Introduction
Femoral head necrosis (FHN) is a type of osteocyte
apoptosis resulting from multiple causes (1). Osteonecrosis may easily cause the
morphological change of caput femoris, subsequently resulting in
the collapse of caput femoris and limiting the joint motion of bone
marrow (1). Blood supply disorder
is a common cause, which presents ischemic necrosis caused by a
femoral neck fracture (2). The
mechanism underlying non-traumatic FHN remains unclear, and
potential etiology includes steroid-induced, alcoholic, idiopathic
and hematological system diseases, amongst others (3). Due to the unclarified mechanism,
there remains a lack of specialized treatment at an early stage. At
later periods, the collapsed caput femoris results in the loss of
joint function of the bone marrow, therefore meaning that joint
replacement of the marrow cannot be adopted (4). However, due to the early onset age of
FHN and unsolved abrasion and dislocation for the joint replacement
prosthesis of marrow, the clinical treatment of FHN remains
difficult (4).
Bone marrow stem cells (BMSCs) are non-hematopoietic
cells with multi-directional differentiation potential in bone
marrow (5). Their self-renewal
ability results in their ability to maintain the versatility of
stem cells. BMSCs may be divided into osteoblast, chondroblast and
adipocyte, which may further secrete a series of growth factors to
promote tissue regeneration (6).
For instance, vascular endothelial growth factor (VEGF) may recruit
endothelial cells and promote the vascularization and
endothelialization of damaged blood vessels in ischemic tissues
(7). It has been demonstrated that
the activity and quantity of near-end BMSCs in patients with
steroid-induced FHN are reduced in comparison with those with
non-femoral head necrosis (8). To
uncover the pathogenesis, researchers have combined medullary core
decompression with autologous BMSC transplant for the early
treatment of FHN, which have yielded a positive clinical effect
(9).
Several studies have revealed that microRNAs
(miRNAs/miRs) are associated with BMSCs (10,11).
miRNAs are a group of small endogenous non-coding RNA molecules,
composed of 21–25 nuclear acids. miRNAs generally target one or
multiple mRNAs which regulate gene expression at the translational
level or by degrading the target Mrna (11). miRNAs serve a notable role in cell
proliferation, differentiation, apoptosis, biological development
and the occurrence and development of diseases (12). The structure of free miRNAs is very
stable and is able to bear the degradation of ribonuclease. Liu
et al (13) reported that
miRNA-15a-5p regulates VEGFA in endometrial mesenchymal stem
cells.
Peroxisome proliferator-activated receptor-γ (PPARγ)
has been confirmed to be an adipogenic transcription factor,
serving a critical regulatory function in adipose cell
differentiation (14). It
possesses a role in adipogenesis and is able to restrain
osteogenesis. CCAAT/enhancer binding protein α (C/EBPα) and PPARγ
are important transcription factors in the process of adipogenic
differentiation (15), with
heterogeneous expression transdifferentiating myoblasts into
adipocytes. PPARγ and C/EBPα are two core transcription factors in
the process of adipogenesis (15).
Substantial attention has been focused on their methylation
situation, however, previous reports on the methylation of PPARγ
and C/EBPα are only limited to the lipogenesis process of adipose
tissues, mesenchymal stem cells and preadipocytes (11,16).
In addition, the C/EBP family and PPAR family are two critical
transcription factor families involved in modulating fat cell
differentiation (17). C/EBP was
initially discovered as a transcription factor family that serves a
vital role in fat cell differentiation, mainly including C/EBPα and
C/EBPβ (18). The transcription
factor family is equipped with a transcription-activated area and
adjacent leucine zipper motif (18).
When BMSCs are differentiated into chondrocytes,
they are affected by multiple factors, including hormones and
cytokines (19). Multiple
signaling pathways regulate the differentiation of mesenchymal stem
cells into chondrocytes, mainly including the fibroblast growth
factor pathway, mitogen-activated protein kinase pathway,
transforming growth factor β/bone morphogenic protein pathway,
SRY-box 9 signaling pathway and the Wnt/β-catenin signal pathway
(20). Wnt/β-catenin is a
newly-discovered pathway that is involved in regulating the
differentiation of chondrocytes (21), which has been reported to regulate
cellular morphology, function, cell-mediated immunity and stress,
cellular carcinogenesis and apoptosis and participate in the
development, differentiation, growth and apoptosis of cells
(21). Shi et al (22) concluded that miR-15a-5p was
involved in human adipocyte differentiation and obesity by
regulating Wnt signaling. The present study examined the molecular
mechanisms of miR-15a-5p in FHN.
Materials and methods
In vivo model
The animal study was ethically approved by the
ethics committee of Hongqi Hospital Affiliated With Mudanjiang
Medical University (Heilongjiang, China). Sprague-Dawley male adult
rats (n=12, 160–180 g, 5–6 weeks old) were obtained from the
Experimental Center of Chinese Medical Sciences University
(Beijing, China) and housed in standard laboratory conditions (12 h
light/dark cycle; 24–25°C; humidity, 50–55%) with ad libitum
access to food and water during the study. All rats were randomly
distributed into sham (n=6) and FHN model groups (n=6). In FHN
model groups, a steroid-induced osteonecrosis model was established
in the rats by the intramuscular injection of 40 mg/kg
methylprednisolone (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
once every 1 week for 5 weeks as previously described (23–25).
Following these 5 weeks, the rats were sacrificed using decollation
under 35 mg/kg pentobarbital sodium injected intraperitoneally. In
the sham group, the rats were sacrificed using decollation under 35
mg/kg pentobarbital sodium injected intraperitoneally.
Histological analysis
The femur specimens were fixed in 10% buffered
neutral formalin solution for 72 h at room temperature. The tissues
were dehydrated in 70–100% graded ethanol, embedded in paraffin and
sliced into 4 µm-thick sections. The sections were stained with
routinely dewaxed and hydrated with gradient ethanol, stained with
hematoxylin for 5 min and washed with tap water for 30 sec. The
sections were disposed with hydrochloric acid ethanol for 20 sec,
washed with distilled water for 1 min and stained with eosin for 1
min. All sections were assessed using a light microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
Cell culture
Male C57 mice (age, 4 weeks; weight, 10–12 g; n=10)
were purchased from the Experimental Center of Chinese Medical
Sciences University (Beijing, China) and housed in standard
laboratory conditions (12 h light/dark cycle; 24–25°C; humidity,
50–55%) with ad libitum access to food and water. BMSCs were
isolated from the femurs and tibias of the mice and cultured with
α-Minimum Essential Media (α-MEM; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% foetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences). BMSCs
(3×105 cells/ml) were trypsinized and plated into 25
cm2 flasks in α-MEM containing 10% FBS once the cells
reached 80% confluence at 37°C.
RNA Isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from BMSCs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to manufacturer's protocol. A total of 1–5 µl RNA
was reverse transcribed using a HiFiMMLV cDNA kit (Tiangen Biotech
Co., Ltd., Beijing, China) according to the manufacturer's
protocol. RT-qPCR was performed according to the manufacturer's
protocol of the Quant SYBR Green PCR kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). PCR cycling conditions used 40 cycles
(95°C for 30 sec and 60°C for 30 sec) subsequent to an initial
denaturation step (94°C for 5 min). Primer sequences were as
follows: miR-15a-5p forward, 5′-TAAGGCACGCGGTGAATGCC-3′ and
reverse, 5′-GCGAGCACAGAATTAATACGACTCAC-3′; U6 forward,
5′-GCTTGCTTCGGCAGCACATATAC-3′ and reverse,
5′-TGCATGTCATCCTTGCTCAGGG-3′. Relative expression levels were
calculated using the 2-ΔΔCq method (26).
Gene expression profiling
cDNA samples were Cy3-labeled using the SureTag DNA
labeling kit (Agilent Technologies, Inc., Santa Clara, CA, USA)
according to the manufacturer's protocol. Robust multi-array
average background removal, quantile normalization, compensation
for systematic technical differences and probe set summary were
executed. The microarray data were submitted to Gene Expression
Omnibus (accession number GSE80754) (27).
Transfection
miRNA-15a-5p (100 ng) and control negative plasmids
(100 ng) were transfected into BMSCs (1×105 cell/ml) in
6 well plates using Lipofectamine RNAiMAX reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. β-catenin plasmid or 1 nM Aleglitazar (PPARγ agonist;
Medchemexpress Co., Ltd., Shanghai, China) were introduced into the
cells by transfection alongside anti-miRNA-15a-5p or miRNA-15a-5p
for 48 h at 37°C.
Cell proliferation assay and lactate
dehydrogenase (LDH) release assay
A total of 20 µl Cell Counting Kit-8 solution
(Beyotime Institute of Biotechnology, Haimen, China) were added to
each well and incubated for 2 h at 37°C. The absorbance of the
samples was measured at 450 nm with a spectrophotometric microplate
reader (Bio-Rad 680; Bio-Rad Laboratories, Inc.). LDH activity
levels were measured using LDH activity kits (C0017; Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's protocols. The absorbance of the samples were
measured at 450 nm with a spectrophotometric microplate reader
(Bio-Rad 680; Bio-Rad Laboratories, Inc.).
Flow cytometry
The Annexin V-fluorescein isothiocyanate (FITC)
early apoptosis detection kit (Cell Signaling Technology, Inc.,
Danvers, MA, USA) according to the manufacturer's protocol was used
to estimate apoptosis. BMSCs (1×106 cell/ml) were fixed
with 4% paraformaldehyde for 15 min at room temperature. The cells
were then stained with 10 µl Annexin V-FITC and 5 µl propidium
iodide for 30 min on ice in the dark at room temperature. Apoptosis
was measured using flow cytometry and analyzed using FlowJo 7.6.1
(FlowJo LLC, Ashland, OR, USA).
Caspase-3/9 activities
BMSCs were washed with ice-cold phosphate buffered
saline and treated with RIPA buffer (Beyotime Institute of
Biotechnology) for 30 min at 4°C. Supernatants were centrifuged at
12,000 × g at 4°C for 10 min and collected to measure the
supernatant proteins using the BCA method (Beyotime Institute of
Biotechnology). Total protein (10 µg) was used to measure
caspase-3/9 activity levels using caspase-3/9 activity kits (C1116
or C1158, Beyotime Institute of Biotechnology). The absorbance of
the samples were measured at 405 nm with a spectrophotometric
microplate reader (Bio-Rad 680; Bio-Rad Laboratories, Inc.).
Western blot analysis
BMSCs were washed with ice-cold phosphate buffered
saline and treated with RIPA buffer (Beyotime Institute of
Biotechnology) for 30 min at 4°C. Supernatants were centrifuged at
12,000 × g at 4°C for 10 min and collected to measure the
supernatant proteins using the BCA method (Beyotime Institute of
Biotechnology). Total protein (50–100 µg) was subjected to
electrophoresis on a 12.5% SDS-PAGE gel and then transferred onto
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.). The
membrane was blocked with tris-buffered saline with 0.1% Tween-20
containing 5% fat-free dried milk at 37°C for 1 h and incubated
with anti-PPARγ (cat no. sc-1981; 1:500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), Wnt-3 (cat no. sc-74537; 1:500; Santa Cruz
Biotechnology, Inc.) anti-β-Catenin (cat no. sc-31001; 1:500; Santa
Cruz Biotechnology, Inc.) and anti-GAPDH (cat no. sc-48166; 1:500;
Santa Cruz Biotechnology, Inc.) at 4°C overnight, followed by
incubation with a goat anti-rabbit IgG secondary antibody (BA1070;
1:5,000; Wuhan Boster Biological Technology, Ltd., Wuhan, China).
Immunodetection was conducted using enhanced chemiluminescence
(Applygen Technologies, Inc., Beijing, China) and analyzed using
Image Lab version 3.0 (Bio-Rad Laboratories, Inc.).
Luciferase reporter assay
miRNA-15a-5p and Wnt3a-3′UTR-phRL-TK (Promega
Corporation, Madison, WI, USA) were cloned by Sangon Biotech Co.,
Ltd. (Shanghai, China). For luciferase activity analysis, HEK-293T
cells (2×105 cells/well) were co-transfected with
luciferase reporter constructs using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following incubation
for 48 h, the luciferase assay was performed using a
dual-luciferase reporter assay system (Promega Corporation). Data
were normalized to Renilla luciferase activity.
Statistical analysis
Data were expressed as the mean ± standard deviation
using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). A Student's
t-test was used for comparisons between two groups. One-way
analysis of variance followed by Tukey's post-hoc test were used
for multiple group comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-15a-5p expression in rat models of
FHN
To investigate the mechanism of miRNAs in the
regulation of the bone cell apoptosis of FHN, the gene expression
of a number of miRNAs were assessed. As presented in Fig. 1A, bone cell apoptosis was observed
in the FHN model group, compared with the sham rat group which did
not present any apoptosis. In addition, caspase-3/9 activities and
Bax protein expression levels were significantly increased in the
FHN model group in comparison with the sham rat group (P<0.01;
Fig. 1B-E). Gene expression
profiling and RT-qPCR were further employed to analyze the changes
in the expression levels of miRNAs, which revealed that miR-15a-5p
expression was significantly increased in the FHN model group
compared with the sham group (P<0.01; Fig. 1F-G).
Overexpression of miR-15a-5p promoted
BMSC apoptosis
Next, the present study investigated whether
miRNA-15a-5p regulated the apoptosis of BMSCs in FHN. miRNA-15a-5p
mimics successfully significantly increase the expression of
miRNA-15a-5p in an in vitro model of FHN compared with
negative control group (P<0.01; Fig. 2A). Consequently, the overexpression
of miRNA-15a-5p significantly reduced cell proliferation at 48 and
72 h post-transfection, significantly increased LDH activity and
the apoptosis rate, and significantly promoted the caspase-3/9
activity levels of BMSCs compared with the negative control group
(P<0.01; Fig. 2B-G).
Downregulation of miR-15a-5p reduced
the apoptosis of BMSCs
Next, the function of miR-15a-5p on the apoptosis of
BMSCs in FHN was analyzed using anti-miR-15a-5p, which successfully
significantly reduced the expression levels of miR-15a-5p in an
in vitro model of FHN in comparison with the negative
control group (P<0.01; Fig.
3A). Furthermore, the downregulation of miR-15a-5p
significantly promoted cell proliferation, significantly decreased
LDH activity and apoptosis and significantly reduced the
caspase-3/9 activity levels of BMSCs compared with the negative
control group (P<0.01; Fig.
3B-G).
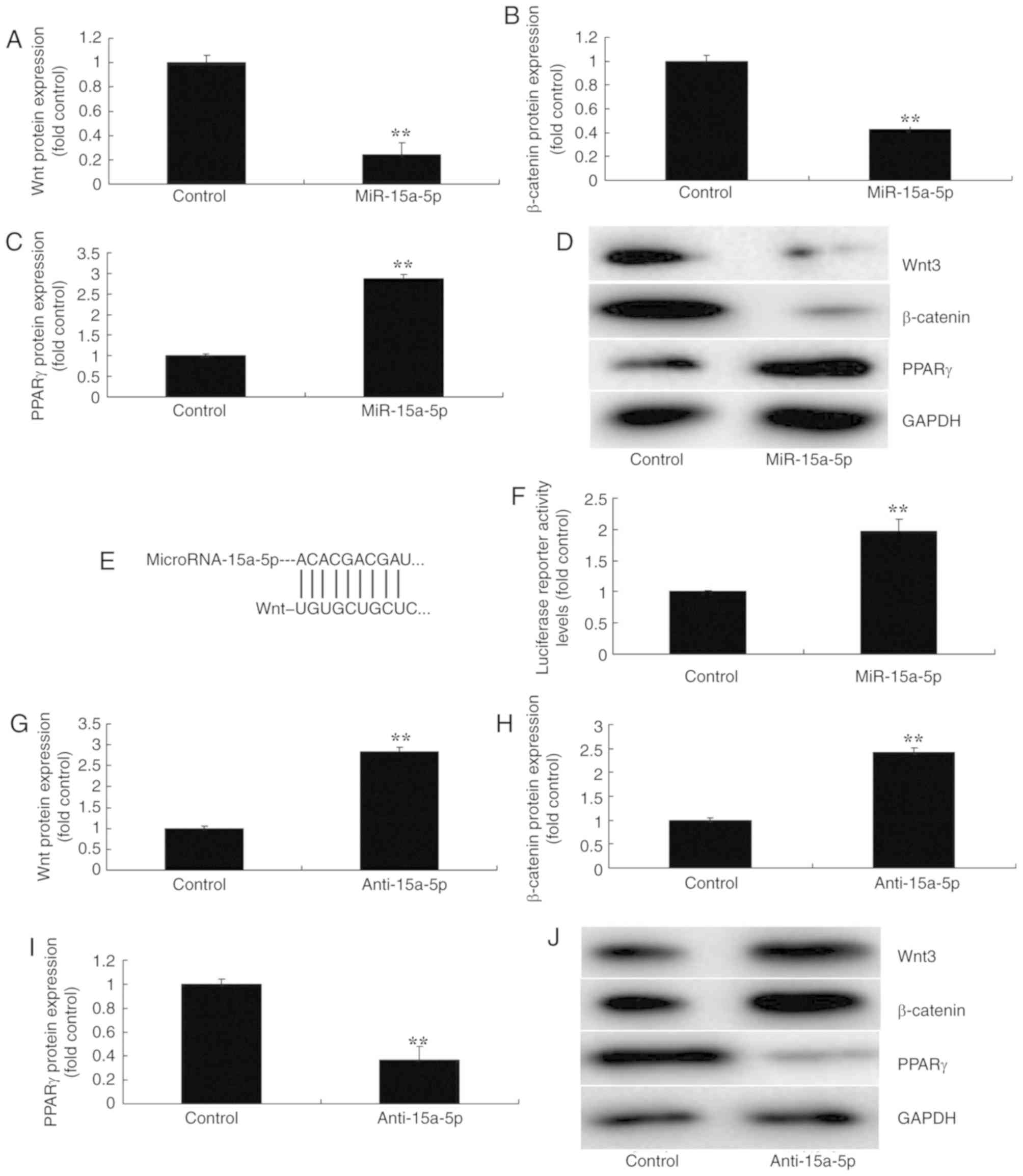
miR-15a-5p regulated the
Wnt/β-catenin/PPARγ signaling pathway
To further investigate the mechanism of miR-15a-5p
in regulating the apoptosis of BMSCs in FHN, western blot analysis
was used to measure the protein expression of the
Wnt/β-catenin/PPARγ signaling pathway. As a result, the
overexpression of miR-15a-5p significantly suppressed the protein
expression of Wnt-3 and β-catenin, and significantly induced that
of PPARγ in the in vitro model of FHN, in comparison with
the negative control group (P<0.01; Fig. 4A-D). Then, a luciferase reporter
assay was performed to analyze a target of miR-15a-5p, which
demonstrated that PPARγ was a direct target of miR-15a-5p; and
luciferase reporter activity levels were significantly increased in
an in vitro model of FHN, compared with the negative control
group (P<0.01; Fig. 4E-F). In
addition, the downregulation of miR-15a-5p significantly induced
the protein expression of Wnt-3 and β-catenin, and suppressed that
of PPARγ, in an in vitro model of FHN compared with the
negative control group (P<0.01; Fig. 4G-J). These results revealed that
the regulation of the β-catenin/PPARγ signaling pathway by Wnt may
be a mechanism of miR-15a-5p in FHN.
Activation of Wnt attenuated the
effects of miR-15a-5p on the apoptosis of BMSCs via the
β-catenin/PPARγ signaling pathway
Considering the above results, in order to examine
the mechanism of miR-15a-5p in regulating the apoptosis of BMSCs, a
Wnt plasmid was used, and significantly induced the protein
expression levels of Wnt-3 and β-catenin and significantly
suppressed that of PPARγ in an in vitro model of BMSCs
following miR-15a-5p, compared with the overexpression of the
miR-15a-5p group (P<0.01; Fig.
5A-5D). Subsequently, the activation of Wnt significantly
attenuated the effects of miR-15a-5p on the inhibition of cell
proliferation, the promotion of LDH activity and apoptosis and the
activation of caspase-3/9 activity levels in BMSCs in comparison
with the overexpression of miR-15a-5p group (P<0.01; Fig. 5E-J).
Discussion
BMSCs are multipotential stem cells existing in bone
marrow and are equipped with the characteristics of adherence
growth. Under specific conditions, they are able to be
differentiated into osteoblasts, chondrocytes and adipocytes
(28). Therefore, the acquisition,
cultivation and expansion of BMSCs are critical steps of stem cell
transplant. In order to verify the functions of certain miRNAs, it
is necessary to introduce the target miRNAs into BMSCs using
adenovirus transfection, lentiviral transfection and lipofection
transfection (12). Suitable
investigative conditions of transfection and the improvement of
acquired efficiency to express miRNAs are preconditions for in
vivo experiments (29). To the
best of our knowledge, the present study is the first to
demonstrate that miR-15a-5p expression is increased in a FHN model
group compared with sham group. Liu et al (13) had previously reported that
miR-15a-5p regulated VEGFA in endometrial mesenchymal stem
cells.
It has been reported that the majority of
osteocalcin composited by BMSCs would be secreted into a nutrient
solution (29). Secretion of
osteocalcin is parallel with osteogenesis (30), which is a characteristic of
differentiating osteoblasts. An early characteristic of the
osteogenic differentiation of BMSCs is increased cellular alkaline
phosphatase (ALP) activity (31).
Therefore, ALP activity in BMSCs and osteocalcin contained in a
nutrient solution is considered as an important indicator used to
test for osteoblast differentiation in vivo (32). In the present study it was revealed
that the overexpression of miR-15a-5p reduced cell proliferation,
increased LDH activity and apoptosis and promoted the caspase-3/9
activity levels of BMSCs. Krejcik et al (33) demonstrated that miR-15a-5p/b-5p was
associated with myelodysplastic syndromes and acute myeloid
leukemia.
PPARγ, mainly present in adipose tissues, is a
adipogenesis transcription factor closely associated with the
generation of adipocytes, with a notable function in adipogenesis
(18). It is able to induce
preadipocytes into adipocytes, affects the storage of fatty acids
in adipose tissues and serves a critical role in adipogenesis and
fat cell differentiation, which is induced by numerous adipocyte
genes prior to transcriptional activation (18). Activated PPARγ in adipose tissues
is able to promote the release of adiponectin and adipocyte
factors, translate accumulated non-esterified fatty acids into
adipose tissues and is eliminated from the liver and skeletal
muscle, in order to protect these organs from loading excessive
lipid matters and protect its normal functions (34). Meanwhile, PPARγ is able to regulate
the expression of relevant genes involved in the release, transfer
and storage of fatty acids, and also exert an important effect on
regulating lipid metabolism (35).
In the present study, the overexpression of miR-15a-5p suppressed
the protein expression of Wnt-3 and β-catenin, and induced that of
PPARγ in an in vitro model of FHN. Shi et al
(22) concluded that miR-15a-5p
was involved in the adipocyte differentiation of human adipocytes
and obesity by regulating Wnt signaling.
The Wnt/β-catenin signal pathway has been
demonstrated to be closely associated with the catabolism of the
cartilage matrix, the dedifferentiation of joint chondrocytes and
the restraint of chondrocyte apoptosis (19). The activation of the Wnt/β-catenin
pathway may protect MSCs from differentiating into chondrocytes,
however the maturity of chondrocytes requires the continuous
activation of β-catenin (36). In
the present study it was revealed that the overexpression of
miR-15a-5p significantly suppressed the protein expression of
β-catenin, and the downexpression of miR-15a-5p significantly
induced β-catenin protein expression in BMSCs compared with their
respective control groups (P<0.01). These results suggest that
the activation of Wnt attenuates the effects of miR-15a-5p on the
apoptosis of BMSCs via the β-catenin/PPARγ signaling pathway. Wang
et al (37) revealed that
miR-15a-5p suppressed endometrial cancer cell growth via the
Wnt/β-catenin signaling pathway. In the present study, only Wnt
activation was used to analyze the function of Wnt in the effects
of miR-15a-5p on the apoptosis of BMSCs. In the future, Wnt
inhibition would also be employed to further analyze the function
of Wnt in the effects of miR-15a-5p on the apoptosis of BMSCs.
In conclusion, it was revealed that miR-15a-5p
facilitated the adipogenesis or osteogenic differentiation of BMSCs
through regulating the PPARγ/β-catenin signaling pathway. These
results have provided insight into the underlying mechanism of the
osteogenic differentiation of BMSCs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL designed the experiments; WZ, CC and XM performed
the experiments; SL and WZ analyzed the data; and SL wrote the
manuscript.
Ethics approval and consent to
participate
The animal study was ethically approved by the
ethics committee of Hongqi Hospital Affiliated With Mudanjiang
Medical University (Heilongjiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Daltro GC, Fortuna V, de Souza ES, Salles
MM, Carreira AC, Meyer R, Freire SM and Borojevic R: Efficacy of
autologous stem cell-based therapy for osteonecrosis of the femoral
head in sickle cell disease: A five-year follow-up study. Stem Cell
Res Ther. 6:1102015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman R, Woodward E, Brown J, Cameron D,
Bell R, Dodwell D, Keane M, Gil M, Davies C, Burkinshaw R, et al:
Safety of zoledronic acid and incidence of osteonecrosis of the jaw
(ONJ) during adjuvant therapy in a randomised phase III trial
(AZURE: BIG 01–04) for women with stage II/III breast cancer.
Breast Cancer Res Treat. 127:429–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ripamonti CI, Cislaghi E, Mariani L and
Maniezzo M: Efficacy and safety of medical ozone (O(3)) delivered
in oil suspension applications for the treatment of osteonecrosis
of the jaw in patients with bone metastases treated with
bisphosphonates: Preliminary results of a phase I–II study. Oral
Oncol. 47:185–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vande Berg BC, Gilon R, Malghem J,
Lecouvet F, Depresseux G and Houssiau FA: Correlation between
baseline femoral neck marrow status and the development of femoral
head osteonecrosis in corticosteroid-treated patients: A
longitudinal study by MR imaging. Eur J Radiol. 58:444–449. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Yuan Z, Wei X, Li H, Zhao G, Miao J,
Wu D, Liu B, Cao S, An D, et al: Application potential of bone
marrow mesenchymal stem cell (BMSCs) based tissue-engineering for
spinal cord defect repair in rat fetuses with spina bifida aperta.
J Mater Sci Mater Med. 27:772016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi S, Wu X, Wang X, Hao W, Miao H, Zhen L
and Nie S: Differentiation of bone marrow mesenchymal stem cells to
cardiomyocyte-like cells is regulated by the combined low dose
treatment of transforming growth factor-β1 and 5-azacytidine. Stem
Cells Int. 2016:38162562016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatzistergos KE, Quevedo H, Oskouei BN, Hu
Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP,
Rodriguez JE, et al: Bone marrow mesenchymal stem cells stimulate
cardiac stem cell proliferation and differentiation. Circ Res.
107:913–922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li YG, Wei JN, Lu J, Wu XT and Teng GJ:
Labeling and tracing of bone marrow mesenchymal stem cells for
tendon-to-bone tunnel healing. Knee Surg Sports Traumatol Arthrosc.
19:2153–2158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rippo MR, Babini L, Prattichizzo F,
Graciotti L, Fulgenzi G, Tomassoni Ardori F, Olivieri F, Borghetti
G, Cinti S, Poloni A, et al: Low FasL levels promote proliferation
of human bone marrow-derived mesenchymal stem cells, higher levels
inhibit their differentiation into adipocytes. Cell Death Dis.
4:e5942013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Luca L, Trino S, Laurenzana I, Simeon
V, Calice G, Raimondo S, Podestà M, Santodirocco M, Di Mauro L, La
Rocca F, et al: MiRNAs and piRNAs from bone marrow mesenchymal stem
cell extracellular vesicles induce cell survival and inhibit cell
differentiation of cord blood hematopoietic stem cells: A new
insight in transplantation. Oncotarget. 7:6676–6692. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ono M, Kosaka N, Tominaga N, Yoshioka Y,
Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K and Ochiya
T: Exosomes from bone marrow mesenchymal stem cells contain a
microRNA that promotes dormancy in metastatic breast cancer cells.
Sci Signal. 7:ra632014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HK, Finniss S, Cazacu S, Bucris E,
Ziv-Av A, Xiang C, Bobbitt K, Rempel SA, Hasselbach L, Mikkelsen T,
et al: Mesenchymal stem cells deliver synthetic microRNA mimics to
glioma cells and glioma stem cells and inhibit their cell migration
and self-renewal. Oncotarget. 4:346–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu XJ, Bai XG, Teng YL, Song L, Lu N and
Yang RQ: miRNA-15a-5p regulates VEGFA in endometrial mesenchymal
stem cells and contributes to the pathogenesis of endometriosis.
Eur Rev Med Pharmacol Sci. 20:3319–3326. 2016.PubMed/NCBI
|
|
14
|
Nakanishi A and Tsukamoto I: n-3
polyunsaturated fatty acids stimulate osteoclastogenesis through
PPARγ-mediated enhancement of c-Fos expression, and suppress
osteoclastogenesis through PPARγ-dependent inhibition of NFκB
activation. J Nutr Biochem. 26:1317–1327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao XY, Chen XY, Zhang ZJ, Kang Y, Liao
WM, Yu WH and Xiang AP: Expression patterns of transcription factor
PPARγ and C/EBP family members during in vitro adipogenesis of
human bone marrow mesenchymal stem cells. Cell Biol Int.
39:457–465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng H, Qiu L, Zhang H, Cheng M, Li W,
Zhao X, Liu K, Lei L and Ma J: Arsenic trioxide promotes senescence
and regulates the balance of adipogenic and osteogenic
differentiation in human mesenchymal stem cells. Acta Biochim
Biophys Sin (Shanghai). 43:204–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He YF, Liu FY and Zhang WX: Tangeritin
inhibits adipogenesis by down-regulating C/EBPα, C/EBPβ, and PPARγ
expression in 3T3-L1 fat cells. Genet Mol Res. 14:13642–13648.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu HF, Gui MX, Dong H, Wang X and Li XW:
Differential expression of AdipoR1, IGFBP3, PPARγ and correlative
genes during porcine preadipocyte differentiation. In Vitro Cell
Dev Biol Anim. 48:54–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Chen L, Yin Q, Gao H, Dong P,
Zhang X and Kang J: Reciprocal interferences of TNF-α and
Wnt1/β-catenin signaling axes shift bone marrow-derived stem cells
towards osteoblast lineage after ethanol exposure. Cell Physiol
Biochem. 32:755–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pai P, Rachagani S, Dhawan P and Batra SK:
Mucins and Wnt/β-catenin signaling in gastrointestinal cancers: An
unholy nexus. Carcinogenesis. 37:223–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Conidi A, van den Berghe V and Huylebroeck
D: Aptamers and their potential to selectively target aspects of
EGF, Wnt/β-catenin and TGFβ-smad family signaling. Int J Mol Sci.
14:6690–6719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi C, Huang F, Gu X, Zhang M, Wen J, Wang
X, You L, Cui X, Ji C and Guo X: Adipogenic miRNA and
meta-signature miRNAs involved in human adipocyte differentiation
and obesity. Oncotarget. 7:40830–40845. 2016.PubMed/NCBI
|
|
23
|
Dong YL, Zhou L, Li YL, Xiao K and Weng
XS: Establishment and assessment of rat models of
glucocorticoid-induced osteonecrosis. Zhongguo Yi Xue Ke Xue Yuan
Xue Bao. 37:152–156. 2015.PubMed/NCBI
|
|
24
|
Karakaplan M, Gülabi D, Topgül H and
Elmali N: Does platelet-rich plasma have a favorable effect in the
early stages of steroid-associated femoral head osteonecrosis in a
rabbit model? Eklem Hastalik Cerrahisi. 28:107–113. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyata N, Kumagai K, Osaki M, Murata M,
Tomita M, Hozumi A, Nozaki Y and Niwa M: Pentosan reduces
osteonecrosis of femoral head in SHRSP. Clin Exp Hypertens.
32:511–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei J, Zhang Y, Luo Y, Wang Z, Bi S, Song
D, Dai Y, Wang T, Qiu L, Wen L, et al: Aldose reductase regulates
miR-200a-3p/141-3p to coordinate Keap1-Nrf2, Tgfβ1/2, and Zeb1/2
signaling in renal mesangial cells and the renal cortex of diabetic
mice. Free Radic Biol Med. 67:91–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duttenhoefer F, de Freitas RL, Loibl M,
Bittermann G, Richards RG, Alini M and Verrier S: Endothelial
progenitor cell fraction contained in bone marrow-derived
mesenchymal stem cell populations impairs osteogenic
differentiation. Biomed Res Int. 2015:6595422015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu G, Feng C, Hui G, Wang Z, Tan J, Luo L,
Xue P, Wang Q and Chen X: Improving the osteogenesis of rat
mesenchymal stem cells by chitosan-based-microRNA nanoparticles.
Carbohydr Polym. 138:49–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang G, Na Z, Ren B, Zhao X and Liu W:
Impacts of fluorescent superparamagnetic iron oxide (SPIO)-labeled
materials on biological characteristics and osteogenesis of bone
marrow mesenchymal stem cells (BMSCs). Int J Clin Exp Med.
8:12172–12181. 2015.PubMed/NCBI
|
|
31
|
Soleimani M, Abbasnia E, Fathi M, Sahraei
H, Fathi Y and Kaka G: The effects of low-level laser irradiation
on differentiation and proliferation of human bone marrow
mesenchymal stem cells into neurons and osteoblasts-an in vitro
study. Lasers Med Sci. 27:423–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi F, Khan M, Gao H, Hao F, Sun M, Zhong
L, Lu C, Feng X and Ma T: Increased differentiation capacity of
bone marrow-derived mesenchymal stem cells in aquaporin-5
deficiency. Stem Cells Dev. 21:2495–2507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krejcik Z, Belickova M, Hrustincova A,
Votavova H, Jonasova A, Cermak J, Dyr JE and Merkerova MD: MicroRNA
profiles as predictive markers of response to azacitidine therapy
in myelodysplastic syndromes and acute myeloid leukemia. Cancer
Biomark. 22:101–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawai M, Sousa KM, MacDougald OA and Rosen
CJ: The many facets of PPARgamma: Novel insights for the skeleton.
Am J Physiol Endocrinol Metab. 299:E3–E9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kolli V, Stechschulte LA, Dowling AR,
Rahman S, Czernik PJ and Lecka-Czernik B: Partial agonist,
telmisartan, maintains PPARg serine 112 phosphorylation, and does
not affect osteoblast differentiation and bone mass. PLoS One.
9:e963232014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Z, Gong X, Zhu H, Wang C, Xu X, Cui D,
Qian W and Han X: Inhibition of Wnt/β-catenin signaling promotes
engraftment of mesenchymal stem cells to repair lung injury. J Cell
Physiol. 229:213–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang ZM, Wan XH, Sang GY, Zhao JD, Zhu QY
and Wang DM: miR-15a-5p suppresses endometrial cancer cell growth
via Wnt/β-catenin signaling pathway by inhibiting WNT3A. Eur Rev
Med Pharmacol Sci. 21:4810–4818. 2017.PubMed/NCBI
|