Introduction
The temporomandibular joint (TMJ) is crucial for
normal mandibular movement, which is associated with numerous vital
physiological processes, including chewing, talking and functions
that require the opening of the mouth (1). TMJ ankylosis (TMJA) restricts the
movement of the mandible, which results in severe health problems
in patients (2). TMJA can result
from various factors including trauma, infection, congenital issues
and arthritis, among these, trauma in the TMJ region is the most
common cause, such as a condylar fracture (3). There are several hypotheses
concerning the pathogenesis of traumatic TMJA. For example, it has
been suggested that the abnormal healing of the fractured condyle
is the principal reason for TMJA in the bone (4). Specifically, injury in the TMJ region
may result in haematoma, fibrosis and the abnormal formation of
excessive bone (5). A previous
study hypothesized that the intra-articular bone fragment may help
to increase the extent of ankylosis (6). Based on the anatomy of the lateral
pterygoid muscle (LPM), it can be inferred that the contraction of
the LPM will generate sufficient distraction force to the condyle
neck during the opening of the mouth and confirm the ‘distraction
osteogenesis (DO) effect of LPM’ hypothesis, which is that the LPM
may exert a DO effect during the healing of the fractured condyle
(7). In addition, the LPM exerts
an important function in the reconstruction of the sagittal
fractured condyle (8) and a
previous study has reported that TMJA will be significantly
alleviated if the LPM is dissected (9). Furthermore, the study of Dai et
al (10) also suggested that
local injection of botulinum toxin A would temporarily block
impulses from the LPM's and may effectively prevent the occurrence
of traumatic TMJA. Consequently, blocking the LPM may serve as a
promising therapeutic strategy for the prevention of traumatic
TMJA. Unfortunately, the associated molecular mechanisms of
traumatic TMJA and the effects of LPM have yet to be completely
elucidated, which has limited the development of suitable
therapeutic strategies.
In the present study, a gene chip technique was used
to systematically investigate any alterations in the expression of
whole mRNA during the development of traumatic TMJA caused by a
condylar fracture. In addition, the influence of LPM in the gene
expression profile was also screened, in an attempt to identify the
key molecules associated with this process.
Materials and methods
Animal model establishment and
examination
Small-tailed Han sheep were provided by the
Laboratory Animal Center of the Fourth Military Medical University
(Xi'an, China) and all animal experiments were approved by the
animal welfare ethics committee of the School of Stomatology, the
Fourth Military Medical University. A total of 6 male sheep (aged 6
months, 25–35 kg) were kept under 20°C, 40–60% humidity with a
circadian rhythm (12:12 h light:dark cycle). The animals were fed
with a standard diet and had free access to water. The traumatic
TMJA model was constructed according to the protocol outlined in
previous publications (9,11,12).
Briefly, the bilateral TMJ capsule was exposed from an incision to
the anterior tragus. The joint capsule was incised and the condyle
was exposed, and underwent a sagittal fracture by piezosurgery.
Meanwhile, the outside quarter of the joint disc was dissected and
a ‘#’ shaped groove was created by piezosurgery on the fossa
surface. In addition, the right side LPM was dissected (LPD) while
the left side was normal (LPN) i.e. without dissection. The tissue
was tightly sutured apart from the capsule and penicillin (1 mg
pure penicillin G sodium per sheep for 3 days) was used to prevent
infection. Sheep who did not receive surgery i.e. untreated normal
sheep (UTR) were used as a blank control. The surgical procedures
are illustrated in Fig. S1. A
total of 6 sheep were used and randomly divided into 2 groups of 3,
which comprised the 4- and 12 weeks evaluation groups. In each
group, one sheep was randomly selected as the UTR and the remaining
sheep were subjected to surgery. For each animal in the group of
surgical sheep, the right side of the TMJ was the LPD group while
the left side was the LPN group.
Gene chip RNA preparation
At either 4 or 12 weeks (8), the sheep were sacrificed using an
overdose injection of pentobarbital sodium, and the bone tissues
within the condylar fractured zones were separated and immediately
frozen in liquid nitrogen (−195.79°C). Then the samples were
subjected to total RNA extraction, purification and quality testing
according to a standard Affymetrix protocol by Shanghai
Biotechnology Corporation (Shanghai, China) as reported elsewhere
(13). The sample quality test is
provided in Table SI.
Gene chip analysis
The prepared RNA samples were subjected to gene chip
hybridization, scanning and normalization according to the standard
Affymetrix protocol. The differential genes were screened by a
threshold method and the genes with a fold-change >2 were
considered as differential genes (e.g. the ratio >2 or ratio
<0.5). In addition, the data was further analyzed by cluster
heat map (Cluster 3.0; http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm),
scatter plot (R 3.5.0; http://www.r-project.org/), Database for Annotation,
Visualization and Integrated Discovery (DAVID) Bioinformatics
Resources 6.8 Platform (https://david.ncifcrf.gov/) and Gene Ontology (GO)
term enrichment (Bioconductor 3.8 in R 3.5.0; http://bioconductor.org/), according to the respective
manufacturer's protocol. The abbreviations for the biological
processes included in the GO term enrichment analysis were listed
in Table SII. Genes were
identified as significantly differentially expressed by GO term
enrichment analysis (P<0.05).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) RNA preparation
The samples were frozen in liquid nitrogen for 48 h
prior to being ground in a cold mortar. The total RNA was extracted
by miRCURY RNA Isolation kit-tissue (Exiqon; Qiagen, Inc.,
Valencia, CA, USA) according to the manufacturer's protocol. The
RNA concentration was measured by NanoDrop 2000 Spectrophotometer
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1 µg total
RNA was used for RT by the PrimeScript RT reagent kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's protocol.
Briefly, RT was conducted at 37°C for 15 min, followed by 5 sec at
85°C to terminate RT. Samples were then stored at 4°C.
RT-qPCR
The cDNA was amplified by SYBR Premix Ex Taq II
(Takara Bio, Inc., Otsu, Japan) in order to perform the RT-qPCR
procedure according to the manufacturer's protocol. qPCR was
performed using an Applied Biosystems 7500 Fast Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) under
the following conditions: 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec and 60°C for 30 sec, and finally the melt curve
stage (95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec). The
associated primers were synthesized by Takara Bio, Inc., and RPL19
was used as endogenous reference, which are listed in Table SIII. The relative gene expression
was calculated by the 2−ΔΔCq method (14). Three independent experiments were
repeated.
Statistical analysis
The quantitative data are presented as the mean ±
standard deviation of at least three experimental repeats. The
one-way analysis of variance with Student-Newman-Keuls post hoc
test was used to compare differences between groups within a
certain gene (GraphPad Prism 5; GraphPad Software, Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
Cluster heat map
The overall gene expression was classified by heat
map analysis and several regions of interest (ROI; the most
different areas) were selected from the complete map (Fig. 1A). The cluster similarity was
similar for the results obtained at 12 and 4 weeks in the same
treatment groups in the majority of clusters (Fig. 1), suggesting that the gene
expression pattern was similar in animals in the same treatment
groups. Of note, the gene expression in LPD was up- or
downregulated in the opposite direction to LPN, at 4 and 12 weeks
(Fig. 1B). In addition, the genes
associated with osteogenesis, including interleukin (IL)1A,
collagen type II α1 chain (COL2A1), cartilage oligomeric matrix
protein (COMP) and C-type lectin domain family 3 member A (CLEC3A)
were included in these differential zones (Fig. 2A-C).
 | Figure 2.Representative osteogenic associated
genes regions. The regions of interest were selected based on
alterations in LPD, which was (A) upregulated, (B) downregulated or
(C) exhibited an average level. The black arrows indicate the
osteogenic associated genes of SLPI, IL1A, COL2A1, COMP and CLEC3A.
LPN, the lateral pterygoid muscle was normal; LPD, the lateral
pterygoid muscle was dissected; UTR, Sham surgery without
treatment. IL, interleukin; C; COL2A1, collagen type II α1 chain,
COMP, cartilage oligomeric matrix protein; CLEC3A, C-type lectin
domain family 3 member A. |
The cluster heat map can directly display the
general information about the expression levels of genes and the
ROI can be easily selected from the differential genes zone. The
data revealed that the cluster similarity was nearly identical
between 4 and 12 weeks in each of the LPN, LPD and UTR groups,
indicating that the different treatments can distinctively alter
the gene expression patterns. There are certain specific muscle
contraction associated genes including myosin light chain 2, myosin
heavy chain (MYH)2, troponin C1 (TNNC1), MYH6 and actin α1 that
were downregulated in LPD, suggesting that the LPM may serve an
important role in the pathogenesis of traumatic TMJA. As bony
ankylosis was the principal focus of the present study, the genes
associated with osteogenesis were of particular interest. Based on
the heat map, the IL1A, COL2A1, COMP and CLEC3A were preliminarily
selected as potential critical genes.
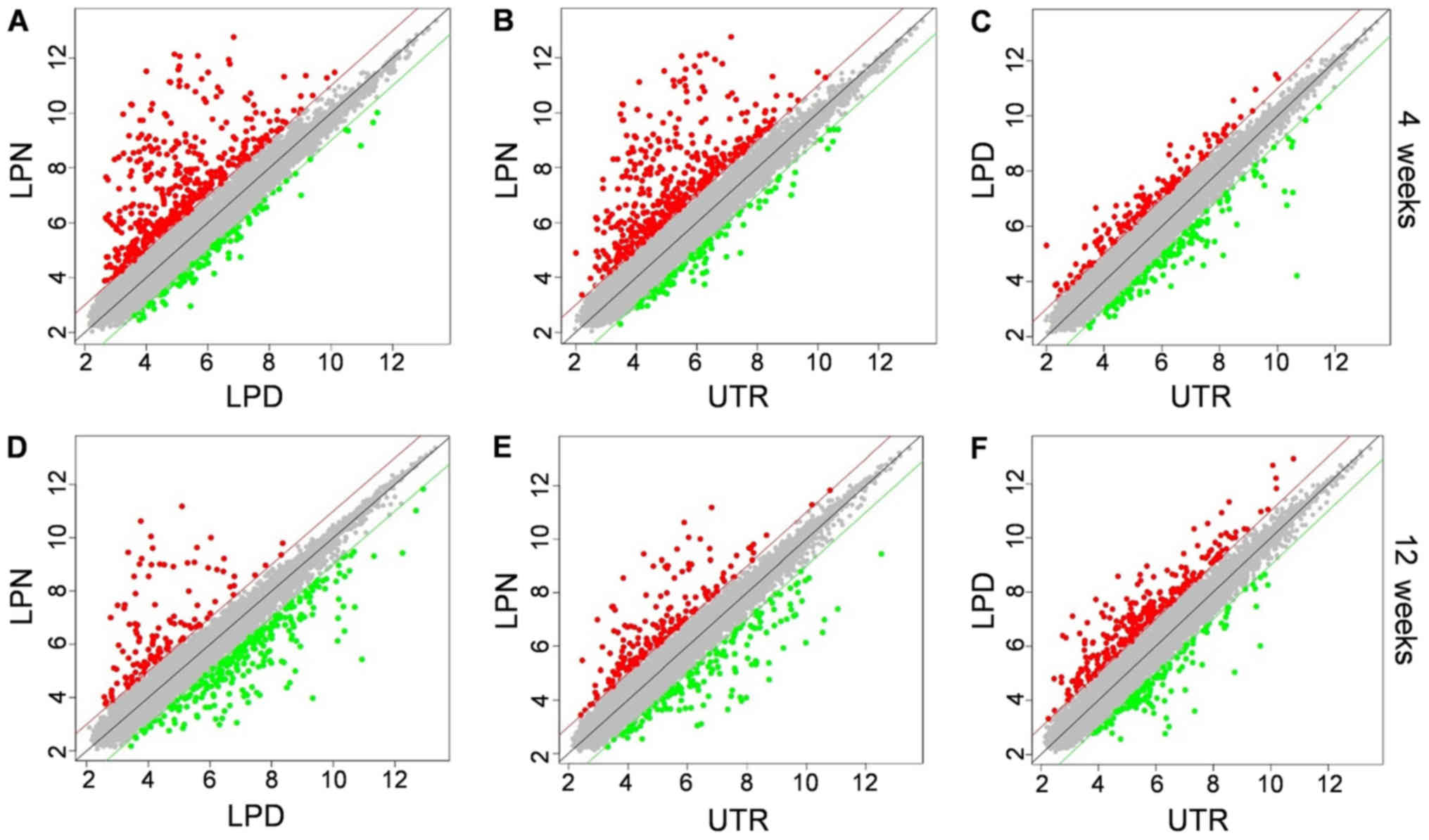
Scatter plot
The scatter plot was used to illustrate the genes
expression distribution between two different groups. At 4 weeks,
it was evident that the majority of genes in LPN were significantly
upregulated, irrespective of whether it was being compared with LPD
or UTR (Fig. 3A and B). However,
the LPD and UTR exhibited near-identical expression patterns
(Fig. 3C). Additionally, at 12
weeks, the LPN group exhibited an increased number of downregulated
genes when compared with the LPD group (Fig. 3D) and there were more upregulated
genes in the LPN group compared with the UTR group (Fig. 3E). Whereas the LPD group had more
upregulated genes compared with UTR at the 12 weeks stage (Fig. 3F).
The scatter plot can give direct insight into the
number of up- and downregulated genes between two groups.
Generally, in the present study, the LPN group exhibited a higher
expression of active genes in the early stage, whereas more genes
were activated in the LPD group at the later stage. Although the
exact number of altered genes cannot be ascertained from the
scatter plot, it corroborated the authors' hypothesis that genes
associated with osteogenesis are dominant. During the process of
DO, rapid osteogenesis is occurring in the first month, which may
limit the degree to which the mouth can open and decrease the force
of LPM (15). As a result, the DO
effect of LPM is weakened and returns to a stable state. Therefore,
most genes are upregulated at an early stage and are then
subsequently downregulated. Conversely, the dissection of LPM makes
it similar to the normal bone fracture healing process and the
majority of the osteogenesis associated genes are activated in a
relatively late stage. However, the exact differential genes
involved in this process require further analysis.
DAVID and GO enrichment analysis
In order to determine the specific differential
genes in differing treatments, DAVID gene classification and GO
enrichment analysis were performed. The DAVID Bioinformatics
Resources can be used to categorize the differential genes through
different filters, which is extensively used for systematical
analysis of large gene lists (16–18).
The GO term enrichment analysis can group the differential genes by
different biological processes and determine which genes are
significant (19,20).
The genes were categorized by DAVID Bioinformatics
Resources and more than 2-fold altered genes were considered
different. Then the representative specific osteogenesis associated
genes were listed. In LPN vs LPD at 4 weeks, the COL2A1, COMP,
CLEC3A, calpain 3 and fibromodulin genes were upregulated up to
>3-fold (Table I). From the GO
term enrichment data, the bone morphogenesis and endochondral bone
growth were the most notable, in which COMP and COL2A1 were
important genes (Table II). It
has previously been reported that the bone formed by DO is formed
via intramembranous ossification (21), in which COL2A1 is involved
(22). Taken together, these data
indicate that the LPN group may be similar to the DO process and
the genes associated with intramembranous ossification are
activated at early stages. In addition, muscle contraction was also
significantly upregulated in the LPN compared with the LPD group
(P<0.05; Table II), which may
be attributed to the function of LPM.
 | Table I.Representative differential genes in 4
weeks by DAVID. |
Table I.
Representative differential genes in 4
weeks by DAVID.
| LPN vs. LPD | LPN vs. UTR | LPD vs. UTR |
|---|
|
|
|
|---|
| Genes | Fold-change | Genes | Fold-change | Genes | Fold-change |
|---|
| COL2A1 | 10.08 | CD180 | 2.26 | FOS | 5.12 |
| COMP | 4.93 | COMP | 2.19 | MMP12 | 7.77 |
| CLEC3A | 13.95 | CLEC3A | 3.11 | IL1RN | 2.44 |
| CAPN3 | 9.19 | TLR7 | 2.03 | CLEC3A | 0.22 |
| FMOD | 3.00 | HRC | 6.90 | COMP | 0.44 |
| S100A12 | 0.20 | RYR1 | 11.48 | COL2A1 | 0.10 |
| OCM | 0.43 | S100A8 | 0.41 | BMP3 | 0.49 |
| SULF1 | 0.49 | ADIPOQ | 0.32 | LECT1 | 0.31 |
| MMP12 | 0.26 | BMP3 | 0.37 | CALCR | 0.36 |
 | Table II.The representative genes of GO-term
enrichment in 4 weeks. |
Table II.
The representative genes of GO-term
enrichment in 4 weeks.
| BP | Genes |
|---|
| LPN vs. LPD |
| BM | COMP, COL2A1,
BGLAP, TGF-β1 |
|
CEBM | COMP |
|
EBG | COMP, COL2A |
| MC | ADRB2, TNNT3, RYR1,
PGAM2, CHRNB1, MYL2, TNNC1, MYH2, MYOM2, CSRP3, TNNT1, TCAP, MYOM1,
PLN, ACTA1, TNNI1, CASQ1, TNNC2, DES, MYLK2, CAV3, MYH6, NEB,
CKMT2, FXYD1 |
| LPN vs. UTR |
|
| NF-kB
TF | PRDX3, S100A8,
S100A12, AR, CAPN3 |
|
OBM | RYR1, THBS3 |
| WNT
pathway | PPP2R3A, SFRP2 |
|
CRCS | AQP1, NR4A2, GATA6,
CD180, S100A9, SOD2, RYR1, FOSB, PRDX3, SLC2A4, YBX3, GOT1, AQP4,
ACKR4, ADIPOQ, THBS4, TUBA4A, ALDH1A2, EGLN3, FABP4, PON3, FBLN5,
SFRP2, FZD7, LHCGR, FOS, ACSS1, ADCY2, LEPR, FMOD, ACADVL, RXRG,
NR4A1, ESRRG, S100A8, S100A12, ANKRD1, TNMD, COMP, AR, RPS6, CAPN3,
CAV3, MYH6, CD36, PGR |
| LPD vs. UTR |
|
|
EBG | COL2A1 |
|
NRO | IGFBP5, CALCR,
LRP4, SOST, MEPE |
|
Ossification | IGFBP5, CYR61,
LRP4, HGF, SFRP2, SOST, THBS3,TP53INP2, COL2A1, MEPE |
| CD | COMP, LOLX2, CYR61,
BMP3, CNMD, SFRP2, EFEMP1,COL2A1 |
|
RES | NR4A2, PLAU, CCR5,
VEGFC, VDR, ADIPOQ, CHL1,LYZ, APOD, HGF, SERPINE1, FABP4, CCNB1,
SLC12A2,KNG1, SEMA3C, SEMA3A, SFRP2, VCAM1, AQP3, SOST, FOS, LPL,
NR4A3, PLAUR, FABP7, NR4A1, DCLK1, S100A8, IFIT2, COL4A1, EDNRB,
COL2A1, ABL2, TLR7 |
In LPN vs. UTR at 4 weeks, not only COMP and CLEC3A
were upregulated but cluster of differentiation (CD)180, Toll-like
receptor 7, histidine rich calcium binding protein and ryanodine
receptor 1 (RYR1) genes were also significantly increased, while
the expression of S100 calcium binding protein A8 (S100A8),
adiponectin (ADIPOQ) and BMP3 were downregulated (Table I). In the GO-term enrichment data,
the nuclear factor-κB transcription factor activity and
ossification in bone mature and cellular response to chemical
stimulus were significantly different, which also contained the
S100A8, CD180, RYR1 and ADIPOQ genes (P<0.05; Table II).
In LPD vs. UTR at 4 weeks, the upregulated genes
were FOS, matrix metalloproteinase 12 and IL1 receptor antagonist
while the CLEC3A, COMP, COL2A1, BMP3, LECT1 and CALCR were markedly
downregulated (Table I). In the
GO-term enrichment data, the ossification, cartilage development
and response to external stimulus were more evident (Table II). The COMP and COL2A genes were
also revealed to be important genes in endochondral bone growth,
ossification and cartilage development (Table II). Since the cartilage
development and endochondral ossification are the main processes
during normal bone fracture healing (23,24),
it may be inferred that LPD is similar to the normal healing of
condylar fractures, which repair slowly over 4 weeks and therefore
this explains why genes associated with osteogenesis including
COMP, COL2A1, and BMP3 were downregulated.
In LPN vs. LPD at 12 weeks, the ADIPOQ, myosin light
chain, phosphorylatable, fast skeletal muscle, TNNC1 and
calsequestrin 1 were upregulated while the CD180, FOS, LECT1 and
IL1A were downregulated (Table
III). In this stage, the response to stress, the immune
response, cellular response to chemical stimulus and response to
external stimulus were significant, among which the FOS and IL1A
exhibited multiple functions in the regulation of this process
(Table IV). Lewinson et al
(25) reported that the expression
of FOS is in line with the DO process, which is upregulated in the
early stages and attenuated when the bone trabecula is formed.
Consequently, the downregulation of FOS in LPN may suggest that the
DO process is entering a stable stage.
 | Table III.The representative differential genes
in 12 weeks by Database for Annotation, Visualization and
Integrated Discovery. |
Table III.
The representative differential genes
in 12 weeks by Database for Annotation, Visualization and
Integrated Discovery.
| LPN vs. LPD | LPN vs. UTR | LPD vs. UTR |
|---|
|
|
|
|---|
| Genes | Fold-change | Genes | Fold-change | Genes | Fold-change |
|---|
| ADIPOQ | 2.22 | COMP | 2.99 | COL2A1 | 2.48 |
| MYLPF | 23.22 | SERPINE1 | 2.67 | IL1A | 12.39 |
| TNNC1 | 29.48 | CLEC3A | 2.87 | CLEC3A | 4.76 |
| CASQ1 | 8.44 | LECT1 | 2.89 | FOS | 4.25 |
| CD180 | 0.43 | LEPR | 0.41 | CD180 | 2.51 |
| TLR7 | 0.48 | BMP7 | 0.40 | COMP | 3.38 |
| IL6 | 0.42 | ADIPOQ | 0.18 | LECT1 | 7.73 |
| FOS | 0.20 | S100A9 | 0.23 | CALCR | 0.49 |
| LECT1 | 0.37 | EDNRB | 0.41 | ADIPOQ | 0.08 |
| IL1A | 0.11 |
|
|
|
|
 | Table IV.The representative genes of Gene
Ontology-term enrichment in 12 weeks. |
Table IV.
The representative genes of Gene
Ontology-term enrichment in 12 weeks.
| BP | Genes |
|---|
| LPN vs. LPD |
| RS | CHI3L1, TNFAIP3,
MB21D1, IL1B, NR4A2, LYZ2, S100A9, SOD2, NOS2, CCL5, TLR2, MX2,
EIF2AK2, DTX3L, IL1A, FAS, IER3, BPI, CD27, LAP, GBP5, RSAD2,
CXCL16, IL1RN, ADIPOQ, CCL20, PARP9, DERL3, XDH, KMO, MT2A, LYZ,
PTAFR, PLAC8, GADD45B, AQP9, MYF6, LY86, MYH2,TUBA4A, DOCK2, HERC5,
FGF10, STC2, PML, SLC7A11,VCAM1, PRKCB, FOS, TCAP, TRIB1, NR4A3,
ATF3, CCRL2,NR4A1, PTGS2, CASQ1, S100A8, S100A12, CD2, IFIT2,
CYSLTR1, ACOD1, CCL7, CYBB, ISG15, DDX58, RFC3, ITGB2, CXCR4,
PARPBP, CXCL8, MX1, TLR7, TLR8 |
| IR | TNFAIP3, MB21D1,
IL1B, S100A9, NOS2, CCL5, TLR2, MX2, IL1A, FAS, GBP5, RSAD2,
CXCL16, IL7R, CCL20, ITK, TNFSF15, AQP9, LY86, MYH2, DOCK2, HERC5,
FGF10, PML, SAMSN1, VCAM1, PRKCB, FOS, CCR8, NR4A1, SEMA4A, S100A8,
S100A12, IFIT2, ACOD1, CCL7, CYBB, ISG15, DDX58, ITGB2,CXCL10, IL6,
CXCL8, MX1, TLR7, TLR8 |
| TLR
pathway | TNFAIP3, TLR2,
RSAD2, FOS, ACOD1, ITGB2, TLR7, TLR8 |
|
CRCS | CHI3L1, TNFAIP3,
CTSL, MB21D1, IL1B, MT1A, CX3CR1,NR4A2, CD180, S100A9, SOD2, NOS2,
RYR1, CCL5, TLR2, FOSB, EIF2AK2, FBP1, IFIT3, IL1A, FAS, GBP5,
CXCL16, IL1RN, ADIPOQ, IL7R, DERL3, XDH, MT2A, CNMD, PTAFR, AQP9,
LY86, TUBA4A, HERC5, FGF10, STC2, WNT2, PML, VCAM1, LHCGR, MZB1,
JUNB, PRKCB, FOS, TR1B1, CCR8,NR4A3, ATF3, CCRL2, NR4A1, PTGS2,
S100A8, S100A12, IFIT2, ACOD1, CCL7, ISG15, ITGB2, MYH6, CXCR4,
EGR2, CXCL8, EGR1 |
|
RES | CHI3L1, TNFAIP3,
MB21D1, IL1B, CX3CR1, NR4A2, LYZ2, CD180, S100A9, NOS2, CCL5, TLR2,
MX2, EIF2AK2, FAS, IER3, BPI, LAP, RSAD2, CXCL16, ADIPOQ, CHL1,
LYZ, PTAFR, PLAC8, LY86, DOCK2, SDS, HERC5, FGF10, STC2, CSRP3,
PML, VCAM1, CDS1, JUNB, FOS, TCAP, TGF-β1, TRIB1, CCR8, NR4A3,
CCRL2, NR4A1, ACTA1, PTGS2, SEMA4A, S100A8, S100A12, IFIT2,
CYSLTR1, ACOD1, CCL7, ISG15, DDX58, ITGB2, CXCR4, EGR2, CXCL8,
TLR7, TLR8, BST-2A, BST-2B |
| LPN vs. UTR |
|
RES | DUSP10, S100A9,
STAT1, MX2, EIF2AK2, RSAD2, ADIPOQ, CHL1, CCL21, PLAC8, PGLYRP1,
HGF, SERPINE1, LTF, SCN1B, HERC5, SEMA3C, TGF-β1, CSRP3, PML,
SFRP2, STAR, VCAM1, TCAP, PLAUR, HP, ACTA1, PTGS2, S100A8, S100A12,
IFIT5, IFIT2,LCN2, EDNRB, ISG15, DDX58, BMP7, CAPN3, RELN |
|
EBM | COMP, PML |
| CD | COMP, LOXL2, CNMD,
PML, SFRP2, BMP7 |
| BD | COMP, HAS2, FREM1,
PML, SFRP2 |
|
EBG | COMP, PML |
| LPD vs. UTR |
|
EBG | COMP |
|
Ossification | CALCR, LRP4, SFRP1,
GF, SFRP2, RSPO2, JUNB, PTGS2, BMP5, COL2A1, EGR2 |
|
CEBM | COMP, COL2A1 |
| BM | COMP, HAS2, FREM1,
SFRP2, COL2A1 |
| RS | CHI3L1, TNFAIP3,
IL1B, DUSP10, CAMP, NR4A2, GATA6, LYZ2, S100A9, SOD2, NOS2, CCL5,
ITIH4, TLR2, EDN1, CXCL13, IL1A, FAS, IER3, LYZ1, BPI, CD27, LAP,
CXCR6, CXCL16, IL1RN, ADIPOQ, HAS2, DERL3, XDH, CCL21, KMO, MT2A,
CATHL2, LYZ,C7, PGLYRP1, SFRP1, HGF, SERPINE1, TNFSF8, LY86,LTF,
MYH2, EGLN3, FABP4, DOCK2, TBX3, FOXF1,SELP, STC2, SFRP2, STAR,
SLC7A11, FOS, CLEC7A, OLR1, TRIB1, NR4A3, DEFB128, LGMN, PLAUR,
ATF3, C5AR1, HP, NR4A1, PTGS2, SGK1, S100A8, S100A12,CTSS, EDNRB,
ACOD1, CCL7, CYBB, IFNA21, CAPN3, ITGB2, CXCR4, MYOCD, CD163, EYA4,
LIPA, TLR10, CXCL8, CD14 |
In LPN vs. UTR at 12 weeks, the osteogenesis
associated genes of COMP, serpin family E member 1, CLEC3A, LECT1
were upregulated, while LEPR, BMP7, ADIPOQ, S100A9 and endothelin
receptor type B were downregulated (Table III). The genes of response to
external stimulus, endochondral bone growth, cartilage development
and bone development were significant, among which COMP was
involved in multiple bone morphogenesis processes (Table IV). This indicates that although
the DO effect at 12 weeks is at a stable stage, the bone
morphogenesis activity in LPN remains increased compared with in
normal sheep.
In LPD vs. UTR at 12 weeks, the osteogenesis
associated genes COL2A1, IL1A, CLEC3A, FOS, CD180, COMP and LECT1
were upregulated and CALCR and ADIPOQ were downregulated (Table III). The GO-term enrichment also
suggested that endochondral bone growth, ossification and bone
morphogenesis were significant processes (Table IV). COMP and COL2A1 were revealed
to be crucial genes in these biological processes (Table IV). The upregulation of genes
associated with osteogenesis indicates that during this stage, the
LPD bone metabolism is more active, which exerts similarities to a
normal bone fracture.
In conclusion, through the gene chip analysis, the
COL2A1, CLEC3A, IL1A, COMP, LECT1, CALCR, FOS and BMP7 genes were
preliminarily selected as potentially critical genes during the
pathogenesis of traumatic TMJA. However, due to the precision
limitations of the gene chip technique, it should be taken into
consideration that these validated genes may only be partially
activated, or not the key vital genes during the establishment of
traumatic TMJA. Therefore, the screened genes still require further
analysis.
Confirmation of validated genes by
RT-qPCR
The genes of interest i.e. BMP7, CALCR, FOS and
LECT1 were confirmed by RT-qPCR. In LPN vs. LPD at 4 weeks, BMP7
(~1.5-fold), CALCR (~2.5-fold) and LECT1 (>5-fold) were
significantly upregulated while FOS (~0.5-fold) was downregulated
(P<0.05; Fig. 4A). In LPN vs.
UTR at 4 weeks, BMP7 (~0.75-fold) was significantly reduced while
the FOS (~5-fold) and LECT1 (~5-fold) were upregulated (P<0.05;
Fig. 4A). The expression of CALCR
exhibited no statistically significant difference between the LPN
and UTR group (Fig. 4A). However,
for LPD vs. UTR at 4 weeks, BMP (<0.5-fold), CALCR (~0.2-fold)
and LECT1 (~0.1-fold) were decreased while the FOS (~10-fold) was
significantly improved (P<0.05; Fig. 4A).
 | Figure 4.Reverse transcription-quantitative
polymerase chain reaction analysis of BMP7, CALCR, FOS and LECT1.
(A) The comparisons between different groups at 4 weeks; (B) The
comparisons between different groups at 12 weeks. *P<0.05. LPN,
the lateral pterygoid muscle was normal; LPD, the lateral pterygoid
muscle was dissected; UTR, Sham surgery without treatment. BMP,
bone morphogenic protein; FOS, Fos proto-oncogene, AP-1
transcription factor subunit; CALCR, calcitonin receptor; LECT1,
chondromodulin; NS, not significant. |
In LPN vs. LPD at 12 weeks, the CALCR (<2-fold)
was upregulated whereas the FOS (~0.1-fold) and LECT1 (~0.5-fold)
were significantly inhibited (P<0.05; Fig. 4B). The BMP7 results exhibited no
statistically significant difference in the LPN group compared with
the UTR (Fig. 4B). In LPN vs. UTR
at 12 weeks, BMP7 (~0.5-fold) was significantly downregulated
whereas FOS (~1.5-fold) and LECT1 (>20-fold) were upregulated
(P<0.05; Fig. 4B). There was no
difference in the expression of CALCR in LPN vs. UTR at 12 weeks
(Fig. 4B). However, in LPD vs. UTR
at 12 weeks, BMP7 (<0.5-fold) was downregulated while the
expression of FOS (>10-fold) and LECT1 (>40-fold) were
markedly upregulated.
In conclusion, the expression of BMP7 and LECT1
confirm the result from the chip data while the CALCR and FOS are
slightly different. It is not uncommon that differences between
Affymetrix GeneChip data and RT-qPCR measurements are detected.
This may be due to the non-specific hybridization of genes and the
fact that these processes rely on different algorithms (26). Nevertheless, the gene chip data is
considered accurate (27) and a
previously study has also suggested that the gene chip data should
be coupled with RT-qPCR analysis (28).
In conclusion, the present study demonstrated that
normal LPM can significantly increase the expression of
osteogenesis associated genes, which is similar to DO gene
expression profiles. However, the dissection of LPM can
significantly delay the expression of these genes, which is similar
to the results observed in normal bone fracture healing. Numerous
genes including COL2A1, CLEC3A, IL1A, COMP, LECT1, CALCR, FOS and
BMP7 may serve vital roles during the pathogenesis of traumatic
TMJA, and warrant further investigation in future studies.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Yang Xue
(Department of Oral Surgery, School of Stomatology, The Fourth
Military Medical University, Xi'an, China), for her help during the
experiment design and data analysis.
Funding
The present study is supported by the China National
Key Research and Development Plan Project (grant no.
2016YFC1102903).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH designed the study and provided financial
support. JZ and XS participated in all the animal experiments as
well as the gene chip analysis. SJ was involved in interpreting the
data and drafted the manuscript. XJ, TD and PL contributed to the
animal surgery and data analysis. All authors reviewed and approved
the manuscript for submission.
Ethics approval and consent to
participate
All the animal experiments were approved by the
animal welfare ethics committee of the School of Stomatology, the
Fourth Military Medical University (Xi'an, China) in accordance
with the relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Chantaracherd P, John MT, Hodges JS and
Schiffman EL: Temporomandibular joint disorders' impact on pain,
function, and disability. J Dent Res. 94 (Suppl 3):S79–S86. 2015.
View Article : Google Scholar
|
|
2
|
Baykul T, Aydin MA, Nasir SN and Toptas O:
Surgical treatment of posttraumatic ankylosis of the TMJ with
different pathogenic mechanisms. Eur J Dent. 6:318–23.
2012.PubMed/NCBI
|
|
3
|
Arakeri G, Kusanale A, Zaki GA and Brennan
PA: Pathogenesis of post-traumatic ankylosis of the
temporomandibular joint: A critical review. Br J Oral Maxillofac
Surg. 50:8–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He D, Cai Y and Yang C: Analysis of
temporomandibular joint ankylosis caused by condylar fracture in
adults. J Oral Maxillofac Surg. 72:763.e1–e9. 2014. View Article : Google Scholar
|
|
5
|
Kaban LB, Perrott DH and Fisher K: A
protocol for management of temporomandibular joint ankylosis. J
Oral Maxillofac Surg. 48:1145–1151. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyamoto H, Kurita K, Ogi N, Ishimaru J
and Goss AN: The effect of an intra-articular bone fragment in the
genesis of temporomandibular joint ankylosis. Int J Oral Maxillofac
Surg. 29:290–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng FW, Zhao JL, Hu KJ and Liu YP: A new
hypothesis of mechanisms of traumatic ankylosis of
temporomandibular joint. Med Hypotheses. 73:92–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu CK, Liu P, Meng FW, Deng BL, Xue Y,
Mao TQ and Hu KJ: The role of the lateral pterygoid muscle in the
sagittal fracture of mandibular condyle (SFMC) healing process. Br
J Oral Maxillofac Surg. 50:356–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng TG, Liu CK, Liu P, Zhang LL, Wu LG,
Zhou HZ, Ding YX and Hu KJ: Influence of the lateral pterygoid
muscle on traumatic temporomandibular joint bony ankylosis. BMC
Oral Health. 16:622016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai J, Yu H, Zhu M and Shen SG: Injection
of botulinum toxin A in lateral pterygoid muscle as a novel method
for prevention of traumatic temporomandibular joint ankylosis. J
Med Hypoth Ideas. 9:5–8. 2015. View Article : Google Scholar
|
|
11
|
Cheung LK, Shi XJ and Zheng LW: Surgical
induction of temporomandibular joint ankylosis: An animal model. J
Oral Maxillofac Surg. 65:993–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyamoto H, Kurita K, Ishimaru JI and Goss
AN: A sheep model for temporomandibular joint ankylosis. J Oral
Maxillofac Surg. 57:812–817. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Skvortsov D, Abdueva D, Curtis C, Schaub B
and Tavaré S: Explaining differences in saturation levels for
Affymetrix GeneChip arrays. Nucleic Acids Res. 35:4154–4163. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lopes SL, Costa ALF, Gamba TdO, Flores IL,
Cruz AD and Min LL: Lateral pterygoid muscle volume and migraine in
patients with temporomandibular disorders. Imaging Sci Dent.
45:1–5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang DaW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID Gene Functional Classifcation Tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC and Lempicki RA:
DAVID Bioinformatics Resources: Expanded annotation database and
novel algorithms to better extract biology from large gene lists.
Nucleic Acids Res. 35:Web Server issue. W169–W175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eden E, Navon R, Steinfeld I, Lipson D and
Yakhini Z: GOrilla: A tool for discovery and visualization of
enriched GO terms in ranked gene lists. BMC Bioinformatics.
10:482009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauer S, Grossmann S, Vingron M and
Robinson PN: Ontologizer 2.0-a multifunctional tool for GO term
enrichment analysis and data exploration. Bioinformatics.
24:1650–1651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Runyan CM and Gabrick KS: Biology of bone
formation, fracture healing, and distraction osteogenesis. J
Craniofac Surg. 28:1380–1389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li G, Simpson AH and Triffitt JT: The role
of chondrocytes in intramembranous and endochondral ossification
during distraction osteogenesis in the rabbit. Calcif Tissue Int.
64:310–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen G, Rabie AB, Zhao ZH and Kaluarachchi
K: Forward deviation of the mandibular condyle enhances
endochondral ossification of condylar cartilage indicated by
increased expression of type X collagen. Arch Oral Biol.
51:315–324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bradaschia-Correa V, Barrence FA, Ferreira
LB, Massa LF and Arana-Chavez VE: Effect of alendronate on
endochondral ossification in mandibular condyles of growing rats.
Eur J Histochem. 56:e242012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lewinson D, Rachmiel A, Rihanibisharat S,
Kraiem Z, Schenzer P, Korem S and Rabinovich Y: Stimulation of Fos-
and Jun-related genes during distraction osteogenesis. J Histochem
Cytochem. 51:1161–1168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mieczkowski J, Tyburczy ME, Dabrowski M
and Pokarowski P: Probe set filtering increases correlation between
Affymetrix GeneChip and qRT-PCR expression measurements. BMC
Bioinformatics. 11:1042010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ishii M, Hashimoto S, Tsutsumi S, Wada Y,
Matsushima K, Kodama T and Aburatani H: Direct comparison of
GeneChip and SAGE on the quantitative accuracy in transcript
profiling analysis. Genomics. 68:136–143. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Juang JL, Chen TC, Jiang SS, Hsiung CA,
Chen WC, Chen GW, Lin SM, Lin JH, Chiu SC and Lai YK: Coupling
multiplex RT-PCR to a gene chip assay for sensitive and
semiquantitative detection of severe acute respiratory
syndrome-coronavirus. Lab Invest. 84:1085–1091. 2004. View Article : Google Scholar : PubMed/NCBI
|