Introduction
Atherosclerosis as a chronic inflammatory disease
caused by the formation of plaques in arteries, causing narrowing
and the consequent development of debilitating conditions, such as
stroke, kidney problems, coronary artery disease and peripheral
artery disease (1,2). Abnormal cholesterol content has been
shown to be a major cause of atherosclerosis, although the exact
mechanisms remain unclear (3).
Risk factors include diabetes, high blood pressure, family history,
obesity and an unhealthy diet (4,5).
Typically, atherosclerosis does not have well-defined symptoms, and
the majority of patients are diagnosed in advanced stages, leading
to poor treatment outcomes (6).
Therefore, early diagnosis is critical for the effective treatment
of atherosclerosis and prevention of atherosclerosis-associated
complications.
Long non-coding RNAs (lncRNAs) are a group of
transcripts composed of >200 nucleotides, with no protein-coding
ability (7). It has been well
established that various lncRNAs participate in the development of
human diseases, by inhibiting or promoting their progression
(8). In effect, certain lncRNAs
have been proven to be a potential therapeutic target for the
treatment of human diseases. The development of atherosclerosis is
also accompanied by alterations in the lncRNA expression profile
(9), indicating the involvement of
certain lncRNAs in the pathogenesis of this disease. lncRNA-ATB has
critical function in several types of human malignancies (10,11).
Altered expression of lncRNA-ATB is closely correlated with the
progression and prognosis of colon cancer (10). During the development of renal cell
carcinoma, lncRNA-ATB is involved in tumor metastases possibly by
promoting cancer cell migration and invasion (11). However, its involvement in
atherosclerosis is unknown. The preliminary microarray analysis
revealed that lncRNA-ATB was altered in atherosclerosis, indicating
its potential involvement in this disease. Therefore, the present
study aimed to investigate the functionality of lncRNA-ATB in
atherosclerosis. The present study provided evidence of a novel
biomarker for the diagnosis of atherosclerosis, and a novel target
for the treatment of this disease.
Materials and methods
Subjects
Serum samples were collected from 56 patients with
atherosclerosis (early stage) (12) and 44 healthy volunteers from the
elbow vein in The Fourth People's Hospital of Jinan from January
2016 to January 2018 (Jinan, China). Patients with other severe
diseases, such as cases of severe heart, lung and liver diseases,
were not included in this study. All participants were willing to
participate in this study. The 56 patients included 29 males and 27
females, with a mean age of 26.8±7.8 (range, 16–34). The 44 healthy
volunteers included 24 males and 20 females, with a mean age of
25.7±9.1 (range, 18–32). No significant differences in age and
gender were demonstrated between the patient group and healthy
control group. The present study was approved by the Ethics
Committee of The Fourth People's Hospital of Jinan (Jinan, China),
and all patients provided written informed consent.
ELISA
Serum TGF-β1 was detected by ELISA using coating
antibody (cat. no. MAB1835) and biotinylated detection antibody
(cat. no. BAF240), obtained from R&D Systems, Inc.
(Minneapolis, MN, USA). All operations were performed in strict
accordance with manufacturer's protocol. Acid activation was
performed to release biologically active TGF-β1, as described
previously (13). Serum samples
were diluted in Dulbecco's PBS (1:75) and added to the ELISA plate
to measure the concentration of active TGF-β1.
Cell culture
Human umbilical vein endothelial cells [HUVECs; cat.
no. CRL-1730; American Type Culture Collection (ATCC), Manassas,
VA, USA] were cultured in F-12K medium (cat. no. 30-2004; ATCC)
supplemented with 0.1 mg/ml heparin (cat. no. H3393; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), endothelial cell growth supplement
(1:100; cat. no. 354006; Corning Corporation, Corning, NY, USA) and
10% fetal bovine serum (cat. no. 30-2020; ATCC) at 37°C with 5%
CO2. Cells were collected during the logarithmic growth
phase for subsequent experiments.
Establishment of lncRNA-ATB
overexpression and siRNA silencing in HUVECs
A EcoRI-EcoRI fragment containing full length
lncRNA-ATB cDNA was inserted into pIRES2-EGFP vector (Clontech
Laboratories, Inc., Mountainview, CA, USA) to establish a vector
expressing lncRNA-ATB. Cells were cultured in the medium mentioned
above at 37°C overnight to reach 70–80% confluence. Transfection
was performed using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) to transfect 10
nM vector, 50 nM lncRNA-ATB siRNA (5′-CCAUGAGGAGUACUGCCAATT-3′) or
non-targeting control siRNA (5′-UUCUCCGAACGUGUCACGUtt-3′; Sangon,
Biotech Co., Ltd., Shanghai, China) into 5×106 HUVECs.
Cell suspensions were prepared by centrifuging cells at 1,000 × g
for 10 min at room temperature to remove the supernatant, followed
by the addition of fresh culture medium. Cells were collected 24 h
following transfection for subsequent experiments. Control cells
were treated with Lipofectamine® 2000 only. Cells
transfected with empty vectors or non-targeting control siRNA were
negative control cells.
MTT assay
Tetraethylammonium (10 mM; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added to cells (5×104
cells/ml) with lncRNA-ATB overexpression and siRNA silencing as
well as control and negative control cells. Each well of a 96-well
plate was filled with 100 µl cell suspension (5×103
cells/well), and cells were cultured at 37°C with 5% CO2
for 4 h, following which 10 µl MTT was added into each well for
another 4 h at 37°C, DMSO was added to dissolve the formazan
crystals, and the optical density was measured at 570 nm with a
microplate reader.
Cell proliferation assay
Treated cells in a 100 µl suspension were added into
each well of 96-well plates (4×104 cells/well). Cells
were cultured in an incubator at 37°C with 5% CO2 and
Cell Counting Kit-8 solution (10 µl) was added into each well 24,
48, 72 and 96 h later, and further cultured at 37°C for another 4
h. Optical density was measured at 450 nm with a microplate reader.
In cases of TGF-β1 treatment, cells were treated with TGF-β1
(Sigma-Aldrich Merck KGaA) at a dose of 10 ng/ml for 1 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) from serum and treated
cells and RNA samples were used to synthesize cDNA through reverse
transcription. Reverse transcription was performed using
SuperScript II Reverse Transcriptase kit (Thermo Fisher Scientific,
Inc.) through the following conditions: 5 min at 25°C, 10 min at
55°C and 5 min at 80°C. Sequences of primers used in PCR reactions
were: 5′-TCTGGCTGAGGCTGGTTGAC-3′ (forward) and
5′-ATCTCTGGGTGCTGGTGAAGG-3′ (reverse) for lncRNA-ATB;
5′-GACCTCTATGCCAACACAGT-3′ (forward) and 5′-AGTACTTGCGCTCAGGAGGA-3′
(reverse) for β-actin. SYBR® Green Quantitative RT-qPCR
kit (Sigma-Aldrich; Merck KGaA) was used to prepare all PCR
reaction systems. The PCR thermocycling conditions were: 95°C for
45 sec, followed by 40 cycles of 95°C for 10 sec and 60°C for 35
sec. Data were quantified using the 2−ΔΔCq method
(14). Relative expression of
lncRNA-ATB was normalized to β-actin.
Western blot analysis
Total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.), and protein concentration was determined by a
bicinchoninic acid protein assay. Proteins (20 µg/lane) were
separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. Membranes were blocked with 5% skimmed milk
for 2 h at room temperature. Following three washes with PBS (10
min each time), membranes were incubated with primary antibodies
including rabbit anti-TGF-β1 (1:2,000; cat. no. ab92486; Abcam,
Cambridge, UK), anti-caspase-3 (1:1,500; cat. no. ab4051; Abcam)
and anti-GAPDH (1:1,000; cat. no. ab9845; Abcam) overnight at 4°C.
Membranes were washed again in PBS three times (10 min each time)
and subsequently incubated with anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:1,000; cat. no.
MBS435036; MyBioSource, Inc., San Diego, CA, USA) at room
temperature for 1.5 h. Following further washes as described above,
bands were visualized with enhanced chemiluminescence reagent
(Sigma-Aldrich; Merck KGaA). The relative expression of TGF-β1 was
normalized to the endogenous control GAPDH using ImageJ v.148
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc., La Jolla,
CA, USA) and Origin v10 (OriginLab Corporation, Northampton, MA,
USA) were used to perform statistical analyses. All experiments
were performed in triplicate manner and data were expressed as mean
± standard deviation. Data comparisons between two groups were
performed with the Student's t-test, and among multiple groups
using one-way analysis of variance, followed by Tukey test.
Chi-square test was used for the analysis of count data. ROC curve
analysis was used to evaluate the diagnostic value of serum active
TGF-β1 and lncRNA-ATB expression in atherosclerosis. P<0.05 was
considered to indicate a statistically significant difference.
Results
TGF-β1 and lncRNA-ATB expression is
increased in patients with atherosclerosis
Serum expression of active TGF-β1 and lncRNA-ATB was
measured in patients with atherosclerosis and healthy controls via
ELISA and RT-qPCR, respectively. As presented in Fig. 1A, active TGF-β1 expression in serum
samples was significantly higher in patients with atherosclerosis,
compared with healthy controls (P<0.05). Similarly,
significantly higher circulating lncRNA-ATB expression was detected
in atherosclerosis patients, compared with healthy controls
(P<0.05; Fig. 1B). It is of
note that no significant differences in serum expression of total
TGF-β1 were demonstrated between patient and control groups (data
not shown). These data suggested that upregulation of TGF-β1 and
lncRNA-ATB may be involved in the pathogenesis of
atherosclerosis.
Serum expression of active TGF-β1 and
lncRNA-ATB may be useful as diagnostic factors in
atherosclerosis
ROC curve analysis was used to evaluate the
diagnostic value of serum active TGF-β1 and lncRNA-ATB expression
in atherosclerosis. As presented in Fig. 2A, the area under the curve (AUC)
for TGF-β1 was 0.9012 with a 95% confidence interval (CI) of
0.8364–0.9660 (P<0.0001). In addition, the AUC for lncRNA-ATB
was 0.8787 with 95% CI of 0.8137–0.9436 (P<0.0001; Fig. 2B). These data indicated that TGF-β1
and lncRNA-ATB expression may be used to effectively distinguish
patients with atherosclerosis from healthy controls.
Effects of lncRNA-ATB overexpression
and TGF-β1 treatment on the proliferation and viability of
HUVECs
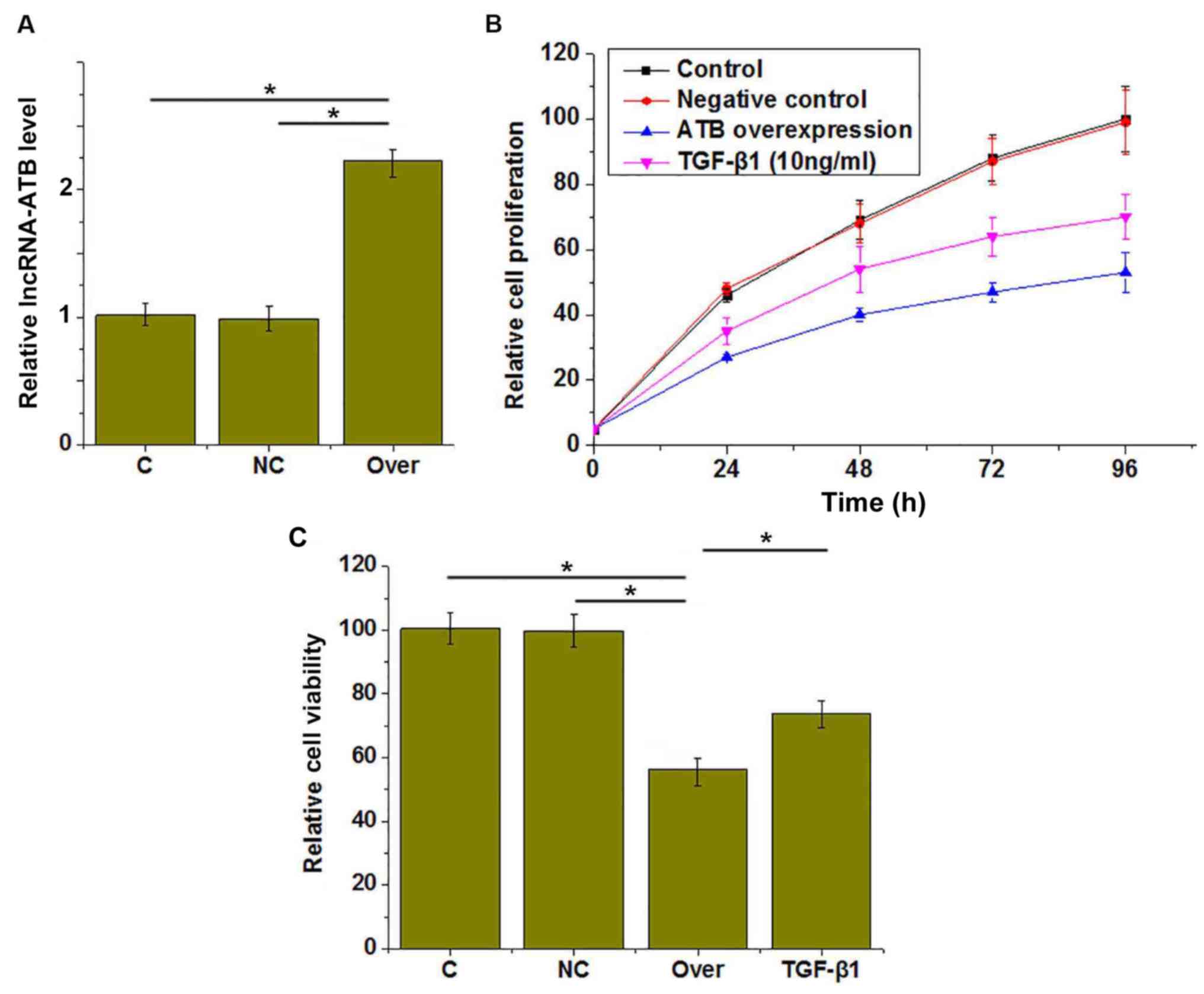
lncRNA-ATB overexpression was confirmed by RT-qPCR
(Fig. 3A). CCK-8 assays were
performed to investigate the effects of lncRNA-ATB overexpression
on the proliferation and viability of HUVECs. The results revealed
that proliferation was markedly inhibited (Fig. 3B) and viability was reduced
(Fig. 3C) in HUVECs by lncRNA-ATB
overexpression, compared with the control groups. In addition,
treatment with TGF-β1 (10 ng/ml for 1 h) also inhibited the
proliferation (Fig. 3B) and
reduced the viability (Fig. 3C) of
HUVECs compared with the control group.
TGF-β1 is an upstream activator of
lncRNA-ATB in HUVECs
As presented in Fig.
4A, treatment with TGF-β1 significantly increased the
expression of lncRNA-ATB in HUVECs, compared with the control
(P<0.05), whereas lncRNA-ATB overexpression had no significant
effect on TGF-β1 expression (Fig.
4B), suggesting that TGF-β1 may be an upstream activator of
lncRNA-ATB in HUVECs.
LncRNA-ATB siRNA inhibits the effects
of TGF-β1 treatment on HUVEC proliferation and viability
The above data demonstrated that TGF-β1 was an
upstream activator of lncRNA-ATB in HUVECs. To further investigate
TGF-β1 signaling in HUVECs, lncRNA-ATB was silenced in HUVECs with
siRNA, as confirmed by RT-qPCR (Fig.
5A). It was demonstrated that lncRNA-ATB siRNA significantly
reduced the effects of TGF-β1 treatment on HUVEC proliferation
(Fig. 5B) and viability (Fig. 5C).
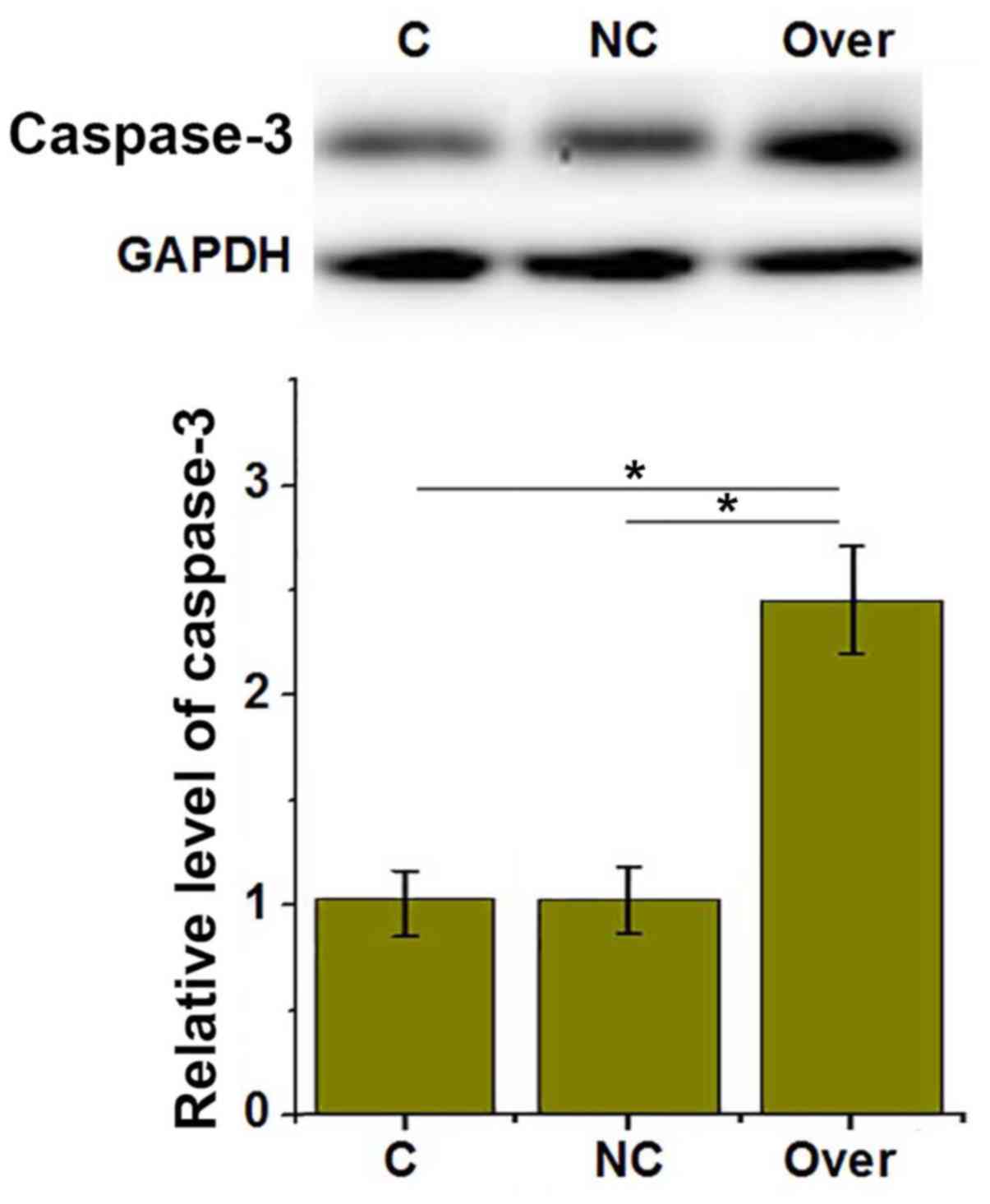
LncRNA-ATB overexpression upregulates
the expression of caspase-3
To investigate the effects of lncRNA-ATB on HUVEC
apoptosis, the expression of pro-apoptotic protein caspase-3 was
detected by western blotting. As presented in Fig. 6, lncRNA-ATB overexpression
significantly upregulated the expression of pro-apoptotic caspase-3
in HUVECs (P<0.05).
Discussion
Although the pathogenesis of atherosclerosis has not
been fully elucidated, the involvement of lncRNAs in the
development and progression of the disease has been studied
extensively. LncRNA-H19 has been reported to be significantly
upregulated in atherosclerosis, and lncRNA-H19 overexpression
promotes the progression of this disease through the activation of
mitogen-activated protein kinase and nuclear factor-kB pathways
(15). In addition, the expression
of lncRNA-RNCR3 is significantly increased in human and mouse
aortic atherosclerotic lesions, and is associated with endothelial
cell dysfunction and vascular smooth muscle cell proliferation
inhibition (16). Furthermore,
lncRNA-TUG1 overexpression may be a promising target for the
treatment of atherosclerosis (17). lncRNA-ATB is upregulated in several
types of human malignancies (10,11).
In the present study, serum lncRNA-ATB expression was significantly
higher in patients with atherosclerosis, compared with healthy
controls, indicating that upregulation of lncRNA-ATB may be
involved in this disease. TGF-β1 signaling has critical function in
the development and progression of atherosclerosis (18). In the present study, serum
expression levels of active TGF-β1 were significantly increased in
atherosclerosis patients compared with healthy controls, further
confirming the involvement of TGF-β1 signaling in
atherosclerosis.
Early diagnosis and treatment of atherosclerosis is
critical, but this is hindered by the lack of classic symptoms
(6). In the present study, all
atherosclerosis patients included were in the early stage of
disease, and ROC curve analysis demonstrated that serum active
TGF-β1 and lncRNA-ATB may be used to active distinguish patients
with atherosclerosis from healthy controls, indicating that serum
active TGF-β1 and lncRNA-ATB may serve as promising biomarkers for
the early diagnosis of atherosclerosis. It is of note that no
significant differences in serum expression of total TGF-β1 between
patient and control groups (data not shown), and ROC curve analysis
suggested that serum total TGF-β1 may not be an effective
diagnostic biomarker for atherosclerosis. Therefore, serum active
TGF-β1 should be used.
TGF-β1 and lncRNA-ATB serve regulatory roles in the
proliferation and apoptosis of various cell types. TGF-β1 has both
antiproliferative and apoptotic effects in bovine mammary
epithelial BME-UV1 cells (19) and
the proapoptotic effect of TGF-β1 on cultured HUVECs has also been
demonstrated (20); whereas
lncRNA-ATB promotes papillary thyroid tumor growth (21). It is well-established that EC
apoptosis serves a pivotal role in the pathogenesis of
atherosclerosis (22). In the
present study, lncRNA-ATB overexpression and TGF-β1 treatment
inhibited the proliferation and reduced the viability of HUVECs,
and promoted the expression of proapoptotic caspase-3, indicating
the involvement of TGF-β1 signaling and lncRNA-ATB in the
regulation of proliferation and apoptosis of key cells involved the
pathogenesis of atherosclerosis. It has been reported that TGF-β
signaling in hepatocellular carcinoma upregulates the expression of
lncRNA-ATB to promote cancer progression (23). In the present study, TGF-β1 was
demonstrated to upregulate lncRNA-ATB expression. Furthermore,
lncRNA-ATB silencing significantly reduced the effect of TGF-β1 on
the proliferation and viability of HUVECs. This suggested that
lncRNA-ATB was upregulated by TGF-β1 to participate in the
pathogenesis of atherosclerosis through apoptosis promotion and
proliferation inhibition in HUVECs.
In conclusion, TGF-β1 and lncRNA-ATB expression was
upregulated in patients with atherosclerosis, and increased
expression levels of TGF-β1 and lncRNA-ATB may be used to
effectively distinguish atherosclerosis patients from normal
healthy individuals. Upregulation of lncRNA-ATB by TGF-β1 inhibited
the proliferation and reduced the viability of HUVECs. The present
study was challenged by a small sample size, and further studies
with a large sample size are required to further confirm the
conclusions. However, the present study failed to construct an
atherosclerosis model using HUVECs due to the limited resources.
Only cell proliferation and apoptosis, but not other cell behaviors
were investigated. This study also failed to detect other
inflammatory markers, such as C-reactive protein, P-selectin and
TNF-α. Further studies are still needed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY and CZ designed experiments. HY and SM performed
experiments. LS and JG analyzed data. CZ wrote the paper. All
autors reviewed and approved the paper.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Fourth People's Hospital of Jinan (Jinan, China),
and all patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li H, Horke S and Förstermann U: Vascular
oxidative stress, nitric oxide and atherosclerosis.
Atherosclerosis. 237:208–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blankenberg S, Barbaux S and Tiret L:
Adhesion molecules and atherosclerosis. Atherosclerosis.
170:191–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinberg D: In celebration of the 100th
anniversary of the lipid hypothesis of atherosclerosis. J Lipid
Res. 54:2946–2949. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parrinello CM, Rastegar I, Godino JG,
Miedema MD, Matsushita K and Selvin E: Prevalence of and racial
disparities in risk factor control in older adults with diabetes:
The Atherosclerosis Risk in Communities Study. Diabetes Care.
38:1290–1298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McEvoy JW, Blaha MJ, DeFilippis AP, Lima
JA, Bluemke DA, Hundley WG, Min JK, Shaw LJ, Lloyd-Jones DM, Barr
RG, et al: Cigarette smoking and cardiovascular events: Role of
inflammation and subclinical atherosclerosis: The multiethnic study
of atherosclerosis. Arterioscler Thromb Vasc Biol. 35:700–709.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kubota Y, London SJ, Cushman M,
Chamberlain AM, Rosamond WD, Heckbert SR, Zakai N and Folsom AR:
Lung function, respiratory symptoms and venous thromboembolism
risk: The atherosclerosis risk in communities study. J Thromb
Haemost. 14:2394–2401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:Spec No 1. R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lalevée S and Feil R: Long noncoding RNAs
in human disease: Emerging mechanisms and therapeutic strategies.
Epigenomics. 7:877–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng F, Yan J, Ma Q, Jiao Y, Han L, Xu J,
Yang F and Liu J: Expression status and clinical significance of
lncRNA APPAT in the progression of atherosclerosis. PeerJ.
6:e42462018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iguchi T, Uchi R, Nambara S, Saito T,
Komatsu H, Hirata H, Ueda M, Sakimura S, Takano Y, Kurashige J, et
al: A long noncoding RNA, lncRNA-ATB, is involved in the
progression and prognosis of colorectal cancer. Anticancer Res.
35:1385–1388. 2015.PubMed/NCBI
|
|
11
|
Xiong J, Liu Y, Jiang L, Zeng Y and Tang
W: High expression of long non-coding RNA lncRNA-ATB is correlated
with metastases and promotes cell migration and invasion in renal
cell carcinoma. Jpn J Clin Oncol. 46:378–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwartz CJ, Valente AJ, Sprague EA,
Kelley JL, Cayatte AJ and Mowery J: Atherosclerosis: Potential
targets for stabilization and regression. Circulation. 86 (Suppl
6):III117–III123. 1992.PubMed/NCBI
|
|
13
|
Roberts AB and Sporn MB: Physiological
actions and clinical applications of transforming growth factor-β
(TGF-β). Growth Factors. 8:1–9. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan JX: LncRNA H19 promotes
atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur
Rev Med Pharmacol Sci. 21:322–328. 2017.PubMed/NCBI
|
|
16
|
Shan K, Jiang Q, Wang XQ, Wang YN, Yang H,
Yao MD, Liu C, Li XM, Yao J, Liu B, et al: Role of long non-coding
RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell
Death Dis. 7:e22482016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen C, Cheng G, Yang X, Li C, Shi R and
Zhao N: Tanshinol suppresses endothelial cells apoptosis in mice
with atherosclerosis via lncRNA TUG1 up-regulating the expression
of miR-26a. Am J Transl Res. 8:2981–2991. 2016.PubMed/NCBI
|
|
18
|
Leonarduzzi G, Sevanian A, Sottero B,
Arkan MC, Biasi F, Chiarpotto E, Basaga H and Poli G: Up-regulation
of the fibrogenic cytokine TGF-β1 by oxysterols: A mechanistic link
between cholesterol and atherosclerosis. FASEB J. 15:1619–1621.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kolek O, Gajkowska B, Godlewski MM and
Motyl T: Antiproliferative and apoptotic effect of TGF-β1 in bovine
mammary epithelial BME-UV1 cells. Comp Biochem Physiol C Toxicol
Pharmacol. 134:417–430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsukada T, Eguchi K, Migita K, Kawabe Y,
Kawakami A, Matsuoka N, Takashima H, Mizokami A and Nagataki S:
Transforming growth factor β1 induces apoptotic cell death in
cultured human umbilical vein endothelial cells with down-regulated
expression of bcl-2. Biochem Biophys Res Commun. 210:1076–1082.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu XM, Guo W, Li N, Liu HZ, Liu J, Qiu SQ,
Zhang Q, Wang LC, Li F and Li CL: The expression and function of
long noncoding RNA lncRNA-ATB in papillary thyroid cancer. Eur Rev
Med Pharmacol Sci. 21:3239–3246. 2017.PubMed/NCBI
|
|
22
|
Choy JC, Granville DJ, Hunt DW and McManus
BM: Endothelial cell apoptosis: Biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|