Introduction
With the rapid development of science and
technology, as well as strong social competition, the nature of
psychological stress has changed significantly (1). Stress is a common risk factor for
75–90% of diseases, including cardiovascular diseases, associated
with the highest morbidity and mortality (2). Accumulating evidence has demonstrated
that severe or chronic stress results in an increased risk for
physical and psychiatric disorders, which is referred to as
stress-associated disease. In stress animal models, accumulation of
oxidative stress has been noted (3). Halliwell (4) reported that overproduction of
reactive oxygen species (ROS) can damage cellular components and
induce functional abnormalities in numerous cell types. Chronic
stress modifies the expression of the genes regulating antioxidant
system and NADPH oxidase (Nox) (5). Furthermore, Nox has been reported to
be a major source of ROS production in different cell types
(6), while experiments from rodent
models suggested that increased levels of ROS in vitro and
in the brain can result in downregulation of various antioxidant
enzymes (7).
Stress aggravates typical clinical symptoms, such as
heartburn in patients with gastroesophageal reflux disease (GERD),
while it also affects visceral sensitivity (8). However, the mechanism underlying
visceral hypersensitivity (VH) in functional gastrointestinal
disorders is not completely understood. The involvement of stress
in VH, specifically in esophageal hypersensitivity, has also been
investigated. Fass et al (9) reported that GERD patients experienced
severe heartburn when exposed to acute auditory stress, while
stress caused by disturbed sleep has also been demonstrated to
cause similar esophageal hypersensitivity (10). Another study indicated that
psychological factors, such as stress, influence VH in humans
(11).
Two main receptors have been reported to contribute
to the mechanism underlying esophageal hypersensitivity, namely
proteinase-activated receptor 2 (PAR-2) and transient receptor
potential channel vanilloid 1 (TRPV-1) (12,13).
A previous study revealed that acid exposure can increase the
expression of PAR-2 on esophageal epithelial cells (14). The role of PAR-2 in the
pathogenesis of GERD has also been assessed. PAR-2 is activated by
mast cell tryptase and trypsin throughout the entire
gastrointestinal tract under physiologic or pathophysiologic
conditions (15). In vitro
experiments of esophageal squamous cell lines demonstrated that
PAR-2 expression was induced by exposure to acidic and weakly
acidic solutions (16), and PAR-2
activation mediated VH and pain (17). In addition to PAR-2, acid-sensitive
receptors, such as TRPV-1, are accountable for one of the important
mechanisms that are involved in peripheral esophageal
hypersensitivity. A study involving TRPV-1 knockout mice revealed
that TRPV-1 is a key receptor that responds to mechanical or acid
irritation, as well as thermal stimulation (18). Several studies have also
demonstrated the existence of neural fibers with TRPV-1 expression
in the esophageal mucosa, as well as increased mRNA and protein
expression in the esophageal mucosa of patients with reflux
esophagitis and non-erosive reflux disease, suggesting the
involvement of TRPV-1 in the mechanism underlying esophageal
hypersensitivity (19,20).
Therefore, in the present study, the aim was to
determine whether chronic stress evokes esophageal inflammation,
ROS production and VH in a murine model.
Materials and methods
Experimental animals
In total, 20 male C57BL/6J mice (8-week-old) were
obtained from the Animal Center of Xinjiang Medical University
(Urumqi, China). Animals were housed (one per cage) under standard
conditions of 21–25°C and 50±5% humidity with a 12/12 h light/dark
cycle (lights on at 8:30 a.m.) in a specific-pathogen-free facility
in the Research Institute of Uygur Pharmaceutics (Urumqi, China).
All mice were provided tap water and standard chow diet ad
libitum (18% fat, 24% protein, 58% carbohydrates). The study
protocol was approved by the Animal Care and Use Committee of the
People's Hospital of Xinjiang Uygur Autonomous Region (protocol no.
KY201803703; Urumqi, China). The study was completed based on the
Guidelines for the Care and Use of Laboratory Animals published by
the National Institutes of Health.
Restraint stress protocol
The mice were randomly divided into the control
(n=10) and chronic restraint stress groups (CRS, n=10). Each of the
control mice were left undisturbed and remained in a single cage,
while stressed mice were isolated in individual cages and subjected
to immobilization stress for 2 h per day (6 days per week, between
10:00 a.m. and 12:00 p.m.) over a period of 14 consecutive days, as
described in detail previously (21,22).
Body weight and food intake were monitored every 2 days during the
stress period.
Sample collection
In the morning following the last restraint stress,
all mice underwent a 16 to 18-h fasting period, anesthetized by
intraperitoneal injection of sodium pentobarbital (150 mg/kg) and
then euthanized. Subsequent to euthanasia, blood samples were
collected from the inferior vena cava, and stored at −80°C.
Esophageal samples were also collected under aseptic conditions and
stored in RNase-free tubes at −80°C for extraction of total RNA,
analysis of biological marker expression levels and
histopathological examination.
Histopathological assay
The esophageal tissues of all mice were excised,
weighed, fixed with 10% formalin, dehydrated at room temperature by
ethanol series and then embedded in paraffin. Next, tissues were
cut into 5-µm sections and stained with hematoxylin and eosin
(H&E) or Masson's trichrome (MT) for the evaluation of
esophageal inflammation and fibrosis, respectively. Images of
H&E and MT staining were obtained from 10 different, randomly
selected microscopic fields per section under a light microscope at
a magnification of ×200 using a digital camera (Eclipse E200; Nikon
Corporation, Tokyo, Japan). The extent of stress-induced
inflammatory damage was evaluated by histologic scoring performed
by an investigator who was blinded to the group. For statistical
analysis, three independent measurements for each specimen were
performed. The histologic findings were scored in terms of the
epithelial damage, submucosal edema and submucosal inflammatory
cells observed (23). The
epithelial damage scores were assigned as follows: 0, normal
morphology; 1, mild surface lifting; 2, intraepithelial separation
and surface lifting; and 3, epithelial cell loss to basal cell
layer or deeper. Submucosal edema was scored as follows: 0, normal
tissue; 1, mild focal edema; 2, moderate diffuse edema; and 3,
severe edema. Finally, the score for submucosal inflammatory cells
was as follows: 0, 0–5 cells/high-power field (HPF); 1, 5–10
cells/HPF; 2, 10–15 cells/HPF; and 3, ≥15 cells/HPF. MT-positive
areas, including the mucosal and epithelial layers, were analyzed
by Adobe Photoshop (Adobe Inc., Mountain View, CA, USA) and
quantified in 10 random fields-of-view per section using NIH ImageJ
version 1.62 software (National Institutes of Health, Bethesda, MD,
USA).
Immunohistochemical assay
Immunohistochemistry was performed according to the
streptavidin-biotin complex method, as described previously
(22,24). Briefly, esophageal sections were
deparaffinized with xylene and dehydrated with ethanol. All
sections were incubated overnight at 4°C with primary antibodies,
including anti-Nox-4 (1:100; cat. no. ab133303; Abcam, Cambridge,
UK), anti-TRPV-1 (1:100; cat. no. NBP1-71774; Novus Biologicals,
Ltd., Cambridge, UK); and anti-PAR-2 (1:100; cat. no. ab180953;
Abcam). The localization of Nox-4, TRPV-1 and PAR-2 was visualized
using 3,3-diaminobenzidine tetrahydrochloride (DAB tablet; Merck
KGaA, Darmstadt, Germany) at a concentration of 30 mg/ml containing
0.03% H2O2. Then, sections were
counterstained in 2% methylene green for 12 min at room
temperature. Sections were dehydrated in descending ethanol series,
washed with xylene for 5 min at room temperature three times, and
mounted in mounting media (Mount-quick; Daido Sangyo Co., Ltd.,
Kawasaki, Japan). Images of all sections were captured under a
light microscope (magnification, ×200) with a digital camera
(Eclipse E200; Nikon Corporation).
Enzyme linked immunosorbent assays
(ELISA)
Plasma samples of all mice were obtained and
properly processed according to the protocols, as described
previously (21,22). Plasma levels of Nox-4, interleukin
(IL)-6, IL-8, interferon-γ (IFN-γ) and tumor necrosis factor-α
(TNF-α) were determined with a competitive ELISA kit (R&D
Systems, Inc., Minneapolis, MN, USA) according to the protocol
provided by the manufacturer.
Cell culture
Normal human esophageal epithelial cells (HEECs)
were purchased from The American Type Culture Collection (cat. no.
ATCC CRL-2629; Manassas, VA, USA), and maintained in keratinocyte
serum-free medium, purchased from Gibco (Thermo Fisher Scientific,
Inc.), containing 10% FBS supplemented with 100 U/ml penicillin and
100 mg/ml streptomycin under standard cell culture conditions of
37°C and a humidified atmosphere with 5% CO2. The medium
was replaced every three days until confluence was reached. Cells
between passages 2 to 5 were used in all cell culture
experiments.
HEEC treatment, and preparation for
Nox-4, PAR-2 and TRPV-1 expression determination
HEECs were seeded at a density of 5×103
cells/well in 96-well plate in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) for 48 h. HEECs were serum-starved for 24 h, and
were subsequently were stimulated with trypsin (0.05 and 0.1 nM),
which served as an endogenous PAR-2 agonist, for 0, 2, 4 and 8 h.
Next, cells were lysed in 200 µl cell lysis buffer, and the lysate
was harvested using a cell scraper and centrifugation for 15 min at
12,000 × g and at 4°C. Finally, the supernatant was collected, and
used to determine the mRNA expression levels of Nox-4, PAR-2 and
TRPV-1 by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR).
Effect of PAR-2 antibody on Nox-4,
TRPV-1, IL-6, IL-8, IFN-γ and TNF-α expression levels
The role of PAR-2 on Nox-4, TRPV-1, IL-6, IL-8,
IFN-γ and TNF-α production by HEECs was investigated using a
blocking antibody against the amino-terminal cleavage region of
PAR-2 (SAM11; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Briefly, HEECs were grown to confluence at a density of
5×105 cells/well on 96-well culture plate. HEECs were
serum-starved for 24 h, and were subsequently treated with or
without anti-PAR-2 blocking antibody (1:20,000) for 2 h, followed
by incubation with trypsin (0.1 nM) for 4 h. Subsequently, culture
supernatants were centrifuged for 15 min at 12,000 × g and at 4°C.
Supernatants were treated with 0.05% trypsin and maintained at 37°C
with 5% CO2 for 5 min. The mRNA expression levels of the
genes of interest were measured by RT-qPCR.
Transfection with small interfering
RNA (siRNA) targeting TRPV-1
siRNA specific to TRPV-1 was purchased from Santa
Cruz Biotechnology, Inc., and Lipofectamine RNA iMAX (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
transfect the siRNA into HEECs according to the manufacturer's
protocol. Briefly, cells were seeded at a density of
5×105 cells/well on a 6-well culture plate in
keratinocyte serum-free medium containing 10% FBS, and grown to
confluence. HEECs were incubated with control siRNA or TRPV-1 siRNA
(20 nmol/l). The siRNAs were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Cells were incubated for 48
h prior to trypsin treatment (0.1 nM) for 4 h. Subsequently,
culture supernatants were harvested, and the mRNA expression levels
of the aforementioned proteins were measured by RT-qPCR.
Western blot analysis
HEECs were lysed in lysis buffer [containing 65
mmol/l Tris-HCl (pH 6.8), 3.3% sodium dodecyl sulfate (SDS), 10%
glycerol and 2.2% bromophenol blue], and the supernatant was
collected and stored at −80°C. The protein concentration was
determined using the bicinchoninic acid protein assay kit. Next,
proteins were fractionated by SDS-polyacrylamide gel
electrophoresis and then transferred to polyvinylidene difluoride
membranes (Immobilon-P; EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 5% bovine serum albumin (BSA) in
Tris-buffered saline Tween-20 (TBS-T) at room temperature for 1 h.
Subsequent to washing with TBS-T, the membranes were treated with
mouse polyclonal anti-TRPV-1 antibody (1:1,000; cat. no.
NBP1-71774; Novus Biologicals, Ltd., Cambridge, UK) and β-actin
(1:1,000; cat. no. 3700; Cell Signaling Technology, Inc., Danvers,
MA, USA). The membranes were further incubated with HRP-linked
secondary antibody (1:10,000; cat. no. 7076; Cell Signaling
Technology, Inc.) at room temperature for 1 h. Following washing
with TBS-T three times, the protein expression levels were
visualized using the Chemi-Lumi One enhanced chemiluminescence
system (Nacalai Tesque, Inc., Kyoto, Japan).
RT-qPCR
Total RNA was extracted from the esophageal tissues
of mice and HEECs using TRIzol reagent (Thermo Fisher Scientific,
Inc.) and subjected to FastLane Cell cDNA Kit (Qiagen GmbH, Hilden,
Germany). Total RNA (1 µg) was reverse transcribed to cDNA
with oligo (dT) primers according to the manufacturer's protocol
(Qiagen GmbH). The obtained cDNA was subjected to qPCR analysis.
The thermocycling conditions were as follows: Initial denaturation
at 95°C for 2 min, followed by 40 cycles of 12 sec at 95°C and 60
sec at 60°C, using the Bio-Rad CFX96 Touch Real-Time PCR detection
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and Power
SYBR Green PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Serial dilutions of a control sample of cDNA
were used as the standard curve for each reaction. All experiments
were performed in triplicate. Changes in gene expression were
calculated by the 2−ΔΔCq method (25), and the values were normalized to
the levels of β-actin and GAPDH. The primer sequences used in the
present study are listed in Tables
I and II. The amount of each
mRNA was normalized to β-actin for the RNA extracted from mice
tissues and GAPDH for the RNA extracted from HEECs,
respectively.
 | Table I.Sequences of mouse primers used in
the present study. |
Table I.
Sequences of mouse primers used in
the present study.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) | Size |
|---|
| Nox-4 |
TGTTGGGCCTAGGATTGTGTT |
AGGGACCTTCTGTGATCCTCG | 125 |
| Cu/Zn-SOD |
CAGCATGGGTTCCACGTCCA |
CACATTGGCCACACCGTCCT | 168 |
| MnSOD |
CACATTAACGCGCAGATCATG |
CCAGAGCCTCGTGGTACTTCTC | 100 |
| GPx |
GGGCAAGGTGCTGCTCATTG |
AGAGCGGGTGAGCCTTCTCA | 269 |
| Catalase |
CCAGCGACCAGATGAAGCAG |
CCACTCTCTCAGGAATCCGC | 198 |
| TRPV-1 |
AGCCATGCTCAATCTGCAC |
TGCTGTCTGGCCCTTGTAG | 133 |
| PAR-2 |
CAAGGTGCTCATTGGCTTTT |
CAGAGGGCGACAAGGTAGAG | 549 |
| IL-6 |
CCAGAGATACAAAGAAATGATGG |
ACTCCAGAAGACCAGAGGAAAT | 88 |
| IL-8 |
CGGCAATGAAGCTTCTGTAT |
CCTTGAAACTCTTTGCCTCA | 224 |
| IFN-γ |
ACACTGCATCTTGGCTTTGC |
GCTTTCAATGACTGTGCCGT | 76 |
| TNF-α |
AGGCTGCCCCGACTACGT |
GACTTTCTCCTGGTATGAGATAGCAA | 70 |
| β-actin |
TATTGGCAACGAGCGGTTC |
ATGCCACAGGATTCCATACCC | 75 |
 | Table II.Sequences of human primers used in
the present study. |
Table II.
Sequences of human primers used in
the present study.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) | Size |
|---|
| Nox-4 |
CTCAGCGGAATCAATCAGCTGTG |
AGAGGAACACGACAATCAGCCTTAG | 286 |
| PAR-2 |
GTTGATGGCACATCCCACGTC |
GTACAGGGCATAGACATGGC | 660 |
| TRPV-1 |
GGCTGTCTTCATCATCCTGCTGCT |
GTTCTTGCTCTCCTGTGCGATCTTGT | 118 |
| IL-6 |
GACAGCCACTCACCTCTTCA |
CCTCTTTGCTGCTTTCACAC | 120 |
| IL-8 |
TCTGCAGCTCTGTGTGAAGGTG |
AATTTCTGTGTTGGCGCAGTG | 153 |
| IFN-γ |
GACCAGAGCATCCAAAAGAGT |
ATTGCTTTGCGTTGGACATTC | 143 |
| TNF-α |
TTGAGGGTTTGCTACAACATGGG |
GCTGCACTTTGGAGTGATCG | 142 |
| GAPDH |
TGCACCACCAACTGCTTAGC |
GGCATGGACTGTGGTCATGAG | 87 |
Statistical analysis
All data are expressed in terms of the mean ±
standard deviation. The quantitative analysis of histological
damage score, MT staining and body weight gain was conducted by
Student's t-test. The mRNA and plasma levels of Nox-4, antioxidant
enzymes, inflammatory cytokines, as well as TRPV-1 and PAR-2, were
also analyzed by Student's t-test. The mRNA levels of Nox-4, PAR-2
and TRPV-1 in cell culture experiments were analyzed by one-way
analysis of variance with Fisher's protected least significant
difference test. Differences between groups were considered as
statistically significant at P<0.05.
Results
Stress-induced esophageal inflammation
in mice
In the current study, 8-week-old male C57BL/6J mice
were subjected to restraint stress for 2 weeks to observe the
stress-induced inflammatory response in the esophagus. The
histopathological analysis by H&E staining revealed that
pathological changes in the esophagus were not observed in the
control group. In the esophagus of the CRS group, however, mild
infiltration of inflammatory cells in the submucosa, basal cell
hyperproliferation, papillary hypertrophy, epithelial
hyperkeratinization, squamous cell expansion and an increase in the
number of fibrin cells were observed (Fig. 1A). The mucosal damage scores in the
esophageal tissue of mice are displayed in Fig. 1B. Compared with the control group,
the histological damage score in the CRS group was markedly
increased (0.38±0.09 in the control vs. 1.23±0.05 in the CRS group;
P<0.01). These results were consistent with the typical
histological findings associated with low-grade reflux esophagitis
(26). In addition, the MT
staining results revealed that stress increased esophageal
interstitial fibrosis as compared with the control mice (Fig. 1C and D).
 | Figure 1.Stress-induced esophageal
inflammation in mice. Mice were subjected to restraint stress by
immobilization for 2 h/day for 2 weeks. Subsequently, esophageal
tissues were collected from CRS and control (non-stressed) mice.
(A) Hematoxylin and eosin staining, showing the accumulation of
mononuclear cells in esophageal tissue from CRS mice
(magnification, ×200; scale bar, 50 µm); (B) Histopathological
damage scores; (C) MT staining (magnification, ×200; scale bar, 50
µm) and (D) MT-positive fibrotic area in esophageal tissue,
indicating interstitial fibrosis (mucosal and epithelial layer) in
CRS mice. Differences between groups were analyzed by the Student's
t-test, and data are expressed as the mean ± standard deviation
(n=10). **P<0.01 vs. control mice. MT, Masson's trichrome; CRS,
chronic restraint stress. |
Stress-induced body weight loss in
mice
The body weight of mice was monitored and measured
during the 2-week stress period. Body weight gain was significantly
reduced in CRS mice as compared with that in the non-stressed
control mice (Table III). Each
group of mice consumed similar amounts of food (~130 mg/g/day;
P>0.05).
 | Table III.Stress-induced weight loss in
mice. |
Table III.
Stress-induced weight loss in
mice.
| Parameter | Control | CRS | P-value |
|---|
| Body weight gain
(g) |
1.37±0.02 |
1.04±0.04 | <0.001 |
| Food intake
(mg) | 133.5±2.46 | 130.7±2.46 |
0.441 |
Stress-induced ROS generation in the
esophagi of mice
It has previously been reported that chronic stress
evokes ROS production in adipose tissue and the colon (22,24).
To determine whether stress induces ROS generation in the
esophagus, the current study analyzed the expression of Nox-4 in
mice by immunohistochemical analysis, RT-qPCR and ELISA. The data
revealed that 2 weeks of restraint stress resulted in an increase
in Nox-4 expression in the mucosal and epithelial layers of the
esophagus (Fig. 2A), significantly
elevated plasma levels of Nox-4 (Fig.
2B) and marked upregulation of Nox-4 mRNA expression (Fig. 2C), when compared with the levels in
control mice.
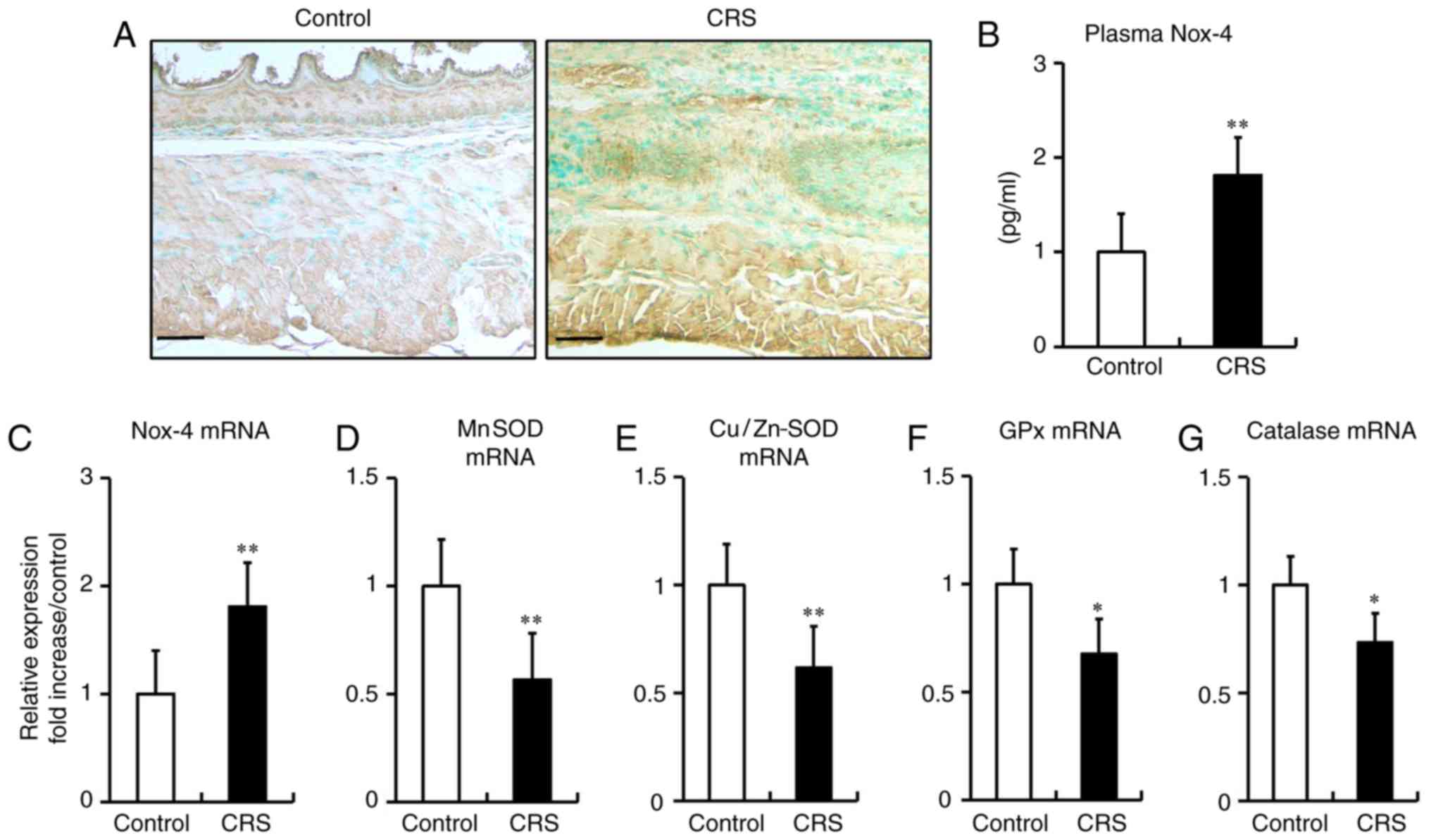 | Figure 2.Stress-induced reactive oxygen
species generation and reduction of antioxidant enzyme levels in
the esophagi of mice. (A) Immunohistochemical staining for Nox-4 in
esophageal tissue (magnification, ×200; scale bar, 50 µm). (B)
Plasma Nox-4 expression examined by ELISA (control, 0.98±0.14; CRS,
1.78±0.18). (C) Nox-4 mRNA, (D) MnSOD, (E) Cu/Zn-SOD, (F) GPx and
(G) catalase mRNA expression levels, assessed by reverse
transcription-quantitative polymerase chain reaction. Differences
between groups were analyzed by the Student's t-test, and data are
expressed as the mean ± standard deviation (n=10). *P<0.05,
**P<0.01 vs. control mice. CRS, chronic restraint stress; Nox-4,
NADPH oxidase 4; SOD, superoxide dismutase; GPx, glutathione
peroxidase. |
Stress reduces the antioxidant enzymes
in the esophagi of mice
Antioxidant enzymes serve an important role against
oxidative stress in various types of cells and tissues (22,24).
Herein, the expression levels of antioxidant enzymes were examined
using RT-qPCR. After a 2-week period of stress, the mRNA expression
levels of antioxidant enzymes, including Mn-superoxide dismutase
(MnSOD), Cu/Zn-SOD, glutathione peroxidase (GPx) and catalase, were
significantly reduced in stressed mice compared with those in
non-stressed control mice (Fig.
2D-G).
Stress increases the esophageal
expression of TRPV-1 and PAR-2
Earlier studies have demonstrated that TRPV-1 and
PAR-2 receptors regulate VH (12).
In the present study, the esophageal expression levels of TRPV-1
and PAR-2 were determined by immunohistochemistry and RT-qPCR. The
results revealed that TRPV-1 and PAR-2 were localized in the
luminal layers of the esophageal squamous epithelia of stressed
mice (Fig. 3A and B). Furthermore,
stress significantly increased the esophageal mRNA expression
levels of TRPV-1 and PAR-2 as compared with those in non-stressed
control mice (Fig. 3C and D).
Stress augments the expression of
inflammatory cytokines in mice
After 2 weeks of restraint stress, significant
increases were observed in the mRNA expression levels of IL-6,
IL-8, IFN-γ and TNF-α in the esophageal tissues of the CRS group,
as compared with those in the non-stressed control group (Fig. 4A, B, C and D). Furthermore, the
plasma concentration levels of IL-6, IL-8, IFN-γ and TNF-α were
significantly elevated in stressed mice, in parallel with the
changes in mRNA expression in the esophagus (Fig. 4E, F, G and H).
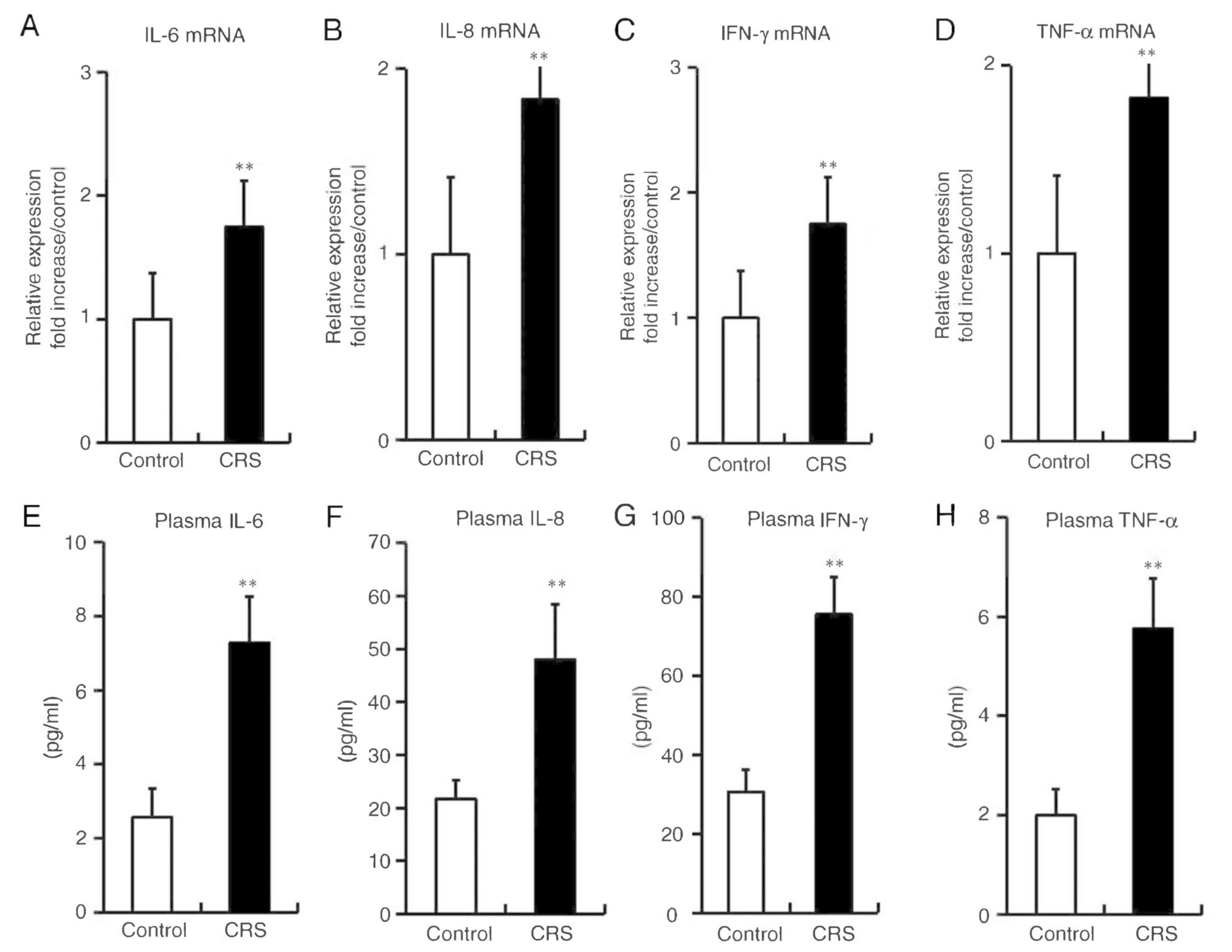 | Figure 4.Stress augmented the expression
levels of inflammatory cytokines in mice. (A) IL-6, (B) IL-8, (C)
IFN-γ and (D) TNF-α mRNA expression levels in esophageal tissues
were analyzed by reverse transcription-quantitative polymerase
chain reaction. (E) IL-6 (control, 2.57±0.77; CRS, 7.27±1.24), (F)
IL-8 (control, 21.68±3.59; CRS, 47.96±10.38), (G) IFN-γ (control,
30.65±5.67; CRS, 75.55±9.39) and (H) TNF-α (control, 2.01±0.53;
CRS, 5.75±1.02) plasma levels were analyzed by ELISA. Differences
between groups were analyzed by the Student's t-test, and data are
expressed as the mean ± standard deviation (n=10). **P<0.01 vs.
control mice. CRS, chronic restraint stress; IL, interleukin;
IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α. |
Expression levels of Nox-4, PAR-2 and
TRPV-1 in cultured HEECs
Reflux of proteases, including trypsin, has been
previously reported to cause damage to the esophageal mucosa
(16,18); however, the mechanism underlying
this effect remains unclear. In the present study, the effect of
trypsin on the expression levels of Nox-4, PAR-2 and TRPV-1 were
investigated. HEECs were incubated with trypsin at the indicated
concentrations and time periods. Nox-4, PAR-2 and TRPV-1 expression
levels were examined at 4 h after incubating with different
concentrations of trypsin (0, 0.05 and 0.1 nM), or at the different
time points (0, 2, 4 and 8) following incubation with trypsin (0.1
nM). It was observed that trypsin increased the mRNA expression
levels of Nox-4, PAR-2 and TRPV-1 in HEECs in a time- and
dose-dependent manner (Fig. 5A-F).
Trypsin at a concentration of 0.1 nM was used in subsequent in
vitro experiments.
Effects of PAR-2 blocking antibody on
Nox-4, TRPV-1, IL-6, IL-8, IFN-γ and TNF-α expression levels
As shown in Fig.
6A-F, the mRNA expression levels of Nox-4, TRPV-1, IL-6, IL-8,
IFN-γ and TNF-α were significantly increased when the cells were
stimulated with trypsin, serving as a natural PAR-2 agonist, for 4
h. Notably, pretreatment with a blocking antibody against PAR-2
resulted in significant inhibition of the mRNA expression of these
inflammatory cytokines in HEECs stimulated with trypsin.
 | Figure 6.Effects of PAR-2 blocking antibody on
Nox-4, TRPV-1, IL-6, IL-8, IFN-γ and TNF-α expression levels in
human esophageal epithelial cells. Serum-starved cells were
preincubated with PAR-2 blocking antibody (20 µg/ml) for 2 h, and
then stimulated by trypsin (0.1 nM) for 4 h. (A) Nox-4, (B) TRPV-1,
(C) IL-6, (D) IL-8, (E) IFN-γ and (F) TNF-α mRNA expression levels
were determined using reverse transcription-quantitative polymerase
chain reaction. Data are expressed as the mean ± standard deviation
(n=5). Differences between groups were analyzed with one-way
analysis of variance, followed by Fisher's protected least
significant difference test. *P<0.5, **P<0.01 vs. control
group; #P<0.05 vs. trypsin-treated group. PAR-2,
protease-activated receptor 2; Nox-4, NADPH oxidase 4; TRPV-1,
transient receptor potential vanilloid 1; IL, interleukin; IFN-γ,
interferon-γ; TNF-α, tumor necrosis factor-α. |
Effects of TRPV-1 knockdown on PAR-2,
Nox-4, IL-6, IL-8, IFN-γ and TNF-α expression levels
The effects of TRPV-1 knockdown on the mRNA levels
of PAR-2, Nox-4 and inflammatory cytokines were examined in
cultured HEECs. HEECs were incubated with or without TRPV-1 siRNA
(20 nmol/l) for 48 h, followed by the stimulation with trypsin (0.1
nM) for 4 h. In cells without trypsin treatment, transfection with
siRNA specific to TRPV-1 suppressed the gene and protein expression
levels of TRPV-1 as compared with that of control-transfected HEECs
(Fig. 7A). The trypsin-treated
cells exhibited significantly increased mRNA expression levels of
Nox-4, PAR-2 and inflammatory cytokines, including IL-6, IL-8,
IFN-γ, and TNF-α, compared with the control group. Importantly,
knockdown of TRPV-1 significantly suppressed the mRNA expression
levels of Nox-4, PAR-2 and inflammatory cytokines (IL-6, IL-8,
IFN-γ, and TNF-α) compared with the trypsin-treated cells.
(Fig. 7B-G).
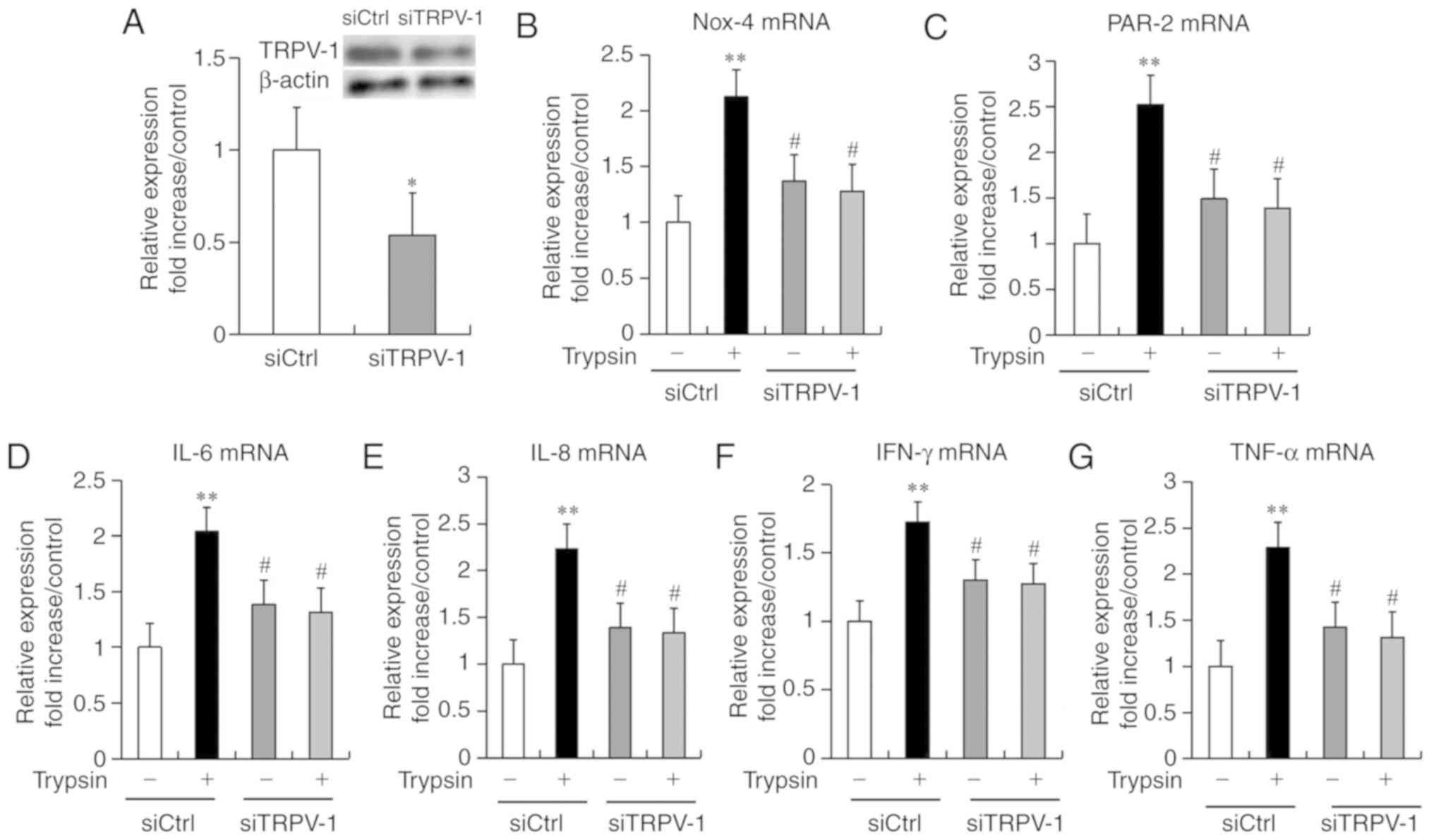 | Figure 7.Effects of siTRPV-1 on Nox-4, PAR-2,
IL-6, IL-8, IFN-γ and TNF-α expression in human esophageal
epithelial cells. Serum-starved cells were preincubated with or
without siTRPV-1 (20 nmol/l) for 48 h before stimulation with
trypsin (0.1 nM) for 4 h. (A) TRPV-1 expression levels in
transfected cells. (B) Nox-4, (C) PAR-2, (D) IL-6, (E) IL-8, (F)
IFN-γ and (G) TNF-α mRNA expression levels were determined using
reverse transcription-quantitative polymerase chain reaction. Data
are expressed as the mean ± standard deviation (n=5). Differences
between groups were analyzed with one-way analysis of variance,
followed by Fisher's protected least significant difference test.
*P<0.05; **P<0.01 vs. siCtrl untreated group;
#P<0.05 vs. siCtrl treated group. TRPV-1, transient
receptor potential vanilloid 1; siTRPV-1, TRPV-1 siRNA; siCtrl,
control siRNA; Nox-4, NADPH oxidase 4; PAR-2, protease-activated
receptor 2; IL, interleukin; IFN-γ, interferon-γ; TNF-α, tumor
necrosis factor-α. |
Collectively, the present findings suggested that
2-week stressed mice showed esophageal inflammation and fibrosis
via ROS accumulation and antioxidants suppression. Furthermore,
stress-activated PAR-2 and TRPV-1 were involved in ROS production
and inflammatory cytokine expression.
Discussion
The present study revealed that chronic restraint
stress conducted in mice over a 2-week period resulted in reduced
body weight gain, and increased esophageal inflammation and ROS
production. Chronic stress also suppressed the expression of genes
regulating the antioxidant system, such as SOD, catalase and GPx.
Furthermore, 2 weeks of stress elevated the levels of TRPV-1 and
PAR-2 receptor in the esophagi of mice, and significantly increased
the inflammatory cytokines in the blood circulation and esophageal
tissue.
Our previous study reported that 2 weeks of
restraint stress induced the shrinkage of inguinal fat pad, and
significant increase in lipolysis and free fatty acid (FFA)
concentration (21). In addition,
our previous results indicated that restraint stress increased FFAs
followed by lipolysis and evoked an inflammatory response in
adipose tissue of non-obese subjects without alterations in diet.
Similar findings were reported in the present study, which
demonstrated that stress reduced the body weight gain by lipolysis
and FFA release in mice, without changes observed in the food
intake.
In a rodent chronic stress model, Nox was reported
to be a key factor in stress-induced oxidative stress, and repeated
restraint stress promoted depression-like behavior through the
upregulation of Nox (27). The
membrane-bound multimeric Nox complex is an enzyme known to
generate ROS. The oxidase family is composed of five Nox members,
including Nox1-5, among which Nox-4 is present in the epithelium.
In the gastrointestinal tract, one of the main sources of ROS is
the Nox enzyme (28). The present
study indicated that the mRNA expression and plasma concentration
of Nox-4 were significantly increased in stressed mice (Fig. 2A-C), while the mRNA expression of
antioxidants, such as MnSOD, Cu/Zn-SOD, GPx and catalase, were
markedly reduced in stressed mice (Fig. 2D-G). Although stress-induced
expression of Nox enzymes (particularly Nox-4) in the esophagus has
rarely been studied, the results of the current study suggested
that Nox-4 may serve an important role in stress-related ROS
accumulation.
Pro- and anti-inflammatory mechanisms clearly depend
on the type and intensity of stressors. Thus far, it is been
reported that acute stressors enhance immune function, whereas
chronic stressors are suppressive (2). Peng et al (29) established a chronic stress model,
and reported that inflammatory cytokines, including TNF-α, IL-18
and IL-1β, were significantly increased in the experimental group
after a 4-week period of stress. Similarly, the current study also
demonstrated that the levels of mRNA expression and circulatory
concentrations of inflammatory cytokines (IL-6, IL-8, IFN-γ and
TNF-α) in the esophageal tissues and plasma of stressed mice were
significantly increased compared to non-stressed control mice
(Fig. 4A-H).
The current study also examined the gene expression
and epithelial localization of TRPV-1 and PAR-2 within the
esophageal mucosa of stressed mice. Compared with the control
group, stressed mice exhibited significantly increased expression
levels of TRPV-1 and PAR-2 (Fig.
3A-D). Subsequent in vitro experiments investigated the
role of PAR-2 activation by trypsin, a PAR-2 agonist. It was
observed that trypsin induced upregulation of Nox-4, PAR-2 and
TRPV-1 in HEECs in a time- and dose-dependent manner (Fig. 5A-F). In addition, the
trypsin-mediated production of inflammatory cytokines in HEECs was
significantly suppressed by pretreatment with a PAR-2 blocking
antibody. These results suggested that reflux of duodenal fluid
containing trypsin, whose release is induced by stress, can
increase ROS-dependent PAR-2, TRPV-1 and cytokine production in
esophageal epithelial cells, consequently leading to esophageal
inflammation (Fig. 6A-F).
To further investigate the role of TRPV-1 in
esophageal inflammation, the present study also measured the mRNA
levels of PAR-2, Nox-4 and inflammatory cytokines (IL-6, IL-8,
IFN-γ and TNF-α) in HEECs using TRPV-1 knockdown by siRNA. It was
observed that siRNA specific to TRPV-1 suppressed the mRNA and
protein expression of TRPV-1 in HEECs without trypsin stimulation
(Fig. 7A). It is known that TRPV-1
is activated by a number of agonists and endogenous chemical
mediators, such as anandamide or leukotrienes. Increased
temperature was a well-established pathological activator of TRPV-1
under certain conditions. In addition, local acidification
(pH<6.0) is also able to activate TRPV-1, and cause pain and
inflammation (18). The role of
TRPV-1 in pain sensation has been clearly demonstrated by the
advent of TRPV-1 knockout mice (30). TRPV-1−/− mice exhibited
impaired thermal sensitivity to pain when triggered by heat or
capsaicin. In addition, TRPV-1−/− mice exhibited
ameliorated edema, vasodilatation and inflammatory cell
infiltration caused by nociceptor overstimulation at the site of
neurogenic injury. In the present study, it was observed that
knockdown of TRPV-1 in cultured HEECs significantly suppressed the
mRNA expression levels of Nox-4, PAR-2 and inflammatory cytokines
(IL-6, IL-8, IFN-γ and TNF-α) (Fig.
7B-G). Taken together, these results indicated that trypsin,
whose release is induced by stress, markedly increased ROS
accumulation and the expression of inflammatory cytokines in HEECs
through a PAR-2-dependent and TRPV-1-dependent manner.
Previous studies have demonstrated that PAR-2 is
expressed in various types of cells and tissues, including the
esophagus (26,31), and is activated by trypsin and mast
cell tryptase, which serve an important role in the pathogenesis of
GERD (31), as well as esophageal
VH (32). Early studies reported
that activation of PAR-2 excites nociceptive neurons and induces
visceral hyperalgesia (33,34).
Amadesi et al (20)
investigated TRPV-1, a cation channel activated by capsaicin,
protons and noxious heat, which contributes to PAR-2-induced
hyperalgesia, and observed that PAR-2 sensitized TRPV-1 through a
PKC-dependent mechanism to cause sustained thermal hyperalgesia.
Similarly, the present study indicated that chronic stress may
result in esophageal hypersensitivity through the activation of
TRPV-1 and PAR-2, which are two essential receptors in the
pathogenesis of VH.
There is a possible mechanism associated with
PAR-induced dilated intercellular spaces (DIS), which are involved
in stress-induced VH. It has been demonstrated that increased PAR-2
expression was observed in all layers of the esophageal mucosa of
GERD patients, and served a potential role in increased epithelial
permeability and DIS, which in result induced PAR-2 expression in
superficial and deep layers of the esophageal squamous epithelium
(26). Consistent with these
findings, activation of PAR-2 has been reported to induce
epithelial barrier dysfunction and disruption of epithelial tight
junctions, leading to dilation of tight junctions and increased
trans-epithelial permeability (35,36).
It has also been suggested that TRPV-1 can be activated by weak
gastric acid (37). Therefore, in
the present study, it was hypothesized that increased epithelial
permeability and DIS induced by PAR-2 further caused activation of
TRPV-1 due to physiologic and/or pathologic gastroesophageal reflux
in mice.
In conclusion, although it is known that stress
induces overproduction of ROS and suppresses the expression of
antioxidants, studies involving the role of Nox-4 in stress-induced
ROS accumulation are limited. Therefore, the present study further
confirmed the notion of stress-induced disruption of
oxidant/antioxidant balance, and investigated the role of Nox-4 in
that process. Furthermore, recent studies have reported that stress
can affect esophageal hypersensitivity; however, the underlying
mechanisms remain unknown. In the present study, the possible
mechanism of stress-activated PAR-2 and TRPV-1 was investigated,
which may provide new insights on understanding the pathogenesis of
VH. However, a corollary study should be conducted in the future to
clearly elucidate the mechanisms underlying stress-induced
esophageal inflammation and VH.
Acknowledgements
The authors would like to thank Professor Zhonggao
Wang and Professor Jiande Chen for the careful reading and editing
of this manuscript.
Funding
Work in the group led by MY and KA was supported by
The Xinjiang Uygur Autonomous Region Natural Science Foundation
Program (grant no. 2018D01C134).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WW, MY, AiA, KT, AzA and KA contributed to the
conception and experimental design of the study, and interpretation
of the results. WW, MY, AiA, AlA, YL, YJ, MA and ZL conducted the
experiments and/or helped with data analysis. WW and MY wrote the
manuscript. WW, MY, AiA, KT, AzA and KA are responsible for the
integrity of the work as a whole. All authors revised the article
and approved the final version to be published.
Ethics approval and consent to
participate
The study protocol was approved by the Animal Care
and Use Committee of the People's Hospital of Xinjiang Uygur
Autonomous Region (protocol no. KY201803703; Urumqi, China). The
study was completed based on the Guidelines for the Care and Use of
Laboratory Animals published by the National Institutes of
Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Landsbergis PA: The changing organization
of work and the safety and health of working people: A commentary.
J Occup Environ Med. 45:61–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu YZ, Wang YX and Jiang CL:
Inflammation: The common pathway of stress-related diseases. Front
Hum Neurosci. 11:3162017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zafir A and Banu N: Modulation of in vivo
oxidative status by exogenous corticosterone and restraint stress
in rats. Stress. 12:167–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halliwell B: Oxidative stress and
neurodegeneration: Where are we now? J Neurochem. 97:1634–1658.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Linares V, Sánchez DJ, Bellés M, Albina L,
Gómez M and Domingo JL: Pro-oxidant effects in the brain of rats
concurrently exposed to uranium and stress. Toxicology. 236:82–91.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee KW, Kim JB, Seo JS, Kim TK, Im JY,
Baek IS, Kim KS, Lee JK and Han PL: Behavioral stress accelerates
plaque pathogenesis in the brain of Tg2576 mice via generation of
metabolic oxidative stress. J Neurochem. 108:165–175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondo T and Miwa H: The role of esophageal
hypersensitivity in functional heartburn. J Clin Gastroenterol.
51:571–578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fass R, Naliboff BD, Fass SS, Peleg N,
Wendel C, Malagon IB and Mayer EA: The effect of auditory stress on
perception of intraesophageal acid in patients with
gastroesophageal reflux disease. Gastroenterology. 134:696–705.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schey R, Dickman R, Parthasarathy S, Quan
SF, Wendel C, Merchant J, Powers J, Han B, van Handel D and Fass R:
Sleep deprivation is hyperalgesic in patients with gastroesophageal
reflux disease. Gastroenterology. 133:1787–1795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradley LA, Richter JE, Pulliam TJ, Haile
JM, Scarinci IC, Schan CA, Dalton CB and Salley AN: The
relationship between stress and symptoms of gastroesophageal
reflux: The influence of psychological factors. Am J Gastroenterol.
88:11–19. 1993.PubMed/NCBI
|
|
12
|
Altomare A, Luca Guarino Sara Emerenziani
MP, Cicala M, Drewes AM, Krarup AL, Brock C, Lottrup C, Frøkjaer
JB, Souza RF, Nardone G and Compare D: Gastrointestinal sensitivity
and gastroesophageal reflux disease. Ann N Y Acad Sci. 1300:80–95.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Souza RF: Bringing GERD management up to
PAR-2. Am J Gastroenterol. 105:1944–1946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kandulski A, Wex T, Mönkemüller K, Kuester
D, Fry LC, Roessner A and Malfertheiner P: Proteinase-activated
receptor-2 in the pathogenesis of gastroesophageal reflux disease.
Am J Gastroenterol. 105:1934–1943. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Brien PJ, Molino M, Kahn M and Brass LF:
Protease activated receptors: Theme and variations. Oncogene.
20:1570–1581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida N, Katada K, Handa O, Takagi T,
Kokura S, Naito Y, Mukaida N, Soma T, Shimada Y, Yoshikawa T and
Okanoue T: Interleukin-8 production via protease-activated receptor
2 in human esophageal epithelial cells. Int J Mol Med. 19:335–340.
2007.PubMed/NCBI
|
|
17
|
Vergnolle N, Bunnett NW, Sharkey KA,
Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI,
Andrade-Gordon P, et al: Proteinase-activated receptor-2 and
hyperalgesia: A novel pain pathway. Nat Med. 7:821–826. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bielefeldt K and Davis BM: Differential
effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation
in mice. Am J Physiol Gastrointest Liver Physiol. 294:G130–G138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matthews PJ, Aziz Q, Facer P, Davis JB,
Thompson DG and Anand P: Increased capsaicin receptor TRPV1 nerve
fibres in the inflamed human oesophagus. Eur J Gastroenterol
Hepatol. 16:897–902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amadesi S, Nie J, Vergnolle N, Cottrell
GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes
H, et al: Protease-activated receptor 2 sensitizes the capsaicin
receptor transient receptor potential vanilloid receptor 1 to
induce hyperalgesia. J Neurosci. 24:4300–4312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yisireyili M, Takeshita K, Hayashi M, Wu
H, Uchida Y, Yamamoto K, Kikuchi R, Hao CN, Nakayama T, Cheng XW,
et al: Dipeptidyl peptidase-IV inhibitor alogliptin improves
stress-induced insulin resistance and prothrombotic state in a
murine model. Psychoneuroendocrinology. 73:186–195. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yisireyili M, Hayashi M, Wu H, Uchida Y,
Yamamoto K, Kikuchi R, Shoaib Hamrah M, Nakayama T, Wu Cheng X,
Matsushita T, et al: Xanthine oxidase inhibition by febuxostat
attenuates stress-induced hyperuricemia, glucose dysmetabolism, and
prothrombotic state in mice. Sci Rep. 7:12662017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Kong L, Zhang S, Zhong Z, Liu X,
Wang J and Kang J: A novel external esophageal perfusion model for
reflux-associated respiratory symptoms. Pathobiology. 77:163–168.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yisireyili M, Uchida Y, Yamamoto K,
Nakayama T, Cheng XW, Matsushita T, Nakamura S, Murohara T and
Takeshita K: Angiotensin receptor blocker irbesartan reduces
stress-induced intestinal inflammation via AT1a signaling and
ACE2-dependent mechanism in mice. Brain Behav Immun. 69:167–179.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abd El-Rehim DM, Fath El-Bab HK and Kamal
EM: Expression of proteinase-activated receptor-2 in the esophageal
mucosa of gastroesophageal reflux disease patients: A
histomorphologic and immunohistochemical study. Appl
Immunohistochem Mol Morphol. 23:646–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seo JS, Park JY, Choi J, Kim TK, Shin JH,
Lee JK and Han PL: NADPH oxidase mediates depressive behavior
induced by chronic stress in mice. J Neurosci. 32:9690–9699. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aviello G and Knaus UG: ROS in
gastrointestinal inflammation: Rescue or sabotage? Br J Pharmacol.
174:1704–1718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng YL, Liu YN, Lei L, Wang X, Jiang CL
and Wang YX: Inducible nitric oxide synthase is involved in the
modulation of depressive behaviors induced by unpredictable chronic
mild stress. J Neuroinflammation. 9:752012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caterina MJ, Leffler A, Malmberg AB,
Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI
and Julius D: Impaired nociception and pain sensation in mice
lacking the capsaicin receptor. Science. 288:306–313. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inci K, Edebo A, Olbe L and Casselbrant A:
Expression of protease-activated-receptor 2 (PAR-2) in human
esophageal mucosa. Scand J Gastroenterol. 44:664–671. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu L, Oshima T, Shan J, Sei H, Tomita T,
Ohda Y, Fukui H, Watari J and Miwa H: PAR-2 activation enhances
weak acid-induced ATP release through TRPV1 and ASIC sensitization
in human esophageal epithelial cells. Am J Physiol Gastrointest
Liver Physiol. 309:G695–G702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoogerwerf WA, Zou L, Shenoy M, Sun D,
Micci MA, Lee-Hellmich H, Xiao SY, Winston JH and Pasricha PJ: The
proteinase-activated receptor 2 is involved in nociception. J
Neurosci. 21:9036–9042. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kirkup AJ, Jiang W, Bunnett NW and Grundy
D: Stimulation of proteinase-activated receptor 2 excites jejunal
afferent nerves in anaesthetised rats. J Physiol. 552:589–601.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Groschwitz KR, Wu D, Osterfeld H, Ahrens R
and Hogan SP: Chymase-mediated intestinal epithelial permeability
is regulated by a protease-activating receptor/matrix
metalloproteinase-2-dependent mechanism. Am J Physiol Gastrointest
Liver Physiol. 304:G479–G489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Enjoji S, Ohama T and Sato K: Regulation
of epithelial cell tight junctions by protease-activated receptor
2. J Vet Med Sci. 76:1225–1229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma J, Altomare A, Guarino M, Cicala M,
Rieder F, Fiocchi C, Li D, Cao W, Behar J, Biancani P and Harnett
KM: HCl-induced and ATP-dependent upregulation of TRPV1 receptor
expression and cytokine production by human esophageal epithelial
cells. Am J Physiol Gastrointest Liver Physiol. 303:G635–G645.
2012. View Article : Google Scholar : PubMed/NCBI
|





















