Introduction
Neovascularization is vital for the progression of
cancer and other diseases, as it provides nutrients and oxygen
supply (1). The development,
growth and spread of cancer has been proposed to be dependent on
the establishment of a vascular network (2). Therefore, anti-angiogenic factors may
be potential candidates to treat cancer and other diseases
involving neovascularization (3).
Autophagy is a conserved catabolic process by which
cytoplasmic components are delivered into lysosomes for degradation
(4). While angiogenic factors,
such as vascular endothelial growth factor, have been reported to
induce endothelial cell autophagy under environmental stress to
sustain cell survival (5),
anti-angiogenic factors, such as endostatin, can also induce
autophagy, in order to promote endothelial cell death (6). Therefore, it has been hypothesized
that angiogenic factors induce basal-level autophagy that is
protective, whereas anti-angiogenic factors induce overactive
autophagy that may cause endothelial cell death.
Pigment epithelium-derived factor (PEDF) is a 50-kDa
secreted glycoprotein, considered to be the most potent inhibitor
of angiogenesis, which is markedly more potent than angiostatin and
endostatin (7). Previous studies
have identified reduced PEDF expression in a number of
angiogenesis-associated diseases, including diabetic retinopathy
(8) and solid tumors (9). Exogenous administration of PEDF has
been reported to decrease tumor volume and intratumoral microvessel
density (10). PEDF exerts
anti-angiogenic activities by targeting multiple pathways that
induce vascular endothelial cell apoptosis and inhibit capillary
morphogenesis (11). While p53
serves an important role in PEDF-induced endothelial cell apoptosis
(12), several earlier studies
have reported p53 to be associated with autophagy (13–15).
Recently, a number of mechanisms have been identified that connect
p53 with autophagy. Sestrin is a novel protein that is reported to
regulate autophagy and is itself regulated by p53 (16). Maiuri reported that p53 induces
autophagy by targeting sestrin2 in cancer cell lines; conversely,
knockdown of sestrin2 reduces autophagy (17). D'Amelio reported that activation of
the p53-sestrin2 signaling pathway leads to autophagy in RAW264.7
macrophages (18). However, the
connection between the p53-sestrin pathway and endothelial
autophagy remains to be elucidated. The present study aimed to
investigate the association between the PEDF-p53-sestrin pathway
and autophagy in human umbilical vein endothelial cells
(HUVECs).
Materials and methods
Cell culture
HUVECs were purchased from AllCells (Alameda, CA,
USA). Cells were cultured in Medium 200 with Low Serum Growth
Supplement (LSGS) kit (supplement contained 1.9% fetal bovine
serum, 3 ng/ml basic fibroblast growth factor, 10 µg/ml heparin, 1
µg/ml hydrocortisone and 10 ng/ml epidermal growth factor; Cascade
Biologics, Inc., Portland, OR, USA). Culture plates were pre-coated
with 2% gelatin. Cells were grown and maintained at 37°C in a
humidified atmosphere containing 5% CO2. Treatment with
PEDF (PeproTech EC, Ltd., London, UK) (200 ng/ml) was performed on
cells (5×105/ml) seeded in LSGS medium for 12 h at
37°C.
Transient transfection and RNA
interference
Specific small interfering (si)RNAs, specific for
p53 and sestrin-2, or control scrambled RNA were purchased from
Shanghai GeneChem Co., Ltd., (Shanghai, China). In total,
4.8×105/well HUVECs were transfected with siRNAs using
10 mg/ml polybrene and enhanced infection solution (cat. no.
REVG0002; Shanghai GeneChem Co., Ltd.). After 24 h, siRNA in the
medium was substituted with normal Medium 200 with LSGS. Western
blot analysis was performed to confirm the effects of p53 and
sestrin-2 gene silencing. The siRNA used were as follows: p53siRNA
forward, 5′-CUACUUCCUGAAAACAACGdTdT-3′ and reverse,
5′-CGUUGUUUUCAGGAAGUAGdTdT-3′; sestrin-2siRNA forward,
5′-GCGAGAUCAACAAAUUACUTT-3′ and reverse,
5′-AGUAAUUUGUUGAUCUCGCTT-3′; scramble siRNA forward,
5′-CUAACUAUCUCGAACGCAAdTdT-3′ and reverse,
5′-UUGCGUUCGAGAUAGUUAGdTdT-3′. All siRNAs were transfected at a
concentration of nmol/ml.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HUVECs, using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. RNA
purity was determined using the 260/280 nm absorbance ratio
(NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
First-strand cDNA was synthesized using the RevertAid First Strand
cDNA Synthesis kit (cat. no. K1622; Fermentas; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA) according to the
manufacturer's protocol. In total, 2 µg total RNA was used as
template and 10% of the total cDNA was used for each PCR reaction
containing Express SYBR Green (Takara Bio, Inc., Otsu, Japan) and
PCR Supermix (Fermentas; Thermo Fisher Scientific, Inc.). The PCR
primers used to amplify p53 and sestrin2 are listed in Table I. The PCR mix was subjected to
45-cycle amplification at 95°C for 15 sec, 95°C for 5 sec and 60°C
for 30 sec. Relative mRNA expression levels of selected genes were
normalized to those of GAPDH and quantified using the
2−ΔΔCq method (19). To
investigate the role of p53 in PEDF-induced autophagy, time-course
analysis of p53 mRNA expression was performed at 0, 4, 8, 12 and 16
h.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Target | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| p53 |
ACACGCTTCCCTGGATTG |
CAAGAAGCCCAGACGGAAAC |
| Sestrin2 |
GCATTACCTGCTGCTGCATA |
AAGGCCTGGATATGCTCCTT |
| GAPDH |
GCTAGGGACGGCCTGAAG |
GCCCAATACGACCAAATCC |
Western blot analysis
Cells were scraped into lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) containing protease
inhibitors (Beyotime Institute of Biotechnology). Subsequently,
protein concentrations were determined using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). Blocking
was performed using 5% non-fat milk at 4°C overnight. Total
proteins (22 µg) were separated by 10% SDSPAGE and transferred onto
polyvinylidene difluoride membranes (PVDF; EMD Millipore,
Billerica, MA, USA). The PVDF membranes were incubated overnight at
4°C with primary antibodies [anti-p53 (1:200; cat. no. 05-224; EMD
Millipore), anti-sestrin2 (1:200; cat. no. sc-101249; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-microtubule-associated
protein light chain 3 (LC3; 1:200; cat. no. 4108; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-p62 (1:200; cat. no.
5114S; Cell Signaling Technology, Inc.), anti-p70S6 kinase (p70S6K;
1:200; cat. no. sc-8418; Santa Cruz Biotechnology, Inc.),
anti-eukaryotic translation initiation factor 4E-binding protein 1
(4E-BP1; 1:200; cat. no. 9452; Cell Signaling Technology, Inc.),
anti-phosphorylated (p)-p70S6K (1:200; cat. no. sc-8416; Santa Cruz
Biotechnology, Inc.) and anti-p-4E-BP1 (1:200; cat. no. 2855; Cell
Signaling Technology, Inc.)]. GAPDH (1:300; cat. no. sc-47724;
Santa Cruz Biotechnology, Inc.) or β-actin (1:300; cat. no.
sc-sc-517582; Santa Cruz Biotechnology, Inc.) served as a protein
loading control. Subsequently, the blots were incubated with an
appropriate secondary antibody (horseradish peroxidase-conjugated;
1:500; cat. no. sc-2350; Santa Cruz Biotechnology, Inc.) at 20°C
for 2 h. Subsequently, the proteins were detected by enhanced
chemiluminescence using BeyoECL Plus (Beyotime Institute of
Biotechnology) and scanned using Quantity One analysis software,
version 4.6 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). To
investigate the involvement of p53 in PEDF-induced autophagy,
time-course analysis of p53 protein expression was performed at 0,
4, 8, 12 and 16 h.
Green fluorescent protein (GFP)-LC3
transient transfection and immunofluorescence microscopy
LC3 translocation was detected using a GFP-fused LC3
construct (cat. no. GM-1314P101H) purchased from Genomeditech
(Shanghai, China). Briefly, 4.8×105 cells/well cells
were plated on glass coverslips in 6-well plates. Following
attachment for 24 h, 13 µg/ml GFP-LC3 expression plasmids were
transfected using Lipofectamine® LTX reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), as shown in Fig. S1. A total of 24 h
post-transfection, the cells were treated with PBS, PEDF, PEDF +
p53 siRNA, PEDF + sestrin2 siRNA or PEDF + cont siRNA. The working
concentration of PEDF was 200 ng/ml, and treatment was performed at
37°C for 12 h. The coverslips containing the attached cells were
stained with DAPI (1 µg/ml at 37°C for 15 min) and rinsed three
times with PBS. The excess buffer was removed and cells were fixed
in 2% paraformaldehyde in PBS for 1 h at 37°C and processed for
imaging using a fluorescence microscope (magnification, ×200). In
total, six randomly-selected fields of view were analyzed.
Statistical analysis
Data from three independent experiments were
analyzed and presented as the means ± standard deviation. Two
groups were compared using Student's t-test. Differences between
groups were analyzed using oneway analysis of variance and the
least significant difference post hoc test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS v.11.0 software
(SPSS, Inc., Chicago, IL, USA).
Results
PEDF can induce autophagy in
HUVECs
To examine the role of PEDF in autophagy, HUVECs
were treated with PEDF for 12 h. A number of GFP-positive
autophagosomes were observed in PEDF-treated cells under a
fluorescence microscope (Fig. 1A).
In addition, compared with control group, PEDF treated group
exhibited higher LC3I and LC3II protein expression levels (Fig. 1B) and lower p62 protein expression
level (Fig. 1C). These data
clearly indicated that PEDF may induce autophagy in HUVECs.
p53 is critical for PEDF-induced
autophagy
To investigate the involvement of p53 in
PEDF-induced autophagy, time-course analysis of p53 mRNA and
protein expression was performed by RT-qPCR and western blotting,
respectively. As demonstrated in Fig.
2A and B, the mRNA and protein expression levels of p53 were
increased at 4 h, peaked at 12 h and were subsequently decreased at
16 h.
To validate the hypothesis that p53 is involved in
mediating PEDF-induced autophagy, p53 siRNA was established and
western blot analysis was performed to confirm the effect of p53
gene silencing (Fig. 2C). HUVECs
were transfected with p53 siRNA, prior to PEDF treatment, to
inhibit p53 expression. Fluorescence microscopy revealed a
punctuate pattern of autophagosomes in PEDF-treated cells and a
diffuse pattern in the PEDF + p53 siRNA-treated cells (Fig. 2D). Furthermore, western blot
analysis of LC3 and p62 demonstrated attenuation of PEDF-induced
LC3 accumulation and p62 downregulation in cells transfected with
p53 siRNA prior to PEDF treatment (Fig. 2E).
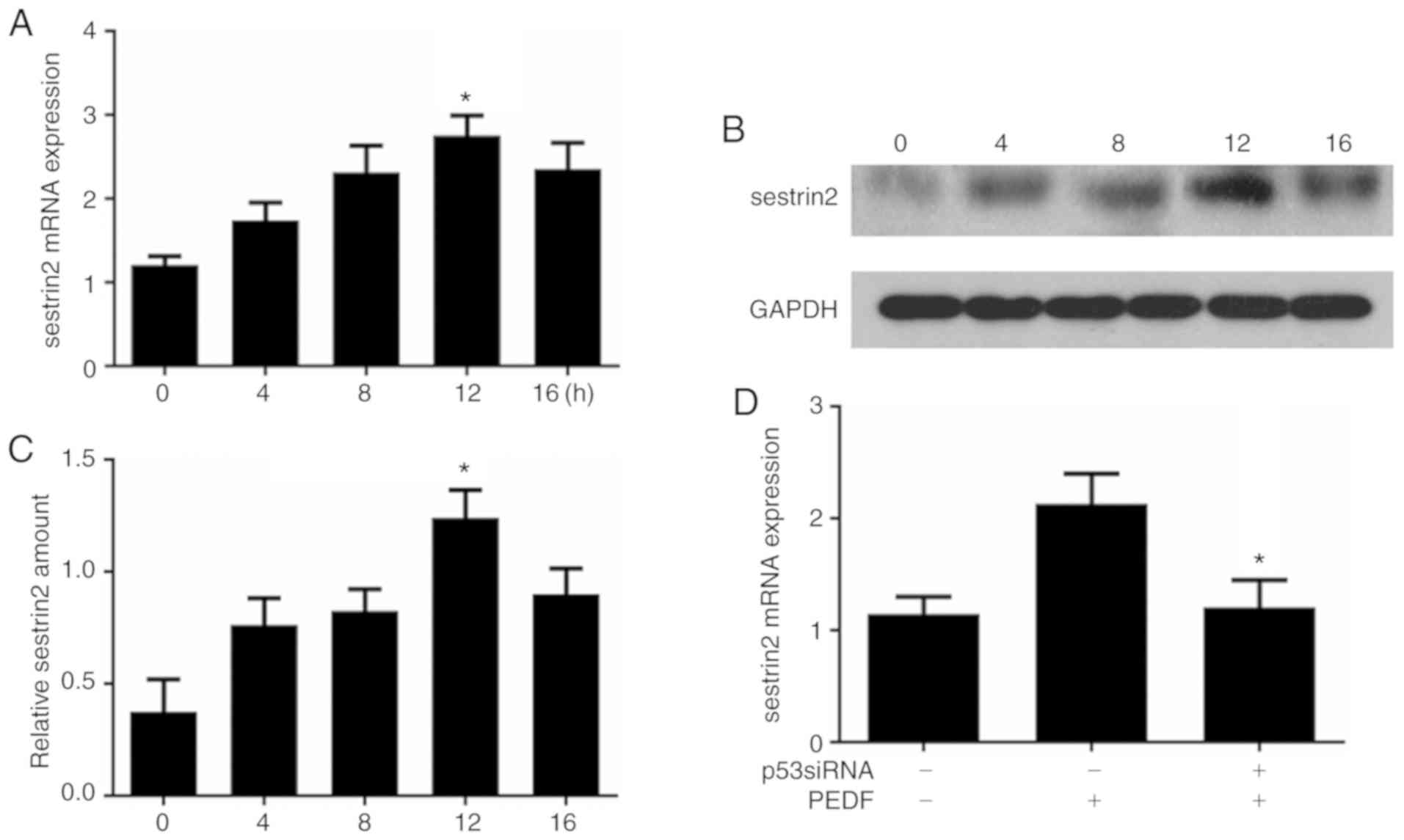
PEDF induces sestrin2 gene expression
and activates sestrin2 transcription via p53
To examine the influence of PEDF on sestrin2
expression, time-course analysis of sestrin2 mRNA expression was
performed by RT-qPCR; the results demonstrated that the mRNA
expression levels of sestrin2 were increased at 4 h, peaked at 12 h
and were subsequently decreased at 16 h (Fig. 3A). Similar results were also
observed for sestrin2 protein expression in HUVECs following PEDF
treatment (Fig. 3B).
Semi-quantitative analysis demonstrated that sestrin2 protein
expression increased at 4 h, peaked at 12 h and was eventually
decreased at 16 h (Fig. 3C). The
effects of p53 on PEDF-induced sestrin2 overexpression were further
investigated; the results revealed that transfection of HUVECs with
p53 siRNA attenuated PEDF-induced sestrin2 overexpression (Fig. 3D).
PEDF triggers sestrin2-dependent
autophagy
To verify that sestrin2 is critical in PEDF-induced
autophagy, HUVECs were transfected with sestrin2 siRNA to inhibit
sestrin2 expression. Western blot analysis confirmed the effect of
sestrin2 gene silencing (Fig. 4A).
Fluorescence microscopy demonstrated fewer GFP-positive
autophagosomes in HUVECs that were transfected with sestrin2 siRNA
prior to PEDF treatment (Fig. 4B).
Western blotting was performed to evaluate LC3 and p62 expression
in HUVECs from different groups (Fig.
4C); semi-quantitative analysis revealed that LC3 II expression
was decreased (Fig. 4D), whereas
p62 expression was increased (Fig.
4E) in the PEDF + sestrin2 siRNA-treated group compared with in
the PEDF-treated group, which indicated that sestrin2 expression
may regulate PEDF-induced autophagy.
PEDF-induced HUVEC autophagy is
mediated by inhibition of mechanistic target of rapamycin
(mTOR)
To explore whether PEDF induces autophagy by
regulating mTOR, p70S6K and 4E-BP1, which are targets of mTOR and
are phosphorylated at a number of sites, were analyzed. Western
blot analysis was conducted to examine alterations in the
phosphorylation of p70S6K and 4E-BP1 in HUVECs following
transfection with p53 siRNA and sestrin2 siRNA (Fig. 5A). The results revealed that
p-p70S6K and p-4E-BP1 expression levels were significantly
upregulated in p53 siRNA- and sestrin2 siRNA-treated groups
(Fig. 5B), thus indicating that
PEDF may regulate mTOR by activating the p53-sestrin2 signaling
pathway.
Discussion
PEDF is an important endogenous anti-angiogenesis
factor that induces apoptosis of endothelial cells and causes
endothelial cell death (20).
Recently, studies have demonstrated that controlled autophagy is a
cytoprotective response (21–23);
however, unchecked autophagy may trigger cell death by activating
p53 (24). While PEDF has been
reported to be associated with autophagy and can induce p53
expression, the mechanism underlying PEDF and autophagy in
endothelial cells is poorly understood. The present study
identified that PEDF can induce autophagy in HUVECs by activating
the p53-sestrin2 signaling pathway.
The current study demonstrated the participation of
p53 in PEDF-induced autophagy in HUVECs. It was identified that
PEDF increased the expression of p53 in HUVECs. To investigate the
role of p53 in PEDF-induced autophagy, p53 siRNA was used to
inhibit p53 signaling; the results demonstrated that p53 siRNA
prevented autophagy, thus indicating that p53 mediated autophagy.
Similarly, other studies have demonstrated the capability of p53 to
induce autophagy; activation of p53 can cause excess autophagy in
cancer cells (25–27). However, inhibition of p53 has also
been reported to enhance autophagy under environmental stress
conditions (28). Therefore, the
present study hypothesized that PEDF may induce excess autophagy
via p53 to induce HUVEC death, which may prevent the progression of
atherosclerotic plaque formation and tumor growth. The association
between PEDF and p53 in HUVEC autophagy requires further
exploration.
The present study also explored the role of a
downstream factor of p53 in autophagy and the results revealed that
PEDF may upregulate sestrin2 expression via p53, thus suggesting
sestrin2 as a major target in p53-mediated autophagy. The present
study also revealed that sestrin2 siRNA was able to reduce the
number of autophagosomes, and attenuate LC3 conversion and p62
degradation, thus suggesting that sestrin2 may serve a critical
role in PEDF-induced autophagy. Furthermore, disruption of the
sestrin2 gene in HUVECs attenuated the inhibition of p70S6K and
4E-BP1 activities. Notably, PEDF treatment alone was not able to
inhibit such effect, possibly due to the influence of PEDF on
p70S6K and 4E-BP1 via other signaling pathways and further studies
are required to understand this effect. Previous studies have
revealed the critical role of sestrin2 in p53 and mTOR signaling
(29–31). An association between p53 and
autophagy, including sestrin2, 5′AMP-activated protein kinase and
DNA damage-regulated autophagy modulator 1, has previously been
reported (17). In addition,
sestrin2 interacts with tuberous sclerosis (TSC)1:TSC2 complex to
regulate mTOR activity in a TSC2-dependent manner (29).
mTOR is a serine/threonine kinase, which acts as a
master regulator of cellular metabolism (32). mTOR regulates cell growth and
proliferation in response to a wide range of cues, and its
signaling pathway is deregulated in a number of human diseases
(33). p53 deficiency and mTOR
signaling activation are hallmarks of human cancer. Several
mechanisms account for mTOR activation in cancer, including the
activation of Ras, phosphatidylinositol 3-kinase and protein kinase
B, and inactivation of tumor suppressors that negatively regulate
these molecules: Phosphatase and tensin homolog, TSC1, TSC2 and
liver kinase B1. mTOR has also been reported to serve a key role in
regulating autophagy (34). In
this study, p70S6K and 4E-BP1, which are targets of mTOR and
phosphorylated at a number of sites, were used to detect mTOR
regulation. It was revealed that p53 siRNA and sestrin2 siRNA
increased p70S6K and 4E-BP1 phosphorylation levels, thus
demonstrating that PEDF inhibits mTOR signaling by activating p53
and sestrin2.
PEDF is involved in in the progression of a number
of diseases. To the best of our knowledge, this is the first study
to reveal that HUVEC autophagy is induced by PEDF via p53-sestrin2
signaling. The findings may contribute to improved understanding of
various diseases, including cancer and arthrosclerosis. p53 has
been revealed to serve an important role in PEDF-induced
endothelial cell apoptosis (20);
therefore, the association between p53 induced-autophagy and p53
induced-apoptosis will be investigated in a future study. The
present study is a preliminary examination that established a link
between the p53 signaling pathway and PEDF-induced autophagy in
HUVECs; in vivo experiments and further in vitro
studies are required to detect the detailed mechanism underlying
PEDF, p53/sestrin2 signaling and autophagy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by funding from the National
Natural Science Foundation of China (grant no. 81473502).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TC and TL conceived and designed the present study.
TC and TL performed the experiments. TL analyzed the data. JW
analyzed the data and provided reagents and materials. TC wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kozin SV, Duda DG, Munn LL and Jain RK:
Neovascularization after irradiation: What is the source of newly
formed vessels in recurring tumors? J Natl Cancer Inst.
104:899–905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Small DM, Burden RE, Jaworski J, Hegarty
SM, Spence S, Burrows JF, McFarlane C, Kissenpfennig A, McCarthy
HO, Johnston JA, et al: Cathepsin S from both tumor and
tumor-associated cells promote cancer growth and
neovascularization. Int J Cancer. 133:2102–2112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee S, Wurtzel JG, Singhal SS, Awasthi S
and Goldfinger LE: RALBP1/RLIP76 depletion in mice suppresses tumor
growth by inhibiting tumor neovascularization. Cancer Res.
72:5165–5173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshida GJ: Therapeutic strategies of drug
repositioning targeting autophagy to induce cancer cell death: From
pathophysiology to treatment. J Hematol Oncol. 10:672017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stanton MJ, Dutta S, Zhang H, Polavaram
NS, Leontovich AA, Hönscheid P, Sinicrope FA, Tindall DJ, Muders MH
and Datta K: Autophagy control by the VEGF-c/NRP-2 axis in cancer
and its implication for treatment resistance. Cancer Res.
73:1602013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen TM, Subramanian IV, Xiao X, Ghosh
G, Nguyen P, Kelekar A and Ramakrishnan S: Endostatin induces
autophagy in endothelial cells by modulating Beclin 1 and
beta-catenin levels. J Cell Mol Med. 13:3687–3698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becerra SP and Notario V: The effects of
PEDF on cancer biology: Mechanisms of action and therapeutic
potential. Nat Rev Cancer. 13:258–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elahy M, Baindur-Hudson S, Cruzat VF,
Newsholme P and Dass CR: Mechanisms of PEDF-mediated protection
against reactive oxygen species damage in diabetic retinopathy and
neuropathy. J Endocrinol. 222:R129–R139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nwani NG, Deguiz ML, Jimenez B, Vinokour
E, Dubrovskyi O, Ugolkov A, Mazar AP and Volpert OV: Melanoma cells
block PEDF production in fibroblasts to induce the tumor-promoting
phenotype of cancer-associated fibroblasts. Cancer Res.
76:2265–2276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujimura T, Yamagishi S, Ueda S, Fukami K,
Shibata R, Matsumoto Y, Kaida Y, Hayashida A, Koike K, Matsui T, et
al: Administration of pigment epithelium-derived factor, (PEDF)
reduces proteinuria by suppressing decreased nephrin and increased
VEGF expression in the glomeruli of adriamycin-injected rats.
Nephrol Dial Transplant. 24:1397–1406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Wei T, Jiang X, Li Z, Cui H, Pan
J, Zhuang W, Sun T, Liu Z, Zhang Z and Dong H: PEDF and 34-mer
inhibit angiogenesis in the heart by inducing tip cells apoptosis
via up-regulating PPAR-γ to increase surface FasL. Apoptosis.
21:60–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Q, Zhu X, Chen J, Mao C, Zhang L and
Xu Z: Upregulation of P53 promoted G1 arrest and apoptosis in human
umbilical cord vein endothelial cells from preeclampsia. J
Hypertens. 34:1380–1388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang A, Rajeshkumar NV, Wang X, Yabuuchi
S, Alexander BM, Chu GC, Von Hoff DD, Maitra A and Kimmelman AC:
Kimmelman, Autophagy is critical for pancreatic tumor growth and
progression in tumors with p53 alterations. Cancer Discov.
4:905–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mrakovcic M and Fröhlich LF: p53-mediated
molecular control of autophagy in tumor cells. Biomolecules.
8:E142018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi J, Xiao H, Li J, Zhang J, Li Y, Zhang
J, Wang X, Bai X, Tao K, Hu D and Guan H: Wild-type p53-modulated
autophagy and autophagic fibroblast apoptosis inhibit hypertrophic
scar formation. Lab Invest. 98:1423–1437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang N, Pan W, Zhu M, Zhang M, Hao X,
Liang G and Feng Y: Fangchinoline induces autophagic cell death via
p53/sestrin2/AMPK signalling in human hepatocellular carcinoma
cells. Br J Pharmacol. 164:731–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maiuri MC, Malik SA, Morselli E, Kepp O,
Criollo A, Mouchel PL, Carnuccio R and Kroemer G: Stimulation of
autophagy by the p53 target gene Sestrin2. Cell Cycle. 8:1571–1576.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Amelio M and Cecconi F: A novel player
in the p53-mediated autophagy: Sestrin2. Cell Cycle.
8:14672009.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ho TC, Chen SL, Yang YC, Liao CL, Cheng HC
and Tsao YP: PEDF induces p53-mediated apoptosis through PPAR gamma
signaling in human umbilical vein endothelial cells. Cardiovasc
Res. 76:213–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sumitomo Y, Koya J, Nakazaki K, Kataoka K,
Tsuruta-Kishino T, Morita K, Sato T and Kurokawa M: Cytoprotective
autophagy maintains leukemia-initiating cells in murine myeloid
leukemia. Blood. 128:1614–1624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao L, Dong Y, Zhou FB, Chang YL, Suo ZG
and Ding HQ: Protective effect of tauroursodeoxycholic acid on the
autophagy of nerve cells in rats with acute spinal cord injury. Eur
Rev Med Pharmacol Sci. 22:1133–1141. 2018.PubMed/NCBI
|
|
23
|
Chen J, Zhu Y, Zhang W, Peng X, Zhou J, Li
F, Han B, Liu X, Ou Y and Yu X: Delphinidin induced protective
autophagy via mTOR pathway suppression and AMPK pathway activation
in HER-2 positive breast cancer cells. BMC Cancer. 18:3422018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ellis M, Stern O and Ashur-Fabian O: The
double benefit of Spalax p53: Surviving underground hypoxia while
defying lung cancer cells in vitro via autophagy and
caspase-dependent cell death. Oncotarget. 7:63242–63251. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jakhar R, Paul S, Bhardwaj M and Kang SC:
Astemizole-Histamine induces Beclin-1-independent autophagy by
targeting p53-dependent crosstalk between autophagy and apoptosis.
Cancer Lett. 372:89–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haque E, Kamil M, Irfan S, Sheikh S, Hasan
A, Nazir A and Mir SS: Blocking mutation independent p53
aggregation by emodin modulates autophagic cell death pathway in
lung cancer. Int J Biochem Cell Biol. 96:90–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu D, Li R, Guo X, Pang L, Zang Y, Liu K
and Chen D: Chen, DNA damage regulated autophagy modulator 1
recovers the function of apoptosis-stimulating of p53 protein 2 on
inducing apoptotic cell death in Huh7.5 cells. Oncol Lett.
15:9333–9328. 2018.PubMed/NCBI
|
|
28
|
Zhang QY, Jin R, Zhang X, Sheng JP, Yu F,
Tan RX, Pan Y, Huang JJ and Kong LD: The putative oncotarget CSN5
controls a transcription-uncorrelated p53-mediated autophagy
implicated in cancer cell survival under curcumin treatment.
Oncotarget. 7:69688–69702. 2016.PubMed/NCBI
|
|
29
|
Budanov AV and Karin M: p53 target genes
sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling.
Cell. 134:451–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jayaraj P, Sen S, Rangarajan S, Ray N,
Vasu K, Singh VK, Phartyal R, Yadav S and Verma A:
Immunohistochemical evaluation of stress-responsive protein
sestrin2 and its correlation with p53 mutational status in eyelid
sebaceous gland carcinoma. Br J Ophthalmol. 102:848–854. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang N, Zhang Q, Luo L, Ning B and Fang Y:
β-asarone inhibited cell growth and promoted autophagy via
P53/Bcl-2/Bclin-1 and P53/AMPK/mTOR pathways in Human Glioma U251
cells. J Cell Physiol. 233:2434–2443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kassem L and Abdel-Rahman O: Targeting
mTOR pathway in gynecological malignancies: Biological rationale
and systematic review of published data. Crit Rev Oncol Hematol.
108:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saxton RA and Sabatini DM: mTOR signaling
in growth, metabolism, and disease. Cell. 168:960–976. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Munson MJ and Ganley IG: MTOR, PIK3C3 and
autophagy: Signaling the beginning from the end. Autophagy.
11:2375–2376. 2015. View Article : Google Scholar : PubMed/NCBI
|