Introduction
Retinoblastoma is an intraocular malignant tumor
derived from immature retinal cells (1). This malignancy is commonly observed
in children <3 years old and poses a severe threat to vision and
life. The intraocular proliferation and migration of retinoblastoma
cells may lead to the necrosis of retinal tissues and subsequent
retinal detachment, in addition to the development of intracranial
metastasis and metastasis to the optic nerve (2). To the best of our knowledge, the
current strategies available for retinoblastoma treatment are
limited, and include surgical resection, radiotherapy and systemic
chemotherapy (3). Although the
5-year survival rate of early diagnosed patients has improved to
>90% over recent years (4), the
side effects of the currently available therapies include facial
defects, optic nerve injury, visual disorders and drug toxicity.
Therefore, novel treatments and therapeutic strategies are required
to improve the outcomes of patients with retinoblastoma.
Arctigenin (ATG) is an active compound extracted
from the seeds of greater burdock (Arctium lappa Linnaeus),
a plant used in traditional Chinese herbal medicine. ATG has been
demonstrated to exhibit pharmacological activities in the treatment
of diabetes (5), obesity (6) and inflammation (7). In addition, in vitro and in
vivo studies demonstrated that ATG possesses antiproliferative,
proapoptotic, antimetastatic and drug-resistance-decreasing effects
in various types of cancer by influencing the activity of numerous
signaling pathways (8,9) and molecular markers (10,11).
However, the effects of ATG on the biological progression of
retinoblastoma remain unclear.
The Notch signaling pathway mediates signal
transduction between adjacent cells and serves an important role in
cancer progression (12,13). Dysregulation of the Notch signaling
pathway was observed in various types of cancer (14,15).
In mammals there are five main ligands: Jagged (JAG)1, JAG2, and
δ-like canonical Notch ligands 1, 3 and 4. The interaction between
one ligand and one of the four Notch receptors (NOTCH1-4) activates
cleavage of the receptor (13).
Following proteolytic cleavage, the Notch intracellular domain
(NICD) is released from the cell membrane and enters the nucleus to
activate transcription of its downstream genes (13). JAG1 is an important Notch ligand
and is able to promote activation of the Notch signaling pathway,
serving as an oncogene in certain types of cancer (16). The present study aimed to
investigate the effects of ATG on retinoblastoma and its underlying
molecular mechanism by examining the involvement of the JAG1-Notch
signaling pathway.
Materials and methods
Reagents and cell culture
ATG (≥95% purity) was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany), dissolved in dimethyl sulfoxide
(DMSO) at a concentration of 50 mM and stored at −20°C. The human
retinoblastoma cell line Y79 was purchased form The Cell Bank of
Type Culture Collection of Chinese Academy of Science (Shanghai,
China) and cultivated in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 20% fetal
bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.),
streptomycin (100 U/ml) and penicillin (100 U/ml) at 37°C in a
humidified atmosphere containing 5% CO2.
Plasmid construction and
transfection
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate RNA from Y79 cells.
Subsequently, cDNA was synthesized from RNA using the PrimeScript™
reverse transcription (RT) reagent kit with genomic DNA Eraser
(Takara Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. The forward
(5′-CCCAAGCTTATGCGTTCCCCACGGACGC-3′) and reverse
(5′-CCGGAATTCCTATACGATGTACTCCATTCGGTTTAAGCTC-3′) primers were
designed for cloning the coding sequence of JAG1 using the cDNA
extracted from Y79 cells as a template. The polymerase chain
reaction (PCR) was performed using the PrimeSTAR HS DNA polymerase
(Takara Biotechnology Co., Ltd.) with the conditions as follows:
Initial denaturation at 94°C for 10 min followed by 30 cycles each
consisting of 98°C for 20 sec, 50°C for 20 sec, and 72°C for 5 min
and a final extension at 72°C for 10 min. The obtained DNA was
subsequently cloned into a pcDNA3.1(+) plasmid (Invitrogen; Thermo
Fisher Scientific, Inc.). The generated recombinant plasmid
pcDNA-JAG1 was sequenced by Sangon Biotech Co., Ltd. (Shanghai,
China). Cells (2×105/well) was transfected with 500 ng
plasmid using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Following incubation for 48 h, cells were harvested for further
experimentation
Reverse transcription-quantitative
(RT-q) PCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate RNA from Y79 cells.
cDNA was synthesized from RNA using the PrimeScript™ RT reagent kit
with genomic DNA Eraser (Takara Biotechnology Co., Ltd.) according
to the manufacturer's protocol. qPCR was conducted using a SYBR
Green kit (Takara Biotechnology Co., Ltd.) and the 7500 Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Primers used were: JAG1, forward, 5′-GGGGCAACACCTTCAACCTC-3′ and
reverse, 5′-CCACGCCTCCACAAGCAAC-3′; GADPH was set as internal
control forward, 5′-GCGACACCCACTCCTCCAC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTAG-3′). Thermocycling conditions were as
follows: Initial denaturation at 95°C for 30 sec followed by 40
cycles each consisting of 95°C for 5 sec and 60°C for 40 sec. The
relative changes in the expression of target genes were calculated
by 2−ΔΔCq method (17).
Cell viability and proliferation
assays
Cells were seeded into a 96-well plate at a density
of 3×103 cells/well. After a 24-h incubation at 37°C,
the medium was replaced with fresh medium containing ATG or the
equivalent volume of DMSO at 37°C for 96 h. Cell viability was
subsequently measured using the Cell Counting kit-8 (CCK-8;
Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's protocol.
For the 5-ethynyl-2′-deoxyuridine (EdU)
incorporation assay, floating cells (1×104/well) were
plated on poly-L-lysine-coated microscope slides. Subsequently,
cell proliferation was measured using an EdU kit (Guangzhou RiboBio
Co., Ltd., Guangzhou, China) according to the manufacturer's
protocol. Images were obtained using a Fluoview1000 laser scanning
confocal microscope (Olympus Corporation, Tokyo, Japan). Cell
counting was performed using ImageJ version 1.8.0 software
(National Institutes of Health, Bethesda, MD, USA).
Apoptosis assay
The treated cells were collected and washed twice
with ice-cold PBS. The apoptotic rate was measured using a
fluorescein isothiocyanate-labeled Annexin V apoptosis detection
kit according to the manufacturer's protocol, and was quantified
using an Accuri C6 flow cytometer and CFlow plus version 1.0.264.15
software (all BD Biosciences, Franklin Lakes, NJ, USA).
Migration assay
Polycarbonate membrane inserts with 8-µm pores and
Transwell plates (Corning Inc., Corning, NY, USA) were used for the
cell migration assay. After a 24-h incubation, cells were cultured
in serum-free medium containing hydroxyurea (1.8 mM) for 12 h at
37°C to synchronize cells and suppress cell proliferation.
Subsequently, cells were trypsinized and reseeded in serum-free
RPMI-1640 medium [containing 10% bovine serum albumin (BSA) (Gibco;
Thermo Fisher Scientific, Inc.) and ATG or the equivalent volume of
DMSO] and transferred to the upper chamber at a density of
5×104 cells/well. RPMI-1640 medium supplemented with 20%
FBS was subsequently added into the lower chamber. After a 48-h
incubation at 37°C, the migratory cells were fixed with methanol at
room temperature for 30 min and stained using 0.1% crystal violet
at room temperature for 20 min. The migrated cells were counted in
five randomly selected fields of view under an inverted light
microscope.
Western blot analysis
The cells were collected, washed twice with PBS and
lysed with radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology) for total protein extraction. The
protein concentration was determined using a bicinchoninic acid
assay kit (Beyotime Institute of Biotechnology). Equal amounts of
protein (50 µg/lane) were subsequently separated by 10% SDS-PAGE
and transferred onto a polyvinylidene difluoride membrane. After
blocking with BSA for 1 h at room temperature, the membranes were
incubated with primary antibodies purchased from Abcam (Cambridge,
UK). The primary antibodies used were as follows: Anti-JAG1 (cat.
no. ab7771; 1:500), anti-NICD (cat. no. ab8925; 1:500),
anti-transcription factor HES (HES)1 (cat. no. ab71559; 1:1,000),
anti-HES5 (cat. no. ab25374, 1:1,000), anti-B-cell lymphoma 2
(BCL2; cat. no. ab32124; 1:1,000), anti-BCL2-associated X protein
(BAX; cat. no. ab32503; 1:2,000) and anti-β-actin (cat. no. ab8227;
1:2,000). The primary antibodies were incubated overnight at 4°C.
The membranes were then incubated with a horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature (cat. no. ab205718; 1:2,000; Abcam) and the bands were
developed using the Ultrasensitive enhanced chemiluminescence kit
(Sangon Biotech Co., Ltd.). Protein quantification were performed
by ImageJ version 1.8.0 software (National Institutes of
Health).
Immunofluorescence assay
Treated cells were collected by centrifugation in
300 × g at 4°C for 5 min and the pellet was washed twice with PBS.
Subsequently, the cells were fixed with 4% paraformaldehyde for at
room temperature 20 min, washed twice with PBS, and treated with
0.5% Triton X-100 for 5 min. The cells were washed twice with PBS
and blocked using 5% BSA at room temperature for 1 h. After
blocking, the cells were incubated with a primary antibody against
JAG1 (cat. no. ab7771; 1:300; Abcam) at 37°C for 1 h and rinsed in
PBS. Subsequently, the cells were incubated with an Alexa
Fluor® 647-conjugated secondary antibody (cat. no.
ab150075; 1:500; Abcam) at 37°C for 1 h. Cells were placed onto
slides and mounted using Fluoromount-G™ with DAPI (Thermo Fisher
Scientific, Inc.). The images were obtained using a laser scanning
confocal microscope (Olympus Corporation).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). The data are presented as the means
± standard deviation of at least three independent experiments.
Statistical differences were analyzed by Student's t-test or
one-way analysis of variance followed by Student-Newman-Keuls post
hoc test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of ATG on the viability of
retinoblastoma cells
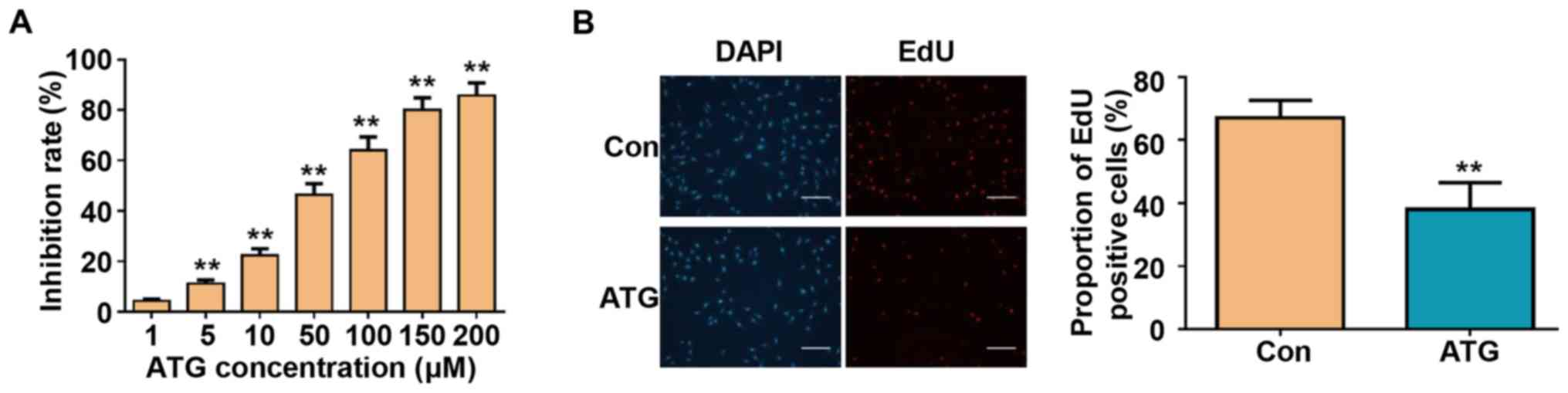
To investigate the effects of ATG on cell viability,
the retinoblastoma cell line Y79 was treated with various
concentrations of ATG for 48 h. ATG significantly decreased cell
viability in a dose-dependent manner, as detected by CCK-8 assay
(Fig. 1A). To further assess the
effects of ATG on cell proliferation, an EdU labeling assay was
performed following treatment with 50 µM ATG, a concentration
identified to exhibit an inhibition rate of 46.5%, as assessed by
CCK-8 assay (Fig. 1A). Treatment
with ATG significantly decreased the number of EdU-positive cells
from 67.9 to 39.4% (Fig. 1B). The
present results suggested that ATG may inhibit the viability and
proliferation of Y79 retinoblastoma cells.
Effects of ATG on apoptosis of human
retinoblastoma cells
Apoptosis was analyzed following treatment with ATG
by performing an Annexin V/propidium iodide assay. The proportion
of cells in the early and late stages of apoptosis was increased by
114 and 468%, respectively (Fig.
2A). Furthermore, western blotting assessed the protein
expression levels of the apoptosis-associated factors BCL2 and BAX.
Treatment with ATG increased the protein expression levels of the
pro-apoptotic factor BAX and decreased the protein expression
levels of the anti-apoptotic protein BCL2 (Fig. 2B). The present results suggested
that treatment with ATG induced apoptosis of Y79 retinoblastoma
cells.
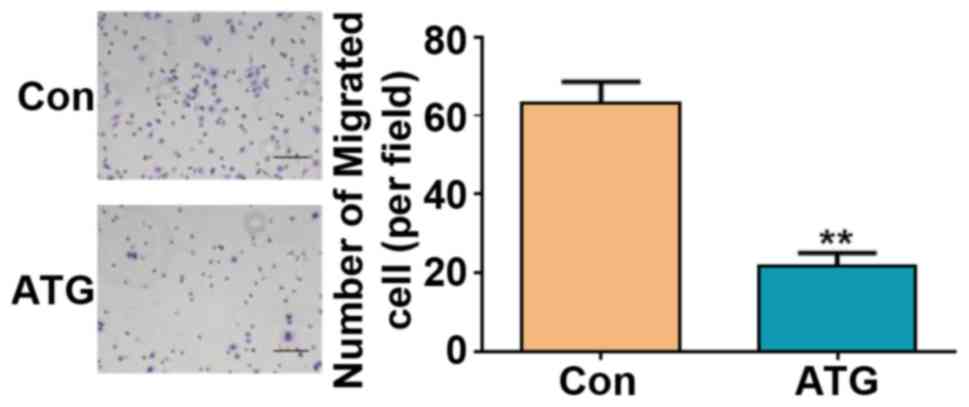
Effects of ATG on retinoblastoma cell
migration
The effects of ATG were also investigated on
retinoblastoma cell migration using a Transwell migration assay.
The average number of migrated cells was significantly decreased by
66.7% following treatment with ATG (Fig. 3). The present results suggested
that treatment with ATG inhibited the migration of Y79
retinoblastoma cells.
Effects of ATG on the Notch signaling
pathway in human retinoblastoma cells
The Notch signaling pathway serves an important role
in cell proliferation, migration and apoptosis. To investigate the
molecular mechanism underlying the anticarcinogenic effects of ATG,
the effects of ATG on JAG1 protein expression were analyzed by
immunofluorescence (Fig. 4A) and
western blotting (Fig. 4B). The
protein expression levels of JAG1 were markedly decreased following
treatment with ATG compared with in the control group (Fig. 4A-C). Additionally, the protein
expression levels of various factors involved in the Notch
signaling pathway, including NICD, HES1 and HES5 were significantly
decreased following treatment with ATG, as assessed by western
blotting (Fig. 4B and C). The
present results suggested that ATG suppressed the protein
expression levels of JAG1 and inhibited the Notch signaling pathway
in Y79 retinoblastoma cells.
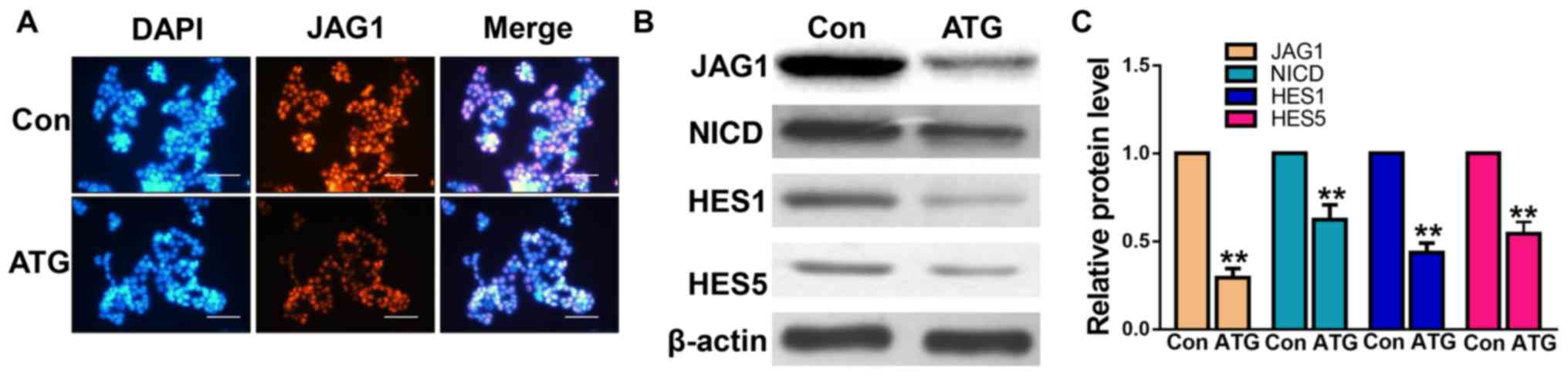 | Figure 4.Effects of ATG on the protein
expression levels of JAG1, NICD, HES1 and HES5 in the
retinoblastoma cell line Y79. (A) Protein expression levels of JAG1
were determined by immunofluorescence assay. (B) Protein expression
levels of JAG1, NICD, HES1 and HES5 were analyzed by western
blotting. (C) Densitometric analysis. Cells were treated with ATG
(50 µM) or the equivalent volume of dimethyl sulfoxide. Scale bar,
100 µm. **P<0.01 vs. Con. ATG, arctigenin; Con, control; HES,
transcription factor HES; JAG1, jagged-1; NICD, Notch intracellular
domain. |
JAG1 mediates the antitumor effects of
ATG in human retinoblastoma cells
To examine the role of JAG1 in the anticarcinogenic
effects of ATG on retinoblastoma, JAG1 was overexpressed by
transfecting Y79 cells with pCDNA-JAG1. The mRNA expression levels
of JAG1 were significantly upregulated in the transfected cells, as
measured by RT-quantitative polymerase chain reaction (Fig. 5A). The immunofluorescence and
western blotting results further suggested that transfection with
the recombinant plasmid significantly increased the expression
levels of JAG1 following treatment with ATG (Fig. 5B and 5C). The protein expression
levels of NICD, HES1 and HES5 were also significantly increased
(Fig. 5D and 5E). Furthermore,
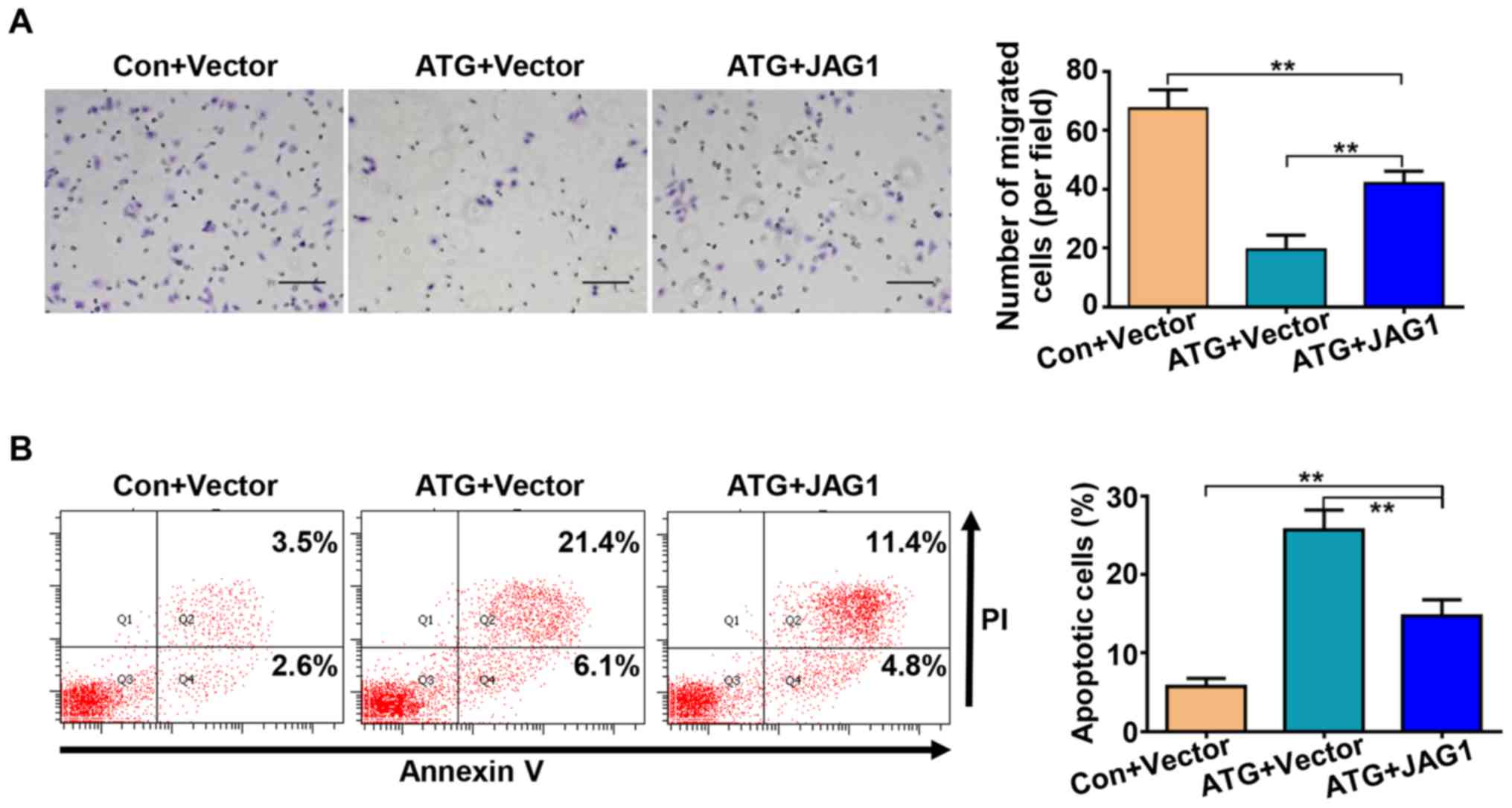
JAG1 overexpression significantly reversed the cell viability
suppressed by treatment with ATG, as determined using the CCK-8
assay (Fig. 5F). The migratory
ability of retinoblastoma cells was also partly restored by
overexpressing JAG1 (Fig. 6A).
Furthermore, overexpression of JAG1 partially abrogated the
pro-apoptotic effects of ATG (Fig.
6B). The present results suggested that ATG may serve as an
antitumor compound partly by downregulating the protein expression
levels of JAG1 in Y79 retinoblastoma cells.
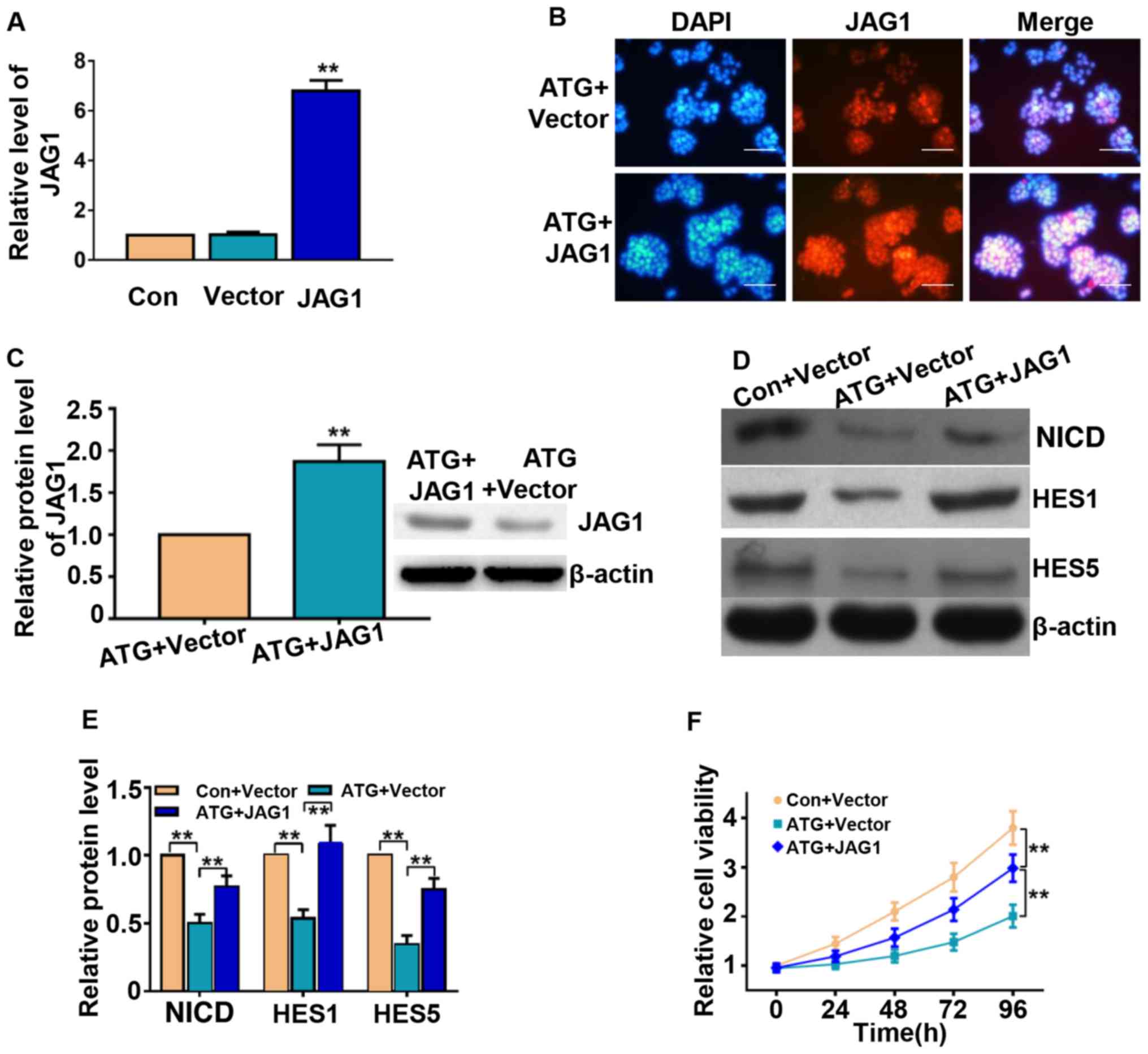 | Figure 5.JAG1 mediates the antitumor effects of
ATG on the retinoblastoma cell line Y79. (A) JAG1 transfection
increased the expression levels of JAG1, as determined by reverse
transcription-quantitative polymerase chain reaction analysis. (B)
JAG1 overexpression increased the protein expression levels of JAG1
in cells treated with ATG, as determined by immunofluorescence
assay. (C) JAG1 overexpression increased the protein expression
levels of JAG1 in cells treated with ATG, as determined by western
blotting. (D) Protein expression levels of NICD, HES1 and HES5
following treatment with ATG and transfection with JAG1 were
determined by western blotting. (E) Densitometric analysis. (F)
JAG1 overexpression increased cell viability following treatment
with ATG, as determined by Cell Counting kit-8 assay. Cells were
transfected with pCDNA3.1(+) vector or pCDNA-JAG1 and were
subsequently treated with ATG (50 µM) or an equivalent volume of
dimethyl sulfoxide. Scale bar, 100 µm. **P<0.01 vs. respective
control. ATG, arctigenin; Con, control; HES, transcription factor
HES; JAG1, jagged-1; NICD, Notch intracellular domain. |
Discussion
In a previous study, ATG exhibited increased
antitumor activity against retinoblastoma cells compared with
carboplatin in vitro (18,19),
indicating its potential clinical value in the treatment of
retinoblastoma; however, ATG does not represent a current available
therapy to treat patients with retinoblastoma, to the best of our
knowledge. The antitumor potential of ATG has been demonstrated in
various types of cancer in vitro and in vivo. In the
HepG2 liver cancer cell line, ATG was revealed to inhibit cell
proliferation and colony formation by recruiting CCAAT-enhancer
binding protein α and peroxisome proliferator-activated receptor α
to the promoter of ankyrin, downregulating its expression levels
(20). In addition, ATG decreases
cell proliferation and promotes apoptosis of the H460 non-small
lung cancer cell line by suppressing the expression level of
surviving (10). Similarly,
treatment with ATG induces cell senescence and inhibits tumor
growth of gallbladder cancer by downregulating the expression
levels of epidermal growth factor receptor (11). Additionally, ATG exhibits
antimetastatic properties in colorectal cancer cells by modulating
the expression of E-cadherin (21)
or by suppressing activity of the Wnt/β-catenin pathway (8). Similar observations have been made in
human lung cancer cells (22).
Although the molecular mechanism underlying ATG function has been
identified to be associated with various signaling pathways in
numerous types of cancer, whether ATG affects the Notch signaling
pathway remains unclear. The present study revealed that treatment
with ATG suppressed activity of the Notch signaling pathway by
inhibiting the protein expression levels of JAG1.
The Notch signaling pathway is highly conserved
among organisms, serving roles in the regulation of cell viability,
apoptosis and migration (12,13,23).
Notably, the Notch signaling pathway is upregulated in cancer cell
lines and tissues, and is able to promote cancer development and
progression (15). Conversely,
activation of the Notch signaling pathway has been identified to
inhibit human tongue carcinoma cells by downregulating the
Wnt/β-catenin pathway (14) and to
suppress the proliferation of small cell lung cancer cells by
inducing cell cycle arrest (24).
The present results suggested that the Notch signaling pathway may
promote the development and progression of retinoblastoma. The
present results are consistent with a previous report that
demonstrated that microRNA-433 may inhibit retinoblastoma
malignancy by suppressing NOTCH1 (25).
In the Notch signaling pathway, the binding between
NOTCH and a ligand leads to the release of the NICD from the cell
membrane into the nucleus, where the NICD is able to serve as a
transcription factor, activating the expression of downstream genes
(26). Treatment with ATG
decreased the protein expression levels of JAG1 and NICD in this
study, indicating a decrease in the activity of the Notch signaling
pathway, which led to a decrease in the protein expression levels
of the downstream factors HES1 and HES5. In addition to the Notch
signaling pathway, treatment with ATG was able to influence the
protein expression levels of two factors associated with apoptosis,
BCL2 and BAX. Previous studies have investigated the molecular
mechanism of ATG, and it has been reported to regulate various
signaling pathways, including transforming growth factor β/SMAD
family member 2/3 (22), p38
mitogen-activated protein kinase (MAPK) (9) and phosphoinositide 3-kinase/AKT
serine/threonine kinase 1 (27),
suggesting that ATG may serve as an anticarcinogenic factor via a
complex regulatory network. Notably, other pathways and molecular
factors may be involved, in addition to JAG1 and the Notch
signaling pathway, in mediating the effects of ATG in
retinoblastoma cells. Furthermore, the Notch signaling pathway was
previously identified to undergo crosstalk with multiple signaling
pathways, including MAPK (28),
Ras (24) and Wnt (29). Therefore, the numerous pathways
interacting with the Notch signaling pathway may be involved in the
anticarcinogenic effects of ATG. Additionally, JAG1 overexpression
may reverse the effects of ATG by influencing multiple pathways
associated with the Notch signaling pathway, thus affecting the
function of ATG on the regulation of various cellular
processes.
In conclusion, the present study suggested that ATG
served anticarcinogenic effects on retinoblastoma cells partly by
negatively regulating JAG1, indicating that ATG may represent a
novel potential therapeutic compound to treat patients with
retinoblastoma. Furthermore, the Notch signaling pathway may be
further investigated as an additional target to treat
retinoblastoma. Nevertheless, further studies are required to
examine the multiple roles of ATG and the Notch signaling pathway
in retinoblastoma, in order to understand the molecular mechanisms
underlying ATG. Notably, only one cell line was investigated in the
present study; therefore, the function of ATG in other
retinoblastoma cell lines requires further investigation in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Traditional
Chinese Medicine Project of Chongqing (grant no. 20160322).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NK and QL designed the study and performed the
experiments. LP analyzed the data and JF, LC and XC helped perform
the experiments.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villegas VM, Hess DJ, Wildner A, Gold AS
and Murray TG: Retinoblastoma. Curr Opin Ophthalmol. 24:581–588.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong FL, Boice JD, Abramson DH, Tarone RE,
Kleinerman RA, Stovall M, Goldman MB, Seddon JM, Tarbell N,
Fraumeni JF Jr and Li FP: Cancer incidence after retinoblastoma:
Radiation dose and sarcoma risk. JAMA. 278:1262–1267. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo C and Deng YP: Retinoblastoma:
Concerning its initiation and treatment. Int J Ophthalmol.
6:397–401. 2013.PubMed/NCBI
|
|
4
|
Yang C, Fan X and Fan S: Effects and
mechanism of puerarin on the human retinoblastoma cells. J Cell
Biochem. 119:4506–4513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Z, Gu C, Wang K, Ju J, Wang H, Ruan K
and Feng Y: Arctigenic acid, the key substance responsible for the
hypoglycemic activity of Fructus Arctii. Phytomedicine. 22:128–137.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han YH, Kee JY, Park J, Kim HL, Jeong MY,
Kim DS, Jeon YD, Jung Y, Youn DH, Kang J, et al: Arctigenin
inhibits adipogenesis by inducing AMPK activation and reduces
weight gain in high-fat diet-induced obese mice. J Cell Biochem.
117:2067–2077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ,
Jang SE, Han MJ and Kim DH: Arctigenin ameliorates inflammation in
vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing
M1 macrophages to M2-like macrophages. Eur J Pharmacol. 708:21–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S, Li J, Song S, Li J, Tong R, Zang
Z, Jiang Q and Cai L: Integrated in silico and experimental methods
revealed that Arctigenin inhibited angiogenesis and HCT116 cell
migration and invasion through regulating the H1F4A and
Wnt/β-catenin pathway. Mol Biosyst. 11:2878–2884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sihai YU and Yang X: Arctigenin inhibits
the proliferation of tongue cancer Tca8113 cells by activation of
p38 MAPK. Med J West China. 5:645–647. 2015.
|
|
10
|
Wang HQ, Jin JJ and Wang J: Arctigenin
enhances chemosensitivity to cisplatin in human nonsmall lung
cancer H460 cells through downregulation of survivin expression. J
Biochem Mol Toxicol. 28:39–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang M, Cai S, Zuo B, Gong W, Tang Z,
Zhou D, Weng M, Qin Y, Wang S, Liu J, et al: Arctigenin induced
gallbladder cancer senescence through modulating epidermal growth
factor receptor pathway. Tumour Biol.
39:10104283176983592017.PubMed/NCBI
|
|
12
|
Qi R, An H, Yu Y, Zhang M, Liu S, Xu H,
Guo Z, Cheng T and Cao X: Notch1 signaling inhibits growth of human
hepatocellular carcinoma through induction of cell cycle arrest and
apoptosis. Cancer Res. 63:8323–8329. 2003.PubMed/NCBI
|
|
13
|
Bolos V, Grego-Bessa J and de la Pompa JL:
Notch signaling in development and cancer. Endocr Rev. 28:339–363.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan L, Yao J, Wu X and Fan M: Growth
suppression induced by Notch1 activation involves Wnt-beta-catenin
down-regulation in human tongue carcinoma cells. Biol Cell.
98:479–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Zhang R, Zhou S and Ji Y:
Overexpression of Notch1 is associated with the progression of
cervical cancer. Oncol Lett. 9:2750–2756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sethi N, Dai X, Winter CG and Kang Y:
Tumor-derived jagged1 promotes osteolytic bone metastasis of breast
cancer by engaging notch signaling in bone cells. Cancer Cell.
19:192–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sreenivasan S and Krishnakumar S:
Synergistic effect of curcumin in combination with anticancer
agents in human retinoblastoma cancer cell Lines. Curr Eye Res.
40:1153–1165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YF, Kunda PE, Lin JW, Wang H, Chen
XM, Liu QL and Liu T: Cytokine-induced killer cells co-cultured
with complete tumor antigen-loaded dendritic cells, have enhanced
selective cytotoxicity on carboplatin-resistant retinoblastoma
cells. Oncol Rep. 29:1841–1850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Y, Tan Y, Lu Z, Li BB, Sun CH, Li T,
Zhao LL, Liu Z, Zhang GM, Yao JC and Li J: Arctigenin inhibits
liver cancer tumorigenesis by inhibiting gankyrin expression via
C/EBPα and PPARα. Front Pharmacol. 9:2682018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han YH, Kee JY, Kim DS, Mun JG, Jeong MY,
Park SH, Choi BM, Park SJ, Kim HJ, Um JY and Hong SH: Arctigenin
inhibits lung metastasis of colorectal cancer by regulating cell
viability and metastatic phenotypes. Molecules. 21:11352016.
View Article : Google Scholar :
|
|
22
|
Xu Y, Lou Z and Lee SH: Arctigenin
represses TGF-β-induced epithelial mesenchymal transition in human
lung cancer cells. Biochem Biophys Res Commun. 493:934–939. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon K and Gaiano N: Notch signaling in
the mammalian central nervous system: Insights from mouse mutants.
Nat Neurosci. 8:709–715. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sriuranpong V, Borges MW, Ravi RK, Arnold
DR, Nelkin BD, Baylin SB and Ball DW: Notch signaling induces cell
cycle arrest in small cell lung cancer cells. Cancer Res.
61:3200–3225. 2001.PubMed/NCBI
|
|
25
|
Li X, Yang L, Shuai T, Piao T and Wang R:
MiR-433 inhibits retinoblastoma malignancy by suppressing Notch1
and PAX6 expression. Biomed Pharmacother. 82:247–255. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Penton AL, Leonard LD and Spinner NB:
Notch signaling in human development and disease. Semin Cell Dev
Biol. 450–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong YH, Park JS, Kim DH and Kim HS:
Arctigenin increases hemeoxygenase-1 gene expression by modulating
PI3K/AKT signaling pathway in rat primary astrocytes. Biomol Ther
(Seoul). 22:497–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pear WS, Aster JC, Scott ML, Hasserjian
RP, Soffer B, Sklar J and Baltimore D: Exclusive development of T
cell neoplasms in mice transplanted with bone marrow expressing
activated Notch alleles. J Exp Med. 183:2283–2291. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo S, Liu M and Gonzalez-Perez RR: Role
of Notch and its oncogenic signaling crosstalk in breast cancer.
Biochim Biophys Acta. 1815:197–213. 2011.PubMed/NCBI
|