Introduction
The incidence of nasopharyngeal carcinoma (NPC) is
particularly high in Southern China, and radiotherapy and
chemotherapy are effective therapeutic strategies for its treatment
(1). Recent advances in the
treatment of NPC have led to an improvement in the local control
rate; however, the distant metastasis rate of NPC remains high
(2,3). Notably, >60% of unsuccessful
treatments are due to distant metastasis (4,5).
Therefore, numerous studies have aimed to investigate the mechanism
of distant metastasis, in order to improve the treatment of locally
advanced NPC. Epithelial-mesenchymal transition (EMT) is one of the
processes involved in distant metastasis of tumor cells, which may
promote uncontrolled growth, migration and invasion of epithelial
cancer cells (6–8). Various extracellular signaling
molecules promote EMT, including transforming growth factor-β
(TGF-β), fibroblast growth factor and epidermal growth factor, via
a series of cascade reactions (9–11).
The mitogen-activated protein kinase (MAPK) signaling pathway is a
signal transduction pathway that is able to induce transcriptional
alterations downstream to the aforementioned extracellular signals
(12). Additionally,
hyperactivation of the MAPK signaling pathway may induce metastasis
in human breast cancer (13).
Amyloid β precursor protein (APP) is a key gene
involved in Alzheimer's disease (14,15).
A previous study identified the upregulation of APP expression in
various types of tumor cells (16). Proteomic profiling of
TGF-β1-treated NPC CNE-2 cells identified an increase in the
protein expression levels of APP (17). Bioinformatics analyses demonstrated
that APP is differentially expressed in NPC tissues compared with
healthy tissues, and it exhibits druggable domains (18). Hérard et al (19) reported that transfection of cells
with a small interfering RNA (siRNA) targeting APP significantly
decreases the protein expression levels of presynaptic APP and
amyloid β precursor-like protein 2. Additionally, siRNA targeting
APP decreases glucose metabolism in neurons in the superior
colliculus (19). In order to
further investigate the mechanism underlying APP function, previous
studies established an APP-siRNA plasmid vector, which laid the
foundation for further studies examining the role of APP in
vitro (20,21). APP silencing is able to effectively
suppress the proliferation, migration and invasion of tumor cells
(22). However, whether APP
silencing may inhibit NPC development remains unclear.
In the present study, an APP-siRNA plasmid was
designed and successfully transfected into NPC cells. The
expression levels of EMT-associated factors were investigated in
NPC cells, and the molecular effects of APP silencing on NPC cells
were investigated.
Materials and methods
Cell culture
Human NPC cell lines C666-1, 6-10B, HNE3 and SUNE-1,
and the normal nasopharyngeal-derived epithelial cell line NP69,
were purchased from Xiangya Medical College Cell Bank (Changsha,
China). Cells were maintained in Dulbecco's modified Eagle's
medium/Ham's F12 nutrient mixture (DMEM/F-12; cat. no. 11320082;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; cat. no. 10099141;
Gibco; Thermo Fisher Scientific, Inc.). All cells were maintained
at 37°C in a humidified incubator containing 5% CO2.
Experimental grouping
Cells were randomly divided into the following three
groups: i) Untransfected SUNE-1 cells (control group), ii) SUNE-1
cells transfected with empty vector (empty vector group) and SUNE-1
cells transfected with APP-siRNA vector (APP-siRNA group).
Cell transfection
The pSUPER vector (Oligoengine, Seattle, WA, USA)
containing APP-siRNA (siRNA sequence: 5′-GAUCCAUCAGGGACCAAAACC-3′)
was purchased from Sangon Biotech Co., Ltd. (Shanghai, China).
SUNE-1 cells were seeded in 6-well plates (3×106
cells/well) and transfected with APP-siRNA (100 nM) and empty
vector (100 nM) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The mixture containing APP-siRNA/empty vector, SUNE-1
cells and transfection reagents was incubated at 37°C for 6 h, and
then the whole mixture was transferred to DMEM/F12 medium
supplemented with 10% FBS. After 48 h, the cells were harvested and
the transfection efficiency of APP-siRNA was examined.
Cell viability
Cell Counting Kit-8 (CCK-8; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) was used to measure
cell viability. Cells were seeded in 96-well plates at a density of
1×103 cells/well. The cells were cultured in a
humidified incubator and were subsequently harvested at 12, 24 and
48 h. Subsequently, CCK-8 solution (10 µl) was added to the culture
medium and incubated at 37°C for 2 h. Optical density (OD) was
measured at 450 nm using a microplate reader (Cany Precision
Instruments Co., Ltd., Shanghai, China). The blank control group
comprised culture medium and CCK-8 solution without cells. The
relative cell viability was measured as follows: Cell
viability=(ODtransfected
cells-ODblank)/(ODcontrol-ODblank).
Wound healing assay
Migration of SUNE-1 cells was quantified using a
wound healing assay. Cells (1×106 cells/well) were
seeded in 6-well plates with complete medium. The cells were
starved in serum-free complete medium for 6–8 h, and a 200-µl
pipette tip was used to create a straight wound in the cell
monolayers. Cell migration was assessed by measuring the relative
size of the wounds at 12 and 24 h compared with at 0 h
post-wounding using a light microscope (Nikon Corporation, Tokyo,
Japan).
Transwell assay
Transwell chambers were used to assess cell
invasion. Briefly, 1×105 cells were resuspended in
serum-free medium and plated into the upper chamber containing
polycarbonate membranes (pore size, 8 µm; Corning Inc., Corning,
NY, USA) coated with Matrigel® (BD Biosciences, San
Jose, CA, USA). DMEM/F12 containing 10% FBS was added to the lower
chamber and cells were incubated at 37°C for 24 h. Cells remaining
on the surface of the upper chamber were removed with a cotton
swab. The invading cells were fixed with 4% paraformaldehyde for 15
min at room temperature, and stained with 0.1% crystal violet
solution for 20 min at room temperature. To assess cell invasion,
three randomly selected fields of view were observed under a light
microscope (magnification, ×200). The invasion rate was calculated
by counting the number of invading cells at 0 and 24 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SUNE-1 cells
(2×104 cells/well in 6-well plates) using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Extracted RNA was treated
with RNase-free DNase (Takara Biotechnology Co., Ltd., Dalian,
China). Prime Script first strand cDNA synthesis kit (Takara
Biotechnology Co., Ltd.) was used to reverse transcribe the RNA to
cDNA, as previously described (23). Briefly, total RNA (2 µg) was used
as template, and the RT reaction was performed at 65°C for 5 min,
followed by incubation at 30°C for 6 min and 50°C for 60 min.
Primer sequences are listed in Table
I. The ABI 7500 Fast Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used for examining the
expression levels of EMT- and MAPK-associated genes in SUNE-1 cells
using a SYBR Green master mix (Fermentas; Thermo Fisher Scientific,
Inc.). The RT-qPCR reaction mixture (20 µl) contained 1 µl forward
and 1 µl reverse primers (concentration, 10 µM), 10 µl SYBR Green
master mix, 2 µl cDNA and RNase-free dH2O. RT-qPCR was
performed using the following thermocycling conditions: Initial
denaturation at 94°C for 3 min, followed by 40 cycles at 94°C for
30 sec, 56°C for 30 sec and 72°C for 2 min, with a final extension
at 72°C for 10 min. GAPDH was used as the internal control. The
relative mRNA expression levels were calculated using the
2−ΔΔCq method (24).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene symbol | Primer
sequence |
|---|
| APP | F:
5′-TGGCCCTGGAGAACTACATC-3′ |
|
| R:
5′-AATCACACGGAGGTGTGTCA-3′ |
| MTA-1 | F:
5′-AGCTACGAGCAGCACAACGGGGT-3′ |
|
| R:
5′-CACGCTTGGTTTCCGAGGAT-3′ |
| TIMP-2 | F:
5′-CCAAAGCAGTGAGCGAGAA-3′ |
|
| R:
5′-CATCCAGAGGCACTCATCC-3 |
| MMP-2 | F:
5′-GGAGGCACGATTGGTCTG-3′ |
|
| R:
5′-TTGGTTTCCGCATGGTCT-3 |
| MMP-9 | F:
5′-TTGACAGCGACAAGAAGTGG-3′ |
|
| R:
5′-GGCACAGTAGTGGCCGTAG-3 |
| GAPDH | F:
5′-ACCACAGTCCATGAAATCAC-3′ |
|
| R:
5′-AGGTTTCTCCAGGCGGCATG-3 |
Western blotting
Total protein was extracted from SUNE-1 cells
(2×104 cells/well in 6-well plates) using RIPA buffer
(Beijing Solarbio Science & Technology Co., Ltd.), and the
protein concentration was determined using a bicinchoninic acid
protein assay kit (Takara Biotechnology Co., Ltd.). Proteins (30
µg/lane) were separated by 10% SDS-PAGE and transferred to
polyvinylidene fluoride (PVDF) membranes (Thermo Fisher Scientific,
Inc.). Subsequently, the PVDF membranes were placed in blocking
buffer (1X TBS with 0.1% Tween-20 and 5% non-fat dry milk) for 1 h
at room temperature. All primary antibodies were diluted to 1:1,000
with blocking buffer, and the membranes were incubated with these
primary antibodies at 4°C overnight. All primary and secondary
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The primary antibodies used were as follows:
Anti-GAPDH (cat. no. 5174), anti-APP (cat. no. 2452),
anti-metastasis-associated 1 (MTA-1; cat. no. 5646), anti-tissue
inhibitor of metalloproteinases 2 (TIMP-2; cat. no. 5738),
anti-matrix metalloproteinase (MMP)-2 (cat. no. 4022), anti-MMP-9
(cat. no. 3852), anti-phosphorylated (p)-p38 (cat. no. 9211),
anti-p38 (cat. no. 9212), anti-p-extracellular signal-regulated
kinases 1/2 (ERK1/2; cat. no. 9102), anti-ERK1/2 (cat. no. 9101),
anti-p-c-Jun N-terminal kinases 1/2 (JNK; cat. no. 9251) and
anti-JNK (cat. no. 9252). Following incubation with primary
antibodies, the PVDF membranes were incubated with the appropriate
secondary antibody for 2 h at room temperature. The secondary
antibodies used were as follows: Anti-rabbit immunoglobulin G (IgG)
horseradish peroxidase (HRP)-conjugated antibody (cat. no. 7074;
1:2,000). The signals were detected using Pierce ECL Western
Blotting Substrate (cat. no. 32106; Pierce; Thermo Fisher
Scientific, Inc.). Optical band density was semi-quantified using
ImageJ software (version 1.46; National Institutes of Health,
Bethesda, MD, USA). GAPDH was used as the internal control.
Statistical analysis
Data are presented as the means ± standard error of
the mean. GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla,
CA, USA) was used to perform statistical analysis. Data were
analyzed using one-way analysis of variance followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
APP-siRNA inhibits viability of SUNE-1
cells
The expression levels of APP were significantly
increased in NPC cells compared with in NP69 cells (Fig. 1A and B). The mRNA expression levels
of APP were highest in SUNE-1 cells among all of the NPC cell lines
tested; therefore, SUNE-1 cells were selected for further
experiments. Post-transfection with APP-siRNA, the mRNA and protein
expression levels of APP were assessed by RT-qPCR and western
blotting, respectively (Fig. 1C and
D). The results revealed that the expression levels of APP were
efficiently decreased following APP-siRNA transfection compared
with in the control groups. Cell viability was assessed using the
CCK-8 assay, and the results suggested that APP-siRNA significantly
inhibited cell viability at 48 h; in addition, a slight effect was
observed at 24 h (Fig. 1E).
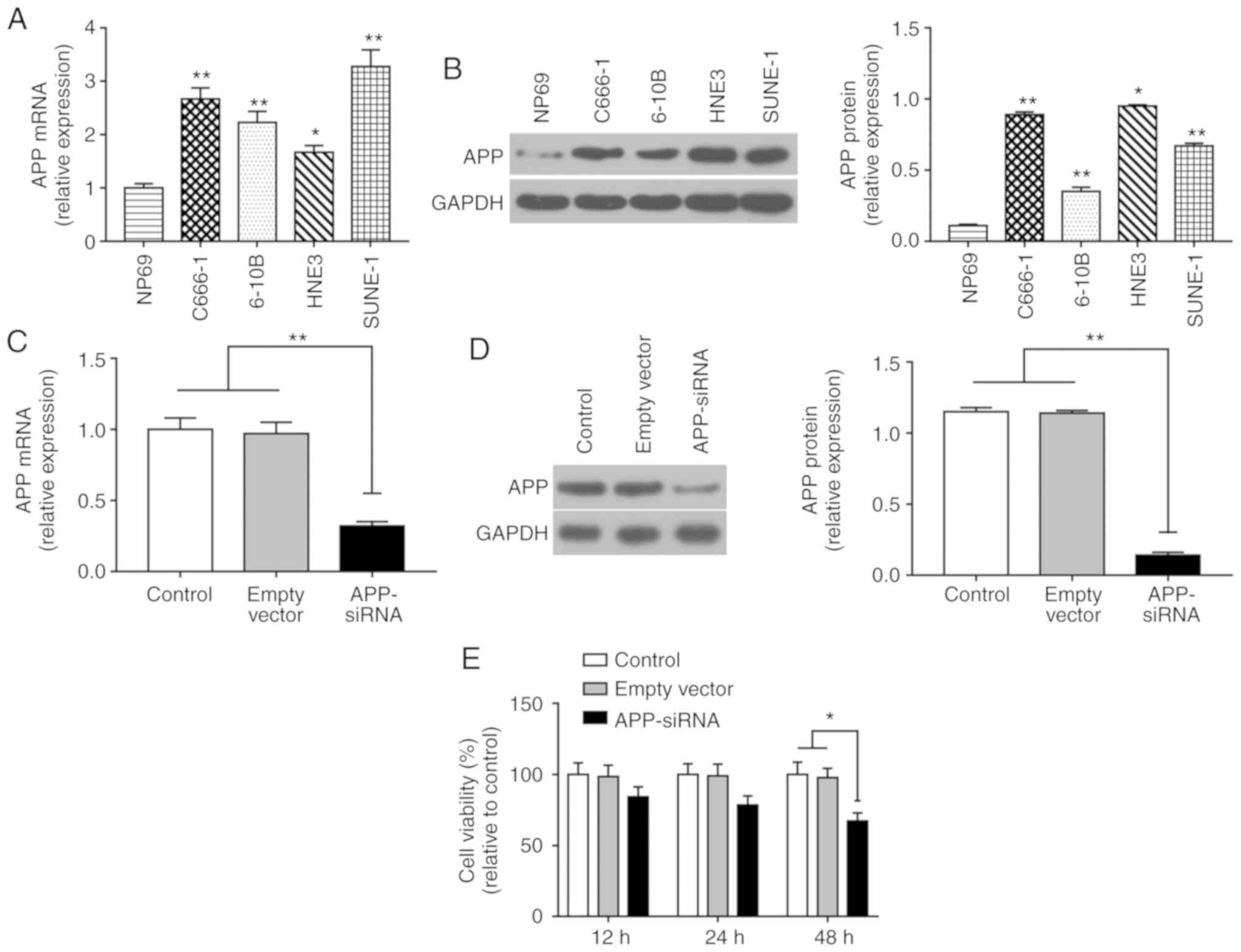 | Figure 1.APP-siRNA inhibits the viability of
SUNE-1 cells. (A) mRNA expression levels of APP in the four NPC
cell lines C666-1, 6-10B, HNE-3 and SUNE-1, and in the normal
nasopharyngeal-derived epithelial cell line NP69, as assessed by
RT-qPCR. (B) Protein expression levels of APP in NP69, C666-1,
6-10B, HNE-3 and SUNE-1 cells, as assessed by western blotting.
*P<0.05, **P<0.01 vs. NP69 cells. (C) mRNA expression levels
of APP following APP knockdown, as assessed by RT-qPCR. (D) Protein
expression levels of APP following APP knockdown, as assessed by
western blotting. (E) Cell Counting Kit-8 analysis was performed to
investigate cell viability following APP knockdown at 12, 24 and 48
h. *P<0.05, **P<0.01. GAPDH was used as the internal control
for western blotting. Data are presented as the means ± standard
error of the mean (n=3). APP, amyloid β precursor protein; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
siRNA, small interfering RNA. |
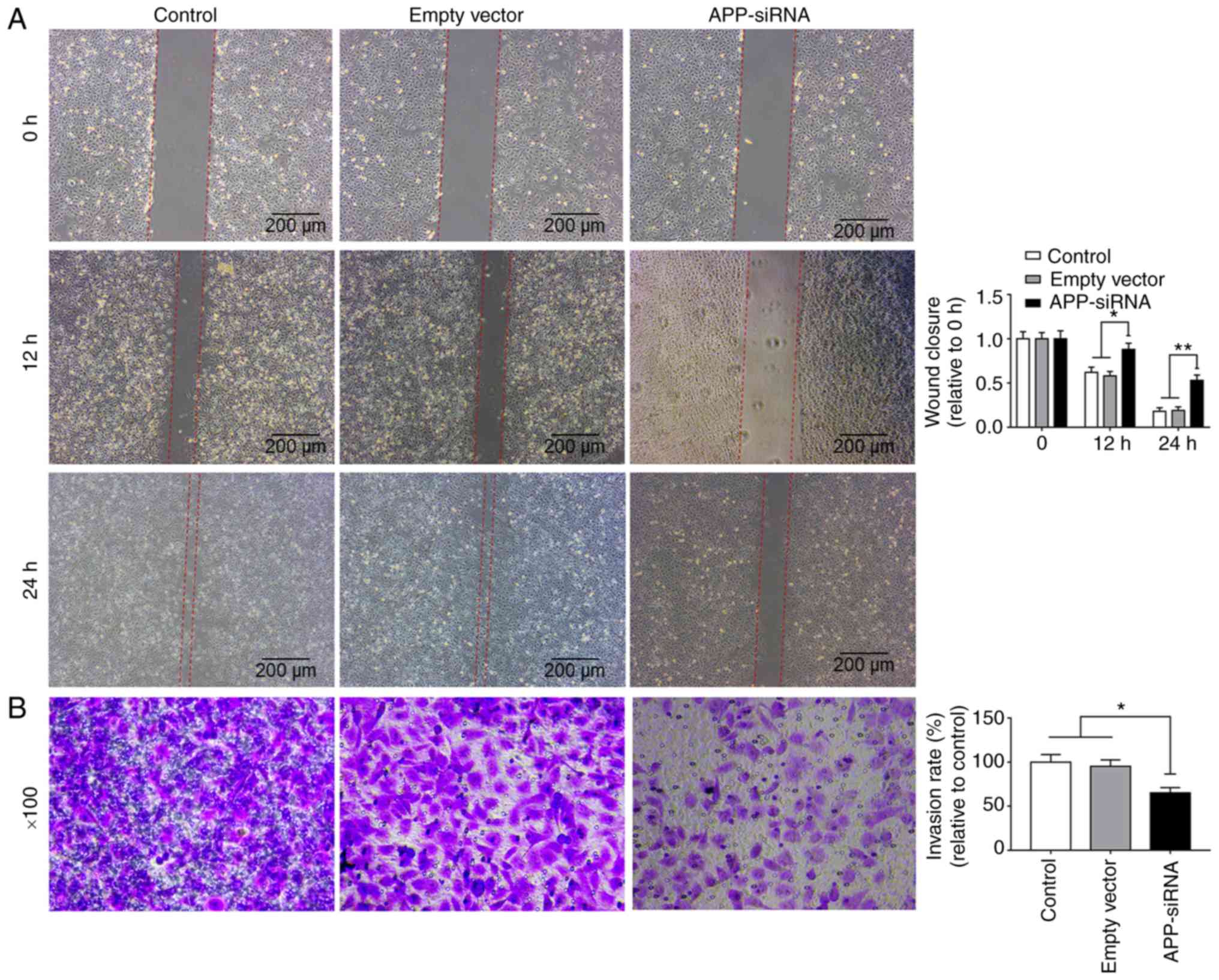
APP-siRNA suppresses migration and
invasion of SUNE-1 cells
Wound healing and Transwell assays were used to
investigate the effects of APP-siRNA on migration and invasion of
SUNE-1 cells, respectively. The present data suggested that
APP-siRNA was able to decrease the migratory and invasive abilities
of SUNE-1 cells compared with in the control groups (Fig. 2A and B). Collectively, the present
results indicated that APP may be involved in migration and
invasion of SUNE-1 cells.
APP-siRNA suppresses EMT in SUNE-1
cells
EMT serves an important role in the invasion and
metastasis of tumor cells (25).
To investigate the effects of APP-siRNA on EMT in SUNE-1 cells, the
expression levels of MTA-1, TIMP-2, MMP-2 and −9 were assessed
using RT-qPCR (Fig. 3A-D) and
western blotting (Fig. 3E).
Compared with the control groups, APP knockdown led to a
significant decrease in the expression levels of MTA-1, MMP-2 and
−9; however, APP silencing increased the mRNA and protein
expression levels of TIMP-2. The present results suggested that
APP-siRNA may inhibit EMT by regulating the expression levels of
associated factors.
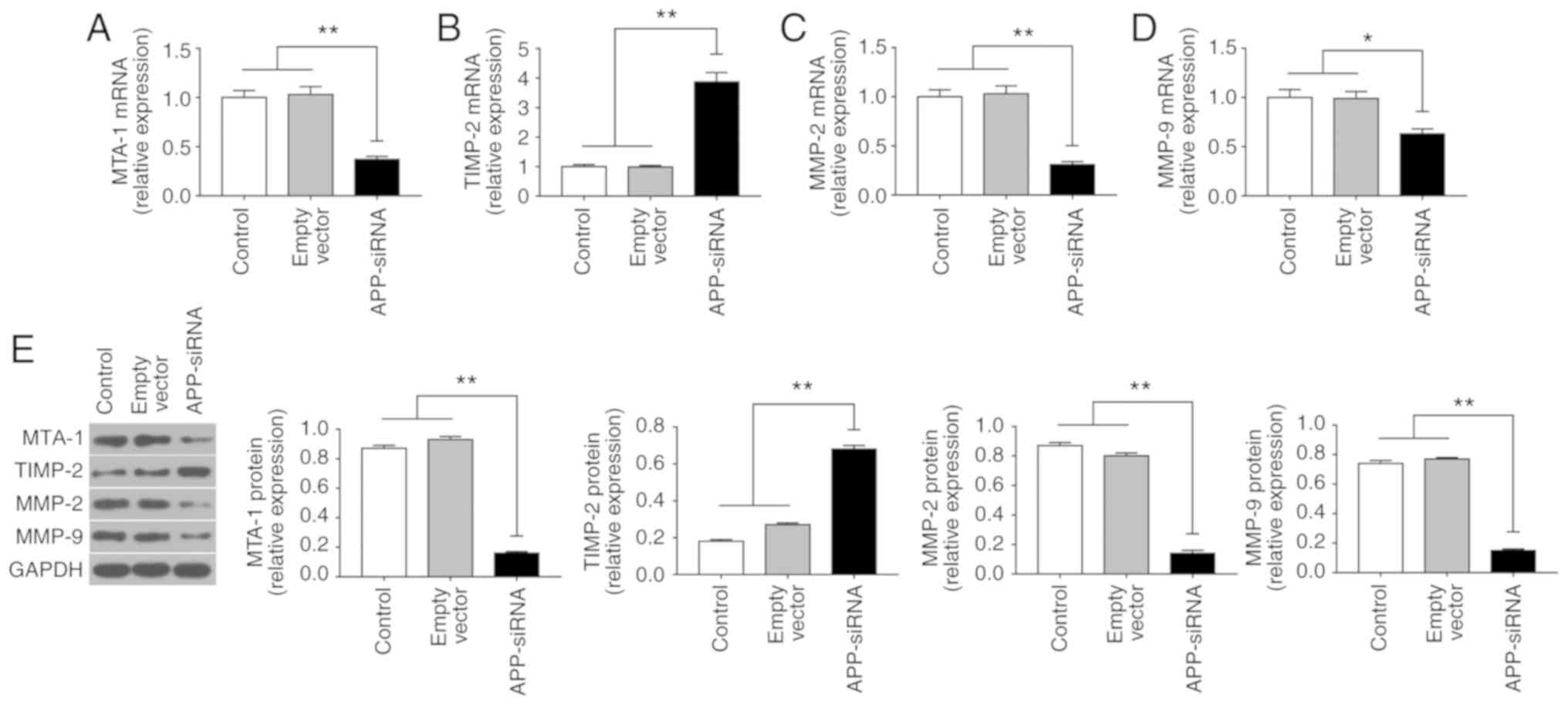 | Figure 3.APP-siRNA suppresses the expression
of MTA-1 and EMT-associated markers in SUNE-1 cells. mRNA
expression levels of (A) MTA-1, (B) TIMP-2, (C) MMP-2 and (D) MMP-9
were assessed using reverse transcription-quantitative polymerase
chain reaction. (E) Protein expression levels of EMT-associated
factors were assessed using western blotting in control, empty
vector and APP-siRNA groups. GAPDH was used as the internal control
for western blotting. Data are presented as the means ± standard
error of the mean (n=3). *P<0.05, **P<0.01. APP, amyloid β
precursor protein; EMT, epithelial-mesenchymal transition; MMP,
matrix metalloproteinase; MTA-1, metastasis-associated 1; siRNA,
small interfering RNA; TIMP-2, tissue inhibitor of
metalloproteinases 2. |
APP-siRNA suppresses activation of the
MAPK signaling pathway
The present study investigated the effects of
APP-siRNA on MAPK pathway. The protein expression levels of ERK1/2,
p38 and JNK1/2, and their corresponding phosphorylated forms, were
measured by western blotting (Fig.
4A). The present results suggested that, compared with the
control groups, APP silencing did not affect the protein expression
levels of total ERK1/2, p38 and JNK1/2; however, it did
significantly decrease their phosphorylation levels (Fig. 4B-D). Collectively, the present
results suggested that APP-siRNA was able to decrease the
phosphorylation of MAPK signaling factors.
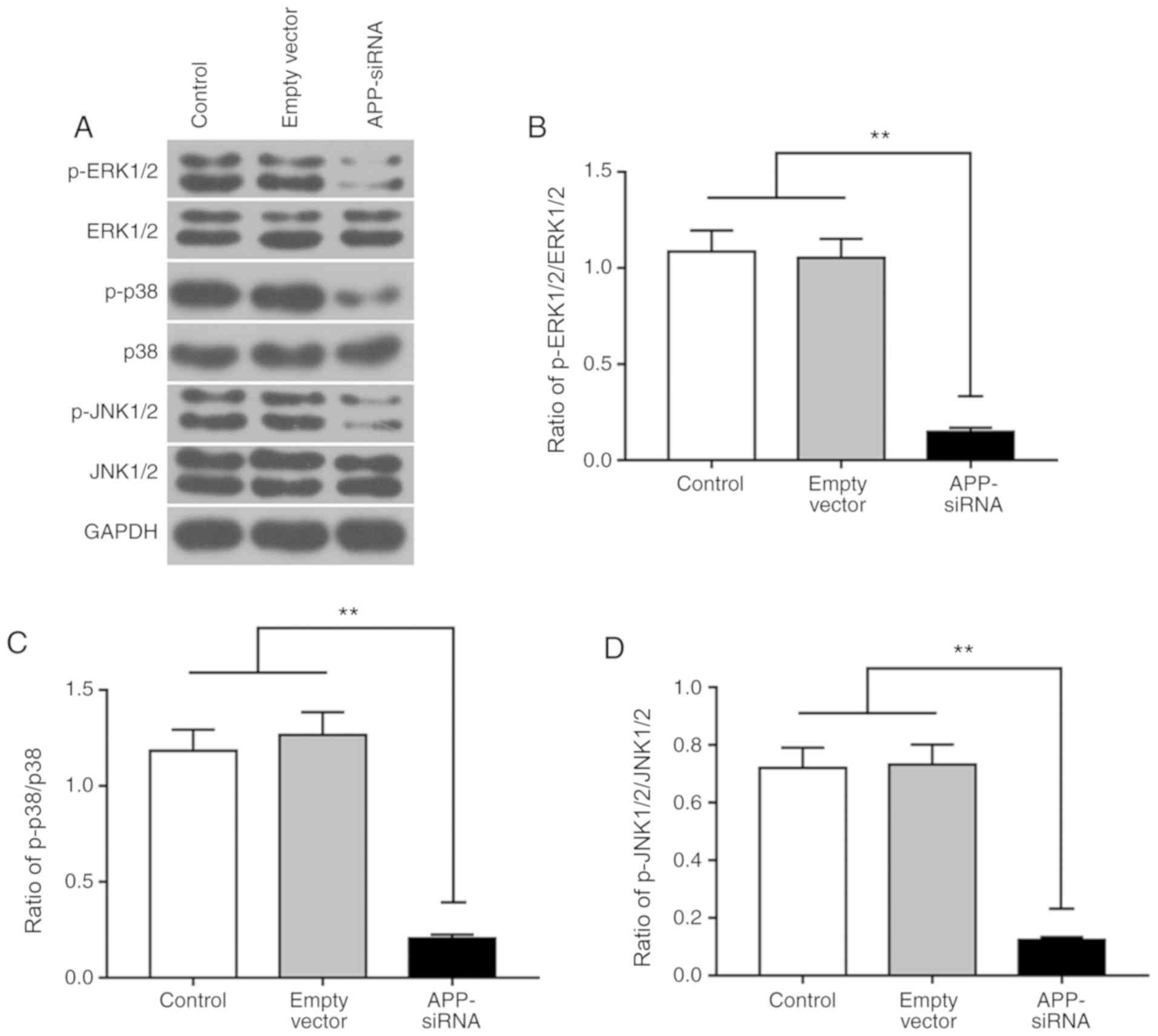 | Figure 4.APP-siRNA suppresses the activation
of the MAPK signaling pathway. Protein expression levels of (A)
ERK1/2, p38 and JNK1/2, and their corresponding phosphorylated
forms, were detected by western blotting in SUNE-1 cells. The ratio
of (B) p-ERK1/2/ERK1/2, (C) p-p38/p38 and (D) p-JNK1/2/JNK1/2
protein expression levels. GAPDH was used as the internal control
for western blotting. Data are presented as the means ± standard
error of the mean (n=3). **P<0.01. APP, amyloid β precursor
protein; ERK, extracellular signal-regulated kinases; JNK, c-Jun
N-terminal kinases; MAPK, mitogen-activated protein kinase; p-,
phosphorylated; siRNA, small interfering RNA. |
Discussion
Previous studies have demonstrated that APP is a
membrane-bound protein present in multiple cell types; notably, the
expression levels of APP are increased in various types of cancer
cells (16,26). A previous study reported that the
proliferation, migration and invasion of CNE-2 cells treated with
anti-APP antibody are significantly decreased compared with in a
control group (27). In addition,
a previous study compared 10 control and 31 NPC cell lines, and
observed that APP is differentially expressed in NPC cells, thus
suggesting an association between APP and the occurrence and
development of NPC (18). In the
present study, the mRNA and protein expression levels of APP were
significantly upregulated in NPC cells. The discrepancy between
mRNA and protein expression levels in various cell lines may be due
to the post-transcriptional regulation of APP, which remains
unclear (28). In the present
study, the protein and mRNA expression levels of APP were increased
in SUNE-1 cells compared with in normal NP69 cells. Therefore,
SUNE-1 cells were selected for further experiments. In the present
study, the effects of APP-siRNA on cell viability, migration and
invasion were investigated. APP knockdown was able to significantly
inhibit the viability, migration and invasion of SUNE-1 cells.
Furthermore, knockdown of APP increased the expression levels of
TIMP-2; however, APP-siRNA decreased the mRNA expression levels of
MTA-1, MMP-2 and −9. Additionally, APP knockdown significantly
decreased the phosphorylation levels of MAPK-associated factors.
Collectively, the present data suggested that APP-siRNA may
suppress the occurrence, development and metastasis of human
NPC.
EMT is a necessary process underlying distant
metastasis of tumor cells (29).
The present findings suggested an association between APP and EMT
in SUNE-1 cells. In the present study, the protein expression
levels of factors involved in EMT were decreased in the APP-siRNA
group compared with in the control group. The EMT process involves
various proteins. β-catenin interacts with E-cadherin to form
complexes that promote intercellular adhesion. β-catenin is able to
enter the nucleus and to induce the expression of
T-cell-factor/lymphoid enhancer binding factor 1, thus increasing
the protein expression levels of vimentin and MMPs, promoting EMT
(30,31). Previous studies have demonstrated
that TIMP-2 inhibits the activity of MMP-2 and −9, thus decreasing
degradation of the extracellular matrix (ECM), and inhibiting tumor
invasion and metastasis (32,33).
In addition, MTA-1 has been identified to be associated with
tumorigenesis, tumor invasion and metastasis (34–36).
The present results suggested that APP-siRNA was able to increase
the expression level of TIMP-2, and to decrease the expression
levels of MMP-2 and −9. These findings suggested that the balance
between TIMP-2 and MMPs may be gradually restored, which may
facilitate recovery of the dynamic equilibrium in ECM and decrease
migration and invasion of SUNE-1 cells. In addition, the expression
levels of MTA-1 were decreased following APP-siRNA transfection,
indicating that APP-siRNA inhibited the metastasis of SUNE-1 cells
by downregulating the expression of MTA-1.
The present results suggested that APP-siRNA was
able to simultaneously suppress multiple factors involved in EMT,
thus regulating tumor cell migration and invasion. Additionally,
the mechanism underlying this effect may involve an upstream
signaling pathway. A previous study observed that overexpression of
aurora kinase A promotes hyperactivation of the MAPK signaling
pathway, thus inducing EMT and invasion of NPC cells (37). The present results suggested that
APP exhibited a similar effect, and may increase EMT and the
activity of the MAPK signaling pathway. Previous studies have
demonstrated that activation of p38 and JNK is involved in EMT in
response to advanced glycation end products. Additionally, EMT may
be induced by activated ERK signaling in renal tubular cell lines
and mouse mammary gland epithelial cells in vitro (38–40).
The present results suggested that APP knockdown decreased the
protein expression levels of p-ERK1/2, p-p38 and p-JNK1/2, thus
decreasing the activity of the MAPK signaling pathway. A large
number of studies have demonstrated that p-ERK1/2 (41–43),
p-JNK (44–46) and p-p38 (40,47,48)
serve an important role in regulating EMT. Taken together,
downregulation of the protein expression levels of p-ERK1/2, p-p38
and p-JNK1/2 in the APP-siRNA group suggested that APP silencing
may inhibit the EMT process by suppressing the MAPK signaling
pathway in SUNE-1 cells. However, the present study was performed
using only one cell line, and further studies are required to
confirm the role of APP in multiple NPC cells and in
vivo.
In conclusion, the present results suggested that
APP knockdown decreased the viability, migration and invasion of
SUNE-1 cells. APP silencing increased the expression levels of
TIMP-2; however, it decreased the expression levels of MTA-1, MMP-2
and −9, thus suggesting that EMT was inhibited in SUNE-1 cells.
Notably, APP silencing may suppress cell migration, invasion and
EMT by inhibiting the MAPK signaling pathway. Therefore, APP may be
considered a novel biomarker for NPC surveillance, and as a
therapeutic target to treat patients with NPC. The present findings
may improve the understanding of NPC and may facilitate the
development of a novel gene therapy for the treatment of NPC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX made substantial contributions to the conception
and design of the study. YYi, GX, LL, QW and YYa were involved in
data acquisition, analysis and interpretation. All authors were
involved in drafting or critically revising the manuscript, and
approved of the final version to be published. All authors agreed
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Teo PM, Kwan WH, Lee WY, Leung SF and
Johnson PJ: Prognosticators determining survival subsequent to
distant metastasis from nasopharyngeal carcinoma. Cancer.
77:2423–2431. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wan G, Peng XU and Lang J: Research
progress of neoadjuvant chemotherapy for locally advanced
nasopharyngeal carcinoma. Cancer Res Prev Treat. 43:2016.(In
Chinese).
|
|
3
|
Zheng LS, Yang JP, Cao Y, Peng LX, Sun R,
Xie P, Wang MY, Meng DF, Luo DH, Zou X, et al: SPINK6 promotes
metastasis of nasopharyngeal carcinoma via binding and activation
of epithelial growth factor receptor. Cancer Res. 77:579–589. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Lu J, He ML, Li Z, Zhang B, Zhou
LH, Li Q, Li G, Wang L, Tian WD, et al: Antitumor effects of
interferon-alpha on cell growth and metastasis in human
nasopharyngeal carcinoma. Current Cancer Drug Targets. 12:561–570.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee CC, Huang TT, Lee MS, Hsiao SH, Lin
HY, Su YC, Hsu FC and Hung SK: Clinical application of tumor volume
in advanced nasopharyngeal carcinoma to predict outcome. Radiat
Oncol. 5:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burns WC and Thomas MC: The molecular
mediators of type 2 epithelial to mesenchymal transition (EMT) and
their role in renal pathophysiology. Expert Rev Mol Med.
12:e172010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chakrabarti R, Hwang J, Andres Blanco M,
Wei Y, Lukačišin M, Romano RA, Smalley K, Liu S, Yang Q, Ibrahim T,
et al: Elf5 inhibits the epithelial-mesenchymal transition in
mammary gland development and breast cancer metastasis by
transcriptionally repressing Snail2. Nat Cell Biol. 14:1212–1222.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai JH, Donaher JL, Murphy DA, Chau S and
Yang J: Spatiotemporal regulation of epithelial-mesenchymal
transition is essential for squamous cell carcinoma metastasis.
Cancer Cell. 22:725–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y and Chen X: miR-4792 inhibits
epithelial-mesenchymal transition and invasion in nasopharyngeal
carcinoma by targeting FOXC1. Biochem Biophys Res Commun.
468:863–869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mccormack N and O'Dea S: Regulation of
epithelial to mesenchymal transition by bone morphogenetic
proteins. Cell Signal. 25:2856–2862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klauzinska M, Castro NP, Rangel MC, Spike
BT, Gray PC, Bertolette D, Cuttitta F and Salomon D: The
multifaceted role of the embryonic gene Cripto-1 in cancer, stem
cells and epithelial-mesenchymal transition. Semin Cancer Biol.
29:51–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kubota T, Maruyama S, Abe D, Tomita T,
Morozumi T, Nakasone N, Saku T and Yoshie H: Amyloid beta (A4)
precursor protein expression in human periodontitis-affected
gingival tissues. Arch Oral Biol. 59:586–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muratore CR, Rice HC, Srikanth P, Callahan
DG, Shin T, Benjamin LN, Walsh DM, Selkoe DJ and Young-Pearse TL:
The familial Alzheimer's disease APPV717I mutation alters APP
processing and Tau expression in iPSC-derived neurons. Hum Mol
Genet. 23:3523–3536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ko SY, Lin SC, Chang KW, Wong YK, Liu CJ,
Chi CW and Liu TY: Increased expression of amyloid precursor
protein in oral squamous cell carcinoma. Int J Cancer. 111:727–732.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang K, Chen ZC, Yi H, Li JL, Zhang P, Li
MY, Li C, Feng XP, Peng F and Xiao ZQ: Screening of EGFR-regulated
secreted proteins in human NPC cell line CNE2. Prog Biochem
Biophys. 34:100–106. 2007.
|
|
18
|
Lai CJ and Tay BH: Pharmacophore-based
screening targeted at upregulated FN1, MMP-9, APP reveals
therapeutic compounds for nasopharyngeal carcinoma. Comput Biol
Med. 69:158–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hérard AS, Besret L, Dubois A, Dauguet J,
Delzescaux T, Hantraye P, Bonvento G and Moya KL: siRNA targeted
against amyloid precursor protein impairs synaptic activity in
vivo. Neurobiol Aging. 27:1740–1750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller VM, Gouvion CM, Davidson BL and
Paulson HL: Targeting Alzheimer's disease genes with RNA
interference: An efficient strategy for silencing mutant alleles.
Nucleic Acids Res. 32:6612004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shyam R, Ren Y, Lee J, Braunstein KE, Mao
HQ and Wong PC: Intraventricular delivery of siRNA nanoparticles to
the central nervous system. Mol Ther Nucleic Acids. 4:e2422015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao YJ, Han HZ, Liang Y, Shi CZ, Zhu QC
and Yang J: Alternative splicing of VEGFA, APP and NUMB genes in
colorectal cancer. World J Gastroenterol. 21:6550–6560. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, An L, Hui KM, Ren Q and Wang W:
An LDLa domain-containing C-type lectin is involved in the innate
immunity of Eriocheir sinensis. Dev Comp Immunol. 42:333–344. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng J, Kataoka H, Itoh H and Koono M:
Amyloid beta protein precursor is involved in the growth of human
colon carcinoma cell in vitro and in vivo. Int J Cancer. 92:31–39.
2015. View Article : Google Scholar
|
|
27
|
Tang CE, Guan YJ, Yi B, Li XH, Liang K,
Zou HY, Yi H, Li MY, Zhang PF, Li C, et al: Identification of the
amyloid β-protein precursor and cystatin C as novel epidermal
growth factor receptor regulated secretory proteins in
nasopharyngeal carcinoma by proteomics. J Proteome Res.
9:6101–6111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang C, Chen K, Liao S, Gu W, Lian X,
Zhang J, Gao X, Liu X, Wang T, He QY, et al: The flightless I
protein interacts with RNA-binding proteins and is involved in the
genome-wide mRNA post-transcriptional regulation in lung carcinoma
cells. Int J Oncol. 51:347–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kraljevic Pavelic S, Sedic M, Bosnjak H,
Spaventi S and Pavelic K: Metastasis: New perspectives on an old
problem. Mol Cancer. 10:222011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi Q, Song X, Wang J, Gu J, Zhang W, Hu
J, Zhou X and Yu R: FRK inhibits migration and invasion of human
glioma cells by promoting N-cadherin/β-catenin complex formation. J
Mol Neurosci. 55:32–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li H, Wang Z, Zhang W, Qian K, Liao G, Xu
W and Zhang S: VGLL4 inhibits EMT in part through suppressing
Wnt/β-catenin signaling pathway in gastric cancer. Med Oncol.
32:832015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eiró N, Fernandezgarcia B, Vázquez J,
Casar JMD, González LO and Vizoso FJ: A phenotype from tumor stroma
based on the expression of metalloproteases and their inhibitors,
associated with prognosis in breast cancer. Oncoimmunology.
4:e9922222015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salimi Sartakhti J, Manshaei MH and
Sadeghi M: MMP-TIMP interactions in cancer invasion: An
evolutionary game-theoretical framework. J Theor Biol. 412:17–26.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu X, Guo Y, Li X, Ding Y and Chen L:
Metastasis-associated protein 1 nuclear expression is associated
with tumor progression and clinical outcome in patients with
non-small cell lung cancer. J Thorac Oncol. 5:1159–1166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Higashijima J, Kurita N, Miyatani T,
Yoshikawa K, Morimoto S, Nishioka M, Iwata T and Shimada M:
Expression of histone deacetylase 1 and metastasis-associated
protein 1 as prognostic factors in colon cancer. Oncol Rep.
26:343–348. 2011.PubMed/NCBI
|
|
36
|
Prisco MG, Zannoni GF, De Stefano I,
Vellone VG, Tortorella L, Fagotti A, Mereu L, Scambia G and Gallo
D: Prognostic role of metastasis tumor antigen 1 in patients with
ovarian cancer: A clinical study. Hum Pathol. 43:282–288. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wan XB, Long ZJ, Yan M, Xu J, Xia LP, Liu
L, Zhao Y, Huang XF, Wang XR, Zhu XF, et al: Inhibition of Aurora-A
suppresses epithelial–mesenchymal transition and invasion by
downregulating MAPK in nasopharyngeal carcinoma cells.
Carcinogenesis. 29:1930–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Wu Z, Pandey V, Chen Y, Zhu T and
Lobie P: GP1-2: Autocrine human growth hormone suppression of
E-CADHERIN via p44/42 MAPK promotes epithelial-tomesenchymal
transition (EMT) of colorectal carcinoma cells. Eur J Cancer. 50
(Suppl):S292014. View Article : Google Scholar
|
|
39
|
Li NY, Weber CE, Wai PY, Cuevas BD, Zhang
J, Kuo PC and Mi Z: An MAPK-dependent pathway induces
epithelial-mesenchymal transition via Twist activation in human
breast cancer cell lines. Surgery. 179:256–257. 2013.
|
|
40
|
Lin Y, Mallen-St CJ, Wang G, Luo J,
Palma-Diaz F, Lai C, Elashoff DA, Sharma S, Dubinett SM and St John
M: p38 MAPK mediates epithelial-mesenchymal transition by
regulating p38IP and Snail in head and neck squamous cell
carcinoma. Oral Oncol. 60:81–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin ZH, Wang L, Zhang JB, Liu Y, Li XQ,
Guo L, Zhang B, Zhu WW and Ye QH: MST4 promotes hepatocellular
carcinoma epithelial-mesenchymal transition and metastasis via
activation of the p-ERK pathway. Int J Oncol. 45:629–640. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu H, Zhang L and Liu P: CXCR7 signaling
induced epithelial-mesenchymal transition by AKT and ERK pathways
in epithelial ovarian carcinomas. Tumour Biol. 36:1679–1683. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi P, Fang C and Pang X: Astrocyte
elevated gene-1 regulates CCL3/CCR5-induced
epithelial-to-mesenchymal transition via Erk1/2 and Akt signaling
in cardiac myxoma. Oncol Rep. 34:1319–1326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Q, Li X, Li X, Li X and Chen Z:
LncRNA H19 promotes epithelial-mesenchymal transition (EMT) by
targeting miR-484 in human lung cancer cells. J Cell Biochem.
119:4447–4457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li H, Li Y, Liu D and Liu J: LPS promotes
epithelial-mesenchymal transition and activation of TLR4/JNK
signaling. Tumour Biol. 35:10429–10435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Epstein Shochet G, Tartakover-Matalon S,
Drucker L, Pasmanik-Chor M, Pomeranz M, Fishman A and Lishner M:
Placenta-breast cancer cell interactions promote cancer cell
epithelial mesenchymal transition via TGFbeta/JNK pathway. Clin Exp
Metastasis. 31:961–975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takahashi E, Haga A and Tanihara H: Merlin
regulates epithelial-to-mesenchymal transition of ARPE-19 Cells via
TAK1-p38MAPK-mediated activation. Invest Ophthalmol Vis Sci.
56:2449–2458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ling G, Ji Q, Ye W, Ma D and Wang Y:
Epithelial-mesenchymal transition regulated by p38/MAPK signaling
pathways participates in vasculogenic mimicry formation in SHG44
cells transfected with TGF-β cDNA loaded lentivirus in vitro
and in vivo. Int J Oncol. 49:2387–2398. 2016. View Article : Google Scholar : PubMed/NCBI
|