Introduction
Individuals, particularly children, undergoing
cardiac surgery with cardiopulmonary bypass (CPB) may suffer from
severe organ injury (1–3). Indeed, CPB induces an inflammatory
response that can cause many serious complications, including renal
failure, liver dysfunction, acute respiratory distress syndrome
(ARDS) and systemic inflammatory response syndrome (SIRS). Among
these fatal comorbidities, respiratory disorders are one of the
most frequent and severe complications following CPB (4). Therefore, preventative strategies to
mitigate the pathogenesis of CPB-induced pulmonary disorders are
important for improving the prognosis of patients undergoing
CPB-related cardiac surgeries.
Many potential pathways have been suggested to
underlie CPB-induced pathogenesis of inflammatory pulmonary injury.
Previous studies indicate that adenosine and its agonists exert an
anti-inflammatory response, which may potentially be used as a
preventative strategy to attenuate CPB-related organ injury
(5,6). Adenosine receptors, including four
G-protein-coupled receptor isoforms (A1, A2a, A2b and A3), are
widely distributed in the nervous, cardiovascular and respiratory
systems, as well as in other tissues. The A2a receptor is
predominantly expressed in inflammatory cells. Increased
intracellular cyclic adenosine monophosphate (cAMP) levels activate
the A2a receptor, which subsequently inhibits activation of
inflammatory cells and the production and release of plasma
inflammation markers, thereby exerting an anti-inflammatory effect
(7). Indeed, a previous study by
Ohta et al (8) showed that
the A2a receptor had a non-redundant role in attenuating
inflammation and tissue damage in vivo. Moreover, activation
of the A2a receptor has been demonstrated to reduce the recruitment
and adhesion of polymorphonuclear neutrophils to pulmonary
endothelial cells in vitro (9).
In a pilot study, activation of the A2a receptor
with agonist ATL313 attenuated lung injury following CPB in adult
rats (6). However, the potential
protective role of A2a receptor activation against CPB-induced
pulmonary injury, particularly in juveniles, has not been
comprehensively evaluated. Investigating the protective mechanisms
of A2a receptor activation against CPB-induced lung injury in
juveniles is of particular significance as the inflammatory
response induced by CPB in children is likely different than the
response in adults (10).
Therefore, in the present study, a juvenile rat model of CPB was
utilized to determine whether the A2a receptor agonist CGS21680
[2-[4-(2-p-carboxyethyl)phenylamino]-5′-N-ethylcarboxamidoadenosine]
attenuates CPB-induced lung injury.
Materials and methods
Animal care
In total, 36 male juvenile Wistar rats (age, 6
weeks; weight, 176.66±8.57 g) were obtained from The Experimental
Animal Center of Anhui Medical University. Animals received
standard laboratory chow and water ad libitum. Rats were
housed in standard stainless steel cages at a constant-temperature
(22°C) and humidity (55%) under a 12-h light/dark cycle (lights on
from 7:30 a.m. to 7:30 p.m.). All animals received humane care in
compliance with the ‘Guide for the Care and Use of Laboratory
Animals’ prepared by the National Institutes of Health (NIH;
Bethesda, MD, USA). Study approval was obtained from the Anhui
Medical University Ethics Committee (Hefei, China) prior to
initiating the study.
Experimental design
Juvenile rats were randomly divided into three
groups with 12 animals per group: The Sham group (underwent
heparinization and cannulation only); the CPB group (underwent 60
min of CPB with normal priming solution); and the CGS group
(underwent 60 min of CPB with CGS21680 added to the normal priming
solution prior to the initiation of CPB).
Surgical procedures
The juvenile rat model of CPB was established as
previously described by Dong et al (11) for adult rats. Briefly, juvenile
rats were anesthetized via intraperitoneal administration of 3%
sodium pentobarbital (50 mg/kg). After achievement of adequate
anesthesia, the right femoral artery was identified through a
1.5-cm groin incision and cannulated with a 24-gauge intravenous
catheter (BD Biosciences, Franklin Lakes, NJ, USA) to serve as the
artery inflow cannula. For the left femoral artery, cannulation
with a 24-gauge intravenous catheter was used to measure mean
arterial pressure (MAP) and obtain blood for arterial blood gas
analysis (i-STAT; Abbott Point of Care, Princeton, NJ, USA). A
modified 20-gauge multi-orifice intravenous catheter (BD
Biosciences) was inserted into the right internal jugular vein and
gently placed into the right atrium. Heparin (3 mg/kg) was
administered via the venous cannula to maintain activated clotting
times (ACT) (~480 sec).
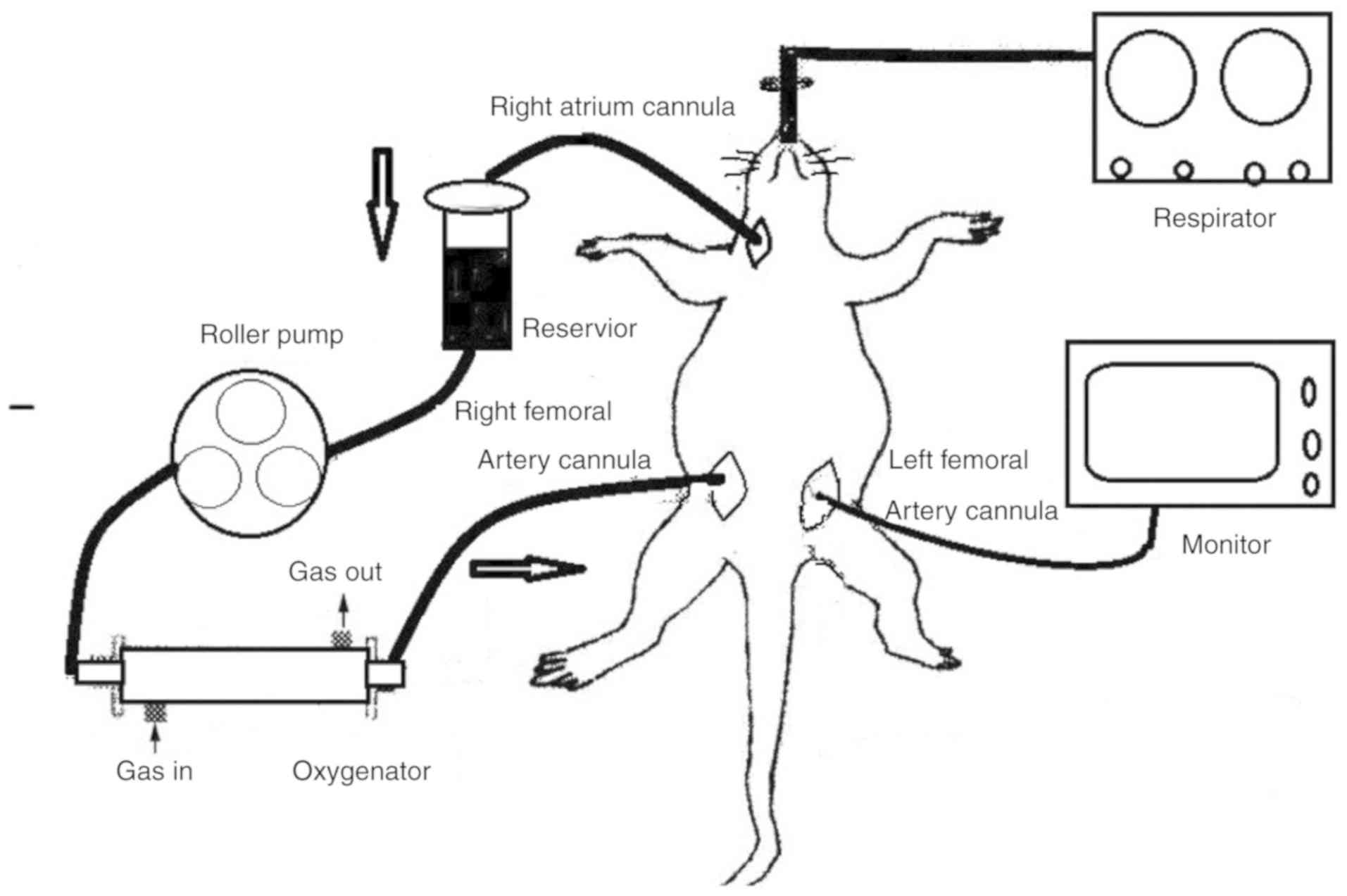
CPB circuit
The CPB circuit included a sterile 20-ml venous
reservoir, a hollow-fiber membrane oxygenator, a roller pump
(BT100-2J; Longer Pump, Baoding, China), a silicon tubing with an
inner diameter of 1.6 mm for the arterial line to monitor blood
pressure during CPB, and a specially designed tubing to connect the
CPB circuit. For the CPB group, the circuit was assembled and
primed with 12 ml whole blood obtained from 2 heparinized (3 mg/kg)
donor rats, 3 ml hydroxyethyl starch, 3 ml Ringer's lactate
solution, 1 ml mannitol and 1 ml sodium bicarbonate. For the CGS
group, CGS21680 (Tocris Bioscience, Bristol, UK) was added to the
priming solution at a dosage of 100 µg/kg. We determined this dose
based on previous reports which showed that 100 µg/kg CGS21680
attenuated oxidative organ injury related to ischemia, which was
consistent with the hypothesized effect of CGS21680 in this study
(12). Moreover, our preliminary
experiments with 200 µg/kg CGS21680 resulted in excessive mortality
in juvenile rats. Based on the literature and our observations, we
chose to use 100 µg/kg CGS21680 in the present study. After
cannulation and heparinization, juvenile rats were connected to the
CPB circuit (Fig. 1). The flow
rate was slowly adjusted to maintain a mean blood pressure of 70
mmHg. After reaching a final flow rate of 100 ml/kg/min, the CPB
procedure was maintained for 60 min. Gas flow to the oxygenator,
which consisted of 100% O2, was set at 1 l/h to sustain
normal values of arterial blood gases and potential of hydrogen
(PH). At the end of the CPB, blood flow was slowly reduced. When
all animals were separated from the CPB circuit, the rats were
mechanically ventilated and monitored for 60 min
post-operatively.
Physiologic data and sample
collection
MAP and arterial blood gas were monitored during the
pre-, intra- and post-operative periods. Blood samples were
collected from the left femoral artery in each group at baseline,
30 and 60 min during CPB, and 60 min after the cessation of CPB.
Plasma was collected by centrifugation at 1,600 × g for 20 min. The
plasma was stored at −80°C, which was subsequently used to
determine levels of interleukin (IL)-1β and glutathione peroxidase
(GSH-PX). The chest was opened after surgery, and the lung was
isolated and divided into two parts. One part was stored at −80°C
for tumor necrosis factor-α (TNF-α) and myeloperoxidase (MPO)
analyses. The remaining lung was fixed in a 4% formaldehyde
solution for histological observations. Moreover, lung injury
severity was quantitatively evaluated according to the number of
macrophages, the amount of interstitial infiltrate, and the
presence of alveolar edema as previously indicated (13).
Cytokine assays
Plasma levels of IL-1β and lung tissue TNF-α levels
were assessed using enzyme-linked immunosorbent assay (ELISA) kits
specific for rats according to the manufacturer's instructions
(Shanghai Crystal Day Biotech Co., Ltd., Shanghai, China).
MPO assay
MPO lung expression, used as an index of tissue
neutrophil accumulation and sequestration, was determined using an
MPO enzyme-linked immunosorbent assay (ELISA) kit (Nanjing
Jiancheng Biological Product, Nanjing, China). MPO activity in lung
tissue was expressed as U/g protein.
GSH-PX assay
GSH-PX activity in the plasma was measured using
both hydrogen peroxide and tert-butyl hydroperoxide as substrates
(Nanjing Jiancheng Biological Product). Plasma GSH-PX activity was
expressed as unit of enzyme activity.
Histologic analysis
Pulmonary tissue fixed in 4% formaldehyde was
processed and paraffin embedded. Tissue sections (5-µm) were then
stained with hematoxylin and eosin (H&E) and examined under a
light microscope. Histological sections were evaluated by an
experienced pathologist blinded to the treatment.
Statistical analysis
Continuous values are expressed as mean ± standard
deviation (SD). The SPSS statistics software package (version 17.0;
SPSS, Inc., Chicago, IL, USA) was used for data analysis. One-way
analysis of variance (ANOVA) was used to determine whether
significant differences existed among the three groups. The post
hoc test used with ANOVA was Student-Newman-Keuls (SNK) method. A
P-value <0.05 was considered to indicate a statistically
significant result.
Results
Physiological data
Surgery was successfully completed in all rats, and
all rats survived the CPB procedure. Physiological parameters,
including blood pressure, oxygen status, carbon dioxide status,
hemoglobulin, and serum electrolytes, measured during the pre-,
intra- and post-operation time-points are shown in Table I. In our animal model, all
physiological parameters remained stable during CPB, which
indicates the potential safety of CGS21680 pretreatment. Moreover,
our CPB juvenile rat model appeared to be consistent with clinical
CPB in children.
 | Table I.Physiological data from juvenile rats
undergoing CPB. |
Table I.
Physiological data from juvenile rats
undergoing CPB.
| Physiological
parameters | Baseline | CPB (30 min) | CPB (60 min) | Post-CPB (60
min) |
|---|
| MAP (mmHg) |
|
|
|
|
| Sham | 85±5.8 | 85±8 | 84±6 | 85±6.8 |
| CPB | 85±6.8 | 72±12.6 | 71±11.3 | 67±13.2 |
| CGS | 84±5.1 | 66±9.6 | 61±18.7 | 60±9.8 |
| Hb (g/dl) |
|
|
|
|
| Sham | 11.7±1.68 | 11.8±1.78 | 11.7±1.76 | 11.6±1.79 |
| CPB | 11.6±1.83 | 6.5±0.78 | 6.5±0.7 | 6.3±0.91 |
| CGS | 11.9±1.89 | 7.7±0.92 | 7.4±0.87 | 6.9±1.16 |
| PaO2
(mmHg) |
|
|
|
|
| Sham | 85.1±6.93 | 86.2±7.96 | 85.4±5.92 | 84.6±4.76 |
| CPB | 85.7±6.72 | 328±54.65 | 331.5±52.68 | 320±64.16 |
| CGS | 86±7.57 | 334.1±40.84 | 328.8±43.05 | 318.3±55.52 |
| Na (mmol/l) |
|
|
|
|
| Sham | 130.6±2.35 | 131.3±2.34 | 130.9±5.57 | 130.3±2.77 |
| CPB | 130.9±3 | 131.4±3.15 | 130.5±2.94 | 130.4±2.50 |
| CGS | 130.8±2.29 | 130.8±2.73 | 131.4±2.81 | 131.8±3.01 |
| K (mmol/l) |
|
|
|
|
| Sham | 4.3±0.35 | 4.2±0.38 | 4.3±0.33 | 4.3±0.36 |
|
CPB | 4.4±0.47 | 4.2±0.44 | 4.3±0.42 | 4.3±0.42 |
|
CGS | 4.4±0.39 | 4.2±0.37 | 4.3±0.36 | 4.1±0.36 |
TNF-α and MPO levels in lung
tissues
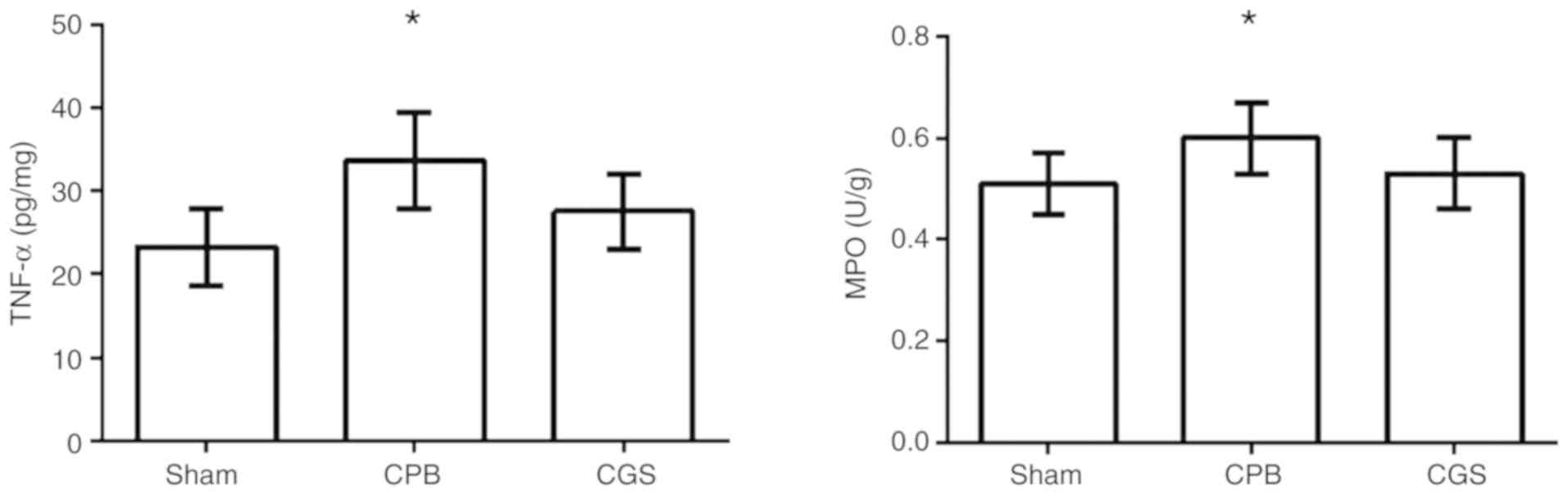
We observed a significant increase in TNF-α and MPO
levels in the lung tissues in the CPB group compared to the Sham
group. However, CGS21680 pretreatment significantly reduced the
TNF-α and MPO levels (Table II
and Fig. 2), indicating a
potential anti-inflammatory effect of CGS21680.
 | Table II.TNF-α (pg/mg) and MPO (U/g) levels in
lung tissue in each group. |
Table II.
TNF-α (pg/mg) and MPO (U/g) levels in
lung tissue in each group.
| Parameters | Sham group | CPB group | CGS group |
|---|
| TNF-α (pg/mg
protein) | 23.26±4.57 |
33.63±5.86a |
27.51±4.51b |
| MPO (U/g
protein) | 0.51±0.06 |
0.60±0.07a |
0.53±0.07b |
Plasma IL-1β and GSH-PX levels
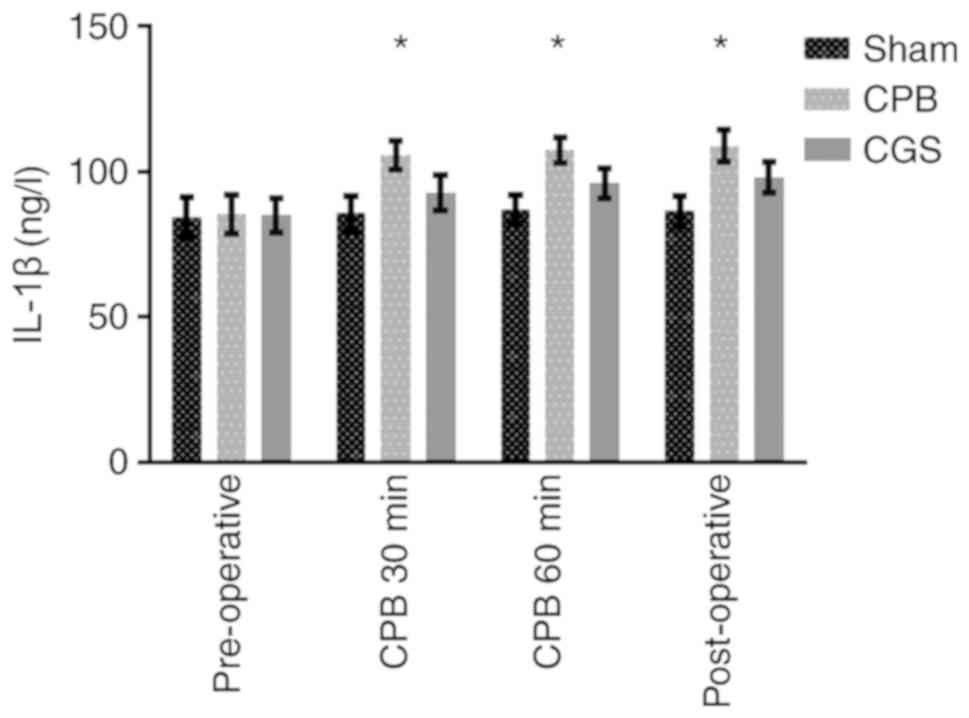
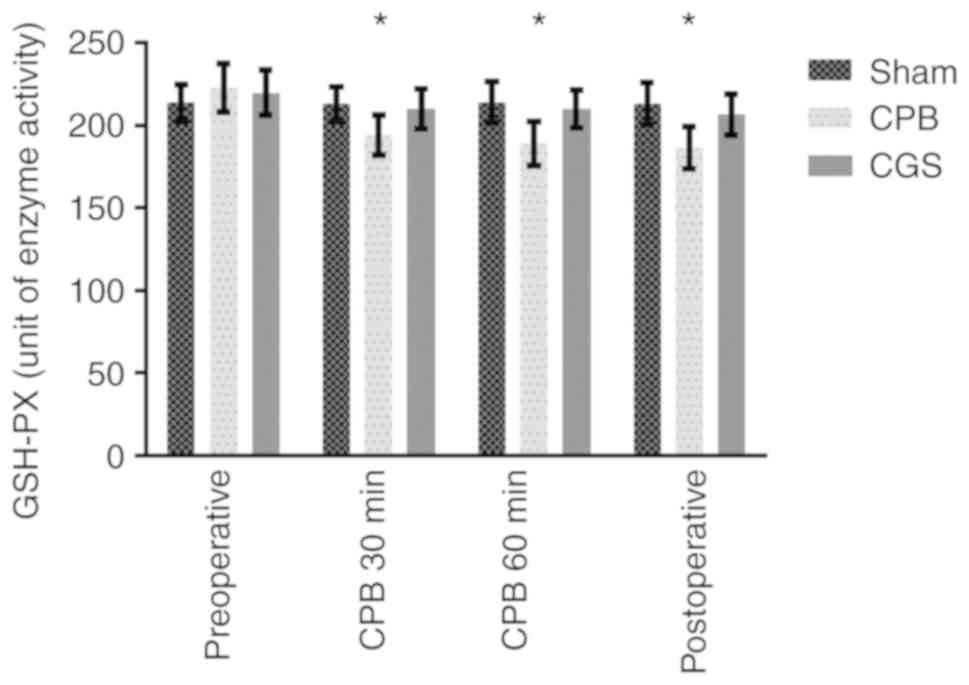
We observed a significant decrease in plasma GSH-PX
in the CPB group compared to the Sham group. CGS21680 treatment
largely reversed this effect. Similarly, plasma IL-1β levels in the
CPB group were significantly increased compared to the Sham group,
which was also reversed by CGS21680 treatment (Table III and Figs. 3 and 4). These results further confirm the
anti-inflammatory effect of CGS21680 pretreatment.
 | Table III.Plasma levels of IL-1β and GSH-PX
activity in each group. |
Table III.
Plasma levels of IL-1β and GSH-PX
activity in each group.
| Parameters | Baseline | CPB (30 min) | CPB (60 min) | Post-CPB (60
min) |
|---|
| IL-1β (ng/l) |
|
|
|
|
|
Sham | 84.44±6.89 | 85.67±6.06 | 86.98±4.95 | 86.55±5.08 |
|
CPB | 85.32±6.69 |
105.68±4.99a |
107.33±4.22a |
108.86±5.61a |
|
CGS | 84.92±5.74 |
92.73±5.98b |
96.10±5.11b |
97.96±5.25b |
| GSH-PX (unit of
enzyme activity) |
|
|
|
|
|
Sham | 213.56±10.93 | 212.96±10.34 | 214.14±12.65 | 213.03±12.77 |
|
CPB | 222.65±14.46 |
194.18±12.11a |
188.95±13.44a |
186.36±12.74a |
|
CGS | 219.77±13.79 |
210.02±12.20b |
210.00±11.50b |
206.74±12.38b |
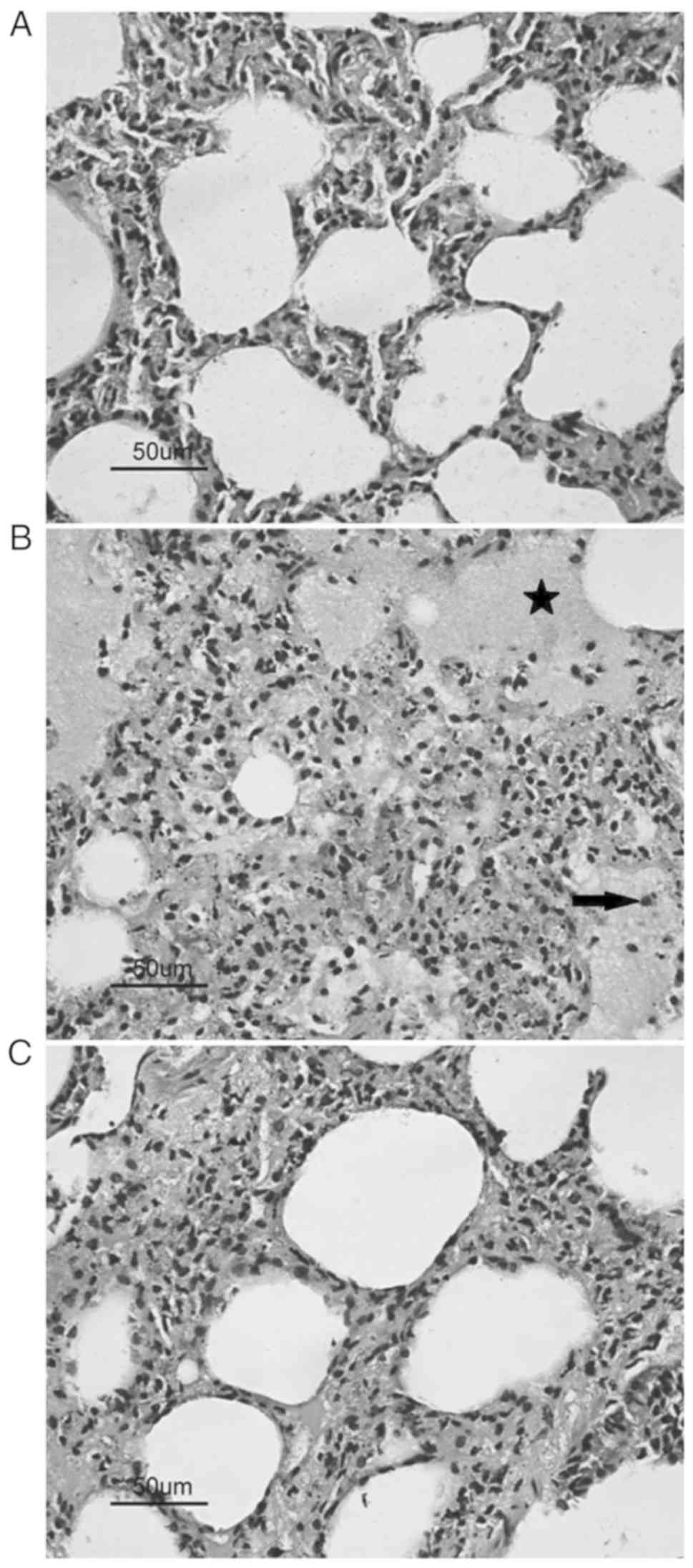
Histological results
Our histological analysis demonstrated a
considerable amount of damaged pneumocytes, severe edema, and
increased alveolar macrophages in pulmonary tissues from the CPB
group compared to the Sham group. Moreover, CGS21680 pretreatment
significantly attenuated pulmonary inflammatory after CPB, as
evidenced by less edema and fewer alveolar macrophages in the
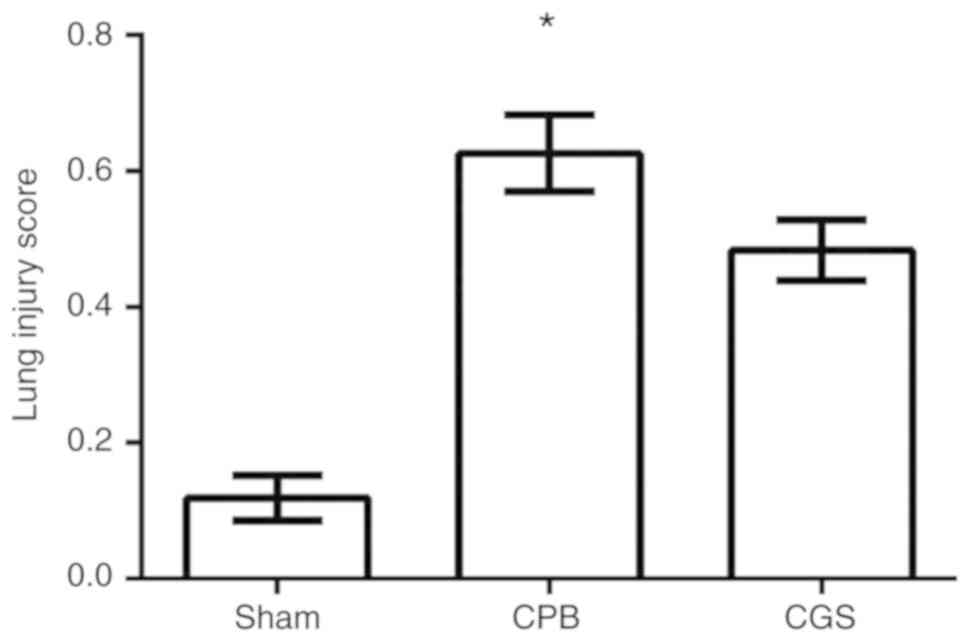
CGS21680-treated group (Fig. 5).
These results are consistent with the lung injury score differences
in each group as presented in Fig.
6.
Discussion
In the present study, it was confirmed that
cardiopulmonary bypass (CPB) is associated with inflammatory lung
injury in juvenile rats. More importantly, it was demonstrated that
pretreatment with an adenosine A2a receptor agonist, CGS21680,
significantly attenuated inflammatory cell infiltration into the
pulmonary tissues following CPB. These anti-inflammatory effects of
CGS21680 pretreatment were further evidenced by upregulation of an
anti-inflammatory biomarker (GSH-PX) and inhibition of inflammatory
cytokines (IL-1β, TNF-α and MPO). CGS21680 pretreatment did not
significantly affect any physiological parameter during CPB,
suggesting the potential safety and feasibility of CGS21680. Taken
together, these results demonstrated that CGS21680 pretreatment
administered in the CBG priming solution attenuated pulmonary
injury in juvenile rats, which supports the use of CGS21680 as a
preventative strategy against CPB-induce pulmonary disorders.
CBG can trigger an abundant inflammatory response,
as evidenced by platelet activation, increased cytokine production,
activated complement and coagulation cascades, and endothelial
dysfunction, in both patients and animal models. These changes, in
turn, increase susceptibility to various levels of multi-organ
dysfunction, including cardiovascular, lung and liver tissues
(14–17). The mechanisms underlying the
CBP-induced inflammatory response have been investigated (14,15),
but still remain unclear. Oxidative/free radicals play an important
role in acute lung injury. GSH-PX is an important protective
antioxidant against free radicals and other oxidants and has been
implicated in attenuating inflammation. An increase in free
radicals can be accompanied by a significant decrease in superoxide
dismutase and GSH-PX activity (18). Adenosine has been reported to have
a strong anti-inflammatory capacity and exert anti-ischemic
properties in response to ischemia/reperfusion induced cell injury
(19,20). The adenosine A2a receptor subtype
is the predominant adenosine receptor expressed on inflammatory
cells, and the majority of the anti-inflammatory effects of
adenosine may depend on regulation of the A2a receptor (19–21).
Therefore, we investigated the anti-inflammatory effect of a highly
selective adenosine A2a receptor agonist, CGS21680.
The A2a receptor has been identified in nearly all
inflammatory cells, including macrophages, eosinophils,
neutrophils, endothelial cells, mast cells and T-lymphocytes, and
the subsequent anti-inflammatory properties of A2a receptor
activation have been shown to inhibit the release of inflammatory
mediators, such as interferon, from these cells (22,23).
In contrast, A2a receptor agonists have been demonstrated to
downregulate the activity of several adhesion molecules, including
vascular cell adhesion molecule-1, intracellular adhesion
molecule-1, P-selectin and platelet cell adhesion molecule
(24–26). Notably, many of these adhesion
molecules have been shown to significantly influence the
propagation of CPB-induced inflammation (26). At the molecular level, the
biological effects triggered by the A2a receptor can increase cAMP
production followed by adenylate cyclase activation. The increase
in cAMP levels can then stimulate cAMP-dependent kinase, which in
turn activates several pathways involving calcium and potassium
channels, cAMP responsive element-binding, and phospholipase C
(27,28). These pathways are proposed to
collectively inhibit inflammation. However, the exact mechanism by
which A2a receptor activation attenuates the inflammatory response
induced by CPB has not been well described. Therefore, more studies
should be performed to further elucidate the causal relationship
between A2a receptor activation and CPB-induced inflammation.
Clinically, children are more susceptible than
adults to CPB-induced inflammatory lung injury. This increased
susceptibility is attributed to higher metabolic demands, reactive
pulmonary vasculature and immature organ systems in children
compared to adults (16,29). Therefore, a juvenile rat model of
CPB was utilized in this study, as it has more practical
implications. Currently, most investigators use young, large
animals, such as sheep, pigs and dogs, in CPB studies. However,
most large animals are not inbred pure species, and therefore yield
poor reproducibility. There are also other limitations of large
animal models, such as inadequate supply of laboratory reagents and
incremental research costs. Based on these limitations, we selected
juvenile rats for this CPB study, which provided the following
advantages. i) The CPB circuit was primed with blood, crystalloid
and colloid solutions, which was to some extent consistent with
clinical pediatric CPB priming. ii) Successful CPB model
establishment in juvenile rats may have direct downstream clinical
implications for pediatric researchers. To the best of our
knowledge, this is the first study to investigate the role of an
adenosine A2a receptor agonist in CPB-induced inflammatory
pulmonary injury in a clinically relevant juvenile rat model.
However, the potential prophylactic role of CGS21680 against
CPB-induced lung injury in children should be confirmed in clinical
trials.
In summary, the present study showed that
pretreatment with the adenosine A2a receptor agonist CGS21680
effectively attenuated CPB-induced inflammatory lung injury in a
juvenile rat model. Activation of the adenosine A2a receptor with
CGS21680 may be a preventative strategy for attenuating
inflammatory lung injury during CPB.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Anhui
Provincial Health Department Foundation of China (grant no.
09B132).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XK and JG designed the study. XK, YZ, and JG
analyzed the data and drafted the manuscript. XK, YZ, and YH
prepared the experimental materials and performed the experiments.
XK, YZ, and YH interpreted data, performed statistical analysis,
and analyzed the results. XK and JG revised the manuscript. All
authors read and approved the final version of manuscript and
agreed to be accountable for all aspects of the research in
insuring that the accuracy and integrity of any part of the work
are appropriately investigated and resolved.
Ethics approval and consent to
participate
Ethical approval was obtained from The Anhui Medical
University Ethics Committee (Hefei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caputo M, Mokhtari A, Miceli A, Ghorbel
MT, Angelini GD, Parry AJ and Suleiman SM: Controlled reoxygenation
during cardiopulmonary bypass decreases markers of organ damage,
inflammation, and oxidative stress in single-ventricle patients
undergoing pediatric heart surgery. J Thorac Cardiovasc Surg.
148:792–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li JA, Liu YL, Liu JP and Li XF: Pulmonary
artery perfusion with HTK solution prevents lung injury in infants
after cardiopulmonary bypass. Chin Med J (Engl). 123:2645–2650.
2010.PubMed/NCBI
|
|
3
|
Pappachan VJ, Brown KL and Tibby SM:
Paediatric cardiopulmonary bypass surgery: The challenges of
heterogeneity and identifying a meaningful endpoint for clinical
trials. Intensive Care Med. 43:113–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salameh A, Greimann W, Vollroth M, Dhein
S, Bahramsoltani M and Dahnert I: Lung protection in
cardio-pulmonary bypass. J Physiol Pharmacol. 68:99–116.
2017.PubMed/NCBI
|
|
5
|
Davidson JA, Urban T, Tong S, Twite M,
Woodruff A, Wischmeyer PE and Klawitter J: Alkaline phosphatase,
soluble extracellular adenine nucleotides, and adenosine production
after infant cardiopulmonary bypass. PLoS One. 11:e01589812016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lisle TC, Gazoni LM, Fernandez LG, Sharma
AK, Bellizzi AM, Shifflett GD, Laubach VE and Kron IL: Inflammatory
lung injury after cardiopulmonary bypass is attenuated by adenosine
A2A receptor activation. J Thorac Cardiovasc Surg. 136:1280–1287.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lappas CM, Sullivan GW and Linden J:
Adenosine A2A agonists in development for the treatment of
inflammation. Expert Opin Investig Drugs. 14:797–806. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohta A and Sitkovsky M: Role of
G-protein-coupled adenosine receptors in downregulation of
inflammation and protection from tissue damage. Nature.
414:916–920. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vuorimaa A, Rissanen E and Airas L: In
vivo PET imaging of adenosine 2A receptors in neuroinflammatory and
neurodegenerative disease. Contrast Media Mol Imaging.
2017:69758412017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jänicke B and Coper H: The effects of
prenatal alcohol exposure on the behavior of rats during their life
span. J Gerontol. 48:B156–B167. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong GH, Xu B, Wang CT, Qian JJ, Liu H,
Huang G and Jing H: A rat model of cardiopulmonary bypass with
excellent survival. J Surg Res. 123:171–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
White PJ, Rose'Meyer RB and Hope W:
Changes in adenosine receptors mediating hypotension in
morphine-dependent rats. Eur J Pharmacol. 294:215–220. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matute-Bello G, Downey G, Moore BB,
Groshong SD, Matthay MA, Slutsky AS and Kuebler WM; Acute Lung
Injury in Animals Study Group, : An official American Thoracic
Society workshop report: Features and measurements of experimental
acute lung injury in animals. Am J Respir Cell Mol Biol.
44:725–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chee YR, Watson RW, McCarthy J, Chughtai
JZ, Nölke L and Healy DG: High dose statin prophylaxis in
cardiopulmonary bypass related surgery: Clinical utility. J
Cardiothorac Surg. 12:202017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujii Y, Tanabe T, Yamashiro T, Shirai M,
Takewa Y and Tatsumi E: Effect of Hydroxyethyl starch priming on
the systemic inflammatory response and lung edema after
cardiopulmonary bypass in a rat model. ASAIO J. 63:618–623. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boehne M, Sasse M, Karch A, Dziuba F,
Horke A, Kaussen T, Mikolajczyk R, Beerbaum P and Jack T: Systemic
inflammatory response syndrome after pediatric congenital heart
surgery: Incidence, risk factors, and clinical outcome. J Card
Surg. 32:116–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bronicki RA and Hall M: Cardiopulmonary
Bypass-induced inflammatory response: Pathophysiology and
treatment. Pediatr Crit Care Med 17 (8 Suppl 1). S272–S278. 2016.
View Article : Google Scholar
|
|
18
|
Nader MA and Baraka HN: Effect of
betulinic acid on neutrophil recruitment and inflammatory mediator
expression in lipopolysaccharide-induced lung inflammation in rats.
Eur J Pharm Sci. 46:106–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sharma AK, LaPar DJ, Stone ML, Zhao Y,
Mehta CK, Kron IL and Laubach VE: NOX2 activation of natural killer
T cells is blocked by the adenosine A2A receptor to inhibit lung
ischemia-reperfusion injury. Am J Respir Crit Care Med.
193:988–999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wagner CE, Pope NH, Charles EJ, Huerter
ME, Sharma AK, Salmon MD, Carter BT, Stoler MH, Lau CL, Laubach VE
and Kron IL: Ex vivo lung perfusion with adenosine A2A receptor
agonist allows prolonged cold preservation of lungs donated after
cardiac death. J Thorac Cardiovasc Surg. 151:538–545. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stone ML, Sharma AK, Mas VR, Gehrau RC,
Mulloy DP, Zhao Y, Lau CL, Kron IL, Huerter ME and Laubach VE: Ex
vivo perfusion with adenosine A2A receptor agonist enhances
rehabilitation of murine donor lungs after circulatory death.
Transplantation. 99:2494–2503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lappas CM, Rieger JM and Linden J: A2A
adenosine receptor induction inhibits IFN-gamma production in
murine CD4+ T cells. J Immunol. 174:1073–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin EY, Wang L, Zemskova M, Deppen J, Xu
K, Strobel F, García AJ, Tirouvanziam R and Levit RD: Adenosine
production by biomaterial-supported mesenchymal stromal cells
reduces the innate inflammatory response in myocardial
ischemia/reperfusion injury. J Am Heart Assoc. 7(pii):
e0069492018.PubMed/NCBI
|
|
24
|
Wang X, Gao M, Schouteden S, Roebroek A,
Eggermont K, van Veldhoven PP, Liu G, Peters T,
Scharffetter-Kochanek K, Verfaillie CM and Feng Y: Hematopoietic
stem/progenitor cells directly contribute to arteriosclerotic
progression via integrin β2. Stem Cells. 33:1230–1240. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chhabra P, Linden J, Lobo P, Okusa MD and
Brayman KL: The immunosuppressive role of adenosine A2A receptors
in ischemia reperfusion injury and islet transplantation. Curr
Diabetes Rev. 8:419–433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi Y, Sawa Y, Nishimura M, Tojo SJ,
Fukuyama N, Nakazawa H and Matsuda H: P-selectin participates in
cardiopulmonary induced inflammatory response in association with
nitric oxide and peroxynitrite production. J Thorac Cardiovasc
Surg. 120:558–565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fredholm BB, Chern Y, Franco R and
Sitkovsky M: Aspects of the general biology of adenosine A2A
signaling. Prog Neurobiol. 83:263–276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ballesteros-Yáñez I, Castillo CA, Merighi
S and Gessi S: The role of adenosine receptors in psychostimulant
addiction. Front Pharmacol. 8:9852018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kozik DJ and Tweddell JS: Characterizing
the inflammatory response to cardiopulmonary bypass in children.
Ann Thorac Surg. 81 (Suppl):S2347–S2354. 2006. View Article : Google Scholar : PubMed/NCBI
|