Introduction
Lung cancer is one of the most common malignancies
worldwide and it is characterized by uncontrolled cell growth in
the lungs. The most common symptoms of lung cancer are coughing,
weight loss, shortness of breath and chest pains (1). The number of novel diagnosed cases of
lung cancer reached 1.8 million in 2012 and 1.6 million mortalities
were reported in the same year, making lung cancer the most common
cause of cancer-associated mortalities in males and the second most
common cause in females, only secondary to breast cancer (2). The five-year survival rate of
patients with lung cancer was 17.4% in the USA in 2008, whereas,
the outcomes were worse in developing countries (3). Limited access to early diagnosis and
treatment is the most important factor for the occurrence of
clinical lung cancer worldwide (4). The majority of patients suffering
from lung cancer additionally present local metastases due to the
high metastatic potential of lung cancer cells (5).
Long non-coding RNAs (lncRNAs) are defined as RNAs
with lengths >200 nucleotides that are not translated into
proteins (6). lncRNA expression
levels are 10-fold lower compared with mRNAs in various cell types,
and the expression levels of lncRNA genes are more variable among
cells, compared with protein-coding genes (7). Transcriptional profiles of various
cell types using next generation sequencing demonstrated that tens
of thousands of lncRNAs exist in mammals (8). Although accumulating evidence has
demonstrated that the majority of these are likely to be
functional, only a small proportion of lncRNAs are biologically
relevant (9).
Previous studies demonstrated that numerous lncRNAs
serve significant roles in tumorigenesis. lncRNA taurine
upregulated gene 1 was downregulated and associated with cell
apoptosis in human breast cancer (10). More specifically, in lung cancer,
lncRNAs CDKN2B antisense RNA 1 and ENST457720 were reported to
regulate cell proliferation in vivo and in vitro
(11,12). However, the detailed mechanisms
underlying the regulatory roles of lncRNAs in human lung cancer
require identification. Furthermore, at present, to the best of the
authors' knowledge, lncRNAs have not been used in the diagnosis and
treatment of lung cancer. Therefore, it is critical to identify
novel lncRNAs involved in the progression of lung cancer.
In the present study, it was identified that a novel
lncRNA, Fer-1-like family member 4 (FER1L4), serves roles in cell
proliferation and metastasis of lung cancer. Furthermore, the
mechanism underlying FER1L4 function in lung cancer was examined.
These results provide novel insight of lung cancer progression, and
may improve clinical diagnosis and treatment of lung cancer in the
future.
Materials and methods
Human samples
The present study was approved by the Ethics
Committee of Xiqing Hospital (Tianjin, China). In total, 100
patients with lung cancer (male:female ratio, 60:40; average age,
59 years old) from the Department of Respiration, Xiqing Hospital,
were enrolled between January 2016 and December 2017. Informed
written consent was obtained from all patients. No chemotherapies
or radiotherapies were performed prior to surgery. During surgery,
the lung cancer tissues and adjacent normal tissues were frozen in
liquid nitrogen as soon as they were dissected from the patients,
and stored until use for subsequent analysis.
Cell culture and transfection
The normal lung cell line BEAS-2B and lung cancer
cell line SPC-A-1 were purchased from The American Type Culture
Collection (Manassas, VA, USA). Other lung cancer cell lines A549,
H1975, H-125 and 95D were obtained from The Cell Bank of Chinese
Academy of Sciences (Shanghai, China). All cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplied with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.)
at 37°C. A FER1L4 expression plasmid was constructed using a pcDNA
3.1 vector by Jie Li Biology (http://www.genebioseq.com/, Shanghai, China) with
Xho I and HindIII restriction enzymes. A549, 95D and
BEAS-2B cells were transfected with 2 µg/ml plasmid using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h, according to the manufacturer's
protocol. SF1670 (Selleck Chemicals, Shanghai, China), a specific
inhibitor of phosphatase and tensin homolog (PTEN), was purchased
to activate the phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt) signaling pathway and diluted in dimethyl sulfoxide to a
concentration of 50 mM. The working concentration of SF1670 was 50
µM and the incubation time was 6 h at 37°C.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the patient tissues and cultured
cells were extracted using TRIzol® (Thermo Fisher
Scientific, Inc.) reagent, using a volume of 1 ml/well in six-well
plates. The RNA amount and quality were measured using Nanodrop
2000 (Thermo Fisher Scientific, Inc., Wilmington, DE, USA). A total
of 500 ng RNA was reverse transcribed into cDNA in a volume of 10
µl using PrimeScript RT Reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China), at 37°C for 15 min and 85°C for 5 sec.
RT-qPCR was performed in an ABI 7900 machine using a SYBR Green kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol. The thermocycling conditions were as follows: Initial
denaturation at 95°C for 5 min, followed by 45 repeats of a
three-step cycling program consisting of 10 sec at 95°C
(denaturation), 10 sec at 60°C (primer annealing) and 10 sec at
72°C (elongation), and a final extension step for 10 min at 72°C.
Primers were synthesized by Jie Li Biology. and the sequences are
listed in Table I. GAPDH was
included as an internal control and 2−ΔΔCq method was
used for statistical analysis (13).
 | Table I.Primers used in the present study. |
Table I.
Primers used in the present study.
| Gene | Direction | Sequences |
|---|
| FER1L4 | Forward | 5′-
AATGTGGGCTTCCAGGAAC-3′ |
|
| Reverse |
5′-CACCAGAAAGTTCCACGTC-3′ |
| PI3K | Forward |
5′-GTCCTATTGTCGTGCATGTGG-3′ |
|
| Reverse |
5′-TGGGTTCTCCCAATTCAACC-3′ |
| Akt | Forward | 5′-
TTCTATGGCGCTGAGATTGTGT-3′ |
|
| Reverse |
5′-GCCGTAGTCATTGTCCTCCAG-3′ |
| GAPDH | Forward |
5′-GGTCGGAGTCAACGGATTTG-3′ |
|
| Reverse |
5′-GGAAGATGGTGATGGGATTTC-3′ |
Colony formation assay
A total of 1×105 cells were seeded into
12-well plates 24 h prior to the transfection with the FER1L4
expression plasmid. A total of 500 A549 and 95D cells were seeded
into separate six-well plates. The plates were incubated at 37°C in
an incubator (5% CO2) for 2 weeks without changing the
culture medium. Subsequently, the colonies were stained with
crystal violet (1%) at room temperature for 10 min and five random
fields were imaged for each group with a Nikon light microscope
(×200; Nikon Corporation, Tokyo, Japan). The number of cells was
counted and statistical analyses were performed.
Cell proliferation assay
An MTT assay (Promega Corporation, Madison, WI, USA)
was performed to examine the effects of FER1L4 on cell growth. A549
and 95D cells were transfected with FER1L4 expression plasmid and
seeded into 96-well plates with an initial concentration of
5×103/well. Each experimental group of cells was seeded
in sextuplicate and the culture medium was replaced every other
day. Cell proliferation was assessed at days 1, 3 and 5, and DMSO
was used to dilute formazan and stop the reaction. During the
experiments, the cell proliferative rate was determined with a
TECAN reader (Tecan Group, Ltd., Männedorf, Switzerland) with an
absorbance of 490 nm.
Transwell and Matrigel assays
For the cell migration assays, A549, 95D cells and
BEAS-2B cells were transfected with the FER1L4-expressing plasmid
for 48 h and collected by low-speed centrifugation (1,000 × g; 4°C;
5 min) with serum-free DMEM. A total of 1×104 cells
(~150 µl) were seeded into the upper chambers (8 µm pore; Corning,
Inc., Corning, NY, USA). The lower chambers were filled with 600 µl
medium containing 10% FBS. The plate was subsequently incubated at
37°C. At 12 h post-seeding, the membrane was washed with PBS, fixed
with precooled methanol (5 min, room temperature) and stained with
crystal violet (1%) for 5 min at room temperature. Cell migration
was determined by counting the cells migrated through the membrane.
In total, five random areas were imaged using a Nikon light
microscope (Nikon Corporation; magnification, ×200). For cell
invasion assays, the membrane was pre-coated with Matrigel
(Corning, Inc.) for 6 h at 37°C.
Western blot analysis
Western blotting analysis was performed with cell
lysates. Total protein from cultured A549 and 95D cells transfected
with or without the FER1L4 expression plasmid were extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The protein amount was determined
with a bicinchoninic acid kit (Thermo Fisher Scientific, Inc.).
Subsequently, a total of 40 µg protein was loaded onto a 10%
SDS-PAGE gel and transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). Subsequent to
blocking with 5% skim milk for 1 h at room temperature, the
membranes were incubated with primary antibodies diluted in 5%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.)
solution. The following primary antibodies were all purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA): PI3K (cat. no.
4257; 1:1,000), Akt (cat. no. 9272; 1:1,000), phosphorylated
(p-)Akt (cat. no. 9271; 1:1,000) and GAPDH (cat. no. 5174;
1:5,000). p-PI3K antibody was purchased from BioWorld Technology
Co., Ltd. (cat. no. BS4605; 1:500; Nanjing, China). The membrane
was incubated with primary antibodies at 4°C overnight, followed by
incubation with secondary antibodies for 1 h at room temperature.
The horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody was purchased from Santa Cruz Biotechnology, Inc. (cat.
no. sc-2004; 1:5,000; Dallas, TX, USA). Immunoreactive bands were
visualized using an enhanced chemiluminescent system (Thermo Fisher
Scientific, Inc.).
Wound healing assays
A549 and 95D cells were transfected with FER1L4
expression plasmid for 48 h and were subsequently cultured in DMEM
in a six-well culture plate at a density of 5×105
cells/well. When the confluence reached 95%, the cells were washed
and the culture medium was replaced with serum-free DMEM. A scratch
was made through the single cell layer using a 10 µl pipette tip
and the cells were washed again with warmed PBS. After an
incubation of 24 h, images of the migrating cells were captured
with a Nikon light microscope (magnification, ×200).
Statistical analysis
All data are presented as the mean ± standard
deviation. GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for analysis. Each experiment was repeated at
least three times, unless otherwise stated. The two-tailed
Student's t-test was used to compare means between two groups,
whereas, one-way analysis of variance was used for comparisons
among multiple groups (more than two groups), followed by Fisher's
least significant difference post-hoc test. χ2 or
Fisher's exact test were used to compare proportion differences of
categorical variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
mRNA expression level of FER1L4 is
downregulated in lung cancer in vivo and in vitro
To examine the role of FER1L4, its expression in
human lung cancer was assessed. In total, 100 patients with lung
cancer were included in the present study and it was demonstrated
by RT-qPCR that the expression level of FER1L4 was significantly
downregulated in patients with lung cancer (Fig. 1A). The clinical features of the 100
patients with lung cancer and the relative expression of FER1L4
were additionally analyzed (Table
II). It was demonstrated that the expression level of FER1L4
was not associated with age, sex or presenting symptoms of the
patients; however, FER1L4 expression level was associated with
tumor size, lymph node metastasis, distant metastasis and tumor,
node and metastasis (TNM) staging. The normal lung epithelia cell
line BEAS-2B and five lung cancer cell lines were assessed for
FER1L4 gene expression. In all of the five cancer cell lines, the
expression levels of FER1L4 were consistently downregulated
compared with the BEAS-2B cell line. In particular, A549 and 95D
cells, the two cell lines with the highest migration potential
(4), exhibited the lowest
expression levels of FER1L4 (Fig.
1B). The present results demonstrated that the expression level
of FER1L4 is downregulated in human lung cancer in vivo and
in vitro, and suggested a potential association between
FER1L4 expression levels and tumor malignancy.
 | Table II.Association of FER1L4 with
clinicopathological characteristics among 100 patients with lung
cancer. |
Table II.
Association of FER1L4 with
clinicopathological characteristics among 100 patients with lung
cancer.
|
|
| Expression of
FER1L4 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Number of
patients | Low, n=57 | High, n=43 | P-value |
|---|
| Age, years |
|
|
| 0.958 |
|
<40 | 15 | 8 | 7 |
|
|
40–50 | 23 | 13 | 10 |
|
|
>50 | 62 | 36 | 26 |
|
| Sex |
|
|
| 0.683 |
|
Male | 60 | 33 | 27 |
|
|
Female | 40 | 24 | 16 |
|
| Presenting
symptoms |
|
|
| 0.121 |
|
Painless lump | 41 | 20 | 21 |
|
| Painful
lump | 35 | 19 | 16 |
|
|
Atypical symptoms | 24 | 18 | 6 |
|
| Tumor size, T |
|
|
|
<0.001a |
| T1, ≤2
cm | 17 | 3 | 14 |
|
| T2,
>2 cm but <5 cm | 32 | 14 | 18 |
|
| T3, ≥5
cm | 28 | 20 | 8 |
|
| T4, any
size with distant metastasis | 23 | 20 | 3 |
|
| Lymph node
metastasis, N |
|
|
| 0.001a |
| N0 | 45 | 17 | 28 |
|
| N1 or
above | 55 | 40 | 15 |
|
| Distant metastasis,
M |
|
|
| 0.002a |
| M0 | 42 | 16 | 26 |
|
| M1 | 58 | 41 | 17 |
|
| TNM stage |
|
|
|
<0.001a |
|
I/II | 34 | 10 | 24 |
|
|
III/IV | 66 | 47 | 19 |
|
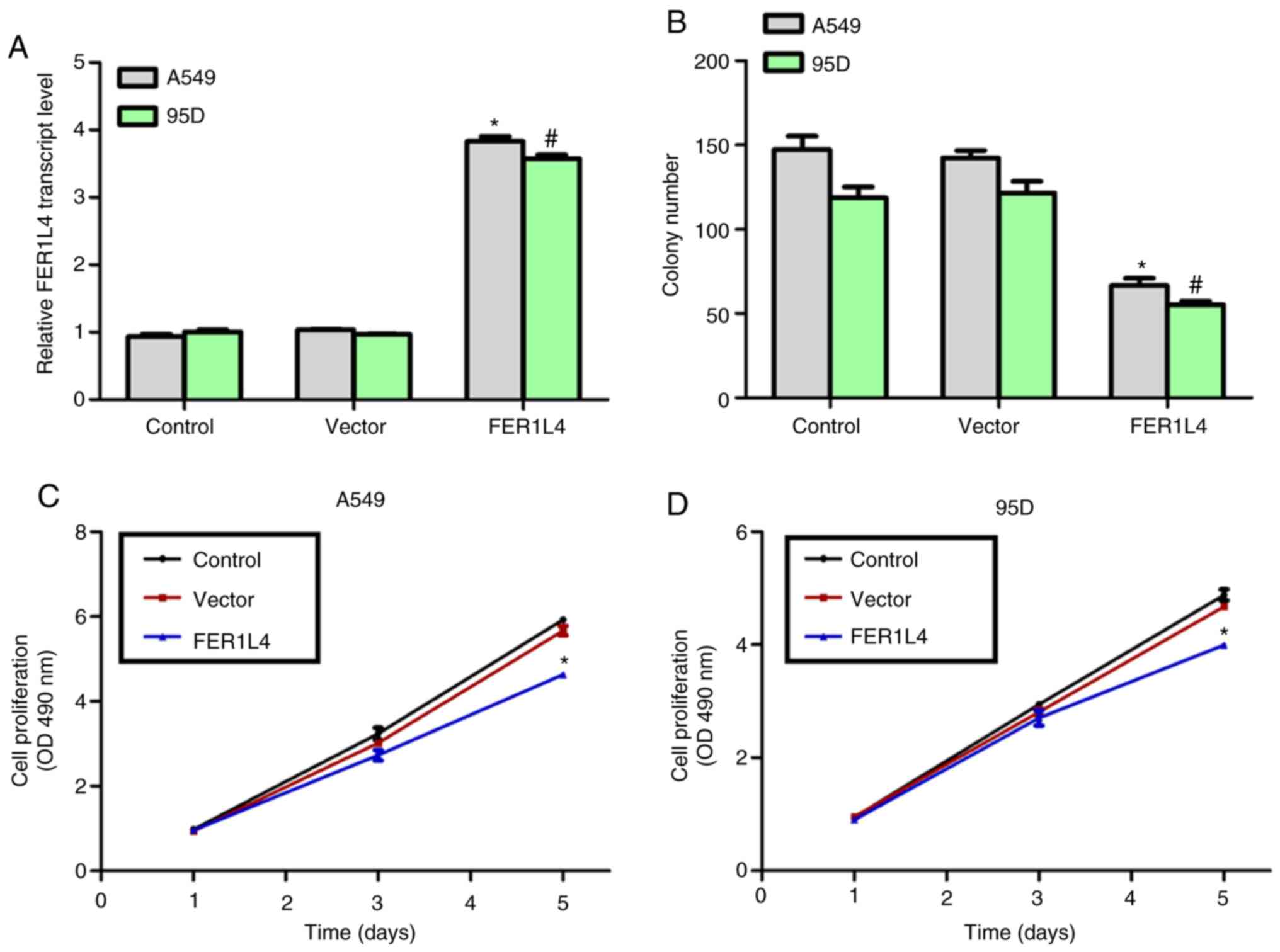
Overexpression of FER1L4 in A549 and
95D cells inhibits cell proliferation in vitro
An expression plasmid of FER1L4 was constructed and
transfected into A549 and 95D cells and a significant increase in
the expression level of FER1L4 was observed following transfection
(Fig. 2A), up to 3.5-fold compared
with the control groups. The effects of FER1L4 were examined by
performing colony formation assays and cell proliferation assays.
In total, ~150 and 125 colonies were detected in the control A549
and 95D cells, respectively. However, only 70 and 60 colonies were
observed for A549 and 95D cells following overexpression of FER1L4
(Fig. 2B). However, a similar
colony number was counted in the control cell line BEAS-2B with or
without overexpression of FER1L4 (data not shown). In addition,
there was no marked difference in the proliferation rate among the
three groups (control, vector and FER1L4 overexpression) in the
first 3 days, whereas, overexpression of FER1L4 led to a reduction
in the cell proliferation rate by 30 and 25% in A549 and 95D cells
at day 5, respectively (Fig. 2C and
D). These data demonstrated that the lncRNA FER1L4 inhibited
cell proliferation in the human lung cancer cell lines A549 and
95D.
Overexpression of FER1L4 in A549 and
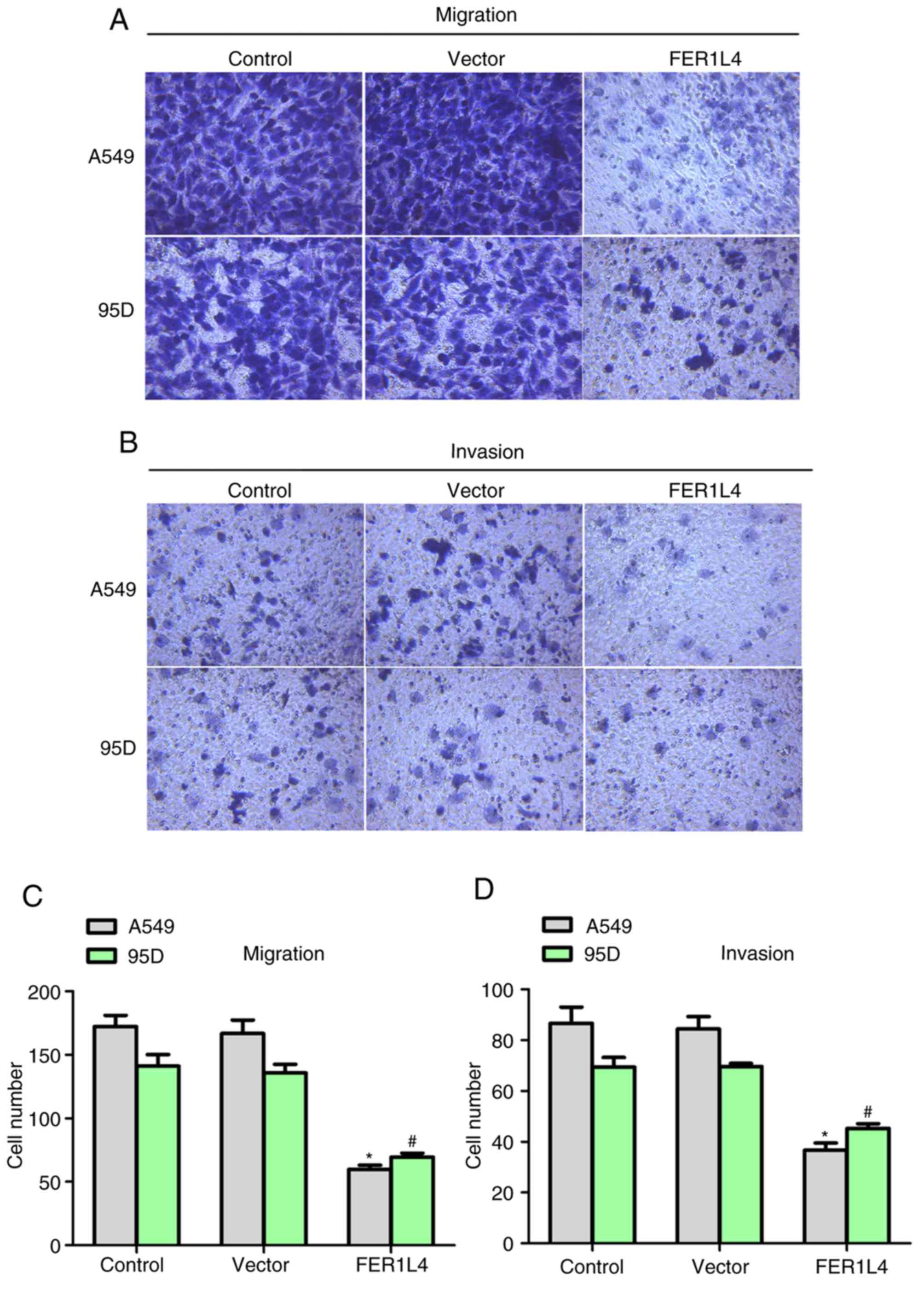
95D cells inhibits cell metastasis in vitro
Uncontrolled cell proliferation and metastasis are
characteristic of malignant tumors (14). Hence, the role of FER1L4 in cell
metastasis was tested (Fig. 3). As
depicted in Fig. 3A and C, ~170
A549 cells and 145 95D cells migrated through the membrane in the
control conditions, whereas, following transfection with the FER1L4
expression plasmid only 60 A549 cells and 65 95D cells were
observed on the lower surface of the chamber. In the invasion
assays, cell invasion was additionally inhibited by ~50% in A549
and 95D cells when FER1L4 was overexpressed (Fig. 3B and D). Notably, the wound closure
rate of BEAS-2B cells was approximately the same in the presence or
absence of the FER1L4 overexpression plasmid (data not shown).
Collectively, the present results demonstrated that overexpression
of FER1L4 in human lung cancer inhibited cell metastasis in A549
and 95D cells.
Upregulation of FER1L4 inhibits the
PI3K/Akt signaling pathway in lung cancer cells
To examine the regulatory mechanism of FER1L4 in
human lung cancer, the downstream signaling of FER1L4 in A549 and
95D cells was investigated. Western blot analysis was performed and
it was identified that the protein expression levels of p-PI3K and
p-AKT were decreased when FER1L4 was overexpressed in A549 and 95D
cells (Fig. 4A and B).
Furthermore, the expression levels of PI3K and Akt were examined.
As depicted in Fig. 4C and D, when
A549 and 95D cells were transfected with the FER1L4 expression
plasmid, the expression levels of PI3K and Akt were downregulated
by ~50%. These results suggested that upregulation of FER1L4
suppressed the PI3K/Akt signaling pathway in lung cancer.
FER1L4 inhibits cell proliferation and
metastasis by downregulating the PI3K/Akt signaling pathway in lung
cancer cells
To test whether FER1L4 regulates cell proliferation
and metastasis through the PI3K/Akt signaling pathway, a specific
inhibitor of PTEN was used, SF1670. Treatment with SF1670 led to an
upregulation of the expression levels of PI3K and Akt by >3-fold
in A549 cells, whereas, overexpression of FER1L4 restored the
expressions of PI3K and Akt to relative normal levels (Fig. 5A). Similar results were obtained in
95D cells (Fig. 5B). Subsequently,
functional experiments were performed in A549 and 95D cells. As
presented in Fig. 5C, treatment
with SF1670 increased the colony numbers in A549 and 95D cells, and
colony formation in the two cell lines was decreased by FER1L4
overexpression, thus indicating that FER1L4 inhibited cell
proliferation via regulation of the PI3K/Akt signaling pathway.
Furthermore, a wound healing assay was performed and it was
identified that treatment with SF1670 increased cell migration,
whereas, overexpression of FER1L4 limited cell migration in A549
and 95D cells (Fig. 5D-F), further
suggesting that FER1L4 inhibited cell metastasis via suppression of
PI3K/Akt signaling pathway. Collectively, the present data
demonstrated that FER1L4 inhibited cell proliferation and
metastasis via downregulation of the PI3K/Akt signaling pathway in
lung cancer cells.
Discussion
Lung cancer is among the most common malignancies in
males and females worldwide (14).
Although substantial efforts have been made in the past decades,
the five-year survival rate of patients with lung cancer is still
low in developed and developing countries due to a late diagnosis
(15). Therefore, it is crucial to
identify novel therapeutic targets against lung cancer.
FER1L4 is a novel lncRNA, which serves significant
roles in various diseases. In 2013, it was identified that FER1L4
is downregulated in human gastric cancer (16) and later, in 2014, its role was
described by Liu et al (17) in gastric cancer. The expression
levels of FER1L4 were subsequently investigated in colon cancer
(18), goat ovarian cancer
(19), hepatocellular carcinoma
(20) and glioma (21). Despite the characterization of its
expression profile, the functional roles of FER1L4 and its
mechanism of action in solid tumors remains unclear (17). In particular, its expression
profile and biological roles in human lung cancer have not yet been
identified. In the present study, it was demonstrated that FER1L4
is downregulated in lung cancer in vivo and in vitro.
Its expression levels were associated with lung cancer
clinicopathological parameters, including TNM staging, lymph node
metastasis, distant metastasis and tumor size. Overexpression of
FER1L4 inhibited cell proliferation and metastasis via regulation
of the PI3K/Akt signaling pathway. Collectively, the present
results suggested that FER1L4 may serve as a potential therapeutic
target for lung cancer.
Numerous signaling pathways are involved in
tumorigenesis, and the PI3K/Akt pathway is an important one
(22). The PI3K/Akt signaling is
aberrantly activated in human malignancies and is associated with
tumor metastasis and drug resistance (23). The PI3K/Akt signaling pathway
regulates the expression of snail family transcriptional repressor
1 and thus epithelial-mesenchymal transition, making the PI3K/Akt
pathway a crucial target in clinical research (24). A principal antagonist of PI3K/Akt
signaling is PTEN, a tumor suppressor that is frequently affected
in a number of types of cancer (25). In the present study, it was
identified that the lncRNA FER1L4 regulated the activity of the
PI3K/Akt signaling pathway in human lung cancer. SF1670, a specific
inhibitor of PTEN, was used as an activator of PI3K/Akt signaling
and the present data suggested that activation of the PI3K/Akt
signaling pathway rescued the inhibitory effects of FER1L4 on cell
proliferation and metastasis in A549 and 95D cells, suggesting that
FER1L4 serves significant roles in human lung cancer via the
repression of the PI3K/Akt signaling pathway.
Collectively, the present study demonstrated that
FER1L4 may serve as a tumor suppressor in lung cancer.
Identification of specific activators of this lncRNA and
identifying the detailed mechanisms underlying its regulatory
effects on cell proliferation and metastasis may guide the
development of novel drugs to treat lung cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG and NW performed the experiments. SW, HC and XA
contributed to the data analysis and discussion. YY designed the
study and prepared the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xiqing Hospital (Tianjin, China). Informed written
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hogan DB: Did Osler suffer from ‘paranoia
antitherapeuticum baltimorensis’? A comparative content analysis of
The Principles and Practice of Medicine and Harrison's Principles
of Internal Medicine, 11th edition. CMAJ. 161:842–845.
1999.PubMed/NCBI
|
|
2
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Majumder S, Chatterjee S, Pal S, Biswas J,
Efferth T and Choudhuri SK: The role of copper in drug-resistant
murine and human tumors. Biometals. 22:377–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng G, Tisch U, Adams O, Hakim M, Shehada
N, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A and Haick H:
Diagnosing lung cancer in exhaled breath using gold nanoparticles.
Nat Nanotechnol. 4:669–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeSantis CE, Siegel RL, Sauer AG, Miller
KD, Fedewa SA, Alcaraz KI and Jemal A: Cancer statistics for
African Americans, 2016: Progress and opportunities in reducing
racial disparities. CA Cancer J Clin. 66:290–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yunusov D, Anderson L, DaSilva LF, Wysocka
J, Ezashi T, Roberts RM and Verjovski-Almeida S: Corrigendum:
HIPSTR and thousands of lncRNAs are heterogeneously expressed in
human embryos, primordial germ cells and stable cell lines. Sci
Rep. 6:327532016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Chen H, Pan T, Jiang C, Zhao Z, Wang
Z, Zhang J, Xu J and Li X: LncRNA ontology: Inferring lncRNA
functions based on chromatin states and expression patterns.
Oncotarget. 6:39793–39805. 2015.PubMed/NCBI
|
|
9
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mourtada-Maarabouni M, Pickard MR, Hedge
VL, Farzaneh F and Williams GT: GAS5, a non-protein-coding RNA,
controls apoptosis and is downregulated in breast cancer. Oncogene.
28:195–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Fang Q and Meng S: Knockdown of Long
noncoding RNA ENST457720 inhibits proliferation of non-small cell
lung cancer cells in vitro and in vivo. Oncol Res.
27:47–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du Y, Hao X and Liu X: Low expression of
long noncoding RNA CDKN2B-AS1 in patients with idiopathic pulmonary
fibrosis predicts lung cancer by regulating the p53-signaling
pathway. Oncol Lett. 15:4912–4918. 2018.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma YM, Peng YM, Zhu QH, Gao AH, Chao B, He
QJ, Li J, Hu YH and Zhou YB: Novel CHOP activator LGH00168 induces
necroptosis in A549 human lung cancer cells via ROS-mediated ER
stress and NF-κB inhibition. Acta Pharmacol Sin. 37:1381–1390.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song H, Sun W, Ye G, Ding X, Liu Z, Zhang
S, Xia T, Xiao B, Xi Y and Guo J: Long non-coding RNA expression
profile in human gastric cancer and its clinical significances. J
Transl Med. 11:2252013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Shao Y, Tan L, Shi H, Chen S and
Guo J: Clinical significance of the low expression of FER1L4 in
gastric cancer patients. Tumour Biol. 35:9613–9617. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yue B, Sun B, Liu C, Zhao S, Zhang D, Yu F
and Yan D: Long non-coding RNA Fer-1-like protein 4 suppresses
oncogenesis and exhibits prognostic value by associating with
miR-106a-5p in colon cancer. Cancer Sci. 106:1323–1332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ling YH, Quan Q, Xiang H, Zhu L, Chu MX,
Zhang XR and Han CY: Expression profiles of differentially
expressed genes affecting fecundity in goat ovarian tissues. Genet
Mol Res. 14:18743–18752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu J, Huang J, Wang W, Xu J, Yin M, Cheng
N and Yin J: Long non-coding RNA Fer-1-like protein 4 acts as a
tumor suppressor via miR-106a-5p and predicts good prognosis in
hepatocellular carcinoma. Cancer Biomark. 20:55–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding F, Tang H, Nie D and Xia L: Long
non-coding RNA Fer-1-like family member 4 is overexpressed in human
glioblastoma and regulates the tumorigenicity of glioma cells.
Oncol Lett. 14:2379–2384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong J, Zhai B, Sun W, Hu F, Cheng H and
Xu J: Activation of phosphatidylinositol 3-kinase/AKT/snail
signaling pathway contributes to epithelial-mesenchymal
transition-induced multi-drug resistance to sorafenib in
hepatocellular carcinoma cells. PLoS One. 12:e1850882017.
View Article : Google Scholar
|
|
25
|
Carracedo A and Pandolfi PP: The PTEN-PI3K
pathway: Of feedbacks and cross-talks. Oncogene. 27:5527–5541.
2008. View Article : Google Scholar : PubMed/NCBI
|