Introduction
Ovarian cancer is one of the most common types of
cancer in women and the most life-threatening gynecologic tumor,
which led to ~151,900 cases of mortality worldwide in 2012
(1,2). There are no obvious clinical symptoms
in the early stages of this disease. Although the international
promotion of treatment protocols consisting of surgery and
neoadjuvant chemotherapy have significantly improved the survival
rate of patients with ovarian cancer, the 5-year-survival rate
remains <45% (3). Drug
resistance is responsible for the failure of platinum-based
chemotherapy, wherein patients initially respond to these agents
but relapse ~6 months following their initial chemotherapy
(4). Therefore, there is an urgent
need to understand the multiple mechanisms underlying the
progression of ovarian cancer and chemotherapy drug resistance to
develop effective targeted therapies.
As a type of gene regulatory factor, microRNAs
(miRNAs) are endogenous, non-coding RNAs with a length of ~22
nucleotides that are widely involved in physiological processes,
including cell growth and replication (5). miRNAs specifically bind to the
3′-untranslated region (UTR) of the target mRNAs (6). In 2006, Chan and Loscalzo (7) found that miRNAs are involved in
ovarian cancer oncogenesis, and numerous subsequent studies have
demonstrated the importance of miRNAs in cell development,
invasion, apoptosis and drug sensitivity in ovarian cancer. For
example, miR-7 exhibited specific methylation in resistant cell
lines and was associated with poor prognosis in patients with
ovarian cancer (8); miR-509-3p
sensitized ovarian cancer cells to cisplatin treatment by targeting
anti-apoptotic genes, including MCL1, B-cell lymphoma 2
(BCL2) and BCL2L2 (9); the overexpression of miR-630 promoted
SKOV3 cell proliferation and migration (10); and the overexpression of miR-18b
was associated with the metastasis of ovarian cancer cells via
phosphatase and tensin homolog (PTEN) (11). In addition, the E2F transcription
factor 3 (E2F3) has been shown to act as a crucial protein
in the cell cycle process and is a known target of miR-210-3p.
Studies have shown that E2F3 is downregulated at the protein
level upon induction of the expression of miR-210 in human ovarian
cancer (12).
The present study aimed to characterize the
expression level of miR-210-3p between a cisplatin-resistant human
ovarian cancer cell line (SKOV-3/DDP) and a cisplatin-sensitive
cell line (SKOV-3) to evaluate the regulatory mechanisms of
miR-210-3p and its target gene, E2F3, in the carcinogenesis
and cisplatin sensitivity of SKOV-3/DDP cells.
Materials and methods
Cell lines, culture conditions and
miRNA transfection
The 293T human embryonic kidney cell line was
purchased from the Chinese Academy of Sciences (Beijing, China) and
was cultured in Dulbecco's modified Eagle's medium (DMEM;
10-013-CVR; Corning Incorporated, Corning, NY, USA) for conducting
a luciferase reporter assay. The SKOV-3/DDP cell line was selected
for the present study following referral to previous studies
(13). The SKOV-3
cisplatin-sensitive human ovarian cancer cell line and the
SKOV-3/DDP cisplatin-resistant cell line were obtained from Huiying
Biological Technology Co., Ltd., (Shanghai, China). The SKOV-3 and
SKOV-3/DDP cells were cultured in Roswell Park Memorial Institute
(RPMI)-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), penicillin (100 U/ml),
and streptomycin (100 µg/ml) in an incubator with 5%
CO2, at 37°C.
The SKOV-3/DDP cells in logarithmic phase were
diluted to a density of 1.0×105/ml, plated in six-well
plates and incubated at 37°C in a humidified atmosphere of 5%
CO2 for 36 h. The SKOV-3/DDP cells were transfected with
50 nM of the miRNA mimics, mimic negative control (NC), miRNA
inhibitor and inhibitor NC using Lipofectamine® 2000
(11668019; Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. For the cell proliferation, apoptosis,
wound healing and migration assays, the cells were divided into the
following mimic and inhibitor groups: i) Mimic groups: Mimic NC
subgroup, transfected with the mimics control sequence; miR-210-3p
mimic experimental subgroup, transfected with miR-210-3p mimics;
ii) inhibitor groups: Inhibitor NC subgroup, transfected with the
inhibitor control sequence; miR-210-3p inhibitor experimental
subgroup, transfected with the miR-210-3p inhibitors sequence.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol. The concentration and purity of the
extracted RNA samples were detected with an ultraviolet
spectrophotometer at a wavelength of 260 nm. Following this, ~1 µg
of RNA and 3 µM primer F+R were transcribed to cDNA using
Thermoscript RT kits (Takara Bio, Inc., Otsu, Japan). RT-qPCR
analysis was performed using a SYBR-Green PCR master mix kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with ~1 µg
cDNA on an ABI 7500 Real-Time PCR system; U6 small nuclear RNA was
used as an internal control. The specific primer sequences are
shown in Table I. PCR
amplification was conducted using the following cycling conditions:
Initial denaturation at 95°C for 5 min; 40 cycles of denaturation
at 95°C for 10 sec, annealing at 60°C for 30 sec, and extension at
72°C for 1 min; followed by a final extension at 72°C for 10 min.
Three independent experiments were performed to detect the relative
gene expression level. The relative expression was quantified using
the 2−ΔΔCq method (14).
 | Table I.Primer sequences for RT-qPCR
analysis. |
Table I.
Primer sequences for RT-qPCR
analysis.
| Primer name | Sequence |
|---|
|
hsa-miR-210-3p-RT |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCAGCCG-3′ |
|
hsa-miR-210-3p-F-qRT |
5′-AGGCTGTGCGTGTGACA-3′ |
|
hsa-miR-210-3p-R-qRT |
5′-AGTGCGTGTCGTGGAGTCG-3′ |
| U6-F |
5′-CGATACAGAGAAGATTAGCATGGC-3′ |
| U6-R |
5′-AACGCTTCACGAATTTGCGT-3′ |
Cell proliferation assay
The cells (~1×103) in the logarithmic
growth phase were plated per well, in 96-well culture plates. Cell
proliferation was examined using a conventional Cell counting kit-8
(CCK-8) assay. CCK-8 solution (Beyotime Institute of Biotechnology,
Shanghai, China) was used in accordance with the manufacturer's
protocol. The plates were incubated in the dark for 3 h, and the
absorbance value at 450 nm wavelength was recorded.
Cell apoptosis assay
The effect of miR-210-3p on apoptosis was detected
through flow cytometry using the Annexin V-fluorescein
isothiocyanate (FITC) cell apoptosis kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
SKOV-3/DDP cells transfected with miR-210-3p mimics, miR-210-3p
inhibitor and corresponding NC controls were cultured in serum-free
Roswell Park Memorial Institute 1640 medium (RPMI-1640; 10-040-CVR;
Corning Incorporated) at 37°C in a humidified atmosphere of 5%
CO2 for 36 h. All cells were collected and washed three
times with phosphate-buffered saline (PBS; pH 7.4) and suspended in
the staining buffer provided in the kit. The cells were mixed with
5 µl of Annexin V-FITC and propidium iodide (PI) and were subjected
to FACScan flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA)
following incubation for 10 min at room temperature. The cells were
pipetted into a test tube and 300 µl of PBS was added to each tube.
PI (~1 µl; 50 µg/ml) was added to each sample and cell apoptosis
was measured within 30 min. Cells that were Annexin V-positive and
PI-negative were considered apoptotic.
Wound healing assay
Following transfection, a scratch was produced in
the monolayer cultures of cells using sterile 200-µl pipette tips.
The detached cells were removed by washing twice with PBS and the
plates were incubated in a medium without FBS. Images were captured
immediately (0 h) and after 24 h. Images of five random fields were
captured under an inverted microscope, and the numbers of migratory
cells were counted. Each experiment was repeated at least three
times.
Invasion assay
Following transfection, the cells were divided and
washed twice with PBS. The cells (5×105) were seeded in
the upper chamber of a Transwell insert (12-µM pores) covered with
Matrigel (0.7 mg/ml; Collaborative Research, Inc., TX, USA). The
lower chamber was filled with RPMI medium (400 ml). Following
incubation for 24 h, the non-migrated cells in the upper chamber
were gently removed and the migrated cells present on the lower
surface of the insert were stained with formaldehyde. Images were
captured under an optical microscope following staining with
crystal violet.
Luciferase reporter assay
To verify whether miR-210-3p directly targets and
inhibits E2F3, the entire 3′-UTR of E2F3 was cloned
downstream of the luciferase gene, into the multiple cloning site
of the pMIR-REPORT (Promega Corporation, Madison, WI, USA) vector,
to construct the pmiR-E2F3-wild-type (WT) plasmid.
TargetScan (http://www.targetscan.org/vert_71/) was used to
predict the binding sites of miR-210-3p on E2F3, and to
introduce sequence mutations to construct the
pmiR-E2F3-mutant (MUT) vectors. pRL-TK, the Renilla
luciferase expression vector (Promega Corporation), was used as an
internal reference. The 293T cells were transfected with 50 nM
miR-210-3p mimics or NC, with the luciferase reporter vector using
Lipofectamine® 2000. The transfected cells were cultured
at 37°C in a humidified atmosphere of 5% CO2 for 24 h.
The activity of luciferase was determined according to the Dual
Luciferase Reporter Gene Assay system (Promega Corporation).
Western blot analysis
The SKOV-3 and SKOV-3/DDP cells (~1×105)
with cisplatin (Meilun Dalian, Liaoning Sheng, China) at
concentrations of 0.5, 1, 2, 5, 10, 20 and 40 mg/l in 12-well
culture plates were then cultured at 37°C in a humidified
atmosphere of 5% CO2 for 48 h. Total cell lysate was
prepared by RIPA lysis (Beyotime Institute of Biotechnology). The
concentration of protein was determined using the Bicinchoninic
Acid Protein Assay kit (Shanghai Solarbio Bioscience &
Technology Co., Ltd., Shanghai, China). The proteins were separated
on a 10% polyacrylamide gel (Shanghai Solarbio Bioscience &
Technology Co., Ltd.) by electrophoresis and the separated proteins
were transferred onto polyvinylidene fluoride membranes. The
membranes were blocked with 5% bovine serum albumin (FBS; Gibco;
Thermo Fisher Scientific, Inc.) for 1 h at room temperature. The
proteins were incubated with primary antibody against E2F3
(rabbit anti-human, 1:1,000, Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and a rabbit anti-human primary antibody against
glyceraldehyde-3-phosphate dehydrogenase (GAPDH, mouse anti-human,
1:1,000, Shanghai Solarbio Bioscience & Technology Co., Ltd.)
overnight at 4°C. Following the addition of horseradish
peroxidase-labeled secondary antibody (1:5,000, Shanghai Solarbio
Bioscience & Technology Co., Ltd.) and incubation for 1 h at
room temperature, the protein signals were detected with
chemiluminescence. The Gel Doc EZ Imager (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) was used to capture the protein image.
ImageJ software version 1.8.0 (National Institutes of Health,
Bethesda, MD, USA) was used to determine the gray value of the
target band.
Cisplatin sensitivity analysis
The viability of the SKOV-3 and SKOV-3/DDP cells
treated with cisplatin at concentrations of 0.5, 1, 2, 5, 10, 20
and 40 mg/l for 48 h was evaluated. The E2F3 sequence was
inserted into a pReceiver vector (GeneCopoeia, Inc., Rockville, MD,
USA) to construct the pReceiver E2F3 vector. The cells in
the experiment were assigned to the five following groups:
SKOV-3/DDP, NC (SKOV-3/DDP with empty vector), miR-210-3p mimics
(SKOV-3/DDP with miR-210-3p mimics), E2F3 (SKOV-3/DDP with
pReceiver E2F3 vector), and miR-210-3p mimic + E2F3
(SKOV-3/DDP with miR-210-3p mimics and pReceiver E2F3
vector). All cells were exposed to cisplatin and the absorbance
value was detected using the CCK-8 assay. The results are expressed
as the cell survival rate, which was determined according to
optical density (OD) as follows: Percentage survival=(mean OD value
of treated group/mean OD value of control group) ×100%.
Statistical analysis
The software SPSS 21.0 (IBM Corp., Armonk, NY, USA)
was used to analyze statistical significance. All assays were
independently performed three times and measurement data are
presented as the mean ± standard deviation. A two-sided Student's
t-test was used to analyze the differences between the two groups.
The significance of differences between groups were evaluated with
one-way analysis of variance followed by Student-Newman-Keuls post
hoc tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of miR-210-3p is
significantly decreased in SKOV-3/DDP cells
RT-qPCR analysis was used to assess the expression
levels of miR-210-3p in SKOV-3/DDP and SKOV-3 cells, which revealed
that the expression of miR-210-3p was significantly lower in the
SKOV-3/DDP cells than in the SKOV-3 cells (Fig. 1A). Following treatment with
cisplatin, the viability of the SKOV-3/DDP cells was significantly
higher compared with that of the SKOV-3 cells (Fig. 1B). These results suggest that the
downregulated expression of miR-210-3p may affect the growth of
ovarian cancer cells and their resistance to cisplatin.
Overexpression of miR-210-3p represses
the ability of cell proliferation
To assess the influence of miR-210-3p on the
proliferation of SKOV-3/DDP cells, miR-210-3p mimics or NC were
transfected into SKOV-3/DDP cells. The expression level of
miR-210-3p was significantly increased in the cells transfected
with miR-210-3p mimics (Fig. 2A).
The CCK-8 analysis revealed that the ability of cells to
proliferate was significantly lower in the SKOV-3/DDP cells than in
the NC group of cells (Fig.
2B).
Overexpression of miR-210-3p promotes
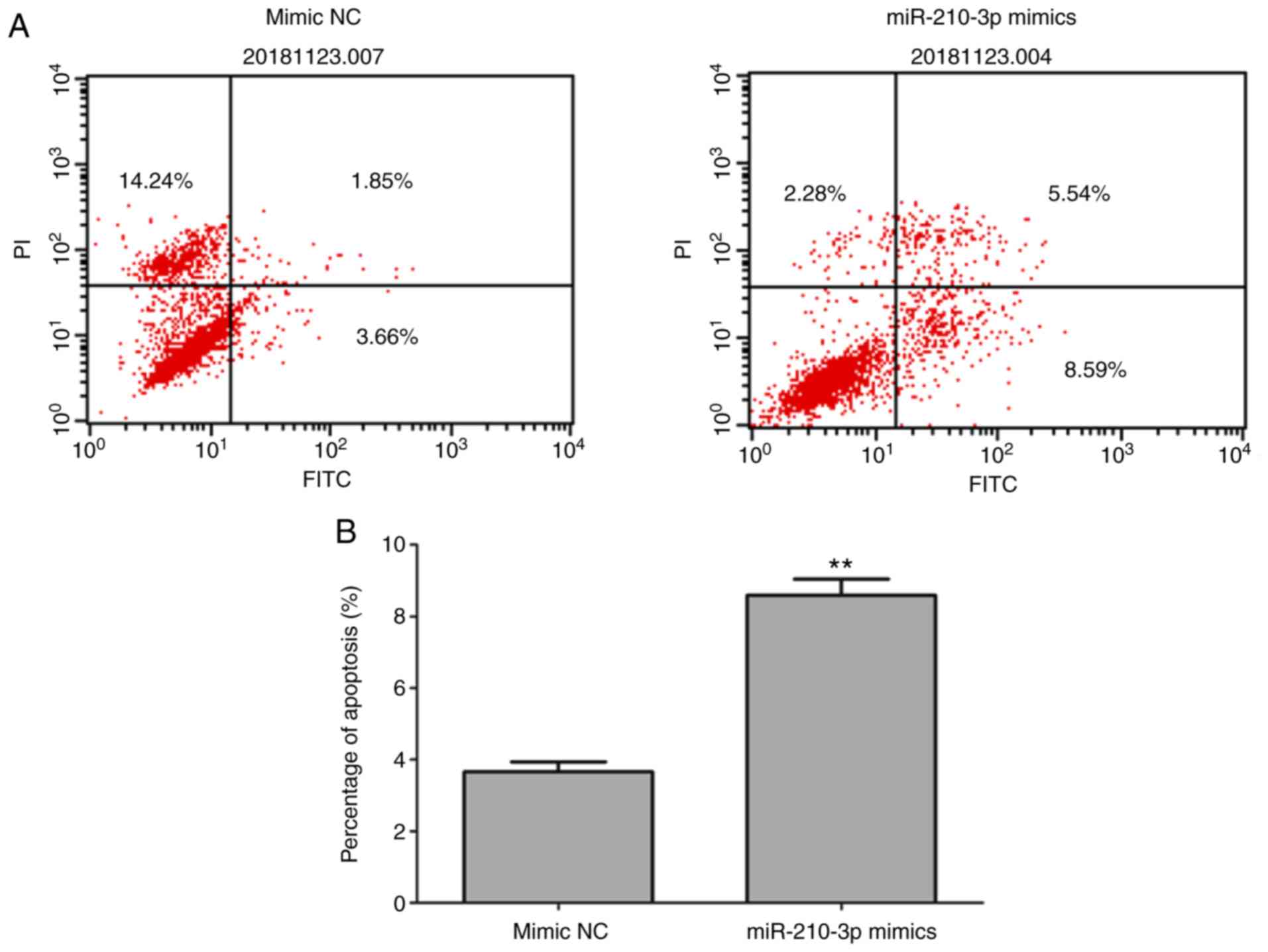
cell apoptosis
To clarify the influence of miR-210-3p on cell
apoptosis, Annexin V/PI staining was performed to detect apoptotic
cells. The proportion of apoptotic cells was significantly higher
in the cells transfected with the miR-210-3p mimics than for those
transfected with the mimics control sequence, suggesting that the
overexpression of miR-210-3p promoted cell apoptosis (Fig. 3A and B).
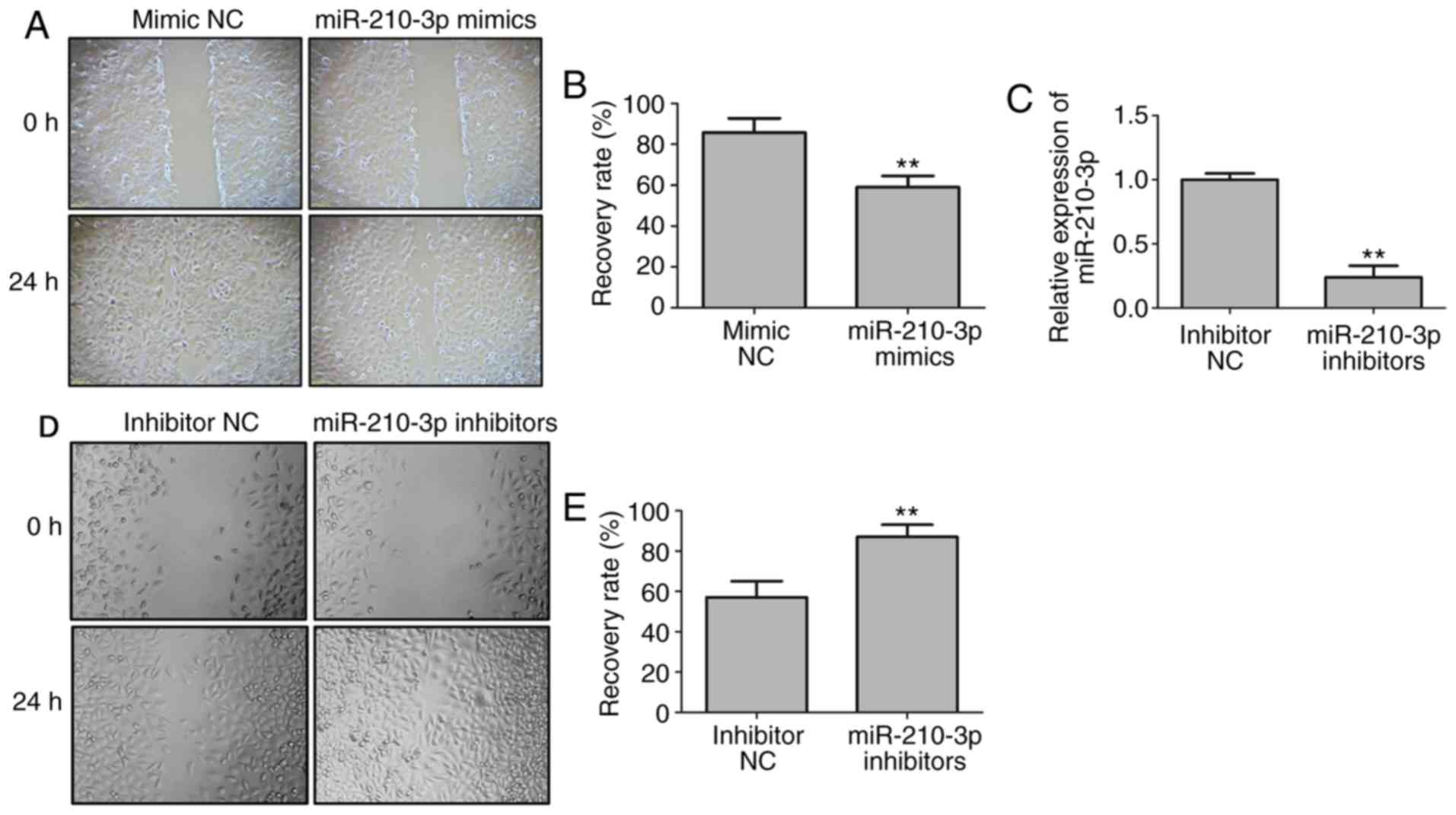
miR-210-3p suppresses the migration of
SKOV-3/DDP cells
To examine the influence of miR-210-3p on cell
metastasis, a wound healing assay was conducted for the SKOV-3/DDP
cells. The results of the Transwell assay suggested that the number
of cells passing through the membrane in the miR-210-3p mimic group
was significantly decreased compared with that in the corresponding
control (P<0.01; Fig. 4A and
B). The expression level of miR-210-3p was significantly
decreased in the cells transfected with miR-210-3p inhibitors
(Fig. 4C). The number of cells
passing through the membrane in the miR-210-3p inhibitor group was
significantly increased compared with that in the corresponding
control (P<0.01; Fig. 4D and
E). These results clearly indicated that the ectopic expression
of miR-210-3p inhibited cell migration.
miR-210-3p inhibits cell invasion in
SKOV-3/DDP cells
The tumor invasion assay revealed that the number of
invasive cells among the SKOV-3/DDP cells transfected with
miR-210-3p mimics was significantly decreased compared that in the
corresponding control (P<0.01; Fig.
5A and B), whereas the miR-210-3p inhibitor promoted cell
invasion compared with that in the corresponding control
(P<0.01; Fig. 5C and D). These
data suggest that the ectopic expression of miR-210-3p inhibited
cell metastasis and invasion.
E2F3 is the target gene of
miR-210-3p
TargetScan was used to predict the potential
miR-210-3p targets. E2F3 was selected for further
examination as it contained a conserved binding site for miR-210-3p
(Fig. 6A). The results of the dual
luciferase reporter assay (Fig.
6B) indicated that the dual luciferase activity of
pmiR-E2F3-WT was significantly lower in the miR-210-3p
mimics group than in the control group (P<0.05), whereas that of
pmiR-E2F3-MUT was not significantly decreased (P>0.05).
The levels of E2F3 in the SKOV-3/DDP and SKOV-3 cells were
significantly decreased with the overexpression of miR-210-3p
(Fig. 6C). Therefore, miR-210-3p
may directly target E2F3 and negatively regulate its
expression.
Overexpression of miR-210-3p affects
the sensitivity of SKOV-3/DDP cells to cisplatin by targeting
E2F3
A CCK-8 assay was performed to assess the viability
of cells following treatment with different concentrations of
cisplatin. In comparison with the SKOV-3/DDP group, the
IC50 value of cisplatin in the miR-210-3p mimics group
was significantly decreased. The analysis of cell survival rate
suggested that, with different concentrations of cisplatin, the
cell survival rates in the SKOV-3/DDP and miR-210-3p mimics groups
were higher compared with those in the SKOV-3 group (P<0.05).
The cell survival rates of the NC and miR-210-3p mimics +
E2F3 groups did not differ significantly (P>0.05) from
that of the SKOV-3/DDP group, whereas that of the miR-210-3p mimics
group was significantly decreased (P<0.05; Fig. 7). In conclusion, transfection with
the miR-210-3p mimic significantly reduced the effect of cisplatin
on the SKOV-3/DDP cells. The overexpression of E2F3 rescued
the miR-210-3p mimics-induced sensitivity to cisplatin.
 | Figure 7.Effects of miR-210-3p and E2F3
on the sensitivity of SKOV-3/DDP cells to cisplatin. SKOV-3/DDP,
NC, miR-210-3p mimic, E2F3, and miR-210-3p mimic +
E2F3 groups refer to SKOV-3/DDP cells transfected with no
sequence, empty vector, miR-210-3p mimics, pReceiver E2F3
vector, and both miR-210-3p mimic and pReceiver E2F3
vectors, respectively. *P<0.05 and **P<0.01 compared with
SKOV-3/DDP, NC, and miR-210-3p mimic + E2F3 (n=3). miR,
microRNA; NC, negative control; E2F3, E2F transcription
factor 3. |
Discussion
In the present study, the role of miR-210-3p in the
carcinogenesis and cisplatin sensitivity of ovarian cancer cells
was investigated. The expression of miR-210-3p was significantly
lower in cisplatin-resistant SKOV3/DDP cells than in chemosensitive
SKOV3 cells. Transfection of the SKOV3/DDP cells with miR-210-3p
mimics resulted in the inhibition of cell proliferation, migration
and invasion, and the induction of cell apoptosis. E2F3 was
verified as the direct target of miR-210-3p. The transfection of
SKOV3/DDP cells with miR-210-3p mimics also increased the
sensitivity of cells to cisplatin. However, the overexpression of
E2F3 attenuated this effect of the overexpression of
miR-210-3p.
Several studies have suggested the important role of
miR-210 in cancer. However, there are contradictory results with
respect to the role of miR-210 as an oncogene or gene suppressor.
miR-210 is upregulated in head and neck cancer, pancreatic tumors
(15), glioma (16), non-small cell lung cancer (17) and prostate cancer (18). By contrast, the expression of
miR-210 is downregulated in esophageal squamous cell carcinoma
(19), bladder cancer (20), angiosarcoma (21) and renal cell carcinoma (22). The function of miR-210 in ovarian
cancer remains to be fully elucidated. Under hypoxic conditions,
miR-210 is upregulated in epithelial ovarian cancer tissues and
ovarian cancer cell lines (23).
miR-210 is located on chromosome 11p15.5, on which allelic loss is
observed in ovarian cancer (24,25).
Giannakakis et al (12),
reported that miR-210 gene copy number was deleted in 64% ovarian
cancer samples and was associated with the expression levels of
mature miR-210. The level of miR-210 is also reduced in primary
ovarian cancer cells compared with that in effusions (26). These results are consistent with
the observations of the present study. Therefore, miR-210-3p is not
only a simple tumor-stimulating miRNA in cancer but may have a dual
role as an oncogene and a tumor suppressor. The results of the
present study revealed that the overexpression of miR-210-3p
suppressed cell multiplication, migration and invasion abilities,
and promoted cell apoptosis in cisplatin-resistant SKOV3/DDP cells.
Therefore, miR-210-3p mainly functions as a tumor suppressor gene
in SKOV3/DDP cells and may prevent the progression of ovarian
cancer.
The protein E2F3 is a key molecule involved
in cell cycle progression. E2F3a acts as an oncogene in
ovarian cancer (27). The
E2F3 isoforms (E2F3a and E2F3b) are
overexpressed in ovarian cancer tissues, compared with normal
tissues; of these, the expression of E2F3a is known to be
associated with tumor stage (28).
The present study verified that miR-210-3p directly targeted the
3′-UTR region of E2F3 and it was hypothesized that
downregulation of the expression of miR-210-3p may affect certain
signaling pathways in ovarian cancer. miR-210-3p suppressed
SKOV3/DDP cell multiplication via E2F3 through cell cycle
arrest, as observed in esophageal squamous cell carcinoma.
miRNAs have been investigated in multiple tumor
types for their roles in drug resistance. For example, the
deregulation of miR-340-5p and miR-128 led to an increase in
cisplatin resistance in osteosarcoma cells and glioma cells,
respectively (29,30). The serum level of miR-210 was
associated with the sensitivity to cisplatin-based chemotherapy in
non-small cell lung cancer (31).
Zhao et al (32), reported
on the association between the expression of miR-9 and increased
response of cancer cells to cisplatin treatment. In the present
study, the overexpression of miR-210-3p reduced the suppressive
effect of cisplatin on resistant cells, however, the overexpression
of E2F3 eliminated the miR-210-3p-induced resistance to
cisplatin. Taken together, the present study highlights the
potential role of miR-210-3p as a novel therapeutic target for
ovarian cancer. miR-210-3p affects the sensitivity of
cisplatin-resistant cells to cisplatin by targeting E2F3 in
ovarian cancer. Further investigations are warranted to evaluate
the specific regulation network of miR-210-3p and its target gene
in ovarian cancer and develop effective drugs for clinical
application.
In conclusion, the overexpression of miR-210-3p
repressed the cell proliferation, apoptosis, migration and invasion
of cells, and the inhibition of miR-210-3p promoted cell migration
and invasion. Th overexpression of miR-210-3p decreased the
sensitivity of SKOV-3/DDP cells to cisplatin treatment via
E2F3. miR-210-3p may serve a tumor suppressor role in
ovarian cancer cells and be a potentially valuable therapeutic
target for improving cisplatin resistance in ovarian cancer
cells.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Medical Science
and Technology Planned Projects of Zhejiang Province (grant no.
2013KYA067).
Availability of data materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HZ made substantial contributions to conception and
design this study. YJ, JW, SX, FG and LY performed all the
experiments, and carried out all the analysis and interpretation of
data. YJ and JW were involved in drafting the manuscript and
revised it critically for important intellectual content. LY gave
final approval of the version to be published. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work is appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davis A, Tinker AV and Friedlander M:
‘Platinum resistant’ ovarian cancer: What is it, who to treat and
how to measure benefit? Gynecol Oncol. 133:624–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J and Sen S: MicroRNA functional
network in pancreatic cancer: From biology to biomarkers of
disease. J Biosci. 36:481–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan SY and Loscalzo J: MicroRNA-210: A
unique and pleiotropic hypoxamir. Cell Cycle. 9:1072–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vera O, Jimenez J, Pernia O,
Rodriguez-Antolin C, Rodriguez C, Sanchez Cabo F, Soto J, Rosas R,
Lopez-Magallon S, Esteban Rodriguez I, et al: DNA methylation of
miR-7 is a mechanism involved in platinum response through MAFG
overexpression in cancer cells. Theranostics. 7:4118–4134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Du J, Li X, Su J, Huang Y, Ding N,
Zhang M and Jiang S: miR-509-3p promotes cisplatin-induced
apoptosis in ovarian cancer cells through the regulation of
anti-apoptotic genes. Pharmacogenomics. 18:1671–1682. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Zhang JY, Lu LJ, Wang CH and Wang
LH: MiR-630 promotes epithelial ovarian cancer proliferation and
invasion via targeting KLF6. Eur Rev Med Pharmacol Sci.
21:4542–4547. 2017.PubMed/NCBI
|
|
11
|
Han X, Zhang Y, Wang D, Fu X, Li M and
Wang A: Upregulation of microRNA-18b induces phosphatase and tensin
homolog to accelerate the migration and invasion abilities of
ovarian cancer. Oncol Lett. 14:5631–5637. 2017.PubMed/NCBI
|
|
12
|
Giannakakis A, Sandaltzopoulos R, Greshock
J, Liang S, Huang J, Hasegawa K, Li C, O'Brien-Jenkins A, Katsaros
D, Weber BL, et al: miR-210 links hypoxia with cell cycle
regulation and is deleted in human epithelial ovarian cancer.
Cancer Biol Ther. 7:255–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Wang C, Su J, Xie Q, Ma L, Zeng L,
Yu Y, Liu S, Li S, Li Z and Sun L: Tolerance to endoplasmic
reticulum stress mediates cisplatin resistance in human ovarian
cancer cells by maintaining endoplasmic reticulum and mitochondrial
homeostasis. Oncol Rep. 34:3051–3060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang X, Ding L, Bennewith KL, Tong RT,
Welford SM, Ang KK, Story M, Le QT and Giaccia AJ:
Hypoxia-inducible mir-210 regulates normoxic gene expression
involved in tumor initiation. Mol Cell. 35:856–867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malzkorn B, Wolter M, Liesenberg F,
Grzendowski M, Stühler K, Meyer HE and Reifenberger G:
Identification and functional characterization of microRNAs
involved in the malignant progression of gliomas. Brain Pathol.
20:539–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puissegur MP, Mazure NM, Bertero T,
Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K,
Cardinaud B, Hofman V, et al: miR-210 is overexpressed in late
stages of lung cancer and mediates mitochondrial alterations
associated with modulation of HIF-1 activity. Cell Death Differ.
18:465–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren D, Yang Q, Dai Y, Guo W, Du H, Song L
and Peng X: Oncogenic miR-210-3p promotes prostate cancer cell EMT
and bone metastasis via NF-κB signaling pathway. Mol Cancer.
16:1172017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsuchiya S, Fujiwara T, Sato F, Shimada Y,
Tanaka E, Sakai Y, Shimizu K and Tsujimoto G: MicroRNA-210
regulates cancer cell proliferation through targeting fibroblast
growth factor receptor-like 1 (FGFRL1). J Biol Chem. 286:420–428.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang X, Shi L, Yi C, Yang Y, Chang L and
Song D: MiR-210-3p inhibits the tumor growth and metastasis of
bladder cancer via targeting fibroblast growth factor receptor-like
1. Am J Cancer Res. 7:1738–1753. 2017.PubMed/NCBI
|
|
21
|
Nakashima S, Jinnin M, Kanemaru H,
Kajihara I, Igata T, Okamoto S, Tazaki Y, Harada M, Masuguchi S,
Fukushima S, et al: The role of miR-210, E2F3 and ephrin A3 in
angiosarcoma cell proliferation. Eur J Dermatol. 27:464–471.
2017.PubMed/NCBI
|
|
22
|
Yoshino H, Yonemori M, Miyamoto K,
Tatarano S, Kofuji S, Nohata N, Nakagawa M and Enokida H:
microRNA-210-3p depletion by CRISPR/Cas9 promoted tumorigenesis
through revival of TWIST1 in renal cell carcinoma. Oncotarget.
8:20881–20894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Huang K, You Y, Fu X, Hu L, Song L
and Meng Y: Hypoxia-induced miR-210 in epithelial ovarian cancer
enhances cancer cell viability via promoting proliferation and
inhibiting apoptosis. Int J Oncol. 44:2111–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vandamme B, Lissens W, Amfo K, De Sutter
P, Bourgain C, Vamos E and De Grève J: Deletion of chromosome
11p13-11p15.5 sequences in invasive human ovarian cancer is a
subclonal progression factor. Cancer Res. 52:6646–6652.
1992.PubMed/NCBI
|
|
25
|
Viel A, Giannini F, Tumiotto L,
Sopracordevole F, Visentin MC and Boiocchi M: Chromosomal
localisation of two putative 11p oncosuppressor genes involved in
human ovarian tumours. Br J Cancer. 66:1030–1036. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vaksman O, Stavnes HT, Kaern J, Trope CG,
Davidson B and Reich R: miRNA profiling along tumour progression in
ovarian carcinoma. J Cell Mol Med. 15:1593–1602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reimer D, Hubalek M, Riedle S, Skvortsov
S, Erdel M, Concin N, Fiegl H, Müller-Holzner E, Marth C, Illmensee
K, et al: E2F3a is critically involved in epidermal growth factor
receptor-directed proliferation in ovarian cancer. Cancer Res.
70:4613–4623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reimer D, Hubalek M, Kiefel H, Riedle S,
Skvortsov S, Erdel M, Hofstetter G, Concin N, Fiegl H,
Müller-Holzner E, et al: Regulation of transcription factor E2F3a
and its clinical relevance in ovarian cancer. Oncogene.
30:4038–4049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song L, Duan P, Gan Y, Li P, Zhao C, Xu J,
Zhang Z and Zhou Q: MicroRNA-340-5p modulates cisplatin resistance
by targeting LPAATbeta in osteosarcoma. Braz J Med Biol Res.
50:e63592017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yi DY, Su Q, Zhang FC, Fu P, Zhang Q, Cen
YC, Zhao HY and Xiang W: Effect of microRNA-128 on cisplatin
resistance of glioma SHG-44 cells by targeting JAG1. J Cell
Biochem. 119:3162–3173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li ZH, Zhang H, Yang ZG, Wen GQ, Cui YB
and Shao GG: Prognostic significance of serum microRNA-210 levels
in nonsmall-cell lung cancer. J Int Med Res. 41:1437–1444. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao HM, Wei W, Sun YH, Gao JH, Wang Q and
Zheng JH: MicroRNA-9 promotes tumorigenesis and mediates
sensitivity to cisplatin in primary epithelial ovarian cancer
cells. Tumour Biol. 36:6867–6873. 2015. View Article : Google Scholar : PubMed/NCBI
|