. ab138492; Abcam) and Bax (1:5,000; cat. no. ab32503;
Abcam) and β-tubulin (1:1,000; cat. no. 10094-1-AP; ProteinTech
Group, Inc., Chicago, IL, USA), washed three times with TBST and
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:5,000; cat. no. SA00001-2; ProteinTech Group,
Inc.) at room temperature for 1.5 h. The specific protein was
detected with an enhanced chemiluminescence (ECL-plus; Thermo
Fisher Scientific, Inc.). Band densities were quantified using an
image analyzer with Quantity One software (version 4.62; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All protein quantifications
were adjusted according to their corresponding β-tubulin level,
which was not varied with different treatment conditions.
Target prediction
TargetScan (www.targetscan.org/vert_72/) and miRanda (www.microrna.org) databases were used to predict the
target genes of miR-29b. The predicted target genes were subjected
to Gene Ontology enrichment analysis and biological pathway
enrichment analysis by the Database for Annotation Visualization
and Integrated Discovery database (david.ncifcrf.gov/).
Apoptosis assay
An Annexin-V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) was used to detect the
apoptosis of LX-2 cells, according to the manufacturer's protocol.
LX-2 cells were seeded in 6-well plates at ~5×104
cells/ml and subsequently serum-starved overnight when cells
reached 30–50% confluence. LX-2 cells were only treated with AST
(ranging between 5 and 80 µM) at 37°C for 12, 24, 48 and 72 h.
However, LX-2 cells were transfected with miR-29b mimics or mimic
negative control or inhibitors or inhibitor negative control at
37°C for 48 h and subsequently AST (40 µM) or the vehicle (DMSO)
were added to the refreshed medium for 48 h. Following treatment,
LX-2 cells were washed with cold PBS, collected by centrifugation
(2,000 × g), and suspended in 500 µl 1× binding buffer and
subsequently incubated with 5 µl Annexin V-FITC and 5 µl PI for 15
min at room temperature in the dark. Subsequently, apoptosis was
analyzed with a FACS Calibur flow cytometer (BD FACSCalibur™; BD
Biosciences, San Jose, CA, USA). A minimum of 10,000 cells per
sample were acquired and analyzed using FlowJo software (version
7.6.1; Tree Star, Inc., Ashland, OR, USA). The experiments were
repeated three times.
Statistical analysis
All experiments were repeated in triplicate and
results are expressed as the mean ± standard deviation and were
analyzed using unpaired Student's t-test and one-way analysis of
variance tests with Duncan's post hoc test. SPSS for Windows
software (version 13.0; IBM Corp., Armonk, NY, USA) was used for
statistical analysis. P<0.05 was used to indicate a
statistically significant difference.
Results
AST increases miR-29b expression in
the LX-2 cells
To investigate whether AST altered miR-29b
expression levels in LX-2 cells, RT-qPCR was performed following 24
and 48 h of treatment with AST (DMSO, 10, 20 and 40 µM). After 24
or 48 h treatment, miR-29b was upregulated in a dose-dependent
manner compared with the DMSO control group (Fig. 1).
Successful experimental transfection
efficiency
The transfection efficiency of miR-29b mimics
(miR-29b; 100 nmol/l) and Con miR group by RT-qPCR were 1.00±0.04
and 22.35±0.84, respectively. Compared with the Con miR group, the
expression of the miR-29b group was significantly increased
(P<0.05; Fig. 2A). The
transfection efficiency of miR-29b inhibitors (anti-miR-29b; 100
nmol/l) and Con Inh group by RT-qPCR were 1.01±0.11 and 0.58±0.08,
respectively. Compared with the Con Inh group, the expression of
anti-miR-29b group was significantly decreased (P<0.05; Fig. 2B). The recommended dose of 100
nmol/l was used as the final concentration for transfection,
according to the manufacturer's protocol.
AST inhibits the proliferation of LX-2
cells by the miR-29b
To investigate the effect of AST on LX-2 cells, MTT
cell viability assay was performed. After treatment with AST for
24, 48 and 72 h, the absorbance in the AST treated groups was
decreased in a dose-dependent manner compared with the DMSO control
group. Results suggested that AST could inhibit the viability of
LX-2 cells and the cell viability decreased by increasing AST
concentration (Fig. 3A).
 | Figure 3.AST inhibits the LX-2 cells
proliferation by regulating miR-29b. (A) LX-2 cells were treated
with various concentration of AST (5, 10, 20, 40 and 80 µM) or the
vehicle (DMSO) for 12, 24, 48 and 72 h. **P<0.01, ***P<0.001,
****P<0.0001 vs. DMSO. (B) LX-2 cells were transfected with
either miR-29b mimics or miR-29b mimic negative control or miR-29b
inhibitors or miR-29b inhibitor negative control for 48 h, and then
treated with AST (40 µM) or the vehicle for 48 h. Data are
expressed as the mean ± standard deviation. **P<0.01,
****P<0.0001 vs. Con miR or Con Inh group. AST, astaxanthin;
Con, control; DMSO, dimethyl sulfoxide; Inh, inhibitor; miR,
microRNA. |
The MTT cell viability assay was additionally used
to examine the effect of miR-29b on viability inhibition of AST on
LX-2 cells. Following transfection with miR-29b mimics (miR-29b),
mimic negative control (Con miR), miR-29b inhibitors (anti-miR-29b)
and inhibitor negative control (Con Inh), 40 µM AST were added to
the culture medium for 48 h. The results demonstrated that the cell
viability in the miR-29b+AST group was significantly decreased
compared with the Con miR group, and the cell viability of the
miR-29b group was additionally significantly decreased compared
with the Con miR group (Fig. 3B).
Conversely, the cell viability of the anti-miR-29b+AST and
anti-miR-29b groups was significantly increased compared with the
Con-Inh group (Fig. 3B).
Therefore, miR-29b may serve a key role in AST anti-viability
effect on the LX-2 cells.
To clarify the roles of AST in collagen deposition,
RT-qPCR and western blot analysis were used to examine the
expression of α-SMA and Col1a1 (Fig.
4). The results demonstrated that AST significantly reduced the
mRNA expression of α-SMA (P<0.05; Fig. 4A) and Col1a1 (P<0.05; Fig. 4B). Using Quantity One software, the
band densities were quantified and it was demonstrated that the
protein expression levels of α-SMA (P<0.05; Fig. 4A and C) and Col1a1 (P<0.05;
Fig. 4B and D) were significantly
decreased in the AST treated group. These results suggested a
notable inhibitory effect of AST. To further determine the role of
miR-29b in collagen deposition, LX-2 cells were transfected with
miR-29b mimics or inhibitors and treated with AST. As presented in
Fig. 5, overexpression of miR-29b
significantly suppressed mRNA and protein expression of α-SMA
(Fig. 5A), which was reversed by
miR-29b inhibition (Fig. 5B).
Similarly, overexpression of miR-29b significantly suppressed mRNA
and protein expression of Col1a1 (Fig.
5C), and AST aggravated this condition. However, this result
was reversed by miR-29b inhibition (Fig. 5D) suggesting that AST can suppress
ECM deposition possibly through miR-29b.
 | Figure 5.AST inhibits the expression of α-SMA
and Col1a1 in LX-2 cells. LX-2 cells were transfected with either
miR-29b mimics or miR-29b mimic negative control or miR-29b
inhibitors or miR-29b inhibitor negative control for 48 h, and
treated with AST (40 µM) or the vehicle for 48 h. (A and B) Protein
and mRNA expression levels of α-SMA and (C and D) protein and mRNA
expression levels of Col1a1 as measured by RT-qPCR and western
blotting. Data are presented as the mean ± standard deviation.
*P<0.05, ***P<0.001, ****P<0.0001 vs. Con miR or Con Inh
group; #P<0.05 vs. miR-29b or anti-miR-29b group.
α-SMA, α-smooth muscle actin; AST, astaxanthin; Col1a1, collagen
α-1(I) chain; Con, control; DMSO, dimethyl sulfoxide; Inh,
inhibitor; miR, microRNA. |
AST induces apoptosis through miR-29b
in LX-2 cells
TargetScan and miRanda databases predicted that
miR-29b had ~1,000 target genes, some of which are involved in cell
apoptosis (data not shown). It was identified that the
3′-untranslated region (UTR) of Bcl-2 contains putative binding
sites for miR-29b (Fig. 6A).
Therefore, the effect of miR-29b on Bcl-2 gene expression in the
AST-treated LX-2 cells was further examined. The Bcl-2 family
serves a key role in apoptosis (23), and RT-qPCR and western blot
analysis were used to detect the mRNA and protein expression levels
of Bcl-2, Bax and Caspase-3, respectively (Fig. 6B-D). AST treatment resulted in a
significant decreased expression of Bcl-2 (Fig. 6B), together with increased
expression of Bax (Fig. 6C) and
Caspase-3 (Fig. 6D). Accompanied
with the upregulation of miR-29b, the mRNA and protein expression
levels of Bcl-2 were decreased (Fig.
7A), whereas following miR-29b inhibition, AST treatment
reversed this (Fig. 7B).
Additionally, the mRNA and protein expression levels of Bax were
increased (Fig. 7C) in the miR-29b
upregulation group, whereas, following miR-29b inhibition, AST
treatment reversed this (Fig. 7D).
Similarly, the mRNA and protein expression levels of caspase-3 were
increased when miR-29b was upregulated (Fig. 7E), whereas following miR-29b
inhibition, AST treatment reversed this (Fig. 7F).
 | Figure 7.AST regulates the expression levels
of apoptosis-associated proteins by regulating miR-29b in LX-2
cells. LX-2 cells were transfected with either miR-29b mimics or
miR-29b mimic negative control or miR-29b inhibitors or miR-29b
inhibitor negative control for 48 h, and then treated with AST (40
µM) or the vehicle for 48 h. mRNA and protein expression levels of
(A and B) Bcl-2, (C and D) Bax and (E and F) Caspase-3 were
investigated by RT-qPCR and western blotting. Data are expressed as
the mean ± standard deviation. *P<0.05, ***P<0.001,
****P<0.0001 vs. Con miR or Con Inh group.
#P<0.05; ##P<0.01 vs. miR-29b or
anti-miR-29b group. AST, astaxanthin; Bax, Bcl-2-associated X
protein; Bcl, B cell lymphoma; Inh, inhibitor; miR, microRNA;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
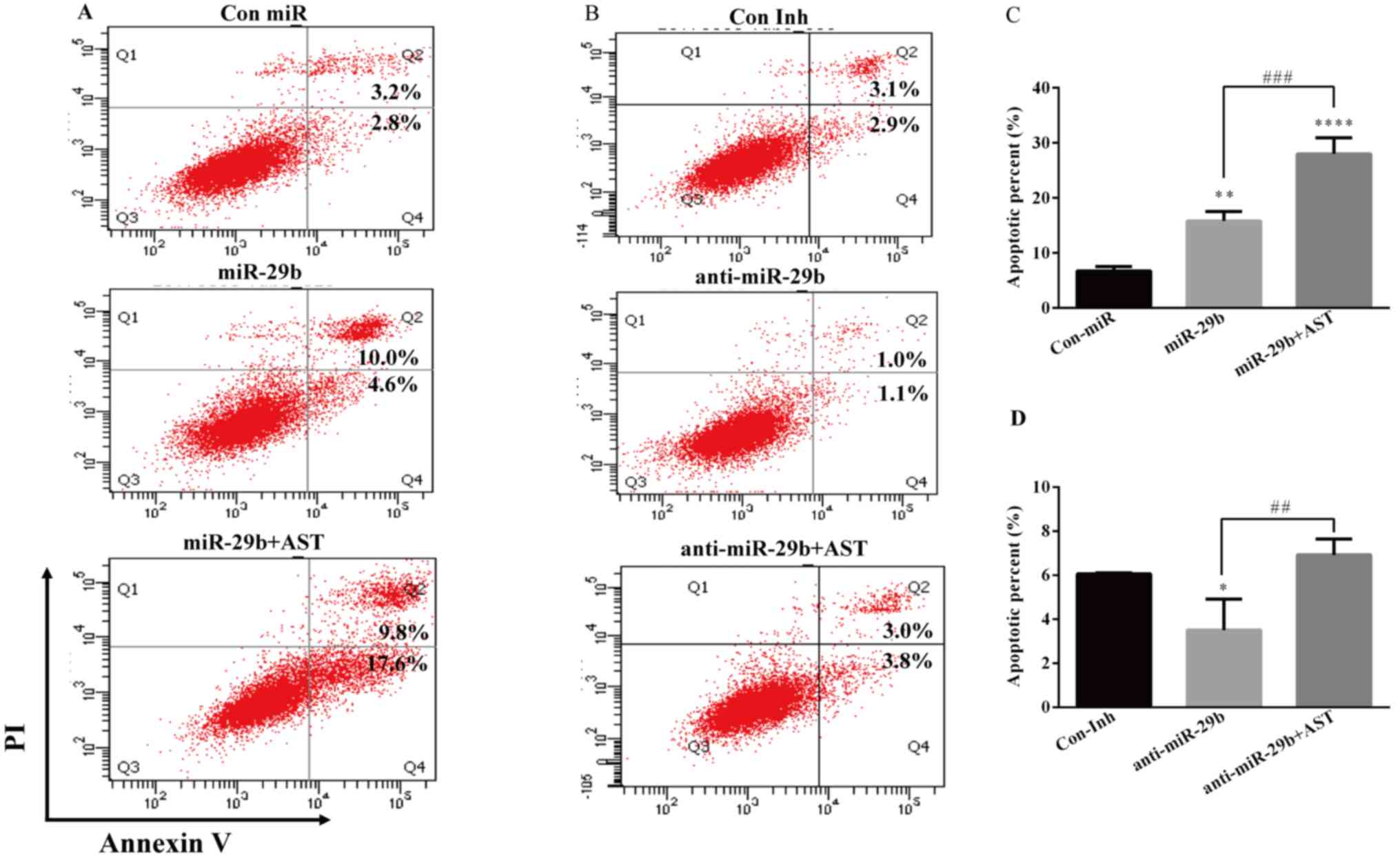
In order to determine the role of miR-29b in the
observed suppressive effect of cell growth by AST, the Annexin
V-FITC/PI double staining and flow cytometry was used to evaluate
the cell apoptosis. When miR-29b is overexpressed, the AST-treated
group demonstrated increased expression of Annexin-V compared with
the Con miR and miR-29b alone groups (Fig. 8A and C). However, following miR-29b
inhibition AST treatment increased the expression of Annexin-V
compared with the Con Inh and anti-miR-29b alone groups (Fig. 8B and D). These findings indicated
that AST induces cell apoptosis potentially through miR-29b.
Discussion
Liver fibrosis is a common cause of chronic liver
disease and HSCs serve an important role in the development of
liver fibrosis (3). Subsequent to
exposing HSCs to various injurious agents, such as pro-inflammatory
cytokines and irradiation, the cells may be transformed into
myofibroblasts, and expression of α-SMA and Col1a1 may be increased
followed by the accumulation of extracellular matrix (24). Therefore, α-SMA and col1a1 are
markers of HSC activation. It was identified that AST inhibits the
activation of HSCs by reducing the expression of α-SMA and Col1a1
(25). Although the abnormal
expression of miRNAs has been reported on various diseases, the
molecular mechanisms by which miRNAs and AST modulate the process
of liver fibrosis and the activation of HSCs is still unknown. The
current study demonstrated that AST may promote apoptosis of HSCs
and inhibit their proliferation through upregulation of miR-29b. In
the present study, it was shown that miR-29b positively regulates
the HSCs apoptosis by suppressing the expression of Bcl-2, whereas
AST increased miR-29b expression levels, leading to enhanced HSCs
apoptosis.
AST is a non-vitamin A carotenoid which can be found
in Haematococcus pluvialis, shrimp, crab and salmon
(16,26). AST is beneficial as a therapeutic
agent for various diseases due to its anti-oxidative property
(27–29). Yang et al (25) observed that AST may be used as a
preventive or therapeutic agent to prevent liver fibrosis by
blocking the tumor growth factor-β1 (TGF-β1) signaling pathway.
Additionally, AST inhibited the activation of HSCs and development
of ECM via decreasing the expression of nuclear factor-κB and
TGF-β1. It also reduced energy production of HSCs by downregulating
the level of autophagy (30).
However, the specific anti-fibrotic mechanism of AST remains
unknown. To date, natural chemical-based drugs, including AST, in
particular, are the main research direction for the treatment of
liver fibrosis (31–33). Despite the protective effect of AST
against liver fibrosis, however, the mechanism needs to be further
explored. Therefore, AST is a crucial clinical component that
requires consider and greater knowledge of the molecular mechanisms
involved in its anti-fibrotic effect will assist the development of
novel treatment targets for eradicating liver fibrosis and other
chronic liver diseases.
Dysregulation of miRNAs contribute to drug
resistance in various cancer types (34), including gastric cancer,
non-small-cell lung cancer, myeloid leukemia and breast cancer
(35–38), as well as hepatocellular carcinoma.
Thus, to determine the mechanism of miRNAs and AST in liver
fibrosis is important. The miRNA-29 family includes miR-29a,
miR-29b, and miR-29c (39).
Previous studies demonstrated that the expression of miR-29b was
decreased in activated HSCs (40,41).
Several studies have revealed that deviant
expression of miR-29b is widespread in the majority of human
cancers and serve as a tumor suppressor affecting the cancer
progression (42). Wang et
al (41) found that miR-29b
can prevent liver fibrogenesis by inhibiting HSC activation and
inducing HSC apoptosis via inhibiting Phosphoinositide 3 kinase
(PI3K)/Akt pathway. Additionally, Li et al (43) reported that AST induces
hepatocellular cells apoptosis through negative activation of
PI3K/Akt. It may be inferred that AST may prevent liver
fibrogenesis by regulating miR-29b/PI3K/Akt (43) (Fig.
9). Bcl-2 and myeloid cell leukemia-1 (Mcl-1) protein, a
potent, multidomain anti-apoptotic protein of the Bcl-2 family, is
downregulated by miR-29b (44,45).
In addition, miR-29b may sensitize HCC cells to apoptosis by
directly targeting the anti-apoptotic molecules Bcl-2 and Mcl-1
using luciferase reporter gene assay (44). These results support that apoptosis
may be reinforced by miR-29 via a mitochondrial pathway involving
Mcl-1 and Bcl-2, and implicate the potential application of miR-29
in prognosis prediction and in cancer therapy, but this needs to be
investigated further. It is important to consider that miR-29b
demonstrated an ability to target apoptosis regulators in the AST
treated HSCs, and it was demonstrated that Bcl-2 serves as a
crucial effector of miR-29b in the AST treated HSCs (Fig. 9).
In the present study, miR-29b was a possible
therapeutic marker for liver fibrosis, and it was identified that
miR-29b is upregulated by AST in LX-2 cells compared with the
vehicle control group. Furthermore, upregulation of miR-29b by AST
prevented LX-2 cells proliferation and induced the LX-2 apoptosis
through modulating expression of Bcl-2. However, the possibility
that the observed effects of AST and miR-29b are additive, as
opposed to that AST is a regulator of miR-29b, requires further
examination. In conclusion, the present data provided evidence that
AST modulates miR-29b in promoting apoptosis and inhibiting
proliferation of HSCs in vitro. The experimental data could
offer a way to pinpoint the miR-29b/Bcl-2 interaction as a novel
therapeutic application to care for people suffering from liver
fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81874260
and 81302416), the Guangdong Science and Technology Planning
Project (grant no. 2014A020212297) and the Dongguan Science and
Technology Planning Project (grant no. 2014108101053).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ, TZ and HW conceived and designed the
experiments. SZ, TW and FL performed the experiments. QJ, HL and TH
analyzed the data. SZ and TZ wrote the paper. HW helped to revise
the manuscript. All authors read and approved the final version of
this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun M and Kisseleva T: Reversibility of
liver fibrosis. Clin Res Hepatol Gastroenterol. 39 (Suppl
1):S60–S63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carloni V, Luong TV and Rombouts K:
Hepatic stellate cells and extracellular matrix in hepatocellular
carcinoma: More complicated than ever. Liver Int. 34:834–843. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campana L and Iredale JP: Regression of
liver fibrosis. Semin Liver Dis. 37:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zoubek ME, Trautwein C and Strnad P:
Reversal of liver fibrosis: From fiction to reality. Best Pract Res
Clin Gastroenterol. 31:129–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Puche JE, Saiman Y and Friedman SL:
Hepatic stellate cells and liver fibrosis. Compr Physiol.
3:1473–1492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elpek GÖ: Cellular and molecular
mechanisms in the pathogenesis of liver fibrosis: An update. World
J Gastroenterol. 20:7260–7276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh TR, Gupta A and Suravajhala P:
Challenges in the miRNA research. Int J Bioinform Res Appl.
9:576–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Q, Yang Z, Shi Y and Fan D: MiRNAs in
human cancers: The diagnostic and therapeutic implications. Curr
Pharm Des. 20:5336–5347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou B, Li Z, Yang H and He N:
Extracellular miRNAs: Origin, function and biomarkers in hepatic
diseases. J Biomed Nanotechnol. 10:2865–2890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Y, Huang C, Sun X, Long XR, Lv XW and
Li J: MicroRNA-146a modulates TGF-beta1-induced hepatic stellate
cell proliferation by targeting SMAD4. Cell Signal. 24:1923–1930.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng J, Lin Z, Dong P, Lu Z, Gao S, Chen
X, Wu C and Yu F: Activation of hepatic stellate cells is
suppressed by microRNA-150. Int J Mol Med. 32:17–24. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Venugopal SK, Jiang J, Kim TH, Li Y, Wang
SS, Torok NJ, Wu J and Zern MA: Liver fibrosis causes
downregulation of miRNA-150 and miRNA-194 in hepatic stellate
cells, and their overexpression causes decreased stellate cell
activation. Am J Physiol Gastrointest Liver Physiol. 298:G101–G106.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitano M and Bloomston PM: Hepatic
stellate cells and microRNAs in pathogenesis of liver fibrosis. J
Clin Med. 5:E382016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He Y, Huang C, Zhang SP, Sun X, Long XR
and Li J: The potential of microRNAs in liver fibrosis. Cell
Signal. 24:2268–2272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ambati RR, Phang SM, Ravi S and
Aswathanarayana RG: Astaxanthin: Sources, extraction, stability,
biological activities and its commercial applications-a review. Mar
Drugs. 12:128–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JT and Kotani K: Astaxanthin as a
potential protector of liver function: A review. J Clin Med Res.
8:701–704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higuera-Ciapara I, Félix-Valenzuela L and
Goycoolea FM: Astaxanthin: A review of its chemistry and
applications. Crit Rev Food Sci Nutr. 46:185–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fakhri S, Abbaszadeh F, Dargahi L and
Jorjani M: Astaxanthin: A mechanistic review on its biological
activities and health benefits. Pharmacol Res. 136:1–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guerin M, Huntley ME and Olaizola M:
Haematococcus astaxanthin: Applications for human health and
nutrition. Trends Biotechnol. 21:210–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roderburg C, Urban GW, Bettermann K, Vucur
M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi
M, et al: Micro-RNA profiling reveals a role for miR-29 in human
and murine liver fibrosis. Hepatology. 53:209–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophy Res Commun. 500:26–34.
2018. View Article : Google Scholar
|
|
24
|
Kisseleva T and Brenner DA: Role of
hepatic stellate cells in fibrogenesis and the reversal of
fibrosis. J Gastroenterol Hepatol. 22 (Suppl 1):S73–S78. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Kim B, Park YK, Koo SI and Lee JY:
Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression
by inhibiting Smad3 activation in hepatic stellate cells. Biochim
Biophys Acta. 1850:178–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou TB, Jia Q, Li HW, Wang CX and Wu HF:
Response surface methodology for ultrasound-assisted extraction of
astaxanthin from Haematococcus pluvialis. Mar Drugs. 11:1644–1655.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang XS, Zhang X, Zhou ML, Zhou XM, Li N,
Li W, Cong ZX, Sun Q, Zhuang Z, Wang CX and Shi JX: Amelioration of
oxidative stress and protection against early brain injury by
astaxanthin after experimental subarachnoid hemorrhage. J
Neurosurg. 121:42–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo SX, Zhou HL, Huang CL, You CG, Fang Q,
Wu P, Wang XG and Han CM: Astaxanthin attenuates early acute kidney
injury following severe burns in rats by ameliorating oxidative
stress and mitochondrial-related apoptosis. Mar Drugs.
13:2105–2123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ravi Kumar S, Narayan B, Sawada Y,
Hosokawa M and Miyashita K: Combined effect of astaxanthin and
squalene on oxidative stress in vivo. Mol Cell Biochem. 417:57–65.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen M, Chen K, Lu J, Cheng P, Xu L, Dai
W, Wang F, He L, Zhang Y, Chengfen W, et al: Protective effect of
astaxanthin on liver fibrosis through modulation of TGF-β1
expression and autophagy. Mediators Inflamm. 2014:9545022014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Böttcher K and Pinzani M: Pathophysiology
of liver fibrosis and the methodological barriers to the
development of anti-fibrogenic agents. Adv Drug Deliv Rev. 121:3–8.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schuppan D and Kim YO: Evolving therapies
for liver fibrosis. J Clin Invest. 123:1887–1901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bansal R, Nagorniewicz B and Prakash J:
Clinical advancements in the targeted therapies against liver
fibrosis. Mediators Inflamm. 2016:76297242016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tutar L, Tutar E and Tutar Y: MicroRNAs
and cancer; an overview. Curr Pharm Biotechnol. 15:430–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin VY and Chu KM: MiRNA as potential
biomarkers and therapeutic targets for gastric cancer. World J
Gastroenterol. 20:10432–10439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng B, Zhang K, Wang R and Chen L:
Non-small-cell lung cancer and miRNAs: Novel biomarkers and
promising tools for treatment. Clin Sci (Lond). 128:619–634. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song SJ and Pandolfi PP: MicroRNAs in the
pathogenesis of myelodysplastic syndromes and myeloid leukaemia.
Curr Opin Hematol. 21:276–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaboli PJ, Rahmat A, Ismail P and Ling KH:
MicroRNA-based therapy and breast cancer: A comprehensive review of
novel therapeutic strategies from diagnosis to treatment. Pharmacol
Res. 97:104–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fiserova B, Kubiczkova L, Sedlarikova L,
Hajek R and Sevcikova S: The miR-29 family in hematological
malignancies. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
159:184–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang C, Bu S and Fan X: Suppressive
effect of microRNA-29b on hepatic stellate cell activation and its
crosstalk with TGF-β1/Smad3. Cell Biochem Funct. 34:326–333. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Chu ES, Chen HY, Man K, Go MY,
Huang XR, Lan HY, Sung JJ and Yu J: microRNA-29b prevents liver
fibrosis by attenuating hepatic stellate cell activation and
inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget.
6:7325–7338. 2015.PubMed/NCBI
|
|
42
|
Yan B, Guo Q, Fu FJ, Wang Z, Yin Z, Wei YB
and Yang JR: The role of miR-29b in cancer: Regulation, function,
and signaling. Onco Targets Ther. 8:539–548. 2015.PubMed/NCBI
|
|
43
|
Li J, Dai W, Xia Y, Chen K, Li S, Liu T,
Zhang R, Wang J, Lu W, Zhou Y, et al: Astaxanthin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells via inhibition of Nf-κb P65 and Wnt/β-catenin in
vitro. Mar Drugs. 13:6064–6081. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y,
Jia WH and Zhuang SM: Effects of microRNA-29 on apoptosis,
tumorigenicity, and prognosis of hepatocellular carcinoma.
Hepatology. 51:836–845. 2010.PubMed/NCBI
|
|
45
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|