Introduction
Ulcerative colitis (UC) is an idiopathic, chronic
inflammatory colitis that manifests as diffuse mucosal inflammation
of the colon and rectum, and interferes with intestinal motility;
however, and the exact pathogenesis of UC remains poorly
understood. UC mainly includes rectal and proximal transmission,
and does not affect the digestive tract beyond the ileocecal valve.
In recent years, the numbers of individuals suffering from UC has
been continuously increasing in several countries (1). UC most commonly affects individuals
aged between 30 and 40 years (2,3). The
pathogenesis of UC is multifactorial, including immune responses
disorders, genetic susceptibility, epithelial barrier defects and
adverse environmental factors, among others (4). Treatments for UC include
aminosalicylates for mild-to-moderate disease, topical and systemic
steroids for disease outbreaks, and immunosuppressants and
biopharmaceuticals for moderate-to-severe disease, whereas patients
with refractory disease or colonic tumors require colectomy
(4). However, the effective
management of UC represents a major challenge.
Artemisinin was isolated from the Artemisia
(genus) plant in 1971 and is a medicinal natural product commonly
used in the treatment of malaria (5). Recent studies have demonstrated that
artemisinin derivatives also exert anti-tumor effects (6–10),
which have attracted attention for use as anticancer drugs. Due to
its anti-inflammatory properties, artesunate (ART), a
semi-synthetic derivative of artemisinin, has been used in the
treatment of a variety of inflammatory diseases. It has also been
reported that ART can inhibit the activation of the Toll-like
receptor 4 (TLR4)-nuclear factor (NF)-κB pathway (11). However, the effect of ART on UC
remains unknown. Therefore, the aim of the present study was to
evaluate the anti-inflammatory effects of ART on DSS-induced
UC.
Tumor necrosis factor (TNF)-α and interleukins (ILs)
serve crucial roles in the inflammatory process of UC. Accumulating
evidence suggests that the TNF-α gene regulates the development of
UC, and increased levels of TNF-α have been detected in patients
with UC; thus, TNF-α is a pro-inflammatory mediator with a key role
in the pathogenesis of inflammatory bowel diseases (12). In addition, pro-inflammatory ILs
serve an important role in the pathogenesis of UC, and high IL
levels secreted by macrophages have been associated with the
severity of inflammation in colitis (13). Nuclear factor (NF)-κB p65 is an
important transcription factor that regulates a large number of
genes involved in immune and inflammatory responses. NF-κB is a key
transcription factor of M1 macrophages and induces a number of
inflammatory genes, including TNF-α, IL-1β, IL-6, IL-10, IL-12, and
cyclooxygenase-2 (14,15). Inactivated NF-κB is potentially
involved in pro-apoptotic signaling pathways (16). Furthermore, NF-κB increases B-cell
lymphoma 2 (Bcl-2) expression, resulting in a decrease in cellular
apoptosis (17). Previous studies
have demonstrated that NF-κB signaling dysfunction is closely
associated with the pathogenesis and progression of UC (18,19).
Since the NF-κB signaling pathway is widely known to be involved in
inflammatory response (20,21),
its inactivation may be critical for the effective therapy of UC
(22).
Another hallmark of UC, namely inflammation limited
to the mucosa, may be associated with TLR4 (23). In mice exposed to DSS, treatment
with anti-TLR4 antibodies resulted in the attenuation of
inflammation of the colon, and downregulated the expression levels
of IL-1β, TNF-α and interferon (IFN)-γ (24). It is well known that the TLR4-NF-κB
signaling pathway is a commonly recognized inflammatory pathway,
which is likely to be activated in DSS-induced UC. These findings
prompted the investigation of the effects of ART on UC.
The aim of the present study was to investigate
whether ART attenuated the DSS-induced colon injury and to further
elucidate whether the underlying mechanism involves regulating the
TLR4-NF-κB signaling pathway, in order to assess the potential of
ART as an effective intervention for UC treatment.
Materials and methods
Materials
ART (SA9720) was purchased from Solarbio Life
Sciences (Beijing, China). TRIzol Reagent (cat. no. 15596026;
Thermo Fisher Scientific, Inc.) was used to extract the total RNA.
The 2X Taq PCR Master Mix was obtained from Beijing Baiao Laibo
Technology Co., Ltd. (Beijing, China). Western blot analysis,
hematoxylin and eosin (H&E) staining, cell RIPA lysis buffer
solution was purchased from Beyotime Institute of Biotechnology and
ELISA assay kits were purchased from Abcam and R&D Systems
China Co., Ltd. The TLR4, NF-κB p65, phosphorylated (p)-p38, Bcl-2,
Bcl-2-associated X protein (Bax) and caspase-9 primary antibodies,
and the horseradish peroxidase-conjugated goat anti-rabbit/mouse
secondary antibodies were obtained from Abcam or Cell Signaling
Technology, Inc. Lipopolysaccharide (LPS; from Escherichia
coli 0111:B4; L2630) and DSS (D4911) powder were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The compound
5-aminosalicylic acid (5-ASA) was purchased from Tokyo Kasei Kogyo
Co., Ltd.
Animals
Male Sprague-Dawley rats weighing 200–250 g (7 weeks
old; n=48) were used in the experiments, which were purchased from
Nanjing Qinglongshan Experimental Animal Breeding Farm. The rats
were housed in a specific-pathogen-free environment, and kept under
an automated 12-h light/dark cycle at a controlled temperature of
24±2°C and relative humidity of 50–60%, with ad libitum
access to food and tap water. All animals received humane care and
the experimental procedures were conducted in strict accordance
with the Guide to the Care and Use of Experimental Animals. All
experimental procedures performed on the rats were approved by the
Animal Experiment Ethics Committee of Nanjing Drum Tower Hospital
(Nanjing, China).
Animal grouping
All animals were randomly divided into the following
six groups, according to different treatments: Normal control group
(CG), DSS-induced UC model group (DG), low-dose ART group (LG),
middle-dose ART group (MG), high-dose ART group (HG) and positive
control drug group (PG). Rats in each group had diets with the same
energy density, macronutrient composition and trace elements.
UC animal model
DSS-induced colitis in rats was induced as described
previously (25). Briefly, DSS was
added to drinking water to a final concentration of 3%. The DSS
solution was freshly prepared and replaced daily. Rats were
randomly divided into six groups (normal control, UC model,
positive control and three ART-treated groups), with 8 rats per
group. Rats in the control group were given normal drinking water,
while drinking water containing 3% DSS was given to the model,
positive control and three ART-treated groups. After 10 days of DSS
administration, the drinking water of all the rats was replaced
with normal drinking water on the day 11. The rats were given free
access to water during the experiment. On day 11 after DSS
induction, the three experimental groups were orally administered
different doses of ART (10, 30 and 50 mg/kg/day) for 5 days, while
the positive control drug group was orally administered 5-ASA (50
mg/kg/day) in l ml 0.9% NaCl saline. Rats in the normal control and
model groups were orally administered 1 ml/kg/day of 0.9%
phosphate-buffered saline (PBS). Subsequent to the last dose, the
rats were fasted for 24 h, and on the following day, rats from each
group were randomly selected for the follow-up experiments.
Measurement of disease activity index
(DAI)
The symptoms of colonic inflammation in all rats
were monitored daily. The stool viscosity rating criteria were as
follows: A rating of 0 indicated normal stool, a rating of 2
indicated thin stool, while a rating of 4 points indicated
diarrhea. Bloody stool was also rated as follows: 0 indicated
normal stool, a rating of 2 indicated fecal occult blood-positive,
and 4 indicated bleeding. The weight loss rating criteria were as
follows: 0, no change; 1, weight loss of 1–5% of the initial body
weight; 2, weight loss of 5–10% of the initial body weight; 3,
weight loss of 10–15% of the initial body weight; and 4, weight
loss of >15% of the initial body weight. Finally, the DAI was
measured as previously described (26). The DAI score was calculated as the
sum of scores assigned for weight loss, stool consistency and
rectal bleeding.
Histological analysis of the colon and
changes in colon length
After the rats were euthanized, the colons were
immediately resected. Next, the mesenteric tissue, blood vessels
and fat were carefully removed, and the colon length was measured.
Subsequent to washing the intestine with PBS, a ~4 cm segment of
the distal colon was removed and weighed. For histological
examination, 1-cm segments from the distal colon were fixed in 10%
formalin, embedded in paraffin, cut into 5-mm sections and placed
on the slides. In order to observe the histological changes, colon
sections were stained with H&E and then examined under an
Olympus microscope (Olympus Corporation, Tokyo, Japan). The
histological score of the H&E-stained colon specimens was
evaluated in terms of the degrees of lymphocyte infiltration,
mucosal erosion, colonic crypt damage and ulcer formation.
Histological scoring was performed in a blinded manner, according
to the classic scoring system described by Cooper et al
(27). The rest of the colon
tissue was immediately frozen in liquid nitrogen and then stored at
−80°C for subsequent western blot and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analyses.
Measurement of myeloperoxidase (MPO)
in colon tissue
Colon tissue (1 g) was mashed with a mortar and
pestle in 1 ml of 20 mM potassium phosphate buffer (pH 7.0). The
supernatants were collected by centrifugation at 16,000 × g for 10
min at 4°C, and the level of MPO was measured using assay kits
purchased from Abcam (Cambridge, MA, USA; cat. no. ab43321).
Cell culture and treatment
RAW264.7 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% (v/v) fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere with 5% CO2 and 95% air, until
70–80% confluence was reached (28). The cells (2×105
cells/well) were then seeded into 24-well plates and incubated for
24 h. Subsequently, RAW264.7 cells were stimulated with LPS (1
µg/ml; Gibco; Thermo Fisher Scientific, Inc.) for 48 h, followed by
treatment with different concentration of ART (5, 10 and 20 µg/ml)
or with 5-ASA (20 mmol/l), which was used as a positive control.
After 24 h, the cells were collected for use in further
experiments. The experimental grouping of cells was as follows:
Control, LPS, 5-ASA, ART 5, ART 10 and ART 20 groups.
Measurement of cytopathological
state
RAW264.7 cells were fixed with 4% paraformaldehyde
for 15 min and washed with distilled water for 2 min, followed by
two 2-min washes with distilled water. The processed samples were
stained with hematoxylin for 10 min and then rinsed with distilled
water for 10 min to remove any excess stain. Next, the samples were
washed for 5 sec with 95% ethanol and then with distilled water.
Finally, the samples were stained with eosin solution for 1 min,
washed twice with 70% ethanol and directly observed under an
Olympus microscope. The affected cells exhibited an increased cell
size, unclear cell borders or cell structure disruptions, and
histological scoring was determined based on the number of abnormal
cells and according to the classic scoring system of Cooper et
al (27).
Measurement of cell migration by a Transwell assay.
The cell migration ability was assayed using 24-transwell inserts
with 8-µm microporous membranes. Briefly, cell suspensions
(2×105 cells/well) were added to the upper chamber of a
transwell migration system (BD Biosciences, Franklin Lakes, NJ,
USA) and incubated for 12 h at 37°C. Following starving for 8 h in
FBS-free DMEM to eliminate the influence of serum, 500 µl DMEM
high-glucose medium supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) was added into the lower Transwell chamber. Then,
the indicated treatments were added and the cells were allowed to
migrate for 24 h. Non-migrating cells were removed from the upper
surface of the insert with a cotton swab. Migrating cells were
fixed with 100% ethanol for 20 min, followed by staining with
crystal violet solution. Images of the migrating cells were
captured using an Olympus microscope.
Measurement of inflammatory factor
levels by ELISA
After the last administration, the rats were fasted
for 24 h and blood samples were collected the next day. The serum
sample (0.5 ml) was obtained from the tail veins of rats, and the
serum was isolated by centrifugation at 500 × g for 5 min. The rat
Hb kit (cat. no. ml0187371; Shanghai Enzyme-linked Biotechnology
Co., Ltd.) was used to measure the Hb content in rat serum.
RAW264.7 cells (2×105 cells/well) were plated into
96-well plates and cultured overnight at 37°C. Subsequently to the
indicated treatments, culture supernatants of RAW264.7 cells were
collected to detect the levels of inflammatory factor. The
concentrations of IL-8 (cat. no. CK-E30583; R&D Systems China
Co., Ltd., Shanghai, China), IFN-γ (cat. no. ab46107; Abcam) and
TNF-α (cat. no. ab46105, Abcam) were measured using their
respective ELISA kits, according to the protocols recommended by
the manufacturer. The inflammatory factor levels were measured in
both the rat serum samples and RAW264.7 cells.
Measurement of cell viability
The viability of RAW264.7 cells was assessed using
the Cell Counting Kit-8 (CCK-8) assay. Cells (5×103
cells/well) were seeded on a 96-well plate. Following the indicated
cell treatments, CCK-8 reagent was added to the culture medium, and
the cells were cultured for 1 h at 37°C in a humidified atmosphere
with 95% air and 5% CO2. Absorbance was measured at 490
nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
RT-qPCR
Total RNA was extracted from colon tissues and
RAW264.7 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and treated with DNase I (Promega Corporation,
Madison, WI, USA). The RNA purity and concentration were measured
using an UV spectrophotometer and RT was then performed using
T7-(dT)24 oligo primers and the Custom SuperScript
Double-Stranded cDNA Synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc.) following the instructions of the manufacturer.
The reverse transcription conditions were 10 min at 25°C, 30 min at
48°C, and a final step of 5 min at 95°C. The cDNA samples were
stored at −20°C for subsequent qPCR analysis. Next, qPCR for the
evaluation of mRNA levels was performed by PowerUp SYBR Green
Master Mix (Thermo Fischer Scientific) according to the
manufacturer's protocol. The qPCR reactions (20 µl) were performed
as follows: 2 min at 95°C, followed by 40 cycles of 15 sec at 95°C
and 60 sec at 60°C. qPCR primers used are listed in Table I. The fold relative expression was
calculated according to the 2−ΔΔCq method (29) and normalized to GAPDH. Three
repeated experiments were performed for each qPCR reaction.
 | Table I.Primers used in quantitative
polymerase chain reaction analysis. |
Table I.
Primers used in quantitative
polymerase chain reaction analysis.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| TLR4 |
AAATGCACTGAGCTTTAGTGGT |
TGGCACTCATAATGATGGCAC |
| NF-κB |
AGGCTTCTGGGCCTTATGTG |
TGCTTCTCTCGCCAGGAATAC |
| TNF-α |
CCCTCACACTCAGATCATCTTCT |
GCTACGACGTGGGCTACAG |
| p38 |
GGGACACCCCCTGCTTATCT |
TCCCTGCTTTCAAAGGACTGG |
| IFN-γ |
ACAGCAAGGCGAAAAAGGATG |
TGGTGGACCACTCGGATGA |
| IL-8 |
TCGAGACCATTTACTGCAACAG |
CATTGCCGGTGGAAATTCCTT |
| Bax |
AGACAGGGGCCTTTTTGCTAC |
AATTCGCCGGAGACACTCG |
| BcL-2 |
GCTACCGTCGTGACTTCGC |
CCCCACCGAACTCAAAGAAGG |
| Caspase-9 |
AGCCAGAGGTTCTCAGACCAG |
ATATCTGCATGTCCCCTGATCT |
| GAPDH |
TGACCTCAACTACATGGTCTACA |
CTTCCCATTCTCGGCCTTG |
Western blot analysis
Total proteins were extracted from the rat colon
tissue and RAW264.7 cells using a protein isolation kit (Beyotime
Institute of Biotechnology), and the protein concentration was
measured using a bicinchoninic assay kit (Beyotime Institute of
Biotechnology). The protein samples were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis (8–12%), and the
protein bands were then transferred onto PVDF membranes. Following
blocking in 5% skim milk for 2 h at room temperature, the membranes
were incubated at 4°C overnight with the designated primary
antibodies against TLR4 (cat. no. ab13556; 1:500), NF-κB p65 (cat.
no. ab16502; 1:1,000), TNF-α (cat. no. ab6671; 1:1,000), Bax (cat.
no. ab199677; 1:1,000), Bcl-2 (cat. no. ab196495; 1:1,000),
Caspase-9 (cat. no. ab25758; 1:1,000) and GAPDH (cat. no. ab37168;
1:10,000), which were purchased from Abcam, and with anti-p-p38
(cat. no. 4511S; 1:1,000) that was purchased from Cell Signaling
Technology (Danvers, MA, USA). Subsequently, the blots were labeled
with horseradish peroxidase-conjugated goat anti-rabbit IgG H&L
(cat. no. ab6721; 1:10,000; Abcam). Then, the membranes were
developed with ECL (Beyotime Institute of Biotechnology). Signals
were quantified using ImageJ software (version 1.47; National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
derivation. Statistical analyses were performed with SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical
differences among groups were determined by one-way analysis of
variance followed by Dunnett's post hoc test, and the quantitative
variables were compared using the paired Student's-t test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of ART on DAI in UC rats
The DAI in the CG remained unchanged until day 14,
whereas the DAI in the DG increased gradually with increasing DSS
treatment time between days 0 and 10 (Fig. 1A). After 10 days, there was a
significant difference in DAI between CG and DG (P<0.001;
Fig. 1A). However, treatment of UC
model rats with ART inhibited the increase in DAI in a
dose-dependent manner when compared with that in untreated model
rats. On day 14, the difference in DAI between the DG and all the
treatment groups (LG, MG, HG and PG) was statistically significant
(LG vs. DG, P<0.01; MG vs. DG, P<0.01; HG vs. DG, P<0.001;
PG vs. DG, P<0.001; Fig.
1A).
 | Figure 1.Effects of ART on (A) DAI and (B)
hemoglobin in a rat ulcerative colitis model. ***P<0.001 vs. CG;
#P<0.05, ##P<0.01 and ###P<0.001, vs. DG. ART, artesunate;
DAI, disease activity index; CG, control group; DG, dextran sulfate
sodium-treated model group; LG, low-dose ART group; MG, middle-dose
ART group; HG, high-dose ART group; PG, positive control group. |
Effect of ART on the serum hemoglobin
(Hb) content of UC rats
ELISA revealed that DSS treatment significantly
reduced the blood Hb level as compared with that in healthy animals
in the CG (P<0.001; Fig. 1B).
However, ART treatment in the model animals increased the blood Hb
level in a dose-dependent manner. The difference in Hb content
between the DG and the treatment groups MG, HG and PG was
statistically significant (MG vs. DG, P<0.05; HG vs. DG,
P<0.01; PG vs. DG, P<0.001; Fig.
1B), whereas no marked difference was observed between the DG
and LG levels.
Effect of ART on DSS-induced colon
length reduction in UC rats
As shown in Fig. 2A and
B, DSS treatment caused a significant decrease in the colon
length of the UC model rats, as compared with that in control rats
(P<0.001). Treatment with ART prevented the DSS-induced colon
length reduction in UC model rats, with statistically significant
differences observed between the DG and the treatment groups MG, HG
and PG (MG vs. DG, P<0.05; HG vs. DG, P<0.001; PG vs. DG,
P<0.001; Fig. 2A and B).
 | Figure 2.Effects of ART on colon length and
MPO in a rat UC model. (A) Representative images of colon sections
from rats in each group. (B) Quantification of the change in colon
length in each group. (C) Change in the activity of MPO in UC rats
in each group. ***P<0.001 vs. CG; #P<0.05, ##P<0.01 and
###P<0.001, vs. DG. ART, artesunate; MPO, myeloperoxidase; UC,
ulcerative colitis; CG, control group; DG, dextran sulfate
sodium-treated model group; LG, low-dose ART group; MG, middle-dose
ART group; HG, high-dose ART group; PG, positive control group. |
Effect of ART on the activity of MPO
in UC rats
DSS treatment increased oxidative stress in UC rats
by markedly increasing the levels of MPO, as compared with those
observed in healthy animals in the CG (P<0.001; Fig. 2C). Comparatively, ART improved the
antioxidant capacities of UC rats by reducing the activity of MPO
in a dose-dependent manner, with significant differences detected
between the DG and the other treatment groups (LG vs. DG,
P<0.01; MG vs. DG, P<0.001; HG vs. DG, P<0.001; PG vs. DG,
P<0.001; Fig. 2C).
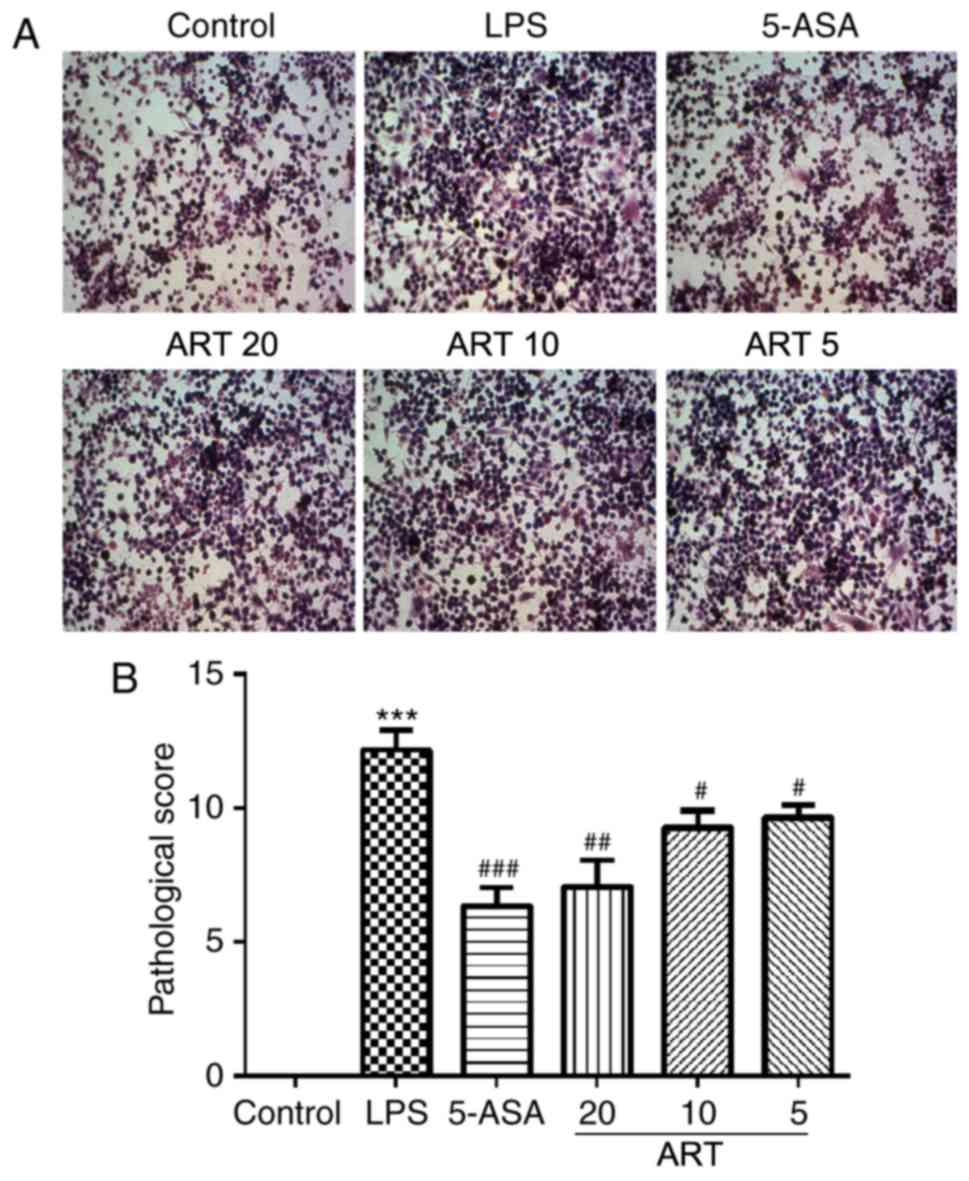
Protective effect of ART against
DSS-induced colon damage
To determine the pathological changes in the colon,
H&E staining was performed. Histological examination revealed a
normal tissue structure, as well as appearance of the cell nuclei
and cytoplasm, in the CG (Fig.
3A). DSS treatment caused tissue damage with cell destruction,
whereas ART treatment in UC rats reduced the severity of the damage
in a dose-dependent manner. In terms of the histological scores,
the differences between the DG and the treatment groups MG, HG and
PG were statistically significant (MG vs. DG, P<0.05; HG vs. DG,
P<0.01; PG vs. DG, P<0.01; Fig.
3B).
 | Figure 3.Effects of ART on the pathological
alterations in the colon. (A) Representative images of hematoxylin
and eosin stained sections (magnification, ×200), and (B)
quantification of the pathological alterations in the colon
according to the histological score. ***P<0.001 vs. CG;
#P<0.05 and ###P<0.001, vs. DG. ART, artesunate; CG, control
group; DG, dextran sulfate sodium-treated model group; LG, low-dose
ART group; MG, middle-dose ART group; HG, high-dose ART group; PG,
positive control group. |
Effects of ART on the serum levels of
inflammatory factors in UC rats
ELISA demonstrated that DSS treatment increased the
serum levels of the inflammatory factors TNF-α, IL-8 and IFN-γ when
compared with those in healthy animals in the CG (P<0.001;
Fig. 4). By contrast, ART
administration in UC rats inhibited the increase in the serum
levels of TNF-α, IL-8 and IFN-γ in a dose-dependent manner, with
statistically significant differences detected between the DG and
the treatment groups MG, HG and PG (MG vs. DG, P<0.05; HG vs.
DG, P<0.01; PG vs. DG, P<0.01; Fig. 4), whereas no differences were
observed between the DG and LG levels.
 | Figure 4.Effects of ART on the serum levels of
inflammatory factors in ulcerative colitis rats. ELISA analysis
indicated that DSS treatment increased the levels of IFN-γ, IL-8
and TNF-α, whereas ART exposure reversed these effects.
***P<0.001 vs. CG; #P<0.05, ##P<0.01 and ###P<0.001,
vs. DG. ART, artesunate; IFN-γ, interferon-γ; IL, interleukin;
TNF-α, tumor necrosis factor α; DSS, dextran sulfate sodium; CG,
control group; DG, DSS-treated model group; LG, low-dose ART group;
MG, middle-dose ART group; HG, high-dose ART group; PG, positive
control group. |
Effects of ART on the regulation of
key molecules involved in the TLR4-NF-κB signaling pathway in UC
rats
As shown in Fig.
5A, compared with the CG, DSS treatment increased the protein
levels of TLR4, p-NF-κB, p-p38, Bax and caspase-9, whereas it
reduced the protein level of Bcl-2. Treatment of UC model rats with
ART inhibited the activity of the TLR4-NF-κB signaling pathway by
reducing the expression levels of TLR4, p-NF-κB, p-p38, Bax and
caspase-9, and increasing the expression of Bcl-2 in a
dose-dependent manner. The difference in the expression was
statistically significant between DG and the treatment groups MG,
HG and PG (all P<0.01 or P<0.001; Fig. 5A). Notably, these levels were not
significantly altered in the LG compared with DG, while the protein
levels of NF-κB and p38 did not differ markedly among all groups.
Comparatively, the results of RT-qPCR on the mRNA levels of TLR4,
p-NF-κB, p-p38, Bax, caspase-9 and Bcl-2 were consistent with the
results of western blotting (Fig.
5B). Furthermore, the results of RT-qPCR verified the ELISA
results, confirming that DSS treatment increased the levels of
IFN-γ, IL-8 and TNF-α compared with the CG, and that ART lowered
these levels in UC rats (DG vs. CG, P<0.001; MG vs. DG,
P<0.01 or P<0.001; HG vs. DG, P<0.001; PG vs. DG,
P<0.001; Fig. 5B).
 | Figure 5.Effects of ART on the levels of key
molecules involved in TLR4-NF-κB signaling pathway in ulcerative
colitis rats. (A) Western blot analysis of the effects of ART on
the levels of key molecules involved in the TLR4-NF-κB signaling
pathway. DSS-treatment increased the protein expression levels of
TLR4, p-NF-κB, p-p38, Bax and caspase-9, and downregulated Bcl-2,
whereas ART reversed these levels. (B) Reverse
transcription-quantitative polymerase chain reaction analysis
yielded similar results to the protein expression observations of
western blot analysis. **P<0.01 and ***P<0.001, vs. CG;
##P<0.01 and ###P<0.001, vs. DG. ART, artesunate; TLR4,
toll-like receptor 4; NF-κB, nuclear factor-κB; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; DSS, dextran sulfate
sodium; CG, control group; DG, DSS-treated model group; LG,
low-dose ART group; MG, middle-dose ART group; HG, high-dose ART
group; PG, positive control group. |
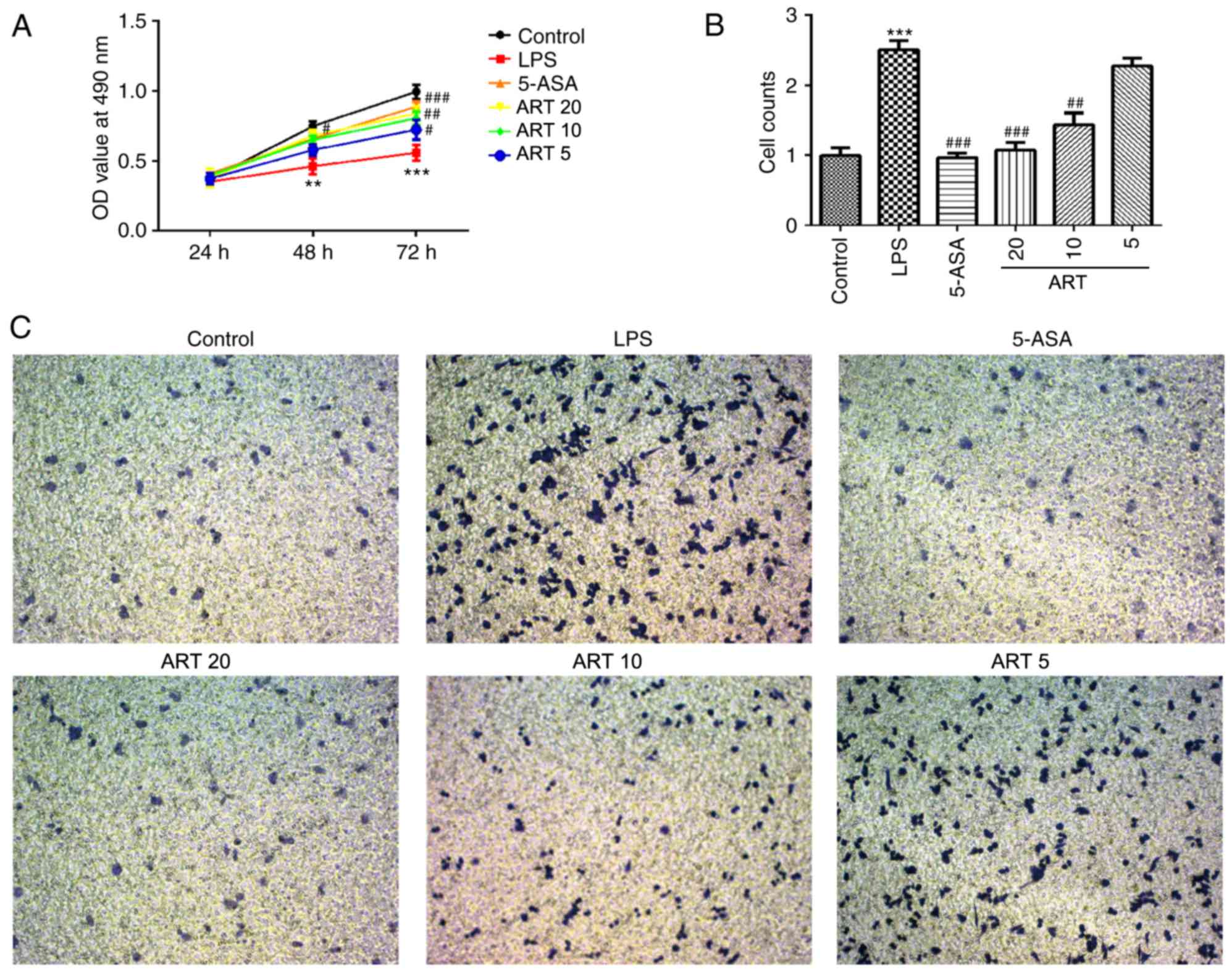
Protective effect of ART against RAW264.7 cell
damage caused by LPS. Following treatment with LPS, used to induce
inflammation, RAW264.7 cell infiltration was markedly increased.
H&E staining revealed that the cells became larger and more
rounded when compared with the control cells (P<0.001); however,
ART treatment reduced cell infiltration, and the cell shape and
size were gradually restored (Fig.
6A). Therefore, ART alleviated cell injury in a dose-dependent
manner, and the pathological score of LPS-treated cells was
significantly higher in comparison with that of the 5-ASA, ART 5,
ART 10 and ART 20 groups (ART 5 vs. LPS, P<0.05; ART 10 vs. LPS,
P<0.01; ART 20 vs. LPS, P<0.001; 5-ASA vs. LPS, P<0.001;
Fig. 6B).
Effects of ART on the levels of
inflammatory factors in RAW264.7 cells
ELISA demonstrated that, when compared with the
control cells, LPS treatment increased the levels of the
inflammatory factors IFN-γ, IL-8 and TNF-α in cells induced by LPS
(P<0.001). However, ART lowered the levels of the abovementioned
inflammatory factors in a dose-dependent manner. The difference in
the levels between the LPS-treated group, and the 5-ASA and ART 20
groups were statistically significant for all factors, while a
marked decrease in IL-8 and TNF-α levels was also observed in ART
10 compared with the LPS group (Fig.
7).
Effect of ART on cell viability
The results of the CCK-8 assay demonstrated that LPS
treatment significantly reduced cell viability when compared with
that in the control (at 48 h, P<0.01; at 72 h, P<0.001). ART
exposure improved the cell viability in a dose-dependent manner,
with statistically significant differences detected between the LPS
group, and the 5-ASA, ART 5, ART 10 and ART 20 groups at 72 h (ART
5 vs. LPS, P<0.05; ART 10 vs. LPS, P<0.01; ART 20 vs. LPS,
P<0.001; 5-ASA vs. LPS, P<0.001; Fig. 8A).
Effect of ART on cell migration
As shown in Fig. 8B and
C, the Transwell assay revealed that LPS treatment
significantly increased cell migration compared with the control
cells (P<0.001). ART reduced cell migration in a dose-dependent
manner, with statistically significant differences observed between
the LPS group, and the 5-ASA, ART 5, ART 10 and ART 20 groups (ART
10 vs. LPS, P<0.01; ART 20 vs. LPS, P<0.001; 5-ASA vs. LPS,
P<0.001; Fig. 8B).
Effect of ART on the regulation of key
molecules involved in the TLR4-NF-κB signaling pathway in RAW264.7
cells
Western blot analysis demonstrated that, when
compared with the control cells, LPS treatment in RAW264.7 cells
significantly increased the protein levels of TLR4, p-NF-κB, p-p38,
Bax and caspase-9, whereas it reduced the protein level of Bcl-2
(P<0.001). Further treatment with ART activated the TLR4-NF-κB
signaling pathway by reducing the expression levels of TLR4,
p-NF-κB, p-p38, Bax and caspase-9, while increasing Bcl-2
expression in a dose-dependent manner. Statistically significant
differences in these levels were observed between the LPS-treated
group, and the 5-ASA, ART 10 and ART 20 groups (ART 10 vs. LPS,
P<0.05; ART 20 vs. LPS, P<0.01; 5-ASA vs. LPS, P<0.01;
Fig. 9).
 | Figure 9.Effects of ART on the levels of key
molecules involved in the TLR4-NF-κB signaling pathway in RAW264.7
cells. (A) Western blot analysis demonstrated the effects of ART on
the levels of key molecules involved in TLR4-NF-κB signaling. (B)
LPS treatment increased the protein expression levels of TLR4,
p-NF-κB, p-p38, Bax and caspase-9, and downregulated Bcl-2, whereas
exposure to ART reverses these effects. No significant differences
in the expression levels of NF-κB and p38 were reported among all
groups. ***P<0.001 vs. control group; #P<0.05 and ##P<0.01
vs. LPS group. ART, artesunate; LPS, lipopolysaccharide; 5-ASA,
5-aminosalicylic acid; TLR4, toll-like receptor 4; NF-κB, nuclear
factor-κB; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein. |
Discussion
UC is a recurrent and prolonged inflammatory disease
of the digestive system. Its pathological manifestations are
diverse, including diarrhea (30),
abdominal pain (25), and tenesmus
(31), among others. Several drugs
are effective in the treatment of UC; however, the majority of
these are accompanied by notable side effects, which significantly
inhibit their widespread clinical application. Although the
aminosalicylate 5-ASA has been widely used as a first-line medicine
for UC therapy, earlier findings have indicated that 5-ASA may
cause symptoms of diarrhea in patients with UC (32). In addition, careful monitoring is
required when 5-ASA is administered to treat UC in elderly patients
with renal impairment or cardiac failure (33).
ART treatment appears to be promising in terms of
tolerance in malaria patients, and the pharmacokinetics of this
agent have been well-characterized (34). A recent studies further revealed
that ART activation of ferroptosis is an effective, novel pathway
for eliminating pancreatic ductal adenocarcinoma cells (10). In addition, previous data
demonstrated that ART may reduce cell proliferation and
angiogenesis, and trigger apoptosis, and that it exerts its
protective effects by enhancing antioxidant activities and
alleviating oxidative stress (35–37).
ART is considered to be suitable for drug development due to its
aqueous solubility. In the present study, it was first hypothesized
that ART may attenuate the DSS-induced inflammatory damage in a UC
animal model, as well as protect RAW264.7 cells against LPS-induced
damage and control their inflammatory status. The results then
revealed that ART reduced the DSS-induced inflammatory damage in UC
rats, and protected RAW264.7 cells against LPS-induced damage and
inflammatory response. To fully elucidate the mechanisms underlying
the anti-inflammatory actions of ART, the present study also
investigated the mechanisms mediating inflammatory damage in UC
rats and the effects of ART on RAW264.7 cells.
The downstream signaling of all TLRs involves three
major signaling pathways: Mitogen-activated protein kinase,
IFN-regulatory factor and NF-κB signaling pathways (38). NF-κB regulates the activation and
differentiation of T cells and inflammation (39); therefore, it was hypothesized that
inactivation of the TLR4-NF-κB signaling pathway may be important
for the effective treatment of UC. LPS activates TLR4 signaling,
leading to the release of TNF-α and IL-6, and the ensuing response
to inflammation (40). Based on
this mounting evidence, a genetic association between TNF-α and UC
was predicted. In the present study, the activity of the TLR4-NF-κB
pathway and the expression of inflammatory genes were found to be
induced by DSS treatment. In addition, the extent of damage to the
colon of UC rats was aggravated, as reflected by alterations in
DAI, MPO, colon length and Hb expression. Furthermore, western
blotting, RT-qPCR analysis and ELISA confirmed that ART inhibited
the TLR4-mediated NF-κB activation, leading to decreased release of
the pro-inflammatory cytokines IFN-γ, IL-8 and TNF-α, markedly
reducing colonic damage in UC rats. The anti-inflammatory effect of
ART in UC rats was similar to its role in LPS-induced RAW264.7
cells. In vitro experiments demonstrated that ART treatment
attenuated the LPS-induced migration, activity and apoptosis of
RAW264.7 cells, as well as the release of inflammatory mediators,
as confirmed by H&E staining, CCK-8 assay, Transwell assay and
ELISA. ART also decreased the LPS-induced expression levels of
factors associated with the TLR4-NF-κB pathway and inflammation, as
determined by western blotting.
As previously reported, upregulation of Bax and
caspase-3, and downregulation of Bcl-2 may result in cell
apoptosis. Bax permeabilizes the mitochondrial outer membrane,
releasing pro-apoptotic factors that activate caspases, and the
apoptosome then activates caspase-9, which in turn leads to the
activation of caspase-3 and thus induces apoptosis (41,42).
In the present study, the apoptosis of colon tissue and RAW264.7
cells following intervention with ART was assessed by H&E
staining, CCK-8 assay and western blotting. Decreases in the
pathological damage of colon tissue and apoptosis were observed in
ART-treated DSS-induced UC rats. In addition, the migration and
apoptosis of ART-treated LPS-induced RAW264.7 cells were decreased.
Similarly, it was observed that ART significantly inhibited the
TLR4-NF-κB pathway and inflammatory genes in LPS-treated RAW264.7
cells. Therefore, ART appears to be beneficial in the treatment of
UC by effectively reducing inflammatory damage.
However, there were certain limitations to the
present study. Firstly, the properties of ART are complex, and the
exact functions of this compound have not been fully elucidated.
Nontprasert et al (43)
reported that ART derivatives were neurotoxic to mice, and the
neurotoxic effects of the artemisinin derivatives used in the
treatment of several diseases have been a matter of concern over
the past years. This point is reflected in the present study; for
instance, the indexes of UC rats failed to return to normal levels
subsequent to treatment with a high dose of ART. Compared with the
positive control group, the therapeutic effect of ART is slightly
inferior. In addition, following ART treatment, rats exhibited a
slightly depressed mental state as compared with normal rats, which
was manifested by weight loss, reduced food intake and reduced
exercise time, suggesting a lack of food reward and euphoria in
rats. These detection indexes were only used as simple daily
observations in this experiment, and were not quantified or formed
part of the experimental results of the present study; thus, more
studies are needed to confirm this point in the future. Secondly,
the present study only focused on the association between different
ART dosage, and the changes of the main molecules participating in
the TLR4-NF-κB signaling pathway and inflammatory responses. Gene
silencing and overexpression of TLR4-NF-κB must be performed to
study the underlying mechanism in more detail. Another limitation
is that the present study only revealed the level change of
caspase-9; however, theoretically the expression levels of both
caspase-3 and −9 should be examined, since numerous studies have
suggested that caspase-3 is more representative of the apoptosis
pathway in comparison with caspase-9. Furthermore, a number of
studies have demonstrated that various cytokines associated with
UC, such as IL-1β and IL-6, are also able to increase inflammatory
response through the TLR4-NF-κB signaling pathway, with the
exception of TNF-α, IL-8 and IFN-γ. Certain studies have further
indicated that NF-κB was a key transcription factor of M1
macrophages and induced a great amount of inflammatory gene
expression, including IL-1β, IL-6, IL-10, IL-12 and
cyclooxygenase-2. Thus, the relevant inflammatory factors mentioned
above can be detected in further experiments. Finally, an
analytical experiment with other tissues of UC rats must be
conducted to investigate whether UC affects other organs as well.
Therefore, in order to obtain more evidence supporting the present
findings, further research is required in the future.
In conclusion, ART effectively controlled the
development of UC by improving the antioxidant and
anti-inflammatory status of UC rats via the TLR4-NF-κB signaling
pathway in vivo. Corresponding cell experiments in
vitro demonstrated that ART affected the TLR4-NF-κB signaling
pathway by reducing cell apoptosis and the expression of
inflammatory factors. The present findings are promising and
support the use of ART as a natural drug with anti-inflammatory,
anti-apoptotic and antioxidants properties. In view of the complex
role of ART and the fact that current research on ART is limited to
animal and cell experiments, further studies and clinical trials
are crucial for determining whether ART may be developed into a
drug for UC therapy in the future. Furthermore, the present study
highlighted the importance of the TLR4-NF-kB signaling pathway in
the development of UC. Inhibition of this signaling may, thus, be a
possible therapeutic strategy for the treatment of UC, and ART may
provide a theoretical basis for the development of new drugs for UC
treatment.
Acknowledgements
The authors would like to express their deepest
gratitude to the chief consultant, Dr Long-dian Chen, who has
provided valuable guidance. The authors are also sincerely grateful
to Prof. Yu-ling Yao, Prof. Yi-yang Zhang, Prof. Ting-sheng Ling,
Prof. Ming Zhang, Dr Chun-yan Peng, Dr Gui-fang Xu and Dr Min Chen
(all Nanjing Drum Tower Hospital) for their help in experimental
techniques and theoretical guidance.
Funding
No funding was received.
Authors' contributions
YXC and XPZ conceived and designed the experiments,
and wrote the paper. YXC, XQZ and CGY performed the experiments.
SLH, YX, XTD and WJL analyzed the data. XPZ and XQZ provided the
reagents, materials and analysis tools.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Ethics approval and consent to
participate
All animals received humane care, and the
experimental procedures were conducted in strict accordance with
the health and care guidelines for experimental animals. All
experimental procedures performed on the rats were approved by the
Animal Experiment Ethics Committee of Nanjing Drum Tower Hospital
(Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ito A, Omori T, Hanafusa N, Tsuchiya K,
Nakamura S and Tokushige K: Efficacy and safety of granulocyte
adsorption apheresis in elderly patients with ulcerative colitis. J
Clin Apher. 33:514–520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Høivik ML, Moum B, Solberg IC, Henriksen
M, Cvancarova M and Bernklev T; IBSEN Group, : Work disability in
inflammatory bowel disease patients 10 years after disease onset:
Results from the IBSEN Study. Gut. 62:368–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torres J, Billioud V, Sachar DB,
Peyrin-Biroulet L and Colombel JF: Ulcerative colitis as a
progressive disease: The forgotten evidence. Inflamm Bowel Dis.
18:1356–1363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ungaro R, Mehandru S, Allen PB,
Peyrin-Biroulet L and Colombel JF: Ulcerative colitis. Lancet.
389:1756–1770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuda K, Miyamoto L, Hamano S, Morimoto Y,
Kangawa Y, Fukue C, Kagawa Y, Horinouchi Y, Xu W, Ikeda Y, et al:
Mechanisms of the pH- and oxygen-dependent oxidation activities of
artesunate. Biol Pharm Bull. 41:555–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Efferth T, Giaisi M, Merling A, Krammer PH
and Li-Weber M: Artesunate induces ROS-mediated apoptosis in
doxorubicin-resistant T leukemia cells. PLoS One. 2:e6932007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ooko E, Saeed ME, Kadioglu O, Sarvi S,
Colak M, Elmasaoudi K, Janah R, Greten HJ and Efferth T:
Artemisinin derivatives induce iron-dependent cell death
(ferroptosis) in tumor cells. Phytomedicine. 22:1045–1054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Button RW, Lin F, Ercolano E, Vincent JH,
Hu B, Hanemann CO and Luo S: Artesunate induces necrotic cell death
in schwannoma cells. Cell Death Dis. 5:e14662014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berdelle N, Nikolova T, Quiros S, Efferth
T and Kaina B: Artesunate induces oxidative DNA damage, sustained
DNA double-strand breaks, and the ATM/ATR damage response in cancer
cells. Mol Cancer Ther. 10:2224–2233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eling N, Reuter L, Hazin J, Hamacher-Brady
A and Brady NR: Identification of artesunate as a specific
activator of ferroptosis in pancreatic cancer cells. Oncoscience.
2:517–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cen Y, Liu C, Li X, Yan Z, Kuang M, Su Y,
Pan X, Qin R, Liu X, Zheng J and Zhou H: Artesunate ameliorates
severe acute pancreatitis (SAP) in rats by inhibiting expression of
pro-inflammatory cytokines and Toll-like receptor 4. Int
Immunopharmacol. 38:252–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sands BE and Kaplan GG: The role of
TNFalpha in ulcerative colitis. J Clin Pharmacol. 47:930–941. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allocca M, Furfaro F, Fiorino G, Gilardi
D, D'Alessio S and Danese S: Can IL-23 be a good target for
ulcerative colitis? Best Pract Res Clin Gastroenterol.
32-33:95–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeoung BR, Lee KD, Na CS, Kim YE, Kim B
and Kim YR: Ganghwaljetongyeum, an anti-arthritic remedy,
attenuates synoviocyte proliferation and reduces the production of
proinflammatory mediators in macrophages: The therapeutic effect of
GHJTY on rheumatoid arthritis. BMC Complement Altern Med.
13:472013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong HY, Choi YS, Lee JK, Lee BJ, Kim WK
and Kang H: Anti-inflammatory activity of citric acid-treated wheat
germ extract in lipopolysaccharide-stimulated macrophages.
Nutrients. 9:2017. View Article : Google Scholar
|
|
16
|
Xiang Y, Ye W, Huang C, Lou B, Zhang J, Yu
D, Huang X, Chen B and Zhou M: Brusatol inhibits growth and induces
apoptosis in pancreatic cancer cells via JNK/p38
MAPK/NF-κb/Stat3/Bcl-2 signaling pathway. Biochem Biophys Res
Commun. 487:820–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie Z, Xiao Z and Wang F: Hepatitis C
virus nonstructural 5A protein (HCV-NS5A) inhibits hepatocyte
apoptosis through the NF-κb/miR-503/bcl-2 pathway. Mol Cells.
40:202–210. 2017.PubMed/NCBI
|
|
18
|
Eissa N, Hussein H, Kermarrec L, Elgazzar
O, Metz-Boutigue MH, Bernstein CN and Ghia JE: Chromofungin (CHR:
CHGA47-66) is downregulated in persons with active
ulcerative colitis and suppresses pro-inflammatory macrophage
function through the inhibition of NF-κB signaling. Biochem
Pharmacol. 145:102–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu P, Zhu L, Liu Y, Zhang L, Liu J and
Shen H: Protective effects of paeoniflorin on TNBS-induced
ulcerative colitis through inhibiting NF-kappaB pathway and
apoptosis in mice. Int Immunopharmacol. 50:152–160. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding X, Liang Y, Peng W, Li R, Lin H,
Zhang Y and Lu D: Intracellular TLR22 acts as an inflammation
equalizer via suppression of NF-κB and selective activation of MAPK
pathway in fish. Fish Shellfish Immunol. 72:646–657. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan T, Shi X, Chen H, Chen R, Wu D, Lin Z,
Zhang J and Pan J: Geniposide suppresses interleukin-1β-induced
inflammation and apoptosis in rat chondrocytes via the
PI3K/Akt/NF-κB signaling pathway. Inflammation. 41:390–399. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu B, Li S, Sui X, Guo L, Liu X, Li H,
Gao L, Cai S, Li Y, Wang T and Piao X: Root extract of polygonum
cuspidatum Siebold & Zucc. ameliorates DSS-induced ulcerative
colitis by affecting NF-kappaB signaling pathway in a mouse model
via synergistic effects of polydatin, resveratrol, and emodin.
Front Pharmacol. 9:3472018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manolakis AC, Kapsoritakis AN, Kapsoritaki
A, Tiaka EK, Oikonomou KA, Lotis V, Vamvakopoulou D, Davidi I,
Vamvakopoulos N and Potamianos SP: Readressing the role of
toll-like receptor-4 alleles in inflammatory bowel disease:
Colitis, smoking, and seroreactivity. Dig Dis Sci. 58:371–380.
2013.PubMed/NCBI
|
|
24
|
Liu Y, Zhang Z, Wang L, Li J, Dong L, Yue
W, Chen J, Sun X, Zhong L and Sun D: TLR4 monoclonal antibody
blockade suppresses dextran-sulfate-sodium-induced colitis in mice.
J Gastroenterol Hepatol. 25:209–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castelli AA, Estrada JJ and Kaminski JP:
Patient with ulcerative colitis and abdominal pain. JAMA Surg.
153:282–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murthy SN, Cooper HS, Shim H, Shah RS,
Ibrahim SA and Sedergran DJ: Treatment of dextran sulfate
sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis
Sci. 38:1722–1734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
28
|
Yang YH, Li DL, Bi XY, Sun L, Yu XJ, Fang
HL, Miao Y, Zhao M, He X, Liu JJ and Zang WJ: Acetylcholine
inhibits LPS-induced MMP-9 production and cell migration via the α7
nAChR-JAK2/STAT3 pathway in RAW264.7 cells. Cell Physiol Biochem.
36:2025–2038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong W, Lu X, Shi H, Zhao G, Song Y, Wang
Y, Zhang J, Jin Y and Wang S: Distinct microbial populations exist
in the mucosa-associated microbiota of diarrhea predominant
irritable bowel syndrome and ulcerative colitis. J Clin
Gastroenterol. 28–Nov;2017.doi: 10.1097/MCG.0000000000000961 (Epub
ahead of print). View Article : Google Scholar
|
|
31
|
Nigg, Kolyvanos N, Käser and Vetter:
Colitis ulcerosa. Praxis. 97:167–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimodate Y, Takanashi K, Waga E, Fujita
T, Katsuki S and Nomura M: Exacerbation of bloody diarrhea as a
side effect of mesalamine treatment of active ulcerative colitis.
Case Rep Gastroenterol. 5:159–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muller AF, Stevens PE, McIntyre AS,
Ellison H and Logan RF: Experience of 5-aminosalicylate
nephrotoxicity in the United Kingdom. Aliment Pharmacol Ther.
21:1217–1224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ericsson T, Blank A, von Hagens C, Ashton
M and Äbelö A: Population pharmacokinetics of artesunate and
dihydroartemisinin during long-term oral administration of
artesunate to patients with metastatic breast cancer. Eur J Clin
Pharmacol. 70:1453–1463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anfosso L, Efferth T, Albini A and Pfeffer
U: Microarray expression profiles of angiogenesis-related genes
predict tumor cell response to artemisinins. Pharmacogenomics J.
6:269–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Efferth T, Sauerbrey A, Olbrich A, Gebhart
E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, et
al: Molecular modes of action of artesunate in tumor cell lines.
Mol Pharmacol. 64:382–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Efferth T, Ramirez T, Gebhart E and
Halatsch ME: Combination treatment of glioblastoma multiforme cell
lines with the anti-malarial artesunate and the epidermal growth
factor receptor tyrosine kinase inhibitor OSI-774. Biochem
Pharmacol. 67:1689–1700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumar H, Kawai T and Akira S: Toll-like
receptors and innate immunity. Biochem Biophys Res Commun.
388:621–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chi W, Chen H, Li F, Zhu Y, Yin W and Zhuo
Y: HMGB1 promotes the activation of NLRP3 and caspase-8
inflammasomes via NF-κB pathway in acute glaucoma. J
Neuroinflammation. 12:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saluk J, Bijak M, Posmyk M and Zbikowska
H: Red cabbage anthocyanins as inhibitors of
lipopolysaccharide-induced oxidative stress in blood platelets. Int
J Biol Macromol. 80:702–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martinou JC and Green DR: Breaking the
mitochondrial barrier. Nat Rev Mol Cell Biol. 2:63–67. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nontprasert A, Pukrittayakamee S, Dondorp
AM, Clemens R, Looareesuwan S and White NJ: Neuropathologic
toxicity of artemisinin derivatives in a mouse model. Am J Trop Med
Hyg. 67:423–429. 2002. View Article : Google Scholar : PubMed/NCBI
|