Introduction
Posterior capsule opacification (PCO) and impaired
vision are complications associated with cataract surgery, and the
high complication rate is due to regeneration of residual lens
epithelial cells (1). PCO remains
a major complication of cataract surgery and the most common cause
of vision loss (2). Despite
current advances in PCO research, including basic research on
cataracts, the development of surgical techniques and the
development of materials used in intraocular lenses, PCO prevalence
is reported to be close to 100% in children and 30–40% in adults
within 5 years post-surgery (3–5).
Pharmacological drugs and small molecules have been used to prevent
PCO in recent years, but their efficiency is poor and they may
cause serious side effects (6).
Therefore, it is necessary to develop novel drugs that may
effectively inhibit the proliferation of HLEs or prevent PCO.
Sanguinarine is a type of benzophenanthridine
alkaloid extracted from the roots of the herbaceous plant
Sanguinaria canadensis (7),
which is widely used for its anti-microbial, anti-inflammatory,
anti-oxidative and tumor-suppressing properties (8–12).
Recent studies have indicated that sanguinarine is able to suppress
inflammation by reducing the expression of inflammatory cytokines,
including tumor necrosis factor-α and interleukins 1β and 6 in rats
(13). Moreover, sanguinarine has
been reported to exhibit anti-tumorigenic activity by promoting
apoptosis in acute lymphoblastic leukemia (ALL), basal-like breast
cancer and colorectal cancer (14–16).
Treatment of ALL cells with sanguinarine was reported to cause
apoptosis through loss of mitochondrial membrane potential (MMP),
activation of caspase pathways and generation of reactive oxygen
species (ROS) (15). Furthermore,
sanguinarine-induced apoptosis is strongly associated with multiple
signaling pathways including the mitogen-activated protein kinase
(MAPK) (17),
phosphoinositide-3-kinase/protein kinase B (18), Wnt/β-catenin (19) and Toll-like receptor 4/nuclear
factor-κ B signaling pathways (20). However, to the best of our
knowledge, few studies have focused on examining its efficacy on
HLEs and its underlying mechanism.
The purpose of the present study was to understand
the effects of sanguinarine on HLEs and the its underlying
mechanisms of action. Firstly, the potential anti-proliferative
effects of sanguinarine on HLEs were examined. Subsequently, the
induction of apoptosis or autophagy was verified to be the
mechanism underlying the anti-proliferative effect of sanguinarine.
In addition, the present study further assessed whether
sanguinarine-induced apoptosis was achieved via mitochondrial and
caspase-dependent pathways, whether ROS generation served a role in
this process and whether MAPK signaling was also involved in human
lens epithelial (HLE) cell death following treatment with
sanguinarine.
Materials and methods
Chemicals
Sanguinarine powder, dimethyl sulfoxide (DMSO) and
the ROS inhibitor N-acetylcysteine (NAC) were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Antibodies against
B-cell lymphoma 2 (Bcl-2) and apoptosis regulator BAX (Bax) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA),
while antibodies against p38, c-Jun N-terminal kinase (JNK),
phosphorylated (phospho)-JNK, phospho-p38 and β-actin were
purchased from Cell Signaling Technology Inc. (Danvers, MA, USA).
The pan-caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp (OMe)
fluoromethylketone (z-VAD-FMK), Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) were purchased from
BD Pharmingen (BD Biosciences, San Jose, CA, USA).
Cell culture and sanguinarine
treatment
HLE B-3 cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA) and cultured in an
incubator containing 5% CO2 at 37°C in RPMI 1640 medium
(cat. no. 21875091; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 20% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 µg/ml streptomycin and 100 U/ml
penicillin (Gibco; Thermo Fisher Scientific, Inc.). Sanguinarine
(0.5 g) was dissolved in 1 ml DMSO and diluted into a 50 ml stock
solution with distilled water, which was used to prepare the
testing concentrations. The experimental groups were treated with
different concentrations of sanguinarine, and with or without
pan-caspase inhibitor z-VAD-FMK or ROS inhibitor NAC. Z-VAD-FMK and
NAC were dissolved in DMSO to obtain stock solutions of 50 mM each,
and added to 1 ml culture wells at a final concentration of 50 µM.
The control groups were treated with DMSO as vehicle.
MTT assay
HLE B-3 cells were seeded at a density of 4,000
cells/well in 96-well plates, and treated with 1, 2, 3 or 4 µM
sanguinarine. The control group was treated with the
sanguinarine-free carrier solution (DMSO). After 24 or 48 h, 10 µl
0.5% MTT solution was added to each well and further incubated for
4 h. Subsequently, 100 µl triple solution (10% SDS, 5% isobutanol
and 0.1% HCl) were added to each well to dissolve the formazan
crystals and incubated overnight. The value of absorbance was
measured at 570 nm using a microplate reader (Tecan Group, Ltd.,
Mannedorf, Switzerland). Cell proliferation was calculated as the
percentage difference in the absorbance of treated cells compared
with untreated cells (21). The
anti-proliferative activity of sanguinarine was expressed as the
half-maximal inhibitory concentration (IC50) and the
calculation formula was as follows: Inhibition
(%)=[1-(ODobserved/ODcontrol)] ×100%.
Detection of apoptosis rate by flow
cytometry
Flow cytometry was used to analyze the apoptosis of
cells treated with 2 µM sanguinarine. A total of 1×105
cells were harvested, washed with precooled PBS, and stained with
Annexin V-FITC and propidium iodide (PI) for 15 min, at room
temperature and in the dark. The percentage of apoptotic cells was
determined via flow cytometry (BD FACSAria™ III; BD Biosciences)
and analyzed with BD CellQuest Pro™ Software (version 5.1, BD
Biosciences). Early stage apoptotic cells were stained only with
Annexin V-FITC, and late stage apoptotic cells were stained with
Annexin V-FITC and PI. Apoptotic cells were considered to be the
sum of all early and late apoptotic cells.
Analysis of MMP
The mitochondria-specific fluorescent dye
tetraethylbenzimidazolylcarbocyanine iodide (JC-1; Abcam,
Cambridge, UK) was used to determine the alterations in the MMP of
HLE B-3 cells treated with 2 µM sanguinarine. Cells were harvested,
washed and stained with JC-1 for 30 min at 37°C in the dark,
followed by washing and re-suspension with 300 µl PBS. Variations
in the MMP levels were measured at 527 and 590 nm by flow cytometry
and data analyses were performed using Summit software (version
5.2, Beckman Coulter, Brea, CA, USA). In cases of mitochondrial
damage, JC-1 will predominantly occur in its monomeric form in the
cytoplasm and will emit green fluorescence, while in healthy cells,
JC-1 forms aggregates and emits red fluorescence (22).
Caspase-3/7 activity assay
The activity of caspase-3/7 was detected by
Caspase-GIo 3/7 Assay kit (Promega Corporation, Madison, WI, USA).
An advantage of this kit is that it provides a luminogenic
substrate of caspase-3/7 containing the tetrapeptide sequence DEVD,
which has optimized caspase activity, luciferase activity and cell
lysis. Following treatment of HLE B-3 cells with different
concentrations of sanguinarine, with or without pan-caspase
inhibitor z-VAD-FMK and ROS inhibitor NAC for 24 h, 100 µl
caspase-GIo 3/7 reagent were added to each well of the 96-well
plates. Cells were incubated with the reagent for 1 h, at 37°C and
in the dark, and the luminescence signal was measured by a
Luminometer (Tecan Group, Ltd.).
Detection of ROS
Following attachment, HLE B-3 cells were pretreated
with 20 µM 2′,7′-dichlorofluorescein diacetate
(CM-H2DCFDA; Invitrogen; Thermo Fisher Scientific,
Inc.), a specific superoxide tracer dye, for 30 min at 37°C prior
to testing. HLE B-3 cells treated with 2 µM sanguinarine, with or
without z-VAD-FMK, were incubated for 1 h, harvested and
re-suspended in PBS. Fluorescence was measured by flow cytometer
with excitation/emission wavelengths of 488/525 nm and data
analysis was performed as described above (23).
Western blot analysis
Following treatment with various concentrations of
sanguinarine for different time intervals, HLE B-3 cells were lysed
with radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc.) with protease and phosphatase inhibitors. The
concentration of protein was determined using the Lowry-based
Bio-Rad DC™ protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Following denaturation, 30 µg of proteins were
loaded per lane and separated via 10% SDS-PAGE. The separated
proteins were transferred to a nitrocellulose membrane. The
membranes were blocked with 5% skimmed milk in TBS-Tween-20 for 1 h
at room temperature. Primary antibodies against Bcl-2 (1:500; cat.
no. sc-23960), Bax (1:500; cat. no. sc-20067), p38 (1:1,000; cat.
no. 9212), JNK (1:1,000; cat. no. 9251), phospho-JNK (p-JNK;
1:1,000; cat. no. 9251), phospho-p38 (1:1,000; cat. no. 9211) and
β-actin (1:1,000; cat. no. 4967) were incubated overnight at 4°C.
Following three 5 min washes with TBS-Tween-20, membranes were
incubated with a horseradish peroxidase-labeled secondary antibody
(1:5,000 dilution; cat. no. sc-2350; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature, followed by enhanced
chemiluminescent detection (Merck KGaA). β-actin was used as a
loading control and for normalization. ImageJ Software version 1.46
(National Institute of Health, Bethesda, MA, USA) was used for
densitometry analysis.
Statistical analysis
Data are expressed as the mean ± standard deviation
of individual groups. Differences between groups were adequately
determined using one-way or two-way analysis of variance followed
by Bonferroni post-hoc tests for multiple comparisons. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using GraphPad Prism
software version 6. (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
Sanguinarine reduces the proliferation
of HLEs
To determine the effects of sanguinarine on the
proliferation/viability of HLE B-3 cells, an MTT assay was
performed. As exhibited in Fig. 1,
the proliferation of HLE B-3 cells tended to decrease with
increasing concentrations of sanguinarine and longer exposure
times. The IC50 values of sanguinarine on HLE B-3 cells
at 24 and 48 h were 3.60±0.68 and 2.02±0.53 µM, respectively. At
the same time points, compared with the control group, the
proliferation of HLE B-3 cells decreased with increasing
concentrations of sanguinarine. Similarly, at the same sanguinarine
concentration, the proliferation of HLE B-3 cells was significantly
decreased following longer treatments. Therefore, these results
indicated that sanguinarine may have significantly inhibited the
proliferation of HLE B-3 cells (P<0.01).
Sanguinarine inhibits the growth of
HLEs by inducing cell apoptosis
To investigate the anti-proliferative mechanism of
sanguinarine on HLEs, the apoptosis of HLE B-3 cells was evaluated
using flow cytometry based on PI and Annexin V-FITC staining
(Fig. 2). A significant number of
HLE B-3 cells underwent apoptosis following treatment with 2 µM
sanguinarine for 24 h. The apoptosis rate [the sum of late
apoptosis (Q2) and early apoptosis (Q3)] of HLE B-3 cells was
30.00±2.36% following treatment with sanguinarine, while the
apoptosis rate in the control group was 7.67±0.98% (Fig. 2). There was a significant
difference between the two groups (P<0.01). These results
demonstrated that treatment with sanguinarine induced apoptosis of
HLEs, which may contribute to the inhibitory effect of sanguinarine
on cell viability.
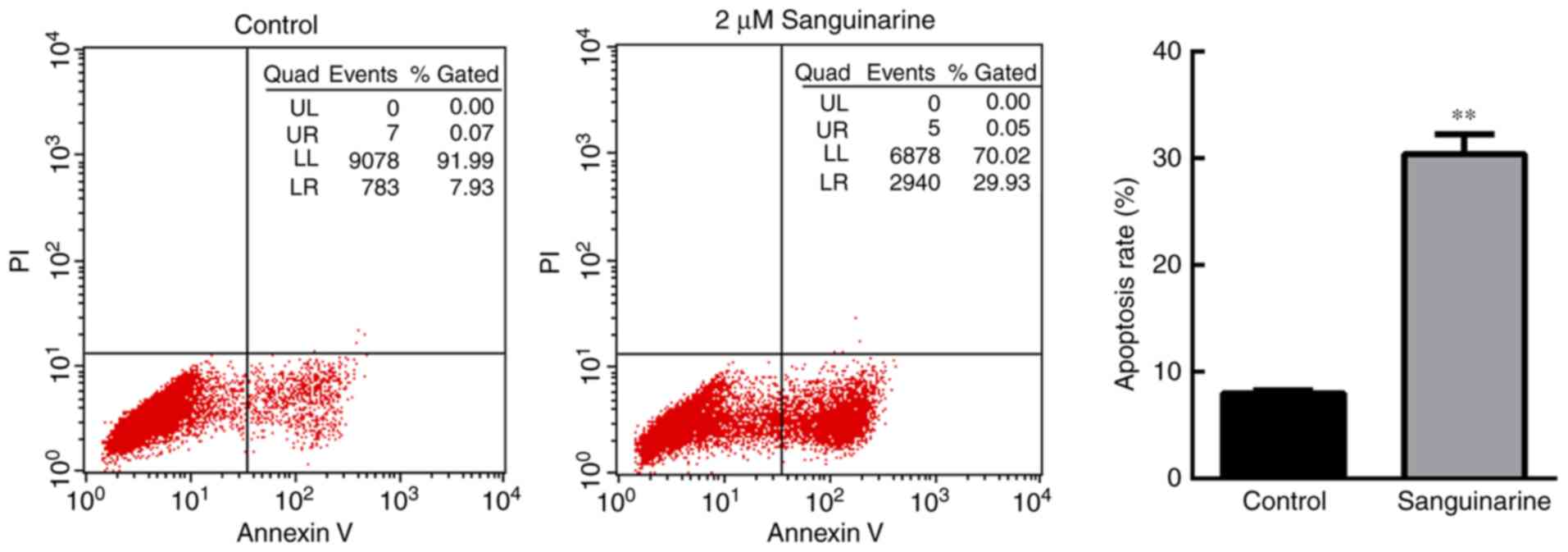 | Figure 2.Sanguinarine induces apoptosis in HLE
cells. Cell apoptosis of HLE B-3 cells was detected via flow
cytometry (n=3). In the control group, >90% of cells were
gathered at the Q4 area, and <10% of cells underwent apoptosis
(Q2+Q3 areas). However, following treatment with 2 µM sanguinarine
for 24 h, cells in the Q4 area decreased to 65.7%, whereas cells in
the Q2 and Q3 quadrants, which represent late and early apoptosis,
respectively, were significantly increased. The results are
expressed as the mean ± standard deviation. **P<0.01 vs. the
control group. HLE, human lens epithelial; PI, propidium
iodide. |
Sanguinarine induces apoptosis through
a mitochondria- dependent pathway
A study reported that mitochondrial dysfunction may
be a critical mediator of apoptosis in cancer (24). To investigate whether mitochondria
are involved in the sanguinarine-induced apoptosis of HLEs, the MMP
was analyzed by JC-1 staining. As exhibited in Fig. 3A, the percentage of cells which
emitted red fluorescence was reduced in the mitochondria of HLE B-3
cells treated with sanguinarine, while the percentage of cells
which emitted green fluorescence increased. These results indicated
that MMP was significantly reduced following treatment of HLE B-3
cells with sanguinarine compared with the control group (P<0.01;
Fig. 3A and B).
 | Figure 3.MMP and HLE cell integrity are
reduced following treatment with sanguinarine. (A) JC-1 dye was
used as an indicator of MMP. Compared with control group, the
number of red fluorescence emitting cells, which is indicative of
healthy mitochondria, was markedly decreased in the sanguinarine
treatment group; however, the green fluorescence emitting cells,
representing damaged mitochondria, were increased. (B)
Quantification of types of fluorescence-emitting HLE cells obtained
following staining with JC-1 (n=3). (C) The western blotting
results demonstrated that the relative proportions of Bcl-2 and
Bax, key regulators of mitochondrial membrane integrity, were
significantly altered following treatment with sanguinarine (n=3).
The results are expressed as the mean ± standard deviation.
*P<0.05 and **P<0.01 vs. respective control. HLE, human lens
epithelial; JC-1, tetraethylbenzimidazolylcarbocyanine iodide dye;
Bcl-2, B-cell lymphoma 2; Bax, apoptosis regulator BAX; MMP,
mitochondrial membrane potential. |
The process of apoptosis involving mitochondria is
regulated via the ratio of Bcl-2 (anti-apoptotic protein) and Bax
(pro-apoptotic protein), and a decrease in this ratio indicates the
activation of the apoptotic pathway (25). This ratio is considered to be a key
factor in controlling the integrity of mitochondrial membranes.
Therefore, the protein levels of Bcl-2 and Bax were detected via
western blotting. Subsequent to treatment with sanguinarine, the
anti-apoptotic protein Bcl-2 was observed to be significantly
decreased (P<0.01), while the pro-apoptotic protein Bax was
increased (P<0.05). Therefore, the ratio of Bcl-2/Bax was
decreased, which further supported the evidence that the
mitochondria in HLE B-3 cells were damaged following treatment with
sanguinarine (Fig. 3C). The flow
cytometry and western blotting results suggested that sanguinarine
may affect mitochondrial function and integrity and that
mitochondria may be involved in sanguinarine-induced apoptosis.
Sanguinarine induces the activation of
caspase in HLEs
Caspases 3 and 7 are considered key mediators of
mitochondrial events of apoptosis, as their activation leads to the
execution stage of apoptosis (26). In order to elucidate whether the
activation of caspases was involved in sanguinarine-induced
apoptosis, the activity of caspases 3 and 7 in control and
treatment groups was analyzed using luminescent signal peaks
proportional to the levels of caspase activity. The results
demonstrated that sanguinarine stimulated caspase-3/7 activity in
HLE B-3 cells in a concentration-dependent manner (Fig. 4A).
 | Figure 4.Caspase and ROS serve essential roles
in sanguinarine-induced apoptosis in HLE cells. (A) Caspase-3/7
activity results demonstrated that following incubation with 1, 2
or 3 µM of sanguinarine for 24 h, caspases 3/7 activity levels were
significantly increased (n=3). (B) In the sanguinarine only
treatment group, 31% of cells underwent apoptosis (Q2+Q3); however,
in HLE B-3 cells co-treated with pan-caspase inhibitor z-VAD-FMK
and sanguinarine, the apoptosis induced by sanguinarine was
reduced; and cells co-treated with the ROS inhibitor NAC and
sanguinarine also exhibited lower levels of apoptosis (n=3). These
results suggested that ROS generation and caspase activity were
required for sanguinarine-induced apoptosis. The results are
expressed as the mean ± standard deviation. **P<0.01 vs.
sanguinarine-only treatment. HLE, human lens epithelial; ROS,
reactive oxygen species; z-VAD-FMK, benzyloxycarbonyl-Val-Ala-Asp
(OMe) fluoromethylketone; NAC, N-acetylcysteine; PI, propidium
iodide. |
Subsequently, the pan-caspase inhibitor z-VAD-FMK
and the ROS inhibitor NAC were used to investigate whether caspase
activation and ROS generation, respectively, are required for
sanguinarine-induced apoptosis. The results of the flow cytometry
analysis indicated that exposure to z-VAD-FMK or NAC significantly
attenuated the effect of sanguinarine in inducing apoptosis
compared with the single sanguinarine treatment group (P<0.01;
Fig. 4B). These results
demonstrated that z-VAD-FMK and NAC significantly reduced the
levels of apoptosis induced by sanguinarine, and that
sanguinarine-induced apoptosis may be caspase-dependent.
Sanguinarine-mediated ROS generation
leads to apoptosis in HLEs
The association between ROS and mitochondria has
been studied for a long time (27). A study has reported that the
accumulation of ROS may be responsible for the mitochondrial
dysfunction (28). Therefore, the
association between the generation of ROS and mitochondrial
dysfunction induced by sanguinarine was evaluated. CM-H2DCFDA was
used to detect the amount of ROS in HLE B-3 cells. As presented in
Fig. 5, following treatment with
sanguinarine, flow cytometry data indicated that more cells
exhibited the peak of fluorescence intensity (the peak shifted to
the right), revealing that sanguinarine greatly increased the
amount of ROS in HLE B-3 cells (P<0.01). To evaluate whether ROS
generation serves an essential role in the process of apoptosis,
the levels of ROS were determined following treatment with
z-VAD-FMK and sanguinarine. This treatment, however, did not
prevent the generation of ROS, suggesting that ROS generation may
be an upstream step in sanguinarine-induced apoptosis.
Sanguinarine induces apoptosis of HLEs
by activating the MAPK pathway
The MAPK pathway has been demonstrated to be closely
associated with the process of apoptosis (29). To test whether the MAPK pathway
serves a similar role in sanguinarine-induced apoptosis, HLE B-3
cells were treated with different concentrations of sanguinarine.
As presented in Fig. 6A, treatment
with sanguinarine promoted the phosphorylation of JNK and p38 in a
dose-dependent manner. Moreover, longer treatments with 2 µM
sanguinarine (2, 4 and 6 h) significantly up-regulated the
expression of p-JNK and p-P38 compared with the control group
(Fig. 6B). Among these exposure
times, the 2 h treatment resulted in higher levels of
phosphorylation. To further determine whether the activation of JNK
and p38 is a key step in sanguinarine-induced apoptosis, HLE B-3
cells were exposed to sanguinarine with or without ROS inhibitor
NAC to assess whether reduced ROS generation prevents the
phosphorylation of p38 and JNK. Co-treatment of HLE B-3 cells with
sanguinarine and ROS inhibitor NAC significantly increased the
expression levels of p-JNK and p-P38 compared with sanguinarine or
NAC alone (Fig. 6C). These results
suggested that the MAPK pathway is associated with
sanguinarine-induced apoptosis, but it is not the primary target of
sanguinarine.
 | Figure 6.MAPK pathway is involved, but serves
a subsidiary role in sanguinarine-induced apoptosis in human lens
epithelial cells. The effect of different concentrations of
sanguinarine (A) and different time treatments (B) on the
activation of MAPK pathway-related proteins (JNK, p-JNK, P38 and
p-P38) was detected using western blotting (n=3). The western
blotting results demonstrated that both JNK and p38 were activated
by sanguinarine in a dose-dependent manner (n=3). (C) NAC, a
specific ROS inhibitor, was applied in a co-treatment with
sanguinarine to determine whether it affected the activation of
MAPK pathway components observed following treatment with
sanguinarine (n=3). Compared with sanguinarine single treatment
group, co-treatment with sanguinarine and ROS inhibitor NAC
significantly increased the levels of p-JNK and p-P38. The results
are expressed as the mean ± standard deviation. *P<0.05 and
**P<0.01. MAPK, mitogen-activated protein kinase; ROS, reactive
oxygen species; NAC, N-acetylcysteine; p, phosphorylated; JNK,
c-Jun N-terminal kinase. |
Discussion
Over 8 million cataract operations are performed
every year in Europe and the United States, and the number is even
higher in China (30). PCO is the
most common complication encountered following cataract surgery,
and it seriously affects the quality of life of the patients
through secondary loss of vision (2). The development of PCO is affected by
various factors, but the proliferation and migration of HLEs is a
common cause of PCO (31).
Discovering novel agents that may effectively suppress the
proliferation of HLEs and prevent PCO is of the utmost
importance.
In the present study, it was verified that
sanguinarine significantly reduced the viability of HLE B-3 cells
in a dose- and time-dependently manner, and that it induced
apoptosis. The apoptosis effects were possibly mediated by early
ROS generation, which may promote the activation of p38 and JNK,
leading to the activation of the caspase pathway and causing
apoptosis. Furthermore, the results seem to suggest that ROS
generation may be essential for sanguinarine-mediated apoptosis.
Thus, sanguinarine may be a potential therapeutic agent in the
treatment of patients with PCO in the future.
ROS, the by-products of ATP production, are
primarily formed by the interaction of oxygen molecules with
electrons escaping from the mitochondrial respiratory chain
(32). Normally, there is an
antioxidant system that controls the basal levels of ROS as, in
excess, these may cause cellular damage by reacting with DNA,
proteins, lipids and even the electron transport chain itself
(33,34). Therefore, ROS generation is closely
associated with mitochondrial function and cell health. Reports
have also demonstrated that elevated levels of basal ROS in cancer
cells are conducive to increased cell proliferation (35). Thus, ROS have complex roles within
the scope of biological processes that occur in cells, making it
difficult to understand how levels of oxidants are involved in cell
growth and maintenance and how ROS may be used therapeutically
(35). The present results seem to
suggest that treatment with sanguinarine may have significantly
increased the generation of ROS in HLE B-3 cells, and inhibiting
its generation may have reduced the levels of apoptosis observed in
these cells.
As mentioned previously, upregulation of ROS in
cells may result in serious mitochondrial and cellular damage
(36,37). In the present study, the MMP of HLE
B-3 cells treated with sanguinarine was significantly reduced
compared with the control group. The pro-apoptotic protein Bax was
also upregulated, while the anti-apoptotic protein Bcl-2 was
downregulated. This alteration in the Bax/Bcl-2 ratio is an
indicator of mitochondrial membrane damage (38,39).
Once mitochondria are damaged, cytochrome c is released and forms a
complex with apoptosis protease-activating factor-1 to further
activate caspase-9, which in turn activates caspases 3 and 7
(40–42). The present study indicated that the
activation of caspases is involved in sanguinarine-induced
apoptosis, as the use of pan-caspase inhibitor z-VAD-FMK resulted
in the reduction of apoptosis levels following treatment with
sanguinarine. Therefore, sanguinarine may have induced apoptosis
through caspase-associated pathways.
Finally, the involvement of the MAPK pathway, which
is closely associated with cell proliferation and survival
(43,44), was evaluated. Sanguinarine promoted
the phosphorylation of JNK and p38 kinases in HLE B-3 cells,
suggesting that MAPK signaling pathway may be involved in
sanguinarine-induced apoptosis.
In conclusion, sanguinarine may significantly affect
the viability of HLE B-3 cells by inducing apoptosis. ROS
generation and caspase activation may serve as key mediators of
sanguinarine-induced apoptosis. The MAPK signaling pathway may be
involved. Sanguinarine may possibly be used as a potential drug for
preventing PCO or even the development of cataracts in the future.
However, the present study is only preliminary. Further in
vivo research and clinical studies should be undertaken to
verify the potential beneficial effects of sanguinarine on PCO
treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Funds (grant no. 81500705).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ and WRH contributed equally to the present study.
YZ and WRH designed the study, performed the experimental work,
collected, analyzed and interpreted the data, and drafted and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang KC and Petrash JM: Aldose reductase
mediates transforming growth factor β2 (TGF-β2)-induced migration
and epithelial-to-mesenchymal transition of lens-derived epithelial
cells. Invest Ophthalmol Vis Sci. 56:4198–4210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meacock WR, Spalton DJ, Boyce J and
Marshall J: The effect of posterior capsule opacification on visual
function. Invest Ophthalmol Vis Sci. 44:4665–4669. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernandez V, Fragoso MA, Billotte C, Lamar
P, Orozco MA, Dubovy S, Willcox M and Parel JM: Efficacy of various
drugs in the prevention of posterior capsule opacification:
Experimental study of rabbit eyes. J Cataract Refract Surg.
30:2598–2605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang YX, Lu Y, Liu TJ, Yang J, Chen Y and
Fang YW: Using HSV-TK/GCV suicide gene therapy to inhibit lens
epithelial cell proliferation for treatment of posterior capsular
opacification. Mol Vis. 17:291–299. 2011.PubMed/NCBI
|
|
5
|
Rönbeck M, Zetterström C, Wejde G and
Kugelberg M: Comparison of posterior capsule opacification
development with 3 intraocular lens types: Five-year prospective
study. J Cataract Refract Surg. 35:1935–1940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang WR, Zhang Y and Tang X: Shikonin
inhibits the proliferation of human lens epithelial cells by
inducing apoptosis through ROS and caspase-dependent pathway.
Molecules. 19:7785–7797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laster LL and Lobene RR: New perspectives
on Sanguinaria clinicals: Individual toothpaste and oral rinse
testing. J Can Dent Assoc. 56 (Suppl 7):S19–S30. 1990.
|
|
8
|
Miao F, Yang XJ, Zhou L, Hu HJ, Zheng F,
Ding XD, Sun DM, Zhou CD and Sun W: Structural modification of
sanguinarine and chelerythrine and their antibacterial activity.
Nat Prod Res. 25:863–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Slaninová I, Pěnčíková K, Urbanová J,
Slanina J and Táborská E: Antitumour activities of sanguinarine and
related alkaloids. Phytochem Rev. 13:51–68. 2014. View Article : Google Scholar
|
|
10
|
Gaziano R, Moroni G, Buè C, Miele MT,
Sinibaldi-Vallebona P and Pica F: Antitumor effects of the
benzophenanthridine alkaloid sanguinarine: Evidence and
perspectives. World J Gastrointest Oncol. 8:30–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Godowski KC: Antimicrobial action of
sanguinarine. J Clin Den. 1:96–101. 1989.
|
|
12
|
Achkar IW, Mraiche F, Mohammad RM and
Uddin S: Anticancer potential of sanguinarine for various human
malignancies. Future Med Chem. 9:933–950. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Q, Dai P, Bao H, Liang P, Wang W,
Xing A and Sun J: Anti-inflammatory and neuroprotective effects of
sanguinarine following cerebral ischemia in rats. Exp Ther Med.
13:263–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalogris C, Garulli C, Pietrella L,
Gambini V, Pucciarelli S, Lucci C, Tilio M, Zabaleta ME, Bartolacci
C, Andreani C, et al: Sanguinarine suppresses basal-like breast
cancer growth through dihydrofolate reductase inhibition. Biochem
Pharmacol. 90:226–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuttikrishnan S, Siveen KS, Prabhu KS,
Khan AQ, Akhtar S, Mateo JM, Merhi M, Taha R, Omri HE, Mraiche F,
et al: Sanguinarine suppresses growth and induces apoptosis in
childhood acute lymphoblastic leukemia. Leuk Lymphoma. 6:1–13.
2018.
|
|
16
|
Gong X, Chen Z, Han Q, Chen C, Jing L, Liu
Y, Zhao L, Yao X and Sun X: Sanguinarine triggers intrinsic
apoptosis to suppress colorectal cancer growth through
disassociation between STRAP and MELK. BMC Cancer. 18:5782018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vogt A, Tamewitz A, Skoko J, Sikorski RP,
Giuliano KA and Lazo JS: The benzo[c]phenanthridine alkaloid,
sanguinarine, is a selective, cell-active inhibitor of
mitogen-activated protein kinase phosphatase-1. J Biol Chem.
280:19078–19086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee TK, Park C, Jeong SJ, Jeong MJ, Kim
GY, Kim WJ and Choi YH: Sanguinarine induces apoptosis of human
oral squamous cell carcinoma KB cells via inactivation of the
PI3K/Akt signaling pathway. Drug Dev Res. 77:227–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Fang Z, Wu J, Yin X, Fang Y, Zhao
F, Zhu S and Li Y: Construction and application of a lung cancer
stem cell model: Antitumor drug screening and molecular mechanism
of the inhibitory effects of sanguinarine. Tumour Biol.
37:13871–13883. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng YY, Liu Y, Hu ZF, Zhang Y, Ni J, Ma
ZG, Liao HH, Wu QQ and Tang QZ: Sanguinarine attenuates
lipopolysaccharide-induced inflammation and apoptosis by inhibiting
the TLR4/NF-κB pathway in H9c2 cardiomyocytes. Curr Med Sci.
38:204–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen KC, Willmore WG and Tayabali AF:
Cadmium telluride quantum dots cause oxidative stress leading to
extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2
cells. Toxicology. 306:114–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang DS, Li YY, Chen XJ, Li YJ, Liu ZY,
Xie WJ and Sun ZL: BCL2 promotor methylation and miR-15a/16-1
upregulation is associated with sanguinarine-induced apoptotic
death in rat HSC-T6 cells. J Pharmacol Sci. 127:135–144. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He D, Ma X, Chen Y, Cai Y, Ru X, Bruce IC,
Xia Q, Shi G and Jin J: Luteolin inhibits pyrogallol-induced
apoptosis through the extracellular signal-regulated kinase
signaling pathway. FEBS J. 279:1834–1843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu PC, Huang YT, Tsai ML, Wang YJ, Lin JK
and Pan MH: Induction of apoptosis by shikonin through coordinative
modulation of the Bcl-2 family, p27, and p53, release of cytochrome
c, and sequential activation of caspases in human colorectal
carcinoma cells. J Agric Food Chem. 52:6330–6337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pradelli LA, Bénéteau M and Ricci JE:
Mitochondrial control of caspase-dependent and -independent cell
death. Cell Mol Life Sci. 67:1589–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lakhani SA, Masud A, Kuida K, Porter GA
Jr, Booth CJ, Mehal WZ, Inayat I and Flavell RA: Caspases 3 and 7:
Key mediators of mitochondrial events of apoptosis. Science.
311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fonseca-Silva F, Inacio JD,
Canto-Cavalheiro MM and Almeida-Amaral EE: Reactive oxygen species
production and mitochondrial dysfunction contribute to quercetin
induced death in leishmania amazonensis. PLoS One. 6:e146662011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang SY, Stem MS, Oren G, Shtein R and
Lichter PR: Patient-centered and visual quality outcomes of premium
cataract surgery: A systematic review. Eur J Ophthalmol.
27:387–401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Awasthi N, Guo S and Wagner BJ: Posterior
capsular opacification: A problem reduced but not yet eradicated.
Arch Ophthalmol. 127:555–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poyton RO, Ball KA and Castello PR:
Mitochondrial generation of free radicals and hypoxic signaling.
Trends Endocrinol Metab. 20:332–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ricci JE, Gottlieb RA and Green DR:
Caspase-mediated loss of mitochondrial function and generation of
reactive oxygen species during apoptosis. J Cell Biol. 160:65–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park SY, Kim DY, Kang JK, Park G and Choi
YW: Involvement of activation of the Nrf2/ARE pathway in protection
against 6-OHDA-induced SH-SY5Y cell death by α-iso-cubebenol.
Neurotoxicology. 44:160–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schumacker PT: Reactive oxygen species in
cancer cells: Live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang B, Xie QY, Quan Y, Pan XM and Liao
DF: Reactive oxygen species induce cell death via Akt signaling in
rat osteoblast-like cell line ROS 17/2.8. Toxicol Ind Health.
31:1236–1242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zubair H, Khan HY, Sohail A, Azim S, Ullah
MF, Ahmad A, Sarkar FH and Hadi SM: Redox cycling of endogenous
copper by thymoquinone leads to ROS-mediated DNA breakage and
consequent cell death: Putative anticancer mechanism of
antioxidants. Cell Death Dis. 4:e6602013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kocic G, Tomovic K, Kocic H, Sokolovic D,
Djordjevic B, Stojanovic S, Arsic I and Smelcerovic A:
Antioxidative, membrane protective and antiapoptotic effects of
melatonin, in silico study of physico-chemical profile and
efficiency of nanoliposome delivery compared to betaine. RSC Adv.
7:1271–1281. 2017. View Article : Google Scholar
|
|
40
|
Zhou J and Tang XC: Huperzine A attenuates
apoptosis and mitochondria-dependent caspase-3 in rat cortical
neurons. FEBS Lett. 526:21–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuyama K, Yamamoto H, Nishizaki I, Kato T,
Sora I and Yamamoto T: Caspase-independent cell death by low
concentrations of nitric oxide in PC12 cells: Involvement of
cytochrome C oxidase inhibition and the production of reactive
oxygen species in mitochondria. J Neurosci Res. 73:351–363. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu JJ, Xu YQ, He JH, Yu HP, Huang CJ, Gao
JM, Dong QX, Xuan YX and Li CQ: Human cardiotoxic drugs delivered
by soaking and microinjection induce cardiovascular toxicity in
zebrafish. J Appl Toxicol. 34:139–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chao JI, Su WC and Liu HF: Baicalein
induces cancer cell death and proliferation retardation by the
inhibition of CDC2 kinase and survivin associated with opposite
role of p38 mitogen-activated protein kinase and AKT. Mol Cancer
Ther. 6:3039–3048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
He XQ, Chen R, Yang P, Li AP, Zhou JW and
Liu QZ: Biphasic effect of arsenite on cell proliferation and
apoptosis is associated with the activation of JNK and ERK1/2 in
human embryo lung fibroblast cells. Toxicol Appl Pharmacol.
220:18–24. 2007. View Article : Google Scholar : PubMed/NCBI
|




















