Introduction
Lung cancer (LC) is the most common malignant tumor
globally, and the leading cause of cancer-associated mortality
(1). The incidence of LC is second
to prostate cancer in all malignant tumors in men and second only
to breast cancer in women (1). LC
is separated into two subtypes, small cell lung carcinoma (SCLC)
and non-small cell lung carcinoma (NSCLC); NSCLC accounts for
70–85% of total LC cases (2). Due
to the absence of clinical symptoms during early stages, patients
are frequently diagnosed with LC at advanced stages, therefore
missing the ideal period for operation; the total surgical removal
rate of NSCLC is only ~25% (3).
Radiotherapy is an effective treatment for malignant tumors,
including LC (4), and is
frequently combined with exeresis and chemotherapy in the treatment
of LC (5).
Radiotherapy mainly involves treatment with ionizing
radiation (IR), which, by direct or indirect actions, leads to the
damage of cellular DNA and subsequently results in cell death
(6,7). The efficacy of radiotherapy depends
on the sensitivity of tumor cells to radiation; tumors can become
insensitive to radiation via the radioresistance of tumor cells or
the protection of the tumor microenvironment, leading to the repair
of damaged cells following IR (7,8).
NSCLC cells frequently develop varying degrees of radiation
tolerance in the middle and later stages of radiotherapy, reducing
its efficacy (9–11). Therefore, it is important to
identify the underlying mechanisms to increase the radiosensitivity
of NSCLC tumors.
Heat shock proteins (HSPs) are a family of important
proteins with molecular chaperone functions in stress situations,
including cold, infection, starvation, trauma, poisoning, and
particularly in high temperature conditions; HSPs are involved in
the folding, assembly and modification of proteins, and promote the
tolerance of cells to stress (12,13).
HSP27 (also known as HSPB1) is an important member of the HSP
family; HSP27 is involved in endoplasmic reticulum stress
associated with protein misfolding, and is a regulator that
contributes to the development of various types of tumors (14–16).
HSP27 expression has been reported to be upregulated in various
tumor cells, including NSCLC, hepatocellular carcinoma and prostate
cancer cells (17–20). Additionally, it was observed that
HSP27 was a radioresistance-associated protein in nasopharyngeal
carcinoma (21); however, whether
HSP27 is involved in radiosensitivity in NSCLC remains unclear.
In the present study, the HSP27 gene was silenced in
human NSCLC A549 cells, and the effects of HSP27 silencing on the
viability, apoptosis, mitochondrial membrane potential (MMP) and
cell cycle of irradiated cells was determined. Additionally, the
underlying molecular mechanisms were investigated.
Materials and methods
Cell culture and transfection
The human lung adenocarcinoma A549 cell line was
purchased from American Type Culture Collection (Manassas, VA,
USA). A549 cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin (Beyotime Institute of Biotechnology,
Shanghai, China) at 37°C with 5% CO2. HSPB1-small
interfering RNA (si-HSPB1) and siRNA negative control (NC) plasmids
were obtained from Genewiz Inc. (Suzhou, China). The sequences of
siRNA used in the current study were as follows: si-HSPB1 sense,
5′-UGAGACUGCCGCCAAGUAAUU-3′ and antisense,
5′-UUACUUGGCGGCAGUCUCAUU-3′; si-NC sense,
5′-UAGCGACUAAACACAUCAAUU-3′ and antisense,
5′-UUGAUGUGUUUAGUCGCUAUU-3′. A549 cells (2×105
cells/well) were transfected with si-HSPB1 (50 nM) or si-NC (50 nM)
using Lipofectamine® 3000 (Thermo Fisher Scientific,
Inc.). The mixture containing si-HSPB1 or si-NC plasmids (15 µg),
transfection reagents and A549 cell was incubated for 48 h at 37°C.
Following transfection for 48 h, the cells were harvested and used
for the subsequent experiments.
Cell Counting Kit-8 (CCK-8) assay
Following transfection, cells (2×103
cells/well, 96-well plates) were subjected to irradiation (0 and 6
Gy) for 24, 48, and 72 h using a PRIMUS™ Linear Accelerator
(Siemens AG, Munich, Germany); the dose rate was 300 cGy/min.
Following treatment, CCK-8 reagent (MedChemExpress, Monmouth
Junction, NJ, USA) was added to the cells, and cells were further
incubated for 4 h at 37°C. The absorbance was detected at 450 nm
using a light absorption microplate reader (SpectraMax iD3;
Molecular Devices, LLC, Sunnyvale, CA, USA).
Cell apoptosis assay
Following transfection and irradiation, cells were
washed three times with PBS. Subsequently, cells were incubated
with 1X Annexin Binding Buffer (BestBio, Shanghai, China) on ice
for 3 min and then stained using Annexin V-fluorescein
isothiocyanate and propidium iodide (PI) (BestBio) in darkness for
25 min at room temperature. Cell apoptosis was detected using a
flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA, USA)
equipped with CellQuest software (version 3.3; BD Biosciences).
Mitochondrial membrane potential (MMP)
assay
Cells were digested by 0.25% EDTA-trypsin (Thermo
Fisher Scientific, Inc.) for 5 min at 37°C and stained with JC-1
solution (MedChemExpress) for 20 min at 37°C. Then, cells were
centrifuged at 800 × g for 2 min at 4°C. Cells were collected and
the MMP was investigated using a flow cytometer.
Cell cycle assay
The cell cycle was investigated using a DNA Content
Quantitation Assay (Cell Cycle) kit (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) according to the
manufacturer's protocols. Briefly, following transfection and
irradiation, cells were treated with 40X RNase A at 37°C for 20
min. Then, cells were stained with PI solution at 4°C for 15 min.
Cell cycle distribution was analyzed using a flow cytometer.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to isolate total RNA from cells. cDNA
was synthesized using the ABScript II cDNA First Strand Synthesis
kit (ABclonal Biotech Co., Ltd., Wuhan, China) according to the
manufacturer's protocols. The RT reaction was performed at 42°C for
15 min, followed by reverse transcriptase inactivation at 85°C for
15 sec. A SYBR Premix Taq™ II kit (Dalian Meilun Biotech Co., Ltd.,
Dalian, China) was used to amplify cDNA according to the
manufacturer's protocols. The qPCR thermocycling conditions were:
Preliminary denaturation at 95°C for 1 min, followed by 30 cycles
of denaturation at 94°C for 30 sec, annealing at 56°C for 40 sec
and extension at 72°C for 60 sec, followed by a final extension
step at 72°C for 7 min. The primers were presented in Table I. GAPDH was used as an internal
reference. The 2−ΔΔCq method was (22) used to quantify relative gene
expression.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Sequence
(5′-3′) | Product size
(bp) |
|---|
| HSPB1-Forward |
AGTGGTCGCAGTGGTTAGG | 223 |
| HSPB1-Reverse |
TCCTTGGTCTTGACCGTCAG |
|
| Cyclin
B1-Forward |
TTGTGTGCCCAAGAAGATGC | 205 |
| Cyclin
B1-Reverse |
GAAGTGCAAAGGTAGAGGCC |
|
| Cyclin
G1-Forward |
CCTTCTGTGTTGGCATTGTCT | 221 |
| Cyclin
G1-Reverse |
GTACGCCCAGAAACAATCCA |
|
| Bcl-2-Forward |
TTCTTTGAGTTCGGTGGGGT | 209 |
| Bcl-2-Reverse |
CTTCAGAGACAGCCAGGAGA |
|
| Bax-Forward |
CATCATGGGCTGGACATTGG | 226 |
| Bax-Reverse |
CCTCAGCCCATCTTCTTCCA |
|
| GAPDH-Forward |
CCATCTTCCAGGAGCGAGAT | 222 |
| GAPDH-Reverse |
TGCTGATGATCTTGAGGCTG |
|
Western blot assay
Total protein was isolated from cells using
radioimmunoprecipitation assay buffer (Beijing Solarbio Science
& Technology Co., Ltd.). Cytosolic and mitochondrial proteins
were extracted using a Cytoplasmic and Mitochondrial Protein
Extraction kit (Sangon Biotech Co., Ltd., Shanghai, China). The
Bradford method was employed to determine the concentration of
protein extracts. Proteins (30 µg/sample) were separated via 10%
SDS-PAGE and then transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% non-fat milk at 37°C for 1 h, and then incubated at
4°C overnight with primary antibodies, including anti-HSPB1 (1:500;
cat. no. ab216610; Abcam, Cambridge, UK), anti-cyclin B1 (1:600;
cat. no. ab32053; Abcam), anti-cyclin G1 (1:800; cat. no. sc-8016;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-B-cell
lymphoma-2 (Bcl-2; 1:1,000; cat. no. MA5-11757, Invitrogen; Thermo
Fisher Scientific, Inc.), anti-Bcl-2 associated X protein (Bax;
1:1,000; cat. no. ab53154; Abcam), anti-pro-caspase-8 (1:700; cat.
no. MAB704; R&D Systems, Inc., Minneapolis, MN, USA),
anti-cytochrome c (cyto c; 1:1,000; cat. no. MAB897;
R&D Systems, Inc.), anti-cleaved caspase-8 (1:600; cat. no.
9429; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-cyto
c oxidase IV (1:100; cat. no. ab33985; Abcam) and anti-GAPDH
(1:800; cat. no. ab8245; Abcam). Membranes were then incubated at
37°C for 90 min with horseradish peroxidase-conjugated secondary
antibodies [mouse anti-rabbit immunoglobulin G (IgG); 1:8,000; cat.
no. 31464, Invitrogen; Thermo Fisher Scientific, Inc.; and goat
anti-mouse IgG; 1:8,000; cat. no. ab97023, Abcam]. Protein bands
were visualized using enhanced chemiluminescence detection reagent
(Thermo Fisher Scientific, Inc.) and the densitometry was performed
using the Bio-Rad ChemiDoc system with Image Lab software version
6.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data were presented as the mean ± standard
deviation. All experiments were performed in triplicate. Data were
analyzed using GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA). Differences were analyzed using Student's t-tests
or one-way analyses of variance followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Silencing of HSPB1 promotes the
radiosensitivity of NSCLC cells by reducing viability, arresting
the cell cycle, depolarizing the MMP and promoting apoptosis
RT-qPCR and western blot analyses demonstrated that
the expression levels of HSPB1 in A549 cells were significantly
downregulated following transfection with si-HSPB1 compared with
the NC (Fig. 1), with a knockdown
efficiency of >40%. A CCK-8 assay revealed that irradiation with
6 Gy significantly decreased the viability of cells at 48 and 72 h
compared with 0 Gy irradiation (Fig.
2A). Furthermore, irradiation with 6 Gy significantly increased
the apoptotic rate by >10% compared with no irradiation (0 Gy),
whereas the number of red fluorescent cells decreased by ~30%
following irradiation (Fig. 2B-E).
In Fig. 2B the upper right
quadrant is the advanced apoptotic cells, and the lower right
quadrant was the early apoptotic cells. The rate of apoptotic cells
is the sum of the rate of early and advanced apoptotic cells.
Furthermore, arrest of the cell cycle in the G2/M phase was
markedly promoted by irradiation when compared with the
corresponding 0 Gy group (Fig. 3).
In si-HSPB1 group, the percentage of cells in S phase was notably
decreased, whereas the percentage of cells in G2/M phase was
markedly increased following irradiation with 6 Gy compared with
the NC group. Furthermore, si-HSPB1 notably enhanced the effects of
radiation on the viability, apoptosis, cell cycle distribution and
MMP of NSCLC cells (Figs. 2 and
3).
 | Figure 2.Silencing HSPB1 increases the
radiosensitivity of non-small cell lung carcinoma cells by
decreasing cell viability, promoting apoptosis and depolarizing the
MMP. (A) The viability of A549 cells following irradiation with 0
or 6 Gy, and transfection with control, NC or si-HSPB1, as
determined by a Cell Counting Kit-8 assay. (B and C) Apoptosis and
(D and E) MMP of A549 cells following irradiation and transfection,
as determined by flow cytometry. Data are presented as the mean ±
standard deviation. #P<0.05 and
##P<0.01, as indicated; **P<0.01 vs. 0 Gy + NC;
^^P<0.01 vs. 6 Gy + NC. FITC, fluorescein
isothiocyanate; HSPB1, heat shock protein 27; MMP, mitochondrial
membrane potential; NC, negative control; PI, propidium iodide;
si-HSPB1, small interfering RNA specific for HSPB1. |
Silencing of HSPB1 increases the
radiosensitivity of NSCLC cells by altering the expression of cell
cycle- and apoptosis-associated genes
To investigate the mechanisms underlying the effects
of irradiation and si-HSPB1 on the cell cycle, the expression of
associated genes and proteins was determined by RT-qPCR and western
blot assays. It was revealed that irradiation of A549 cells with 6
Gy significantly downregulated the mRNA levels of cyclin B1 and
cyclin G1 compared with the corresponding 0 Gy control group
(Fig. 4A and B). Additionally,
transfection of cells with si-HSPB1 significantly decreased the
levels of cyclin B1 and cyclin G1 mRNA compared with the NC, and
promoted the effects of irradiation on expression. The expression
of apoptosis-associated genes was also investigated. Irradiation
significantly increased the expression of Bax and decreased that of
Bcl-2 compared with 0 Gy, and transfection with si-HSPB1 exhibited
similar effects on expression when compared with the NC group
(Fig. 4C and D). Additionally,
transfection of irradiated cells with si-HSPB1 further promoted the
effects of irradiation. Furthermore, western blotting revealed that
6 Gy irradiation and si-HSPB1 similarly affected the expression of
cyclin B1, cyclin G1, Bax and Bcl-2 at the protein level (Fig. 5). It was also demonstrated that
irradiation and si-HSPB1 significantly decreased the expression of
pro-caspase-8 and increased that of cleaved caspase-8 compared with
their respective controls.
 | Figure 4.Silencing of HSPB1 increases the
radiosensitivity of non-small cell lung carcinoma cells by
regulating the expression of cell cycle- and apoptosis-associated
genes. mRNA expression of (A) cyclin B1, (B) cyclin G1, (C) Bax and
(D) Bcl-2 in A549 cells following irradiation with 0 or 6 Gy, and
transfection with control, NC or si-HSPB1, as determined by reverse
transcription-quantitative polymerase chain reaction. Data are
presented as the mean ± standard deviation. #P<0.05
and ##P<0.01, as indicated; *P<0.05 and
**P<0.01 vs. 0 Gy + NC; ^P<0.05 and
^^P<0.01 vs. 6 Gy + NC. Bcl-2, B-cell lymphoma-2;
Bax, Bcl-2-associated X protein; HSPB1, heat shock protein 27; NC,
negative control; si-HSPB1, small interfering RNA specific for
HSPB1. |
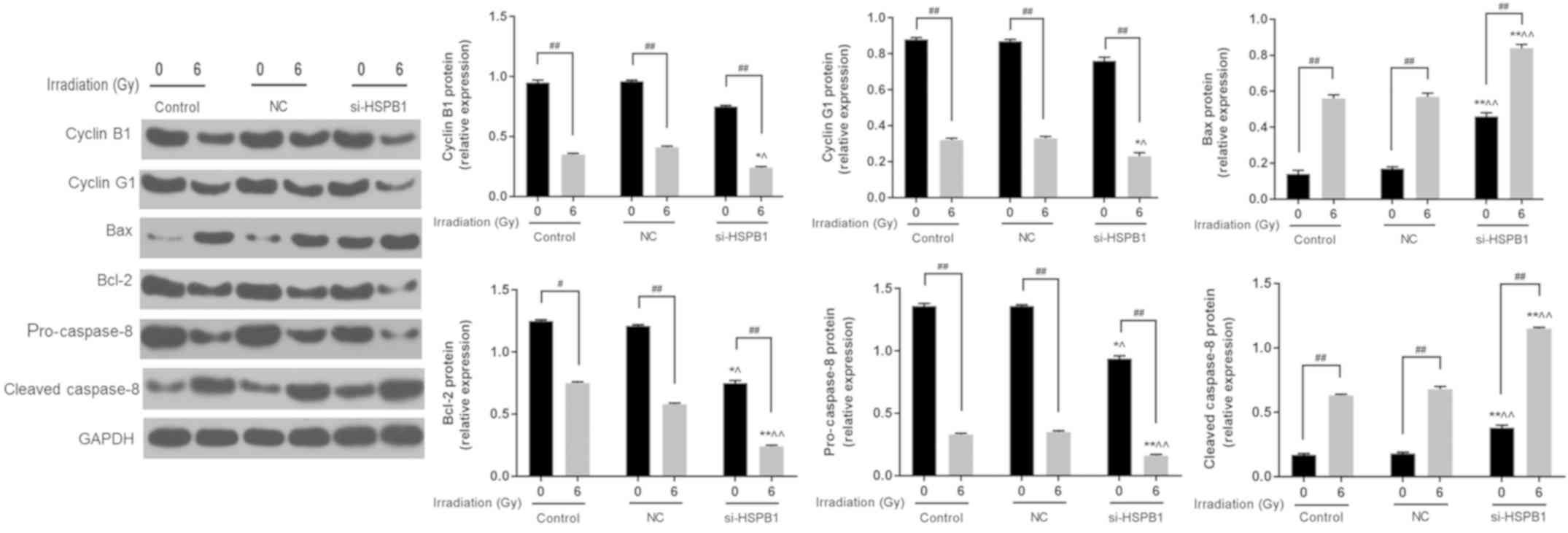 | Figure 5.Silencing of HSPB1 increases the
radiosensitivity of non-small cell lung carcinoma cells by
regulating the expression of cell cycle- and apoptosis-associated
proteins. Representative western blot and protein expression of
cyclin B1, cyclin G1, Bax, Bcl-2, pro-caspase-3 and cleaved
caspase-3 in A549 cells following irradiation with 0 or 6 Gy, and
transfection with control, NC or si-HSPB1. Data are presented as
the mean ± standard deviation. #P<0.05 and
##P<0.01, as indicated; *P<0.05 and **P<0.01
vs. 0 Gy + NC; ^P<0.05 and ^^P<0.01 vs.
6 Gy + NC. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X
protein; HSPB1, heat shock protein 27; NC, negative control;
si-HSPB1, small interfering RNA specific for HSPB1. |
Silencing of HSPB1 and irradiation
increase the expression of cytosolic cyto c and reduce the
expression of mitochondrial cyto c
Increased mitochondrial membrane permeability is
associated with the opening of the mitochondrial permeability
transition pore (23). To
investigate the molecular mechanisms underlying the effects of
HSPB1 silencing and irradiation on the MMP, the expression of cyto
c was determined by western blotting. It was revealed that 6
Gy irradiation significantly decreased the mitochondrial levels of
cyto c and increased those of cytosolic cyto c
compared with 0 Gy; si-HSPB1 also had similar significant effects
on cyto c expression (Fig.
6). Furthermore, the effects of irradiation were promoted by
transfection with si-HSPB1 (Fig.
6).
Discussion
At present, radiation therapy is an effective
treatment of LC in clinical settings (4,5);
however, radiosensitivity and radioresistance serve important roles
in the therapeutic effects of radiotherapy (24–26).
Compared with SCLC, NSCLC is frequently insensitive to radiation,
reducing the efficacy of radiotherapy as a treatment of NSCLC
(27). Therefore, the aims of the
present study were to investigate the mechanisms underlying the
radioresistance of NSCLC cells and identify novel targets for
improving radiosensitivity. It has been reported that HSPB1 was
upregulated in NSCLC (20). In
addition, it was demonstrated that knockdown of HSPB1 increased the
radiosensitivity of nasopharyngeal carcinoma and head-neck cancer
cells (21,28). Therefore, it was hypothesized that
silencing HSPB1 may increase the radiosensitivity of NSCLC
cells.
Transfection with si-HSPB1 markedly decreased the
viability of A549 cells following irradiation. One of the main
effects of radiation on tumor cells is the induction of apoptosis
(29). Additionally,
depolarization of the MMP is associated with apoptosis (30). Thus, the apoptosis and MMP of A549
cells following irradiation and silencing of HSPB1 was
investigated. It was demonstrated that irradiation promoted the
apoptosis and depolarized the MMP of NSCLC cells, with si-HSPB1
further enhancing the effects of irradiation. These findings
indicated that downregulation of HSPB1 increased the
radiosensitivity of NSCLC via the induction of apoptosis. The
molecular mechanisms underlying radiosensitization include
alterations in the tumor microenvironment, fixation of free
radicals, cell cycle arrest, inhibition of DNA damage repair and
the promotion of apoptosis (31–33).
Cell cycle distribution is an important factor influencing
radiosensitivity. The sensitivity of tumor cells to irradiation
depends on their cell cycle phase (34); cells in the G2/M phase are more
sensitive to radiation than those in other phases (35–37).
It has been reported that drugs and gene manipulations increased
the radiosensitivity of NSCLC cells by promoting G2/M phase cell
cycle arrest (38–41). Consistent with these previous
studies, the present study demonstrated that HSPB1 silencing
markedly increased irradiation-induced cell cycle arrest in the
G2/M phase.
As important regulators of the cell cycle, the roles
of cyclins in the development and progression of tumors have been
investigated; the abnormal expression of cyclins is a common
mechanism underlying the dysregulation of cell cycle in malignant
tumors (42–44). Cyclins are involved in cell cycle
arrest (45,46). Cyclin B1 and cyclin G1 regulate
G2/M phase transition (47). In
the present study, cyclin B1 and cyclin G1 levels were detected by
RT-qPCR and western blot analyses in A549 cells transfected with
si-HSPB1 and exposed to radiation. It was demonstrated that the
combination of si-HSPB1 and irradiation significantly decreased the
expression levels of cyclin B1 and cyclin G1, suggesting that
silencing HSPB1 increases the radiosensitivity of HSPB1 cells by
arresting the cell cycle in the G2/M phase via the downregulation
of cyclins.
Apoptosis is associated with radiosensitivity;
increased apoptosis indicates elevated radiosensitivity (48–50).
Numerous molecules and pathways are involved in apoptotic
processes, including the mitochondria, which serve key roles
(51–53). For example, the voltage-dependent
anion channel 1 (VDAC1) plays a key role in mitochondrial mediated
apoptosis, VDAC1 oligomerization leads to the formation of a large
pore that allows the release of pro-apoptotic proteins to cytosol,
thereby activating apoptosis (51); Mitogen-activated protein
kinases/extracellular signal-regulated kinase pathway has been
reported to be associated with cell differentiation and apoptosis
(54). Depolarization of the MMP
is an early event in apoptotic cascades, leading to a series of
biochemical alterations in the mitochondrial membrane that induce
proapoptotic pathways (55,56).
In the present study, it was observed that downregulation of HSPB1
increased apoptosis and altered the MMP in A549 cells following
irradiation.
Apoptosis pathways can be divided into extracellular
and intracellular apoptotic pathways (57,58).
The external pathway is mainly regulated by caspase-8, which is
activated by apoptotic receptors on the surface of the cell
membrane (59,60). The internal pathway involves Bcl-2
and Bax, which regulate the permeability of the outer membrane of
mitochondria (61). Bcl-2 exhibits
antiapoptotic effects and prevents the release of cyto c
from mitochondria into the cytoplasm (62). Activation of the proapoptotic
protein Bax accelerates cell death (58). Therefore, the expression of Bcl-2,
Bax, cyto c and caspase-8 was investigated in A549 cells
subjected to si-HSPB1 transfection and irradiation. It was
demonstrated that si-HSPB1 upregulated the levels of Bax, cytosol
cyto c and cleaved caspase-8, and downregulated those of
Bcl-2, mitochondrial cyto c and pro-caspase-8 in cells
exposed to irradiation. These findings indicated that HSPB1
silencing promoted the apoptosis of NSCLC cells following radiation
treatment.
There are certain points to consider for future
experiments. NSCLC accounts for the majority of lung cancers
(63); however, the present study
did not investigate the effects of HSPB1 silencing on SCLC cells,
which should be determined in future studies. Additionally, a
previous study reported that HSPs did not regulate the
radioresistance of NSCLC cells, with no effect following the
downregulation of HSP72 or heat shock treatment (64). The reasons for the discrepancy
between the absence of a reported effect of heat shock treatment on
NSCLC cell radiosensitivity in the previous study and the increase
in radiosensitivity following silencing of HSPB1 in the present
study require further investigation.
In conclusion, the results of the present study
demonstrated that silencing HSPB1 increased the radiosensitivity of
NSCLC cells via reductions in cell viability, depolarization of the
MMP, cell cycle arrest in the G2/M phase and the promotion of
apoptosis. Therefore, HSPB1 may be a potential target for the
treatment of radiation-insensitive NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX made substantial contributions to the conception
and design of the present study; XL, YZ and HZ performed data
acquisition, analysis and interpretation; LX drafted the article or
critically revised it for important intellectual content; all
authors agreed to be accountable for all aspects of the work and
ensuring that questions related to the accuracy or integrity of the
work were appropriately investigated and resolved; all authors
approved the final published version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ellis PM and Vandermeer R: Delays in the
diagnosis of lung cancer. J Thorac Dis. 3:183–188. 2011.PubMed/NCBI
|
|
4
|
Benveniste MF, Welsh J, Godoy MC,
Betancourt SL, Mawlawi OR and Munden RF: New era of radiotherapy:
An update in radiation-induced lung disease. Clin Radiol.
68:e275–e290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fox W and Scadding JG: Medical research
council comparative trial of surgery and radiotherapy for primary
treatment of small-celled or oat-celled carcinoma of bronchus.
Ten-year follow-up. Lancet. 2:63–65. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Changizi V, Bahrami M, Esfahani M and
Shetab-Boushehri SV: Prevention of γ-radiation-induced DNA damage
in human lymphocytes using a serine-magnesium sulfate mixture.
Sultan Qaboos Univ Med J. 17:e162–e167. 2017.PubMed/NCBI
|
|
7
|
Triggiani L, Colosini A, Buglione M,
Pasinetti N, Orizio F, Bardoscia L, Borghetti P, Maddalo M, Spiazzi
L, Magrini SM and Bresciani R: Exploring the role of enzalutamide
in combination with radiation therapy: An in vitro study.
Anticancer Res. 38:3487–3492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murad H, Alghamian Y, Aljapawe A and
Madania A: Effects of ionizing radiation on the viability and
proliferative behavior of the human glioblastoma T98G cell line.
BMC Res Notes. 11:3302018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kased N, Erasmus JJ, Komaki R and Cox JD:
Prognostic value of posttreatment [18F] fluorodeoxyglucose uptake
of primary non-small cell lung carcinoma treated with radiation
therapy with or without chemotherapy: A brief review. J Thorac
Oncol. 3:534–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagata Y and Kimura T: Stereotactic body
radiotherapy (SBRT) for stage I lung cancer. Jpn J Clin Oncol.
48:405–409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Skrzypski M and Jassem J: Consolidation
systemic treatment after radiochemotherapy for unresectable stage
III non-small cell lung cancer. Cancer Treat Rev. 66:114–121. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deniset JF and Pierce GN: Heat shock
proteins: Mediators of atherosclerotic development. Curr Drug
Targets. 16:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Fok JH and Davies FE: Heat shock
proteins in multiple myeloma. Oncotarget. 5:1132–1148. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Albany C and Hahn NM: Heat shock and other
apoptosis-related proteins as therapeutic targets in prostate
cancer. Asian J Androl. 16:359–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi B, Choi SK, Park YN, Kwak SY, Lee HJ,
Kwon Y, Na Y and Lee YS: Sensitization of lung cancer cells by
altered dimerization of HSP27. Oncotarget. 8:105372–105382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McConnell JR and McAlpine SR: Heat shock
proteins 27, 40, and 70 as combinational and dual therapeutic
cancer targets. Bioorg Med Chem Lett. 23:1923–1928. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Z, Yang C, Sun S, Nan Y, Lang Z,
Wang X, Zhao J and Liu Y: Heat shock protein 27, a novel regulator
of transforming growth factor β induced resistance to cisplatin in
A549 cell. Pharmacology. 100:283–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stope MB, Weiss M, Preuss M, Streitbörger
A, Ritter CA, Zimmermann U, Walther R and Burchardt M: Immediate
and transient phosphorylation of the heat shock protein 27
initiates chemoresistance in prostate cancer cells. Oncol Rep.
32:2380–2386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Tao X, Jin G, Jin H, Wang N, Hu
F, Luo Q, Shu H, Zhao F, Yao M, et al: A targetable molecular
chaperone Hsp27 confers aggressiveness in hepatocellular carcinoma.
Theranostics. 6:558–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao GY, Ding JY, Lu CL, Lin ZW and Guo J:
The overexpression of 14-3-3ζ and Hsp27 promotes non-small cell
lung cancer progression. Cancer. 120:652–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang B, Qu JQ, Xiao L, Yi H, Zhang PF, Li
MY, Hu R, Wan XX, He QY, Li JH, et al: Identification of heat shock
protein 27 as a radioresistance-related protein in nasopharyngeal
carcinoma cells. J Cancer Res Clin Oncol. 138:2117–2125. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bernardi P, Rasola A, Forte M and Lippe G:
The mitochondrial permeability transition pore: Channel formation
by F-ATP synthase, integration in signal transduction, and role in
pathophysiology. Physiol Rev. 95:1111–1155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Wang P, Guo F, Wang X, Wang J, Xu
J, Yuan D, Zhang J and Shao C: Autophagy enhanced the
radioresistance of non-small cell lung cancer by regulating ROS
level under hypoxia condition. Int J Radiat Biol. 93:764–770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saitoh JI, Shirai K, Abe T, Kubo N, Ebara
T, Ohno T, Minato K, Saito R, Yamada M and Nakano T; Working Group
of The Lung Tumor, : A phase I study of hypofractionated Carbon-ion
radiotherapy for stage III Non-small cell lung cancer. Anticancer
Res. 38:885–891. 2018.PubMed/NCBI
|
|
26
|
Song Y, Zuo Y, Qian XL, Chen ZP, Wang SK,
Song L and Peng LP: Inhibition of MicroRNA-21-5p promotes the
radiation sensitivity of non-small cell lung cancer through HMSH2.
Cell Physiol Biochem. 43:1258–1272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carmichael J, Degraff WG, Gamson J, Russo
D, Gazdar AF, Levitt ML, Minna JD and Mitchell JB: Radiation
sensitivity of human lung cancer cell lines. Eur J Cancer Clin
Oncol. 25:527–534. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guttmann DM, Hart L, Du K, Seletsky A and
Koumenis C: Inhibition of Hsp27 radiosensitizes head-and-neck
cancer by modulating deoxyribonucleic acid repair. Int J Radiat
Oncol Biol Phys. 87:168–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimura K, Bowen C, Spiegel S and Gelmann
EP: Tumor necrosis factor-alpha sensitizes prostate cancer cells to
gamma-irradiation- induced apoptosis. Cancer Res. 59:1606–1614.
1999.PubMed/NCBI
|
|
30
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; An
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dittmann KH, Rothmund MC, Paasch A, Mayer
C, Fehrenbacher B, Schaller M, Frauenstein K, Fritsche E,
Haarmann-Stemmann T, Braeuning A and Rodemann HP: The nuclear aryl
hydocarbon receptor is involved in regulation of DNA repair and
cell survival following treatment with ionizing radiation. Toxicol
Lett. 240:122–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raviraj J, Bokkasam VK, Kumar VS, Reddy US
and Suman V: Radiosensitizers, radioprotectors, and radiation
mitigators. Indian J Dent Res. 25:83–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saleh EM: Inhibition of topoisomerase IIα
sensitizes FaDu cells to ionizing radiation by diminishing DNA
repair. Tumour Biol. 36:8985–8992. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Medema RH and Macurek L: Checkpoint
control and cancer. Oncogene. 31:2601–2613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Adeberg S, Bernhardt D, Harrabi SB,
Nicolay NH, Hörner-Rieber J, König L, Repka M, Mohr A, Abdollahi A,
Weber KJ, et al: Metformin enhanced in vitro radiosensitivity
associates with G2/M cell cycle arrest and elevated
Adenosine-5′-monophosphate-activated protein Kinase levels in
glioblastoma. Radiol Oncol. 51:431–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lesueur P, Chevalier F, El-Habr EA, Junier
MP, Chneiweiss H, Castera L, Müller E, Stefan D and Saintigny Y:
Radiosensitization effect of talazoparib, a parp inhibitor, on
glioblastoma stem cells exposed to low and high linear energy
transfer radiation. Sci Rep. 8:36642018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo H, Xi Y, Li W, Li J, Li Y, Dong S,
Peng L, Liu Y and Yu W: Cell identity bookmarking through
heterogeneous chromatin landscape maintenance during the cell
cycle. Hum Mol Genet. 26:4231–4243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Xia Y, Chen T, Zeng Y, Li L, Hou
Y, Li W and Liu Z: Sanyang Xuedai enhances the radiosensitivity of
human non-small cell lung cancer cells via increasing iNOS/NO
production. Biomed Pharmacother. 102:618–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Hu L, Zhang X, Zhao H, Xu H, Wei
Y, Jiang H, Xie C, Zhou Y and Zhou F: Downregulation of
mitochondrial single stranded DNA binding protein (SSBP1) induces
mitochondrial dysfunction and increases the radiosensitivity in
non-small cell lung cancer cells. J Cancer. 8:1400–1409. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhan N, Li B, Xu X, Xu J and Hu S:
Inhibition of FASN expression enhances radiosensitivity in human
non-small cell lung cancer. Oncol Lett. 15:4578–4584.
2018.PubMed/NCBI
|
|
41
|
Zhao Y, Jiang W, Li B, Yao Q, Dong J, Cen
Y, Pan X, Li J, Zheng J, Pang X and Zhou H: Artesunate enhances
radiosensitivity of human non-small cell lung cancer A549 cells via
increasing NO production to induce cell cycle arrest at G2/M phase.
Int Immunopharmacol. 11:2039–2046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun X, Zhangyuan G, Shi L, Wang Y, Sun B
and Ding Q: Prognostic and clinicopathological significance of
cyclin B expression in patients with breast cancer: A
meta-analysis. Medicine (Baltimore). 96:e68602017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye C, Wang J, Wu P, Li X and Chai Y:
Prognostic role of cyclin B1 in solid tumors: A meta-analysis.
Oncotarget. 8:2224–2232. 2017.PubMed/NCBI
|
|
44
|
Tashiro E, Tsuchiya A and Imoto M:
Functions of cyclin D1 as an oncogene and regulation of cyclin D1
expression. Cancer Sci. 98:629–635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bandopadhyay M, Sarkar N, Datta S, Das D,
Pal A, Panigrahi R, Banerjee A, Panda CK, Das C, Chakrabarti S and
Chakravarty R: Hepatitis B virus X protein mediated suppression of
miRNA-122 expression enhances hepatoblastoma cell proliferation
through cyclin G1-p53 axis. Infect Agent Cancer. 11:402016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Min J, Wang X, Tong Y, Li X, Tao D, Hu J,
Xie D and Gong J: Expression of cyclins in high-density cultured
cells and in vivo tumor cells. Cytometry A. 81:874–882. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Seo HR, Lee DH, Lee HJ, Baek M, Bae S, Soh
JW, Lee SJ, Kim J and Lee YS: Cyclin G1 overcomes radiation-induced
G2 arrest and increases cell death through transcriptional
activation of cyclin B1. Cell Death Differ. 13:1475–1484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: PI3K/Akt/mTOR pathway inhibitors
enhance radiosensitivity in radioresistant prostate cancer cells
through inducing apoptosis, reducing autophagy, suppressing NHEJ
and HR repair pathways. Cell Death Dis. 5:e14372014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Song L, Peng L, Hua S, Li X, Ma L, Jie J,
Chen D, Wang Y and Li D: miR-144-5p enhances the radiosensitivity
of non-small-cell lung cancer cells via targeting ATF2. Biomed Res
Int. 2018:51094972018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou HP, Qian LX, Zhang N, Gu JJ, Ding K,
Wu J, Lu ZW, Du MY, Zhu HM, Wu JZ, et al: MIIP gene expression is
associated with radiosensitivity in human nasopharyngeal carcinoma
cells. Oncol Lett. 15:9471–9479. 2018.PubMed/NCBI
|
|
51
|
Shoshan-Barmatz V, Krelin Y and Chen Q:
VDAC1 as a player in mitochondria-mediated apoptosis and target for
modulating apoptosis. Curr Med Chem. 24:4435–4446. 2017.PubMed/NCBI
|
|
52
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Y, Xia C, Lv Y, Li C, Mei Q, Li H,
Wang H and Li S: Crosstalk influence between P38MAPK and autophagy
on mitochondria-mediated apoptosis induced by Anti-Fas
Antibody/Actinomycin D in human hepatoma Bel-7402 cells. Molecules.
22:E17052017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bishayee K, Chakraborty D, Ghosh S,
Boujedaini N and Khuda-Bukhsh AR: Lycopodine triggers apoptosis by
modulating 5-lipoxygenase, and depolarizing mitochondrial membrane
potential in androgen sensitive and refractory prostate cancer
cells without modulating p53 activity: Signaling cascade and
drug-DNA interaction. Eur J Pharmacol. 698:110–121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li D, Dai C, Yang X, Wang F, Yu X, Xiao X
and Tang S: Critical role of p21 on olaquindox-induced
mitochondrial apoptosis and S-phase arrest involves activation of
PI3K/AKT and inhibition of Nrf2/HO-1pathway. Food Chem Toxicol.
108:148–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang C, Yuan Y, Hu F, Wang Q, Zhang K,
Wang Y, Gu J, Liu X, Bian J and Liu Z: Cadmium induces PC12 cells
apoptosis via an extracellular signal-regulated kinase and c-Jun
N-terminal kinase-mediated mitochondrial apoptotic pathway. Biol
Trace Elem Res. 158:249–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shang HS, Shih YL, Lu TJ, Lee CH, Hsueh
SC, Chou YC, Lu HF, Liao NC and Chung JG: Benzyl isothiocyanate
(BITC) induces apoptosis of GBM 8401 human brain glioblastoma
multiforms cells via activation of caspase-8/Bid and the reactive
oxygen species-dependent mitochondrial pathway. Environ Toxicol.
31:1751–1760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tan M, Rong Y, Su Q and Chen Y: Artesunate
induces apoptosis via inhibition of STAT3 in THP-1 cells. Leuk Res.
62:98–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Soriano ME and Scorrano L: The interplay
between BCL-2 family proteins and mitochondrial morphology in the
regulation of apoptosis. Adv Exp Med Biol. 687:97–114. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yanagisawa K and Takahashi T: Protein
expression profiling for identification of molecular mechanism in
human NSCLC by mass spectrometry (The 20th Lung Cancer Workshop).
Jpn J Lung Cancer. 46:231–236. 2006. View Article : Google Scholar
|
|
64
|
Ekedahl J, Joseph B, Marchetti P, Fauvel
H, Formstecher P, Lewensohn R and Zhivotovsky B: Heat shock protein
72 does not modulate ionizing radiation-induced apoptosis in U1810
non-small cell lung carcinoma cells. Cancer Biol Ther. 2:663–669.
2003. View Article : Google Scholar : PubMed/NCBI
|




















