Introduction
Bladder cancer is one of the most common malignant
tumors of the genitourinary system worldwide (1). Researchers have identified that
75–85% of patients with bladder cancer have non-muscle-invasive
bladder cancer (NMIBC), while 15–25% of cases progressed to
muscle-invasive bladder cancer (MIBC) (1). At present, radical cystectomy with
urinary diversion is the standard treatment for patients with NMIBC
and MIBC (2); however, ~50% of
patients with MIBC develop metastatic disease, which is likely to
be fatal. The prognosis following recurrence after cystectomy is
poor (3). Therefore, in order to
gain a comprehensive understanding of the pathogenesis of bladder
cancer, the molecular mechanisms underlying the occurrence and
development of this disease must be identified. This may provide
insight into novel and effective treatment strategies for the
treatment of bladder cancer.
Feline sarcoma-related protein (Fer) is a unique Src
homology 2 non-receptor tyrosine kinase, which is expressed in
certain mammalian cell subpopulations, and resides in the cytoplasm
and nucleus (4). Fer is highly
expressed in numerous types of cancer, including lung (5), hepatic (6), prostate (7), breast (8) and bladder cancer (9). Previous studies have demonstrated
that Fer expression is associated with the proliferation of certain
cancer cell lines cultures; the poor prognosis of cancer has been
associated with increased Fer expression levels (10–12).
In addition, Fer is involved in the signaling downstream of the
receptor systems of cell proliferation and invasion in several cell
types (13). These studies
indicate a potential function of Fer in the progression of cancer;
however, the exact roles and underlying mechanisms of Fer in the
proliferation and apoptosis of bladder cancer remain to be fully
elucidated. To the best of our knowledge, the present study is the
first to determine the role of Fer in the viability and apoptosis
of bladder cancer cells.
In the present study, the effects of transfection of
bladder cancer cells with short interfering RNA against Fer
(Fer-siRNA) and Fer overexpression vector (Vector-Fer) on the
expression of Fer mRNA and protein. Furthermore, the underlying
mechanism of Fer in the proliferation and apoptosis of bladder
cancer cells was investigated. The results of this study
demonstrated that Fer serves a role in development of the bladder
cancer.
Materials and methods
Cell line culture and maintenance
Bladder cancer cell lines T24, 5637 and an
immortalized normal human epithelial cell line SV-HUC-1 were
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). T24 and 5637 cell lines were
cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C under 5%
CO2 and 95% humidified air. SV-HUC-1 cells were cultured
under an atmosphere of 5% CO2 at 37°C in F12k medium
(F12K; WISENT Inc., Saint-Jean-Baptiste, QC, Canada) with 10% fetal
bovine serum (PAA Laboratories; GE Healthcare, Chicago, IL, USA),
100 U/ml penicillin, and 100 lg/ml streptomycin.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). Total RNA was isolated from T24, 5637 and
SV-HUC-1 cells (2×106 cells/ml) by RNAiso Plus (Takara
Bio, Inc., Otsu, Japan), according to the manufacturer's protocols,
and 5 µg of each sample was reverse-transcribed using the M-MLV
First-strand Synthesis System (Promega Corporation, Madison, WI,
USA) as follows: 37°C for 25 min, followed by incubation at 85°C
for 5 sec in 20 µl of reaction volume. All reactions were performed
in triplicate using the MJ Real-Time PCR System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). qPCR was performed using
the Power SYBR Green Master Mix (Takara Bio, Inc.) and an ABI 7300
real-time PCR detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.), using the following primers (all primers were
synthesized by Invitrogen; Thermo Fisher Scientific, Inc.): Fer,
forward 5′-TTCGAGGGCACTGGGTTTTC-3′, reverse
5′-TTCCCTTGCCCAGTAATTCTCC-3′; GAPDH, forward
5′-GGTGAAGGTCGGAGTCAACGGA-3′, reverse 5′-GAGGGATCTCGCTCCTGGAAGA-3′;
GAPDH served as an internal control. qPCR was performed under the
following thermocycling conditions: 96°C for 2 min; followed by 21
cycles of 96°C for 30 sec, 55°C for 30 sec, 68°C for 30 sec, and a
final elongation at 68°C for 30 sec. The relative levels of
individual mRNA in each sample were normalized to GAPDH and
calculated using the 2−∆∆Cq method (14).
Western blotting
Cells (5×107 cells/ml) were harvested 72
h following infection and lysed in Radioimmunoprecipitation Assay
buffer (Fermentas; Thermo Fisher Scientific, Inc.) supplemented
with 1% protease inhibitors (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) on ice, followed by centrifugation at 12,000 × g for 15
min at 4°C. Protein concentrations were measured with the
Bicinchoninic Protein Assay (Pierce; Thermo Fisher Scientific,
Inc.). Subsequently, proteins were diluted to equal concentrations
(20 or 30 mg), boiled for 5 min and separated by 7.5–10% SDS-PAGE,
followed by transblotting to an Immun-Blot polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc.). The membranes
were blocked with 5% defatted milk in TBS + 0.1% Tween-20 and
probed with primary antibodies overnight at 4°C. Membranes were
subsequently incubated with horseradish peroxidase (HRP)-conjugated
secondary antibody for 1 h at room temperature. Protein bands were
visualized using an Enhanced Chemiluminescence Detection kit (GE
Healthcare). The following rabbit monoclonal antibodies were used:
Anti-Fer (1:500; catalog no. ab191060; Abcam, Cambridge, UK);
anti-Bcl-2 monoclonal antibody [1:500; catalog no. 4223; Cell
Signaling Technology (CST), Inc., Danvers, MA, USA]; CyclinD1
(1:500; catalog no. 2978; CST); p21(1:500; catalog no. 2947; CST);
cleaved Caspase-3 (1:500; catalog no. 9661; CST); phosphorylated
(p)-p38 MAPK (Thr180/Tyr182) (1:500; catalog no. 4511; CST); Rabbit
anti-GAPDH polyclonal antibody (1:500; catalog no. Ab9485; Abcam)
was used as an internal control protein. Densitometric analysis to
quantify protein expression levels was performed using ImageJ
software v1.46 (National Institutes of Health, Bethesda, MD,
USA).
Small interfering RNA (siRNA)
transfection
siRNAs targeting Fer and a negative control (NC)
siRNA were purchased from Shanghai GenePharma Co., Ltd. (Shanghai,
China). The targeting sequences of three Fer-siRNAs (siRNA1,
5′-AAAGAAATTTATGGCCCTGAG-3′; siRNA2, 5′-CAGATAGATCCTAGTACAGAA-3′;
siRNA3, 5′-AACTACGGTTGCTGGAGACAG-3′) and one NC-siRNA
(5′-UUCUCCGAACGUGUCACGU-3′) were designed using an RNAi algorithm
available online at The RNAi Web (http://www.rnaiweb.com). For transfection, the siRNAs
(100 nmol/l) were transfected into T24 using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. T24 cells (4×105 cells/cm2) were
grown in regular medium for 72 h at 37°C and subsequently
transfected with siRNAs, control cells were transfected with the NC
siRNA. Cells were transfected at 37°C for 48 and 72 h at which time
they were harvested for RNA and protein extraction,
respectively.
Generation of plasmid constructs and
establishment of Fer overexpression cell lines
To generate Fer overexpression vectors, Fer coding
sequences were obtained by RT-PCR (primers: Forward
5′-TTCGAGGGCACTGGGTTTTC-3′, reverse 5′-TTCCCTTGCCCAGTAATTCTCC-3′)
and cloned into the pEGFP-N1 vector (Clontech Laboratories, Inc.,
Mountainview, CA, USA). The resulting plasmid was designated as
pEGFP-N1-Fer and was transfected into 5637 bladder cancer cells to
induce Fer overexpression, 5637 cells were plated in regular medium
at a density of 1×104 cells/cm2 for 72 h at
37°C and then transfected with pEGFP-N1-Fer; pEGFP-N1 empty vector
was used as a control, and the resulting cell lines were designated
as 5637/pEGFP-N1-Fer and 5637/pEGFP-N1, respectively. After 24 h
post-transfection, G418 solution was added to cells for the
selection of stable clones, which were then cultured in medium
containing G418. Both 5637/pEGFP-N1-Fer and 5637/pEGFP-N1 cells
were maintained in fresh regular medium for 2 days and then
harvested for cell number counting.
MTT assay
Bladder cancer cells were seeded (5×103
cells/well) in flat-bottomed 96-well plates. After 24 h, cells were
transfected for 1, 2, 3 or 4 days, as aforementioned. Following
culture, 10 µl MTT (5 mg/ml) was added to each well and plates were
incubated at 37°C for 4 h. The medium was removed and 100 µl
dimethyl sulfoxide solution was added to each well to dissolve the
purple formazan crystals. Absorbance was measured at 490 nm using a
microplate reader to determine cell viability; three replicate
wells were analyzed per assay and each experiment was repeated
three times.
Cell cycle and apoptosis assays
Transfected cells (3×105 cells/well) were
harvested using 0.25% trypsin at 37°C for 30 min, and were
subsequently added to 1 ml of 70% cold ethanol overnight at 4°C.
The next day, cells were centrifuged at 12,000 × g for 5 min at
room temperature, treated with 100 µl RNase at 37°C for 30 min, and
stained with 400 µl propidium iodide (PI; catalog no. KGA511;
Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China) at 4°C for 30
min. The percentage of cells in G0/G1, S and G2/M phase was
determined by DNA flow cytometry with Cell Quest software v5.1 (BD
Biosciences, San Jose, CA, USA).
Annexin V-fluorescein isothiocyanate (FITC)/PI
Apoptosis Detection kit (catalog no. KGA108; Nanjing KeyGen Biotech
Co., Ltd.) was used to analyze apoptosis; culture medium without
FBS was added to the cells which had been transfected at 37°C,
followed by further culture for 24 h. Cells were harvested and
centrifuged at 12,000 × g for 5 min at room temperature. The medium
was removed, and cells were washed once in PBS. Cells were
resuspended in in 500 µl Annexin V binding buffer, and 5 µl Annexin
V-FITC and 10 µl PI were added; cells were incubated for 15 min at
room temperature in the dark and analyzed by flow cytometry
(Beckman Coulter, Inc., Brea CA, USA) with Cell Quest software v5.1
(BD Biosciences). Non-viable cells were stained only by PI (Q1);
live cells possessed no staining of PI or Annexin V-FITC (Q2);
early apoptotic cells exhibited a high degree of Annexin V-FITC
staining without PI staining (Q3); and late apoptotic cells had a
high degree of PI and Annexin V-FITC staining (Q4). The apoptotic
rate was calculated as: [(Q3+Q4)/(Q1+Q2+Q3+Q4)] × 100. Each
experiment was performed in triplicate.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used to
perform statistical analysis. All the experiments were repeated
three times independently and data are presented as the mean ±
standard deviation. Statistical significance was compared between
the treatment and controls groups using the one-way analysis of
variance followed by a Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Fer expression in bladder cancer cell
lines
The mRNA and protein expression levels of Fer in
SV-HUC-1, 5637 and T24 cells were examined by RT-qPCR and western
blot analysis, respectively. The expression levels of Fer mRNA and
protein were significantly upregulated in the two bladder cancer
cell lines (5637 and T24) compared with expression in the SV-HUC-1
normal bladder epithelium cell line (Fig. 1A and B; P<0.05). Furthermore,
the expression of Fer in the highly invasive and mesenchymal-like
bladder cancer T24 cells was significantly higher compared with in
5637 cells, and these two bladder cancer cell lines were used in
subsequent experiments to determine the biological roles of
Fer.
 | Figure 1.Efficacy of Fer-siRNA and vector
overexpression of Fer in bladder cancer cells. (A and B) Fer mRNA
and protein expression levels were higher in two bladder cancer
cell lines (5637 and T24) compared with in the normal bladder
epithelium cell line SV-HUC-1, as evaluated by RT-qPCR and western
blot analysis, respectively. GAPDH was used as an internal control.
Data are presented the mean ± standard deviation; n=3; *P<0.05,
5637 vs. SV-HUC-1 cells; **P<0.01, T24 vs. SV-HUC-1 cells;
##P<0.01, T24 vs. 5637). (C and D) Relative Fer mRNA
relative levels in the Fer-siRNA1 and Fer-siRNA2 groups were
significantly decreased compared with the NC-siRNA group, as
evaluated by RT-qPCR. Similar results were obtained for protein
expression as determined by western blot analysis. Data are
presented as the mean ± standard deviation; n=3; **P<0.01 vs.
NC-siRNA. (E and F) Fer mRNA and protein expression was upregulated
by ~2-fold in the Vector-Fer group. Data are presented as the mean
± standard deviation; n=3; **P<0.01 vs. Empty vector and
Control. Fer, feline sarcoma-related protein; NC, negative control;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; siRNA, small interfering RNA; Vector-Fer, Fer
overexpression plasmid. |
Efficacy of siRNA and overexpression
plasmid transfection of Fer in bladder cancer cells
To investigate the biological function of Fer in the
progression of bladder cancer, T24 cells were transfected with one
of three siRNAs against Fer (siRNA1, siRNA2 or siRNA3), whereas
5637 cells were transfected with a Fer overexpression plasmid;
Nc-siRNA and empty vectors were used as the respective negative
controls. RT-qPCR results demonstrated that the relative Fer mRNA
expression levels in the Fer-siRNA1 and Fer-siRNA2 groups were
significantly decreased compared with the NC-siRNA group
(P<0.01; Fig. 1C); similar
results were observed for Fer protein levels (P<0.01; Fig. 1D). However, no significant
differences in expression levels were observed in the
Fer-siRNA3-transfected cells compared with the NC-siRNA group.
Conversely, Fer overexpression in 5637 cells by plasmid
transfection. As demonstrated by RT-qPCR and western blot analysis,
the expression of Fer was significantly increased compared with the
untreated control and the empty vector group (P<0.05; Fig. 1E and F).
Effects of down- or upregulation of
Fer on cell viability and the cell cycle
To investigate the role of Fer on the viability of
bladder cancer cells, the viability of T24 and 5637 cells
transfected with Fer-siRNAs or Vector-Fer was determined by an MTT
assay at days 1, 2, 3 and 4 post-transfection. The results
demonstrated that the viability of Fer-siRNA-transfected T24 cells
was significantly decreased compared with that of
Nc-siRNA-transfected cells (P<0.01; Fig. 2A); the viability of
Vector-Fer-transfected 5637 cells was significantly increased
compared with that of the control and empty vector groups at day 4
(P<0.01; Fig. 2B). Furthermore,
flow cytometric analysis was conducted to determine the potential
mechanism underlying the effects of Fer on bladder cancer cell
viability and cell cycle following Fer knockdown and
overexpression. As presented in Fig.
2C and D, Fer-siRNA significantly increased the proportion of
cells in G0/G1 phase and reduced the proportion in S phase compared
with Nc-siRNA-transfected cells. Conversely, treatment with
Vector-Fer significantly reduced the proportion of cells in G0/G1
phase and elevated the number of cells in G2/M phase compared with
the controls. Furthermore, the proportion of Vector-Fer-transfected
cells at S phase was significantly decreased compared with the
control and empty vector groups (P<0.05). These results
indicated that Fer may be closely associated with cell viability
and the cell cycle in bladder cancer cells.
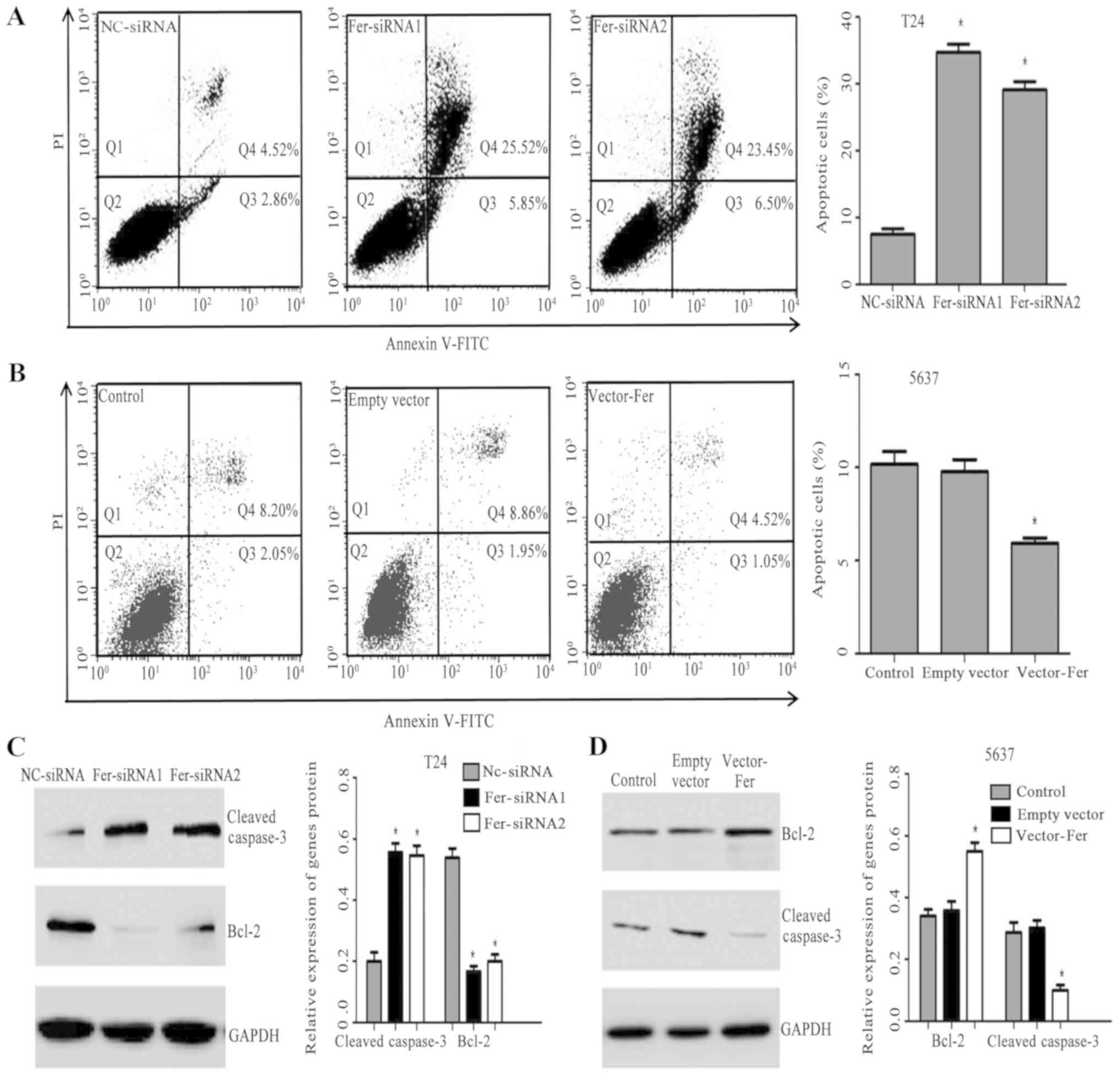
Effects of down- or upregulation of
Fer on cell apoptosis
To further study the effects of Fer on the apoptosis
of bladder cancer cells following transfection an Annexin V/FITC
kit and flow cytometry were employed. As presented in Fig. 3A and B, the apoptotic rate of T24
cells in the Fer-siRNA groups was significantly higher compared
with the Nc-siRNA group (P<0.05). However, the apoptotic rate of
the Vector-Fer group of 5637 cells was significantly lower compared
with the control and empty vector groups (P<0.05). The results
indicated that downregulation of Fer promoted the apoptosis of T24
cells, while overexpression of Fer inhibited 5637 cell
apoptosis.
Bcl-2 family proteins regulate cell apoptosis
through promoters or inhibitors (15). Cleaved caspase-3, as a prognostic
predictor involved in the ‘execution’ phase of apoptosis, is a key
regulator of promoting tumor repopulation induced by dying cells
(16). Therefore, the expression
of Bcl-2 and cleaved caspase-3, key mediators of apoptosis, was
investigated. As presented in Fig. 3C
and D, downregulated Fer was associated with significantly
increased cleaved caspase-3 expression, whereas the expression of
Bcl-2 was significantly downregulated compared with the Nc-siRNA
group. On the contrary, Fer overexpression significantly increased
Bcl-2 expression and downregulated that of cleaved capsase-2
compared with the controls.
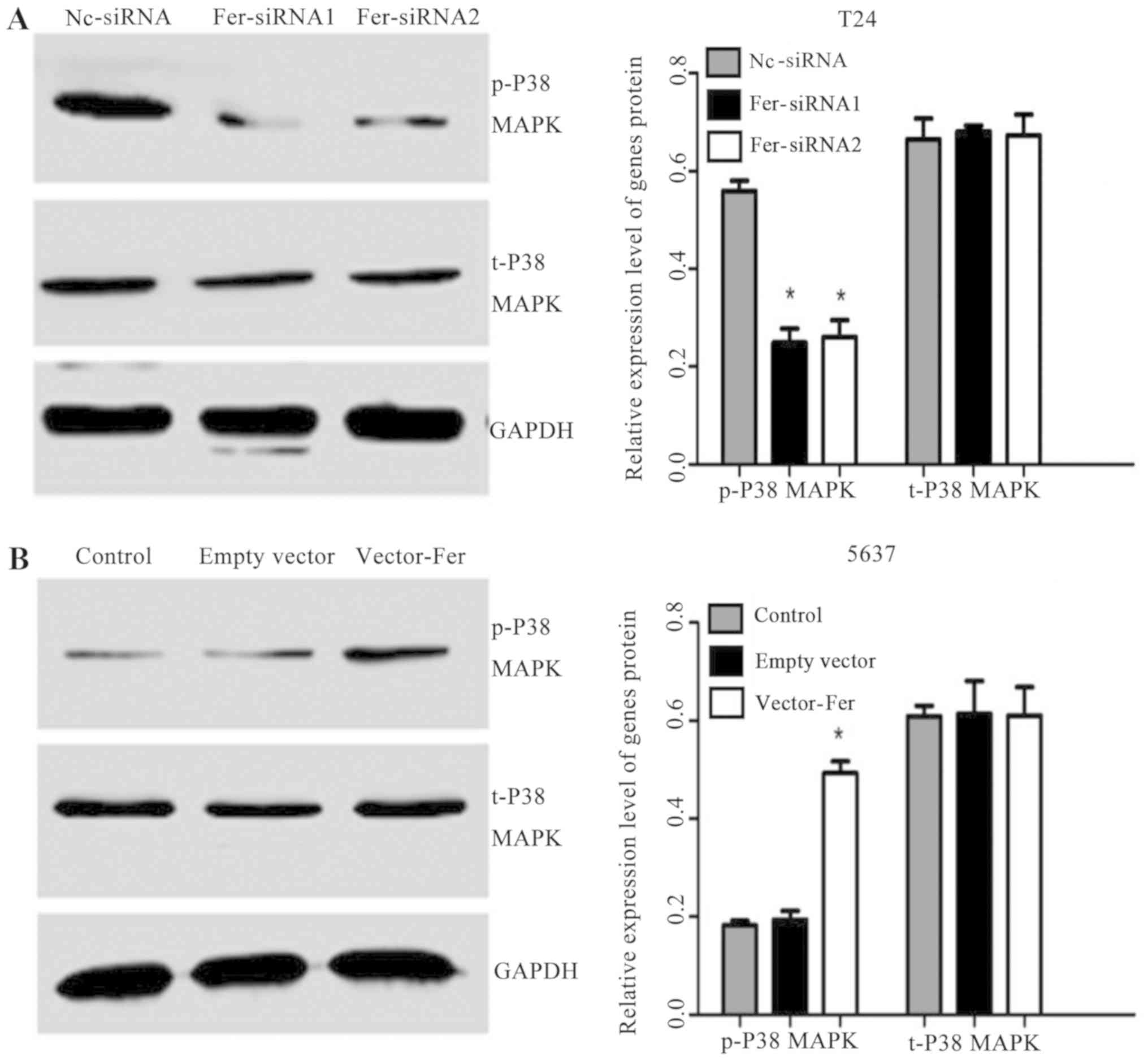
Effects of down- or upregulation of
Fer on P38 MAPK phosphorylation
P38 MAPK signaling is one of the main pathways
undertaken by MAPK, and serves a key role in regulating cell
apoptosis, growth, differentiation and oncogenic transformation
(17). Therefore, the effects of
Fer down- or upregulation on the P38 MAPK signaling pathway were
investigated. As presented in Fig. 4A
and B, a significant decrease in the expression of p-P38 MAPK
following Fer knockdown in T24 cells compared with in the Nc-siRNA
group was observed. Conversely, overexpression of Fer significantly
upregulated the expression of p-P38 MAPK in 5637 cells compared
with the controls; however, the levels of total P38 MAPK were
markedly unaffected in either bladder cancer cell group. These
findings suggested that Fer may regulate cell proliferation and
apoptosis via P38 MAPK modulation.
Discussion
Previously, several studies have reported that Fer
is widely expressed in proliferating mammalian cells (18,19).
Overexpression of Fer has also been associated with poor prognosis
in various types of human cancer and serves as a prognostic marker
(5,7–9);
however, the biological role and underlying mechanism of Fer in
bladder cancer cell viability and apoptosis require further
investigation. In the present study, Fer expression in normal and
bladder cancer cells was analyzed by RT-qPCR and western blotting.
The findings revealed that Fer was significantly overexpressed in
bladder cancer cells compared with in normal bladder cells. This
was consistent with our previous findings in bladder cancer tissues
(9). These results indicate that
Fer may be involved in the progression of bladder cancer.
A recent report has indicated that Fer activation is
required in tumorigenesis and the invasiveness of certain cancer
cells in which C-Src is upregulated (20); however, the molecular mechanisms of
Fer remain unknown. In the present study, to reveal the biological
effects of Fer on cell viability and apoptosis in bladder cancer,
the transfection of bladder cancer cells with siRNA or plasmid was
conducted to knock down or overexpress Fer, respectively. The
effects of downregulation or upregulation of Fer on bladder cancer
cell viability and apoptosis were determined. The results revealed
that Fer siRNA suppressed the viability and G1/S transition of T24
cells. This resulted in cell cycle arrest at G1, which may explain
the inhibition of cell growth induced by Fer knockdown in T24 cells
(21). In addition, Annexin
FITC/PI staining was used to observe the rate of apoptosis; cell
death is the process by which aged or damaged cells are eliminated
and serves a key role in carcinogenesis (22). The present study reported that
compared with Nc-siRNA-transfected cells, the fluorescence
intensity of Fer-siRNA cells increased significantly, indicating
that Fer knockdown could induce the apoptosis of T24 bladder cancer
cells. Overexpression of Fer in 5637 cells exhibited opposing
effects on cell viability, cell cycle and apoptosis in
vitro. Bcl-2 is a member of the regulatory Bcl-2 protein family
(15). Overexpression of Bcl-2 is
associated with cell cycle arrest (23), and the inhibitory effects of Bcl-2
could increase the rate of apoptosis (24,25).
Bcl-2 also increases the expression levels of activated caspase-3
(cleaved caspase-3), which is a key mediator of programmed cell
death (16). Therefore, the
present study also investigated the association between the
activity of Fer, and the expression of the Bcl-2 and caspase-3. Fer
knockdown downregulated Bcl-2 and upregulated cleaved caspase-3
expression in T24 cells. The overexpression of Fer in 5637 cells
had opposing effects, which may explain the inhibition of the cell
cycle and apoptosis. Therefore, these findings indicate that Fer
serves an important role in the biological behavior of T24 cells,
which is mediated by regulating the expression of certain
genes.
Additionally, present study reported that knockdown
or overexpression of Fer disrupted the MAPK signaling pathway by
altering the expression of phosphorylated p38 MAPK, whereas the
expression of total p38 MAPK protein did not notably change. Senis
et al (26) revealed that
many small G protein/MAPK cascades are involved in downstream
signal transduction of FPS/FES tyrosine kinase. Craig and Greer
(27) also revealed that Fer
kinase is required for sustained p38 kinase activation and the
maximal chemotaxis of activated mast cells. Of note, p38 MAPK is
activated via sequential phosphorylation in the MAPK signaling
pathway (28). Therefore, the
results of the present study suggest that Fer may affect cell
viability and apoptosis via the P38 MAPK signaling pathway.
In conclusion, these findings contribute to the
increasing evidence that Fer is involved in the development and
progression of cancer. To the best of our knowledge, the present
study is the first to report of Fer as a novel regulator of cell
viability and apoptosis in bladder cancer. Fer was indicated to
exert its effects by regulating the expression of certain genes and
inhibiting the p38 MAPK signaling pathway. In addition, the
association between Fer, and viability and apoptosis of bladder
cancer cells was determined. Future investigation into the
association between phosphorylated P38 and apoptosis is under way.
Collectively, the results of the present study indicated that Fer
serves a role in the biological behavior of bladder cancer cells,
and suggest that Fer may be considered as a novel molecular target
for the treatment of bladder cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from The National
Natural Science Foundation of China (grant nos. 81373005, 81072330
and 81202194) and by The Priority Academic Program Development of
Jiangsu Higher Education Institutions.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, DY and CZ conceived and designed the study. ZG,
JX and XH performed cell culture and cell transfection. XM and XH
performed reverse transcription-quantitative polymerase chain
reaction, western blotting, MTT assay and flow cytometry. MB, FJ
and XH provided reagents and interpreted the data. XH performed
data analysis and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A, Palou-Redorta J and Rouprêt M; Asociación
Europea de Urología, : EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder, the 2011 update. Actas Urol
Esp. 36:389–402. 2012.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stenzl A, Cowan NC, De Santis M, Jakse G,
Kuczyk MA, Merseburger AS, Ribal MJ, Sherif A and Witjes JA: The
updated EAU guidelines on muscle-invasive and metastatic bladder
cancer. Eur Urol. 55:815–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeGraff DJ, Clark PE, Cates JM, Yamashita
H, Robinson VL, Yu X, Smolkin ME, Chang SS, Cookson MS, Herrick MK,
et al: Loss of the urothelial differentiation marker FOXA1 is
associated with high grade, late stage bladder cancer and increased
tumor proliferation. PLoS One. 7:e366692012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greer P: Closing in on the biological
functions of Fps⁄Fes and Fer. Nat Rev Mol Cell Biol. 3:278–289.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahn J, Truesdell P, Meens J, Kadish C,
Yang X, Boag AH and Craig AW: Fer protein-tyrosine kinase promotes
lung adenocarcinoma cell invasion and tumor metastasis. Mol Cancer
Res. 11:952–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Ren Z, Kang X, Zhang L, Li X, Wang
Y, Xue T, Shen Y and Liu Y: Identification of
tyrosine-phosphorylated proteins associated with metastasis and
functional analysis of FER in human hepatocellular carcinoma cells.
BMC Cancer. 9:3662009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rocha J, Zouanat FZ, Zoubeidi A, Hamel L,
Benidir T, Scarlata E, Brimo F, Aprikian A and Chevalier S: The Fer
tyrosine kinase acts as a downstream interleukin-6 effector of
androgen receptor activation in prostate cancer. Mol Cell
Endocrinol. 381:140–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Albeck JG and Brugge JS: Uncovering a
tumor suppressor for triple-negative breast cancers. Cell.
144:638–640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu X, Zhang Z, Liang Z, Xie D, Zhang T, Yu
D and Zhong C: Downregulation of feline sarcoma-related protein
inhibits cell migration, invasion and epithelial-mesenchymal
transition via the ERK/AP-1 pathway in bladder urothelial cell
carcinoma. Oncol Lett. 13:686–694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allard P, Zoubeidi A, Nguyen LT, Tessier
S, Tanguay S, Chevrette M, Aprikian A and Chevalier S: Links
between Fer tyrosine kinase expression levels and prostate cell
proliferation. Mol Cell Endocrinol. 159:63–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pasder O, Shpungin S, Salem Y, Makovsky A,
Vilchick S, Michaeli S, Malovani H and Nir U: Downregulation of Fer
induces PP1 activation and cell-cycle arrest in malignant cells.
Oncogene. 25:4194–4206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu G, Craig AW, Greer P, Miller M,
Anastasiadis PZ, Lilien J and Balsamo J: Continuous association of
cadherin with beta-catenin requires the non-receptor
tyrosine-kinase Fer. J Cell Sci. 117:3207–3219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sangrar W, Gao Y, Scott M, Truesdell P and
Greer PA: Fer-mediated cortactin phosphorylation is associated with
efficient fibroblast migration and is dependent on reactive oxygen
species generation during integrin-mediated cell adhesion. Mol Cell
Biol. 27:6140–6152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alnemri ES, Livingston DJ, Nicholson DW,
Salvesen G, Thornberry NA, Wong WW and Yuan J: Human ICE/CED-3
protease nomenclature. Cell. 87:1711996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Letwin K, Yee SP and Pawson T: Novel
protein-tyrosine kinase cDNAs related to fps/fes and eph cloned
using anti-phosphotyrosine antibody. Oncogene. 3:621–627.
1988.PubMed/NCBI
|
|
19
|
Hao QL, Heisterkamp N and Groffen J:
Isolation and sequence analysis of a novel human tyrosine kinase
gene. Mol Cell Biol. 9:1587–1593. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oneyama C, Yoshikawa Y, Ninomiya Y, Iino
T, Tsukita S and Okada M: Fer tyrosine kinase oligomer mediates and
amplifies Src-induced tumor progression. Oncogene. 35:501–512.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Wang T, Han Y, Wu H, Zhao W, Tong
D, Wei L, Zhong Z, An R and Wang Y: Reduced ING4 Expression Is
Associated with the Malignancy of Human Bladder. Urol Int.
94:464–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelekar A and Thompson CB: Bcl-2-family
proteins: The role of the BH3 domain in apoptosis. Trends Cell
Biol. 8:324–330. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simpson NH, Singh RP, Emery AN and
Al-Rubeai M: Bcl-2 over-expression reduces growth rate and prolongs
G1 phase in continuous chemostat cultures of hybridoma cells.
Biotechnol Bioeng. 64:174–186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cleary ML, Smith SD and Sklar J: Cloning
and structural analysis of cDNAs for bcl-2 and a hybrid
bcl-2/immunoglobulin transcript resulting from the t(14;18)
translocation. Cell. 47:19–28. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Wu X, Wang D, Luo C and Chen L:
Renal carcinoma cell-derived exosomes induce human immortalized
line of Jurkat T lymphocyte apoptosis in vitro. Urol Int.
91:363–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Senis YA, Sangrar W, Zirngibl RA, Craig
AW, Lee DH and Greer PA: Fps/Fes and Fer non-receptor
protein-tyrosine kinases regulate collagen- and ADP-induced
platelet aggregation. J Thromb Haemost. 1:1062–1070. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Craig AW and Greer PA: Fer kinase is
required for sustained p38 kinase activation and maximal chemotaxis
of activated mast cells. Mol Cell Biol. 22:6363–6374. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|