Introduction
Stress urinary incontinence (SUI) is defined as the
involuntary leakage of urine during laughing, coughing, sneezing or
physical exercise (1), and is the
most common type of urinary incontinence affecting up to 35% of
women (2). The predisposing risk
factors include pregnancy, vaginal delivery, obesity, long-term
high abdominal pressure and age (3). It is known to have adverse effects on
quality of life in ~54.3% of all pregnant women in four aspects:
Physical activity, travel, social relationships and emotional
health (4). These aspects lead
women to isolate themselves and to stop participating in the
routine activities of daily living. In 1995 it was estimated that
more than $16 billion was spent on SUI (5); this figure may take into account the
costs of nursing home admissions, incontinence pads, medical and
surgical treatment and time lost from work. With an increase in the
incidence of SUI among middle-aged and elderly women, the social
and economic burden of SUI may be more serious in a context of
increased global life expectancy (6,7).
Currently, surgical and non-surgical strategies are
used to treat SUI. The use of a midurethral sling (MUS) is the
preferred surgical treatment for women with SUI (8). MUS is a one-time, effective and
long-term treatment of SUI, but it carries a greater risk of
complications (9). MUS is not
suitable for patients with mild SUI. Treatment of SUI must balance
the efficacy, adverse events and costs. Pelvic electrical
stimulation (PES) is a conservative treatment strategy that can
inhibit the parasympathetic motor nerve to relax the bladder and
promote repeated pelvic floor muscle contraction to enhance muscle
contraction while strengthening a full bladder (10,11).
Numerous reports have confirmed that PES can treat SUI and relieve
symptoms (12–15). A large clinical, randomized
controlled study published by the UK National Health Service in
2016 showed that the cure rate for PES treatment combined with
pelvic floor muscle exercises was 40% higher than that of simple
pelvic floor exercises (14).
However, the specific mechanism of action of PES in treating SUI
remains unknown.
Previous studies have confirmed that extracellular
matrix (ECM) remodeling is an important step in the pathogenesis of
pelvic floor dysfunction (15,16).
A large number of studies suggest that integrin β1 is a key
component of the cytoskeleton and is involved in the regulation of
cell adhesion, migration, proliferation and apoptosis; however, to
the best of our knowledge there is no research about the role of
integrin β1 in the treatment of SUI by electrical stimulation. In
this study, the mechanism of electrical stimulation in the
treatment of SUI through integrin β1/transforming growth factor
(TGF)-β/ECM was investigated, providing a theoretical basis for the
prevention and treatment of SUI.
Materials and methods
Patient selection and tissue
collection
All protocols were approved by the Ethics Committee
of Renmin Hospital of Wuhan University. All samples were obtained
from patients undergoing routine hysterectomy after obtaining
signed informed consent forms. A total of 8 patients (age between
55 to 65) who underwent hysterectomy surgery (for reasons excluding
the presence of malignant tumors and SUI) served as controls, and
18 patients (age between 55 to 65) who underwent gynecological
surgery only for SUI (grade II and III) in the Obstetric and
Gynecological Department of Renmin Hospital of Wuhan University
between January 2017 and January 2018, were enrolled in the present
study. SUI severity was classified using the Ingelman-Sundberg
scale: Grade I urinary incontinence was characterized as being
induced by coughing or sneezing, grade II urinary incontinence was
induced by running or picking up an object from the floor and grade
III urinary incontinence was induced by walking or climbing stairs
(17). If a patient had
overlapping symptoms, the higher grade was chosen as the severity
of urinary incontinence. None of the women recruited had any
connective tissue diseases, patholog-ically confirmed
endometriosis, estrogen-associated ovarian tumors, pelvic
inflammatory conditions, or advanced pelvic organ prolapse [greater
than stage II by the Pelvic Organ Prolapse Quantification scale
(18)]. Furthermore, these
patients were free from any compli-cations that may lead to
diseases associated with collagen depletion, including diabetes and
hyperthyroidism. Patients who had received any pelvic surgery or
had a history of estrogen application within the past three months
were excluded from the present study. All participants were
postmenopausal. After informed consent was obtained, approximately
1 cm2 of a full-thickness excision was made of the
periurethral vaginal wall 1 cm lateral to the urethrovesical
junction (identified by a Foley balloon) from patients undergoing
surgery for SUI. Smaller 0.5-cm2 biopsy samples of the
vaginal wall from a similar area were excised in postmenopausal
continent control subjects who underwent benign gynecologic
surgeries for fibroids, dysfunctional bleeding and ovarian cysts.
The epithelial layer was removed with a razor blade when the
tissues were collected, after which primary cell culture was
performed immediately. The remainder of the tissue was frozen
immediately in liquid nitrogen and then stored at −80°C for further
processing.
Primary cell culture
A modified enzyme digestion method was used in the
present study for the establishment of the primary cell culture,
which was similar to previous research methods (19). The tissues were washed 3–5 times
with PBS (HyClone; GE Healthcare Life Sciences) containing 1%
double-antibiotic solution (100K U/ml penicillin G and 100 mg/ml
streptomycin; Hangzhou Ginom Biomedical Technology Co., Ltd.) in
order to clear the underlying blood, axungia and necrotic tissue.
Tissues were then sectioned into 1 mm3 fragments.
Sections were digested with 1% collagenase I (Invitrogen; Thermo
Fisher Scientific, Inc.) for 3 h at 37°C in 5% CO2,
followed by further digestion with 0.25% trypsin (Sigma-Aldrich;
Merck KGaA) for 5 min. Subsequently, 2 ml of FBS (Gibco; Thermo
Fisher Scientific, Inc.) was added to stop digestion. DMEM
(Hangzhou Ginom Biomedical Technology Co., Ltd.) supplemented with
15% FBS was slowly added to the culture flask. The culture medium
was replaced every two days and FVWFs were cultured to 70%
confluence for passage. The stable primary cells were obtained
after ~15 days. The FVWFs were used at passages 4–8 for subsequent
experiments.
Immunofluorescence staining
Vimentin is commonly used as a fibroblast marker to
confirm the purity of cultured fibroblasts, as described in a
previous study (19). After cells
had migrated out of the vaginal wall tissue and become stable, the
cells were applied to a glass slide. The cells were then fixed with
4% paraformaldehyde (Sinopharm Chemical Reagents Co., Ltd.) for 15
min at 4°C and 0.5% Triton X-100 (Beyotime Institute of
Biotechnology) for 20 min at 4°C. After washing with PBS and
blocking with 5% goat serum for 30 min at room temperature (cat.
no. AR1009; Wuhan Boster Biological Technology, Ltd.), the cells
were incubated with rabbit anti-vimentin (1:50; cat. no.
SAB1305433; Sigma-Aldrich; Merck KGaA) and mouse
anti-cytokeratin-19 (1:50; cat. no. ab7755; Abcam) overnight at
4°C, followed by fluorescence-marked goat anti-rabbit IgG (FITC,
1:200; cat. no. BA1032; Wuhan Boster Biological Technology, Ltd.)
and goat anti-mouse (Cy3, 1:200; cat. no. BA1101; Wuhan Boster
Biological Technology, Ltd.) at room temperature for 1 h. DAPI (1
µg/ml, Beyotime Institute of Biotechnology) staining conducted for
5 min at room temperature was used to observe the nuclei. Finally,
images (magnification, ×100) were captured using a fluorescence
microscope (Olympus BX53; Olympus Corporation).
Electrical stimulation and drug
incubation
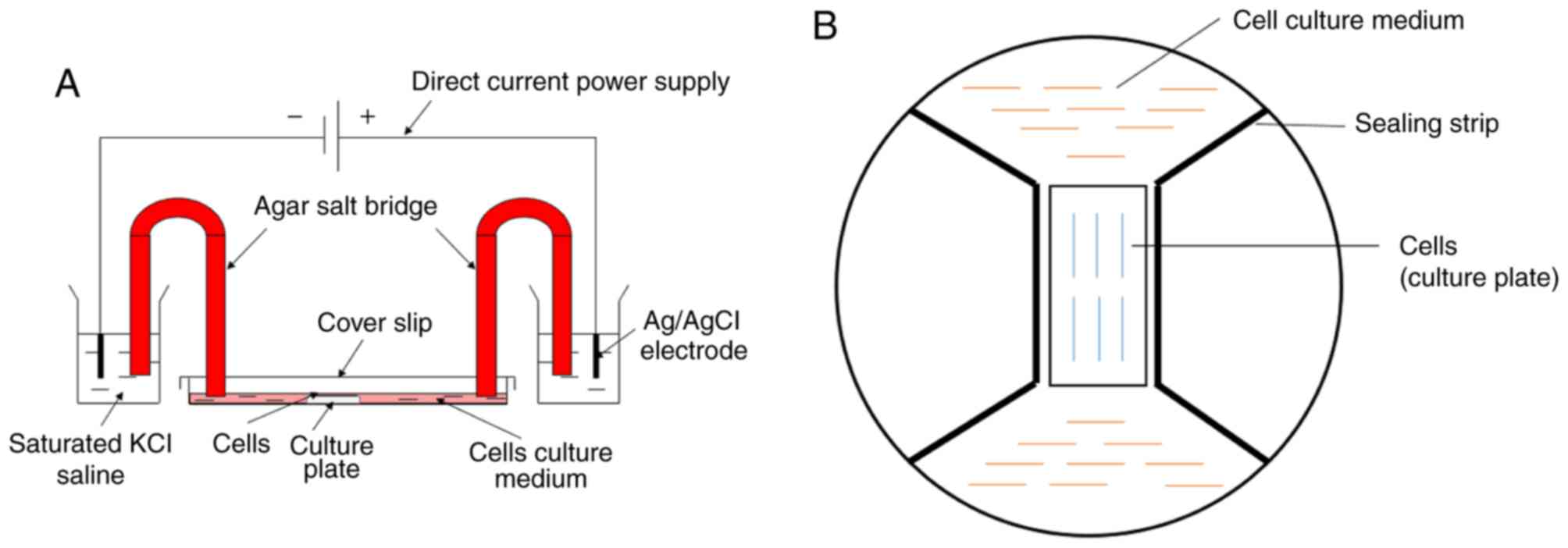
The model of cellular electrical stimulation was
modified from Song et al (20). The cover glass (24×50 mm;
thickness, 0.13–0.17 mm) was cut into two equal parts as a sealing
strip. Dow Corning 4 (Dow Silicones Corp.) electrical insulating
compound was applied under the sealing strip to form a rectangular
area between the sealing strips that was used to inoculate cells.
Then, DC4 was used to connect the sealing strip and the inner edge
of the culture plate to block the free flow of the culture medium
and to prepare the electric chamber (Fig. 1B). Agar salt bridges (2%) of 15 cm
in length were used to connect Ag/AgCl electrodes in beakers of
saturated KCl solution to the chamber of culture medium. Direct
current was provided by a direct current power supply (Ever
Prosperous Instruments, Inc.), which was connected with the Ag/AgCl
electrodes in the beakers (Fig.
1A). The FVWFs were exposed to the electrical stimulation at
100 mV/mm and incubated in a 5% CO2 incubator at 37°C
for 2 h. Cells without exposure to electrical stimulation served as
controls. In order to inhibit integrin β1, anti-integrin β1
antibody (10 µg/ml; cat. no. ab24693; Abcam) was added to the FVWF
culture medium both 2 h prior to and during the treatment with
electrical stimulation in the electrostatic chamber. For the
non-specific antibody control, Purified Mouse Monoclonal IgG 1 (10
µg/ml; cat. no. ab91353; Abcam) was used. In addition, recombinant
human integrin β1 (0.1 µg/ml; cat. no. NBP2-59570; Novus
Biologicals, LLC) was also used for incubation with FVWFs for 24 h
in a 5% CO2 cell incubator at 37°C.
Western blot analysis
After exposure to electrical stimulation and/or the
inhibitor, FVWFs were harvested using RIPA lysis buffer containing
a proteinase inhibitor (cat. no. AR0102 and AR1178; Wuhan Boster
Biological Technology, Ltd.) for 30 min on ice. Following this,
total protein was extracted by centrifugation for 10 min at 4°C and
12,000 × g, followed by protein concentration quantification with a
bicinchoninic acid assay kit (cat. no. P0010; Beyotime Institute of
Biotechnology). A total of 20 µg of total cellular protein was
mixed with gel loading buffer, separated by 10% SDS-PAGE and
transferred onto PVDF membrane (Merck KGaA). Membranes were blocked
in 5% non-fat milk in TBS with 0.1% Tween 20 (TBS-T) for 1 h at
room temperature and washed with TBS-T twice. Membranes were
incubated with the following rabbit primary antibodies:
Anti-integrin β1 (1:1,000; cat. no. 34971; Cell Signaling
Technology, Inc.), anti-TGF-β1 (1:1,000; cat. no. ab92486; Abcam),
anti- collagen (COL) I (1:1,000; cat. no. ab34710; Abcam) and
anti-COL III (1:1,000; cat. no. ab7778; Abcam), overnight at 4°C,
followed by incubation with goat anti-rabbit fluorescence-labeled
secondary antibodies (1:10,000; IRDye700 and IRDye800; cat. no.
926-32211; LI-COR Biosciences) for 1 h at 37°C after washing. A
rabbit anti-GAPDH primary antibody (1:2,000; cat. no. ab9485;
Abcam) served as an internal reference control. The reactive bands
were detected with an Odyssey® infrared imaging system
(LI-COR Biosciences). For each sample, the band densities were
quantified using LI-COR Odyssey v.3.0 software (ODY-0102; LI-COR
Biosciences) were normalized against that of GAPDH and the data was
obtained from three experiments.
Reverse transcription-quantitative
(RT-q) PCR
The mRNA expression levels of integrin β1, TGF-β1,
and COL I and III in cells were evaluated by RT-qPCR after
electrical stimulation or/and drug incubation. The primers used for
amplification were purchased from Beijing SBS Genetech Co., Ltd.
Total RNA from FVWFs was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), following the
manufacturer's protocol. RNA (2 µg) was reverse transcribed to cDNA
(n=3; temperature protocol: 25°C for 5 min, 42°C for 30 min and
85°C for 5 min) using a Revert Aid First Strand cDNA Synthesis kit
(cat. no. k1622; Thermo Fisher Scientific, Inc.) and reaction
mixture aliquots (1 µl) were used as templates for PCR. Primer
sequences for TIMP-1, MMP-2, MMP-9, COL I and III, and GAPDH were
purchased from SBS Genetech Co., Ltd. (Table I). qPCR was performed using
SYBR® Premix Ex Taq™ reagent (cat. no.
DRR041; Takara Bio, Inc.) and an Applied Biosystems 7500 Real-Time
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were: 40 cycles of initial denaturation,
95°C for 5 min; denaturation, 95°C for 10 sec; anneal, 55°C for 20
sec; and extension, 72°C for 20 sec. Normalized quantification
cycle (Cq) values were used for comparison (21). Each sample was analyzed in
triplicate.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Species | Forward primer;
5′-3′ | Reverse primer;
5′-3′ |
|---|
| GAPDH | Human |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| Integrin β1 | Human |
GCCTGTTTACAAGGAGCTGAA |
CTGACAATTTGCCGTTTTCC |
| TGF-β1 | Human |
ACCTGAACCCGTGTTGCTCT | CTAAGGCGAAA
GCCCTCAAT |
| COL I | Human |
CAAGACGAAGACATCCCACCAATC |
ACAGATCACGTCATCGCACAACA |
| COL III | Human |
TCGCTCTGCTTCATCCCACTAT |
CTTCCAGACATCTCTATCCGCAT |
Statistical analysis
Data are presented as the mean ± SD for each group
and were analyzed by one-way ANOVA using GraphPad Prism 5.0
(GraphPad Software, Inc.). Multiple means were compared by Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
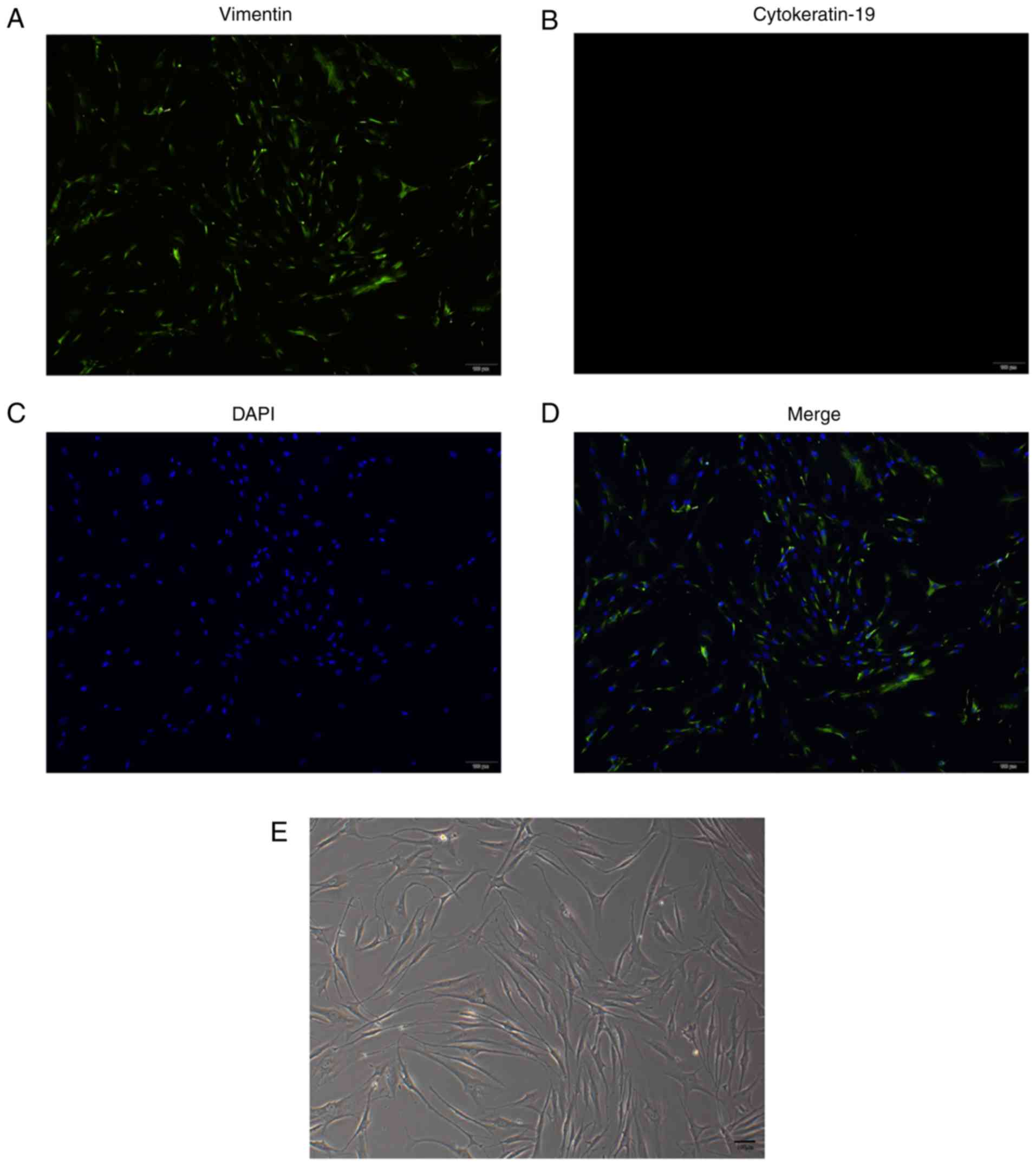
Identification of FVWFs
A previous study by the authors accurately
identified stable fibroblasts by positive staining of vimentin and
negative staining of cytokeratin (22) (Fig.
2A-D). As observed by light microscopy, the cells had long
spindles contacting each other to form a network structure with a
similar behavior to fibroblasts (Fig.
2E).
Response of FVWFs to electrical
stimulation
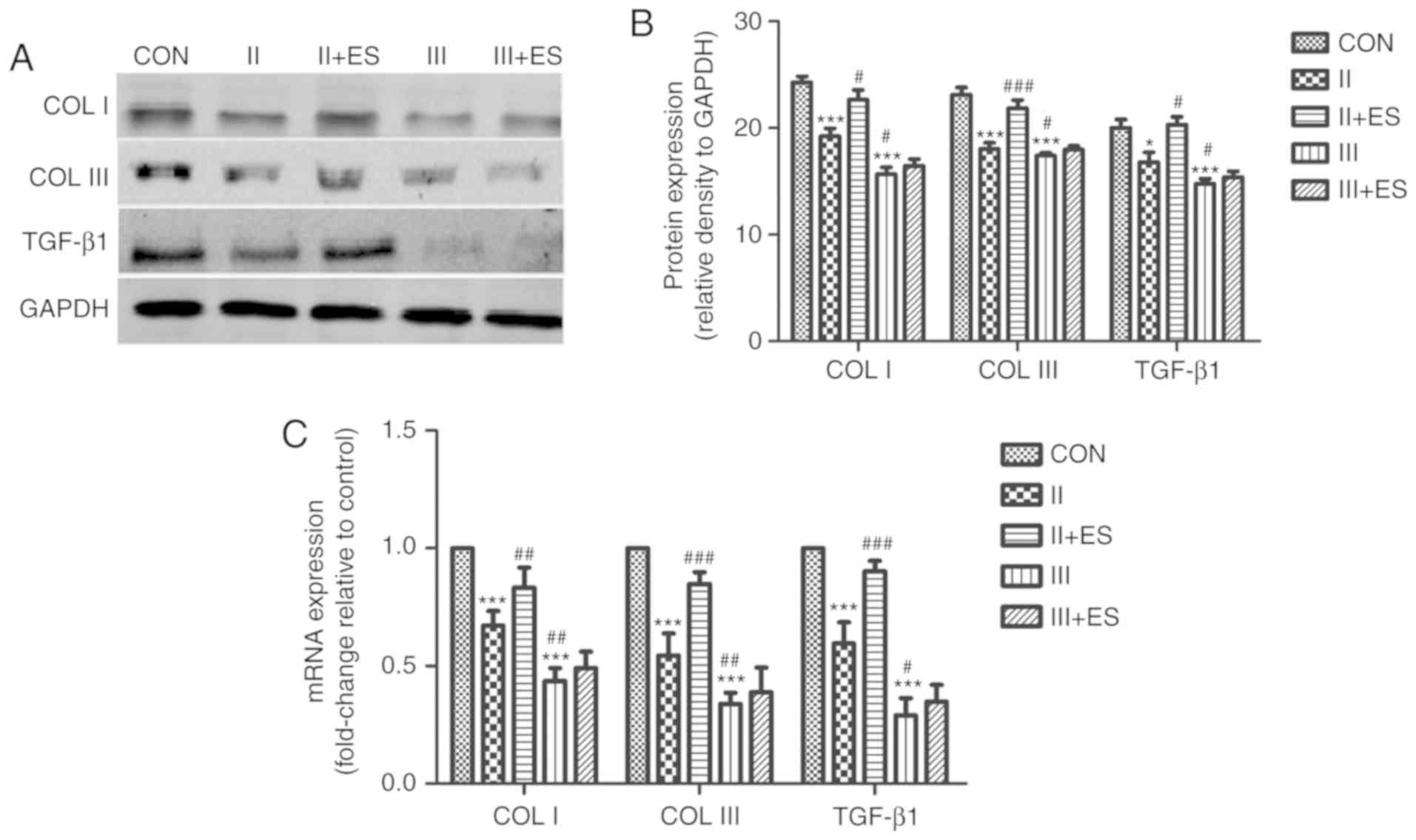
To verify the protective effect of electrical
stimulation on FVWFs, protein and mRNA expression of TGF-β1, COL I
and III were analyzed before and after electrical stimulation. When
compared to the control group, the protein and mRNA expression of
TGF-β1, COL I and III in the grade II and grade III groups
decreased significantly, with the reduction being more severe in
the grade III group (Fig. 3). The
results showed that the protein expression levels of TGF-β1, COL I
and III were increased in the grade II group after electrical
stimulation. Therefore, electrical stimulation could reverse the
reduction of TGF-β1, COL I and III at the mRNA and protein level in
the grade II group. However, the protein and mRNA expression levels
of TGF-β1, COL I and III were not changed by electrical stimulation
in the grade III group. This is consistent with the fact that
electrical stimulation is an effective treatment strategy for mild
and moderate SUI, but not for severe SUI.
Integrin β1 plays an important role in
the treatment of SUI by electrical stimulation
Integrins are cell surface receptors with diverse
functions that allow cells to bind to and respond to each other and
to the ECM (23). Integrins also
contribute to the control of cell proliferation, shape and motility
(24). Integrin β1 plays an
important role in the activation of downstream signals that
regulate several cellular mechanisms. Previously, studies found
that integrin β1 and TGF-β1 are involved in a variety of diseases,
including fibrosis. TGF-β1 can induce the expression of integrin β1
and the activation of integrin β1 can enhance the effect of
TGF-β1-mediated collagen synthesis (25,26).
In a study investigating the central nervous system,
oligodendrocyte progenitor cells were exposed to physiological
levels of electrical stimulation and were found to display a marked
electrotactic response that was dependent on integrin β1 (27). In the present study, it was found
that the levels of integrin β1 were lower in the grade II and grade
III groups compared to the control group, while the grade III group
displayed a more marked reduction than that in the grade II group
(Fig. 4A). Therefore, a link
between integrin β1 and the electrical stimulation-guided synthesis
of the ECM was examined. It was found that inhibiting integrin β1
led to a reduction in the protein levels of TGF-β1, COL I and III,
with a reduction at the mRNA level also observed in the grade II
group (Fig. 4B). The protective
effect of electrical stimulation was suppressed by the inhibitor of
integrin β1. These results show that integrin β1 is important for
the electrical stimulation-guided synthesis of the ECM. To further
investigate the role of integrin β1, recombinant human integrin β1
was incubated with FVWFs from the grade III group. A protective
effect of electrical stimulation in the grade III group was
observed. After incubation with recombinant human integrin β1
followed by electrical stimulation, the FVWFs of the grade III
group showed a significant increase in the expression of TGF-β1,
COL I and III (Fig. 4C).
 | Figure 4.Effect of integrin β1 in the
treatment of SUI by electrical stimulation. (A) Western blot
analysis was used to detect the different expression levels of
integrin β1 in three groups. (B) The proteins and mRNA levels of
TGF-β1, COL I and III were measured after incubation with the
inhibitor of integrin β1 and/or electrical stimulation in the grade
II group. (C) After incubation with recombinant human integrin β1
and/or electrical stimulation in the grade III group, the
expression levels of TGF-β1, COL I and III were analyzed.
***P<0.001 vs. CON, #P<0.05 ##P<0.01 and ###P<0.001 vs.
group II., &&P<0.01 and &&&P<0.001 vs.
ES. ҰҰҰP<0.001 vs. group III. TGF-β1, transforming growth
factor-β1; COL I and III, collagen I and III; CON, control group
without SUI; SUI, stress urinary incontinence; II, grade II group;
III, grade III group; i, inhibitor (anti-integrin β1 antibody); ic,
inhibitor control (non-specific antibody control); ES, electrical
stimulation; RH-integrin β1, recombinant human integrin β1. |
Discussion
Electrical stimulation is a versatile treatment with
a poorly understood therapeutic mechanism. Pelvic floor electrical
stimulation is one of the first-line conservative treatments for
female urinary incontinence. The effects of electrical stimulation
on collagen metabolism have been extensively studied in many
fields, including wound healing (28,29),
rehabilitation of osteoarthritis and fractures (30), and periodontal tissue remodeling
(31). Furthermore, studies
reported that electrical stimulation could upregulate the
expression of collagen (15,32).
In the present study, the importance of integrin β1 in the
treatment of SUI via TGF-β1/collagen in electrical stimulation was
demonstrated.
The pathophysiology of SUI involves defects in the
supporting tissues, including the suburethral vaginal wall, pelvic
floor muscles, fascia and pubo-urethral ligaments (33). The connective tissue linking these
structures is also an important factor (34). The pelvic supporting tissues are
composed primarily of connective tissue in which collagen and
elastic fibers are the predominant ECM components. Altered collagen
and elastin metabolism has been documented in tissues from women
with SUI (35). Collagen provides
strength and stability to the supporting tissue. Therefore, the
collagens in pelvic tissue, COL I and III, provide powerful tension
and strength for the pelvic tissue. In addition, fibroblasts serve
a pivotal role, particularly in pelvic connective tissue, ECM
remodeling and mechanical force resistance, by regulating the
balance between collagen synthesis and degradation following tissue
injury (36,37). Therefore, the present study
selected FVWFs to examine the phenotype of COL I and III. In this
study, ECM metabolism and associated molecules including TGF-β1,
COL I and III were altered in the FVWFs of SUI patients compared to
patients without SUI. Loss of the ECM leads to the dysfunction of
attachments and support of pelvic connective tissue (38,39).
In addition, a previous study demonstrated that the expression of
COL I and III are decreased in SUI in the periurethral vaginal wall
tissue (16).
TGF-β1 is a key factor in ECM metabolism and is
involved in the pathomechanism of SUI (40,41).
Low expression of TGF-β1 is closely related to the occurrence of
retrograde venereal degeneration of pelvic support structures such
as in SUI (42). A recent study
reported that the TGF-β1 pathway is activated and the expression of
COL I and III are significantly decreased in rats with SUI
(40). Integrins were originally
named to denote their role as integral membrane complexes linking
the ECM to the actin cytoskeleton. However, it is now clear that
integrins alone or in combination with other cell surface receptors
mediate many key intracellular signals and are important for
survival. Integrins exist in several activation states on the cell
surface, where activation induces integrin clustering that leads to
the recruitment of multiple signaling molecules and the regulation
of different signaling pathways (43). The regulatory function of integrin
can be achieved in several ways, including ligand engagement and
binding of intracellular proteins. Evidence indicates that there is
extensive crosstalk between integrins and TGF-β. Wang et al
(40) demonstrated that a subset
of integrins is responsible for a large proportion of TGF-β
activation in the epithelial-mesenchymal trophic unit. In addition,
it has been reported that blocking integrin β1 function inhibits
TGF-β-mediated mitogen activated protein kinase 1/p38 activation
and epithelial to mesenchymal trans-differentiation progression.
Therefore, integrin β1 can affect the activation and expression of
TGF-β1. In a study by Li et al (41), inhibition of TGF-β1 signaling by
SB-431542 blocked electrical stimulation-driven condensation and
significantly suppressed electrical stimulation-driven increases in
COL II A1, aggrecan, and SOX9 expression in mesenchymal stem cells
(40–44). The present study showed that
electrical stimulation with decreased integrin β1 could not
upregulate the expression of TGF-β1 in the grade III group.
However, the expression levels of TGF-β1, COL I and III were
enhanced by electrical stimulation after adding recombinant human
integrin β1. Furthermore, electrical stimulation had no effect on
the ECM when integrin β1 was inhibited by an antibody in the grade
II group. These data suggest that the upregulation of TGF-β1 and
the ECM by electrical stimulation may be dependent on integrin
β1.
Electrical stimulation not only induces a directed
accumulation of the integrin β1 subunit in human retinal pigment
epithelial cells, but also upregulates its mRNA and protein levels
(45). Furthermore,
integrin-mediated TGF-β activity could promote enhanced collagen
deposition in epithelial cells (46). In the present study, electrical
stimulation reversed the reduction of the ECM in the grade II
group. However, this was only effective when electrical stimulation
was combined with recombinant human integrin β1 in the grade III
group. Endogenous integrin β1 alone is not sufficient to improve
the expression of the ECM under conditions of electrical
stimulation. These data suggest that the lack of integrin β1 may be
a reason why electrical stimulation is ineffective at treating
severe SUI.
In conclusion, it has been shown that electrical
stimulation can improve collagen expression when integrin β1 levels
are sufficient via the integrin β1/TGF-β1 pathway, which may play
an important role in the treatment of SUI by electrical
stimulation. This work offers a mechanistic insight into SUI that
may facilitate therapeutic intervention and contribute to the
efficient prevention and treatment of SUI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81771562).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
YL was involved in project development, data
collection, analysis and manuscript writing. BSL, CL, SSH and JM
were involved with study development, supervision, manuscript
editing. MH, JMT and STL were involved with manuscript editing and
data analysis. TTW and HXZ were involved with project development,
data collection and analysis. LH was involved with project
development, manuscript editing and supervision.
Ethics approval and consent to
participate
The study was performed in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
Renmin Hospital of Wuhan University. The participants provided
written informed consent to participate in this study.
Patient consent for publication
Consent for publication was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Haylen BT, de Ridder D, Freeman RM, Swift
SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK and
Schaer GN: An International Urogynecological Association
(IUGA)/International Continence Society (ICS) joint report on the
terminology for female pelvic floor dysfunction. Int Urogynecol J.
21:5–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson L, Brown JS, Shin GP, Luc KO and
Subak LL: Annual direct cost of urinary incontinence. Obstet
Gynecol. 98:398–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sievert KD, Amend B, Toomey PA, Robinson
D, Milsom I, Koelbl H, Abrams P, Cardozo L, Wein A, Smith AL and
Newman DK: Can we prevent incontinence? ICI-RS 2011. Neurourol
Urodyn. 31:390–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dolan LM, Walsh D, Hamilton S, Marshall K,
Thompson K and Ashe RG: A study of quality of life in primigravidae
with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct.
15:160–164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hannestad YS, Rortveit G, Sandvik H and
Hunskaar S; Norwegian EPINCONT study. Epidemiology of Incontinence
in the County of Nord-Trøndelag, : A community-based
epidemiological survey of female urinary incontinence: The
Norwegian EPINCONT study. Epidemiology of Incontinence in the
County of Nord-Trondelag. J Clin Epidemiol. 53:1150–1157. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Norton P and Brubaker L: Urinary
incontinence in women. Lancet. 367:57–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abrams P, Andersson KE, Birder L, Brubaker
L, Cardozo L, Chapple C, Cottenden A, Davila W, de Ridder D,
Dmochowski R, et al: Fourth International Consultation on
Incontinence Recommendations of the International Scientific
Committee: Evaluation and treatment of urinary incontinence, pelvic
organ prolapse, and fecal incontinence. Neurourol Urodyn.
29:213–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leach GE, Dmochowski RR, Appell RA,
Blaivas JG, Hadley HR, Luber KM, Mostwin JL, O'Donnell PD and
Roehrborn CG: Female stress urinary incontinence clinical
guidelines panel summary report on surgical management of female
stress urinary incontinence. The American Urological Association. J
Urol. 158:875–880. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oliphant SS, Wang L, Bunker CH and Lowder
JL: Trends in stress urinary incontinence inpatient procedures in
the United States, 1979–2004. Am J Obstet Gynecol. 200:521.e1–e6.
2009. View Article : Google Scholar
|
|
10
|
Monga AK, Tracey MR and Subbaroyan J: A
systematic review of clinical studies of electrical stimulation for
treatment of lower urinary tract dysfunction. Int Urogynecol J.
23:993–1005. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt AP, Sanches PR, Silva DP Jr, Ramos
JG and Nohama P: A new pelvic muscle trainer for the treatment of
urinary incontinence. Int J Gynaecol Obstet. 105:218–222. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terlikowski R, Dobrzycka B, Kinalski M,
Kuryliszyn-Moskal A and Terlikowski SJ: Transvaginal electrical
stimulation with surface-EMG biofeedback in managing stress urinary
incontinence in women of premenopausal age: A double-blind,
placebo-controlled, randomized clinical trial. Int Urogynecol J.
24:1631–1638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shamliyan T, Wyman J and Kane RL:
Nonsurgical treatments for urinary incontinence in adult women:
Diagnosis and comparative effectiveness [Internet]. Rockville (MD):
Agency for Healthcare Research and Quality (US); 2012,
|
|
14
|
Richmond CF, Martin DK, Yip SO, Dick MA
and Erekson EA: Effect of supervised pelvic floor biofeedback and
electrical stimulation in women with mixed and stress urinary
incontinence. Female Pelvic Med Reconstr Surg. 22:324–327. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Min J, Li B, Liu C, Hong S, Tang J, Hu M,
Liu Y, Li S and Hong L: Therapeutic effect and mechanism of
electrical stimulation in female stress urinary incontinence.
Urology. 104:45–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang J, Li B, Liu C, Li Y, Li Q, Wang L,
Min J, Hu M, Hong S and Hong L: Mechanism of mechanical
trauma-induced extracellular matrix remodeling of fibroblasts in
association with Nrf2/ARE signaling suppression mediating
TGF-β1/Smad3 signaling inhibition. Oxid Med Cell Longev.
2017:85243532017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ingelman-Sundberg A and Ulmsten U:
Surgical treatment of female urinary stress incontinence. Contrib
Gynecol Obstet. 10:51–69. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YT, Jiang JY and Han JS: A review of
the pelvic organ prolapse quantification system in China. Int
Urogynecol J. 27:287–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Hong L, Liu C, Min J, Hong S, Hu M,
Zhao Y, Yang Q, Tang J and He S: Effect of puerarin on collagen
metabolism of fibroblasts in pelvic tissue of women with pelvic
organ prolapse. Mol Med Rep. 17:2705–2711. 2018.PubMed/NCBI
|
|
20
|
Song B, Gu Y, Pu J, Reid B, Zhao Z and
Zhao M: Application of direct current electric fields to cells and
tissues in vitro and modulation of wound electric field in vivo.
Nat Protoc. 2:1479–1489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong S, Li H, Wu D, Li B, Liu C, Guo W,
Min J, Hu M, Zhao Y and Yang Q: Oxidative damage to human
parametrial ligament fibroblasts induced by mechanical stress. Mol
Med Rep. 12:5342–5348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arous C and Wehrle-Haller B: Role and
impact of the extracellular matrix on integrin-mediated pancreatic
β-cell functions. Biol Cell. 109:223–237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen HM, Lin YH, Cheng YM, Wing LC and
Tsai SJ: Overexpression of integrin-β1 in leiomyoma promotes cell
spreading and proliferation. J Clin Endocrinol Metab. 98:E837–E846.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayashida T, Jones JC, Lee CK and Schnaper
HW: Loss of beta1-integrin enhances TGF-beta1-induced collagen
expression in epithelial cells via increased alphavbeta3-integrin
and Rac1 activity. J Biol Chem. 285:30741–30751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Girgert R, Martin M, Kruegel J, Miosge N,
Temme J, Eckes B, Muller GA and Gross O: Integrin α2-deficient mice
provide insights into specific functions of collagen receptors in
the kidney. Fibrogenesis Tissue Repair. 3:192010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu B, Nicholls M, Gu Y, Zhang G, Zhao C,
Franklin RJ and Song B: Electric signals regulate the directional
migration of oligodendrocyte progenitor cells (OPCs) via β1
integrin. Int J Mol Sci. 17:2016. View Article : Google Scholar
|
|
28
|
Ashrafi M, Alonso-Rasgado T, Baguneid M
and Bayat A: The efficacy of electrical stimulation in
experimentally induced cutaneous wounds in animals. Vet Dermatol.
27:235–e57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim TH, Cho HY and Lee SM: High-voltage
pulsed current stimulation enhances wound healing in diabetic rats
by restoring the expression of collagen, α-smooth muscle actin, and
TGF-β1. Tohoku J Exp Med. 234:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brighton CT, Wang W, Seldes R, Zhang G and
Pollack SR: Signal transduction in electrically stimulated bone
cells. J Bone Joint Surg Am 83-A. 1514–1523. 2001. View Article : Google Scholar
|
|
31
|
Spadari GS, Zaniboni E, Vedovello SA,
Santamaria MP, do Amaral ME, Dos Santos GM, Esquisatto MA, Mendonca
FA and Santamaria M Jr: Electrical stimulation enhances tissue
reorganization during orthodontic tooth movement in rats. Clin Oral
Investig. 21:111–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Rouabhia M, Lavertu D and Zhang Z:
Pulsed electrical stimulation modulates fibroblasts' behaviour
through the Smad signalling pathway. J Tissue Eng Regen Med.
11:1110–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wein AJ: Re: A model for predicting the
risk of de novo stress urinary incontinence in women undergoing
pelvic organ prolapse surgery. J Urol. 194:4702015. View Article : Google Scholar
|
|
34
|
Falconer C, Blomgren B, Johansson O,
Ulmsten U, Malmström A, Westergren-Thorsson G and Ekman-Ordeberg G:
Different organization of collagen fibrils in stress-incontinent
women of fertile age. Acta Obstet Gynecol Scand. 77:87–94. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen B, Wen Y, Yu X and Polan ML: Elastin
metabolism in pelvic tissues: Is it modulated by reproductive
hormones? Am J Obstet Gynecol. 192:1605–1613. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao E, Lei YH, Shang X, Huang ZM, Zuo L,
Boucher M, Fan Q, Chuprun JK, Ma XL and Koch WJ: A novel and
efficient model of coronary artery ligation and myocardial
infarction in the mouse. Circ Res. 107:1445–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sassoli C, Chellini F, Pini A, Tani A,
Nistri S, Nosi D, Zecchi-Orlandini S, Bani D and Formigli L:
Relaxin prevents cardiac fibroblast-myofibroblast transition via
notch-1-mediated inhibition of TGF-β/Smad3 signaling. PLoS One.
8:e638962013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Wang S, Wu S, Hao Q, Li Y, Guo Z
and Wang W: Exosomes secreted by adipose-derived mesenchymal stem
cells regulate type I collagen metabolism in fibroblasts from women
with stress urinary incontinence. Stem Cell Res Ther. 9:1592018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han L, Wang L, Wang Q, Li H and Zang H:
Association between pelvic organ prolapse and stress urinary
incontinence with collagen. Exp Ther Med. 7:1337–1341. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Liu J, Zeng J, Zeng C and Zhou Y:
Expression of TβR-2, Smad3 and Smad7 in the vaginal anterior wall
of postpartum rats with stress urinary incontinence. Arch Gynecol
Obstet. 291:869–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li GY, Cui WS, Zhou F, Gao ZZ, Xin H, Liu
T, Li WR, Gong YQ, Bai GY, Guo YL and Xin ZC: Pathology of urethral
fibromuscular system related to parturition-induced stress urinary
incontinence and TGF-β1/Smad pathway. Mol Cell Biochem.
364:329–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Border WA and Noble NA: Transforming
growth factor beta in tissue fibrosis. N Engl J Med. 331:1286–1292.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Smerling C, Tang K, Hofmann W and Danker
K: Role of the alpha(1) integrin cytoplasmic tail in the formation
of focal complexes, actin organization, and in the control of cell
migration. Exp Cell Res. 313:3153–3165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kwon HJ, Lee GS and Chun H: Electrical
stimulation drives chondrogenesis of mesenchymal stem cells in the
absence of exogenous growth factors. Sci Rep. 6:393022016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han J, Yan XL, Han QH, Li YJ, Du ZJ and
Hui YN: Integrin β1 subunit signaling is involved in the directed
migration of human retinal pigment epithelial cells following
electric field stimulation. Ophthalmic Res. 45:15–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jolly L, Stavrou A, Vanderstoken G,
Meliopoulos VA, Habgood A, Tatler AL, Porte J, Knox A, Weinreb P,
Violette S, et al: Influenza promotes collagen deposition via αvβ6
integrin-mediated transforming growth factor β activation. J Biol
Chem. 289:35246–35263. 2014. View Article : Google Scholar : PubMed/NCBI
|