Introduction
Non-small cell lung cancer (NSCLC), which accounts
for >85% of newly diagnosed cases of lung cancer, is the leading
cause of morbidity and cancer-associated mortality worldwide
(1). Although there are a large
number of clinical treatment options, the effectiveness of
treatment for patients with NSCLC is unfavorable, with a 5-year
relative survival rate of 18% (1).
Chemotherapy, adjuvant radiotherapy and biological targeted therapy
following surgical excision have improved the survival rate of
patients with cancer. Chemotherapy is the most effective and common
treatment method. Although a significant number of patients
initially respond favorably to chemotherapy, the majority of them
exhibit severe side-effects and de novo or acquired
resistance to chemotherapeutic drugs, and conventional
chemotherapeutic drugs do not provide a marked survival advantage
for patients (2,3). Therefore, there is an urgent
requirement to develop a novel drug that can effectively treat
NSCLC with fewer side-effects.
Metastasis and proliferation of cancer cells are the
main causes of deterioration of patients with NSCLC as cancer cells
can survive beyond the normal life span of a cell, have increased
proliferation and resistance to chemotherapy and facilitate
metastatic activity (4). In
addition, defective apoptosis is recognized as the major criterion
that contributes to the initiation and progression of cancer. The
key proteins in this process are BCL2 associated X (Bax) and B-cell
lymphoma (Bcl-2). Consequently, induction of apoptosis and
inhibition of cell viability are promising strategies for treatment
of cancer. The process is associated with various signaling
pathways, including that of phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR).
A previous study reported that the PI3K/Akt/mTOR pathway is
involved with different cellular processes, from cell growth or
survival, to cell necrosis or apoptosis (5). Notably, natural products are
considered a promising source for the development of novel
anticancer drugs due to their potential effectiveness and low
toxicity (6).
Chinese herbal medicine has gradually become an
important modern clinical therapeutic approach for human diseases
due to the strong pharmacological properties, which contribute to
cancer chemotherapy (7). Alisol B
23-acetate (AB23A), a triterpenoid compound, exists naturally in
the rhizomes of Alisma orientalis (8) and has been identified to have
anti-cancer biological functions (9). Furthermore, AB23A had been
demonstrated to possess anti-proliferative activity (10) and induced Bax gene nuclear
translocation and apoptotic in PC-3 cells (4). In addition, a number of studies have
demonstrated that AB23A has anti-hepatitis virus (11) and anti-bacterial (12) pharmacological activity. In human
renal proximal tubular cells, alisol B-induced autophagy mediates
apoptosis and nephrotoxicity through the PI3K/AKT/mTOR signaling
pathway (13). However, the
anticancer mechanism of AB23A remains unclear.
In the present study, the effects of AB23A on A549
cells were systematically investigated, including those on cell
viability, migration and invasion, the cell cycle, apoptosis and
the activity of the PI3K/AKT/mTOR signaling pathways. The results
demonstrated that AB23A may be a promising compound for the
treatment of NSCLC. To the best of our knowledge, this study is the
first to demonstrate that AB23A exerts anticancer effects on NSCLC
and to investigate the possible corresponding molecular
mechanism.
Materials and methods
Materials
AB23A (High pressure liquid chromatography ≥98%) was
purchased from Shanghai Moqi Biological Technology Co., Ltd.
(Shanghai, China). The Cell Counting Kit-8 (CCK-8; cat. no. C0039)
was purchased from Beyotime Institute of Biotechnology (Haimen,
China). The propidium iodide (PI)/RNase staining kit and the
Annexin V-FITC/7AAD kit were all purchased from BD Biosciences (San
Jose, CA, USA); All primary antibodies, including Bax (cat. no.
ab53154; 1:1,000), Bcl-2 (cat. no. ab196495; 1:1,000), AKT (cat.
no. ab38449; 1:1,000), phosphorylated (p)-AKT (cat. no. ab18206;
1:500), PI3K (cat. no. ab86714; 1:1,000), p-PI3K (cat. no.
ab125633; 1:1,000), mTOR (cat. no. ab63552; 1:500), p-mTOR (cat.
no. ab1093; 1:1,000) and GAPDH (cat. no. ab9484; 1:5,000), and
horseradish peroxidase-conjugated anti-mouse IgG (cat. no.
ab205719; 1:10,000) or anti-rabbit IgG (cat. no. ab205718; 1:5,000)
secondary antibodies were purchased from Abcam (Cambridge, UK).
Cell culture
The human NSCLC cell line A549 and normal human lung
epithelial cell line BEAS-2B were obtained from the American Type
Culture Collection (Manassas, VA, USA). BEAS-2B cells were cultured
in bronchial epithelial cell growth medium (Lonza Group, Ltd.,
Basel, Switzerland). A549 cells were cultured in Dulbecco's
Modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin in a
standard incubator supplied with 5% CO2 at 37°C.
AB23A treatment experiment
AB23A were dissolved in dimethyl sulfoxide (DMSO).
The A549 cells and BEAS-2B cells were seeded in 12-well plates at a
density of 6×105 cells/well. AB23A at concentrations of
6 and 9 mM or the vehicle (vehicle control, 1% DMSO) was added to
the culture medium. The cells were then harvested for each
experiment.
Cell growth rate assay
A CCK-8 assay was conducted to measure cell
viability and proliferation. Briefly, A549 cells (2×104
cells/well) and BEAS-2B cells (5×103 cells/well) in the
exponential growth were placed in a 96-well plate overnight. At a
confluence of 70–80%, the A549 and BEAS-2B cells were incubated
with different concentrations of AB23B (0, 6 and 9 mM) for
different times (12, 24 and 48 h). Following treatment, the CCK-8
reagent was added to each well for 2 h at 37°C according to the
manufacturer's protocol. The growth rate of the A549 and BEAS-2B
cells was determined by measuring the absorbance at 450 nm among
the three group under a microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and was calculated as follows: Growth rate
(%)=(mean experimental absorbance/mean control absorbance)
×100.
Cell apoptosis assay
Quantification of apoptotic cells was conducted
using flow cytometry. Briefly, A549 cells were pretreated with
AB23A for 24 h and then were harvested and stained with an
Annexin-V-FITC/7-AAD kit (BD Biosciences) according to the
manufacturer's protocol. The data acquisition and analysis were
performed with CellQuest 5.0 software (BD Biosciences).
Cell cycle analysis
The cell cycle distributions of the A549 cells was
determined using flow cytometry. A total of 1×106 cells
were cultured in combination with various concentration of AB23A
for 24 h. Subsequently, the cells were harvested and washed with
ice-cold PBS, then fixed in 70% ethanol for 2 h at −20°C. The cells
were then washed again with PBS and incubated with the PI/RNase
solution for 25 min at 37°C. The cell cycle distributions were
detected using a FACSCAN laser flow cytometer equipped with
CellQuest software version 5.0 (BD Biosciences).
Wound healing assay
The migration ability of the A549 cells was
evaluated using a wound healing assay. When 70–80% of the 6-well
plate was covered, the cells were vertically scraped with a 200 µl
pipette tip. Subsequently, the cells were treated with various
concentrations of AB23A. Images of three randomly selected fields
along the scraped line in each well were captured using a camera
(Nikon Corporation, Tokyo, Japan). Following incubation for 24 and
48 h, further images of the selected fields were captured. The
relative width of the wound in the 6 and 9 mM groups compared with
the vehicle control (0 mM) group at 0, 24 and 48 h was determined
using ImageJ version 1.51j8 software (National Institutes of
Health, Bethesda, MD, USA).
Transwell assay
The invasion activity of the A549 cells were
assessed using a 24-well Transwell assay. An insert that was
precoated with 100 µl Matrigel and dried for 30 min at 37°C was
placed in the upper chamber. Subsequently, A549 cells
(2×104 cells/well) were suspended in DMEM with 0.5% FBS
and various concentrations of AB23A, and deposited into the upper
chamber of each well; DMEM with 10% FBS was added to the lower
chambers. After 24 and 48 h incubation, the cells remaining on the
upper surface of the membranes were scraped off and the invasive
cells on the lower surface of the membranes were fixed in 4%
paraformaldehyde at 4°C and stained with 0.1% crystal violet
(Beyotime Institute of Biotechnology) for 25 min at room
temperature. Images of three randomly selected fields were captured
with a camera (Nikon Corporation). The invasion activity of the
A549 cells was assessed by counting the number of cells under a
light microscope (magnification, ×200).
Protein isolation and western blot
analysis
Following treatment with various concentrations of
AB23A (0, 6 and 9 mM) for 24 and 48 h, the A549 cells were
harvested, washed with ice-cold PBS and lysed in
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.) for 30 min. The cell lysates were separated with
centrifugation at 3,000 × g at 4°C for 15 min and then the
supernatants were collected. The protein concentration was
determined by a bicinchoninic acid (BCA) protein assay (Thermo
Fisher Scientific, Inc.). Equivalent amounts of samples containing
40 µg protein from each lysate were resolved by 10% SDS-PAGE.
Subsequently, the protein was transferred onto a polyvinylidene
difluoride membrane (Thermo Fisher Scientific, Inc.). After
blocking with 5% non-fat milk in PBS containing 0.1% Tween-20
(TPBS) at room temperature for 2 h, the membranes were incubated
overnight at 4°C with specific primary antibodies. The membranes
were then washed with TPBS and incubated with the corresponding
secondary antibodies for 1 h at room temperature. After washing the
membranes again with TPBS, the protein bands were detected by an
enhanced chemiluminescence method using a Tanon 4200R automatic
chemiluminescence image analysis system (Tanon Science and
Technology Co., Ltd., Shanghai, China) and ImageJ version 1.51j8
software was used to quantify protein expression.
Statistical analysis
Statistical analyses were performed with SPSS 19.0
software (IBM, Corps., Armonk, NY, USA). Data were presented as the
mean ± standard deviation of at least three independent
experiments. Statistical differences were determined by a Student's
t-test or one-way analysis of variance with Dunnett's multiple
comparison post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of AB23A on the growth rate of
human NSCLC cells, A549 and normal human lung epithelial cells,
BEAS-2B
To evaluate the cytotoxicity of AB23A on A549 and
BEAS-2B cells, a CCK-8 assay was used to measure cell growth. As
presented in Fig. 1A, the growth
rate of the A549 cells was significantly reduced by the various
concentrations of AB23A (6 and 9 mM) in a time-dependent manner
(P<0.01). Therefore, AB23A was cytotoxic to the A549 cells. The
results also revealed that AB23A inhibited the growth rate of the
A549 cells in a dose-dependent manner. The growth rate of the A549
cells was reduced to 50% following treatment with 9 mM AB23A for 24
h (Fig. 1B). As presented in
Fig. 1C, the growth rate of the
BEAS-2B cells exhibited no significant change following treatment
with the various concentrations of AB23A (6 and 9 mM) in a
time-dependent manner. However, AB23A also demonstrated some
cytotoxicity on the BEAS-2B cells, which lead to a slight decrease
in its activity, although there was no statistically significance
difference.
AB23A induces arrest of A549 cell
cycle progression
AB23A induces an inhibitory effect through specific
disturbances of cell cycle-associated events. To determine the
effect of AB23A treatment on the progression of A549 cells cycle,
flow cytometry was performed. As presented in Fig. 2, treatment with AB23A (6 and 9 mM)
for 24 h led to a significant increase in the proportion of cells
at the G0/G1 phase (P<0.01) and significantly reduced arrest in
the S phase in a dose-dependent manner (P<0.05).
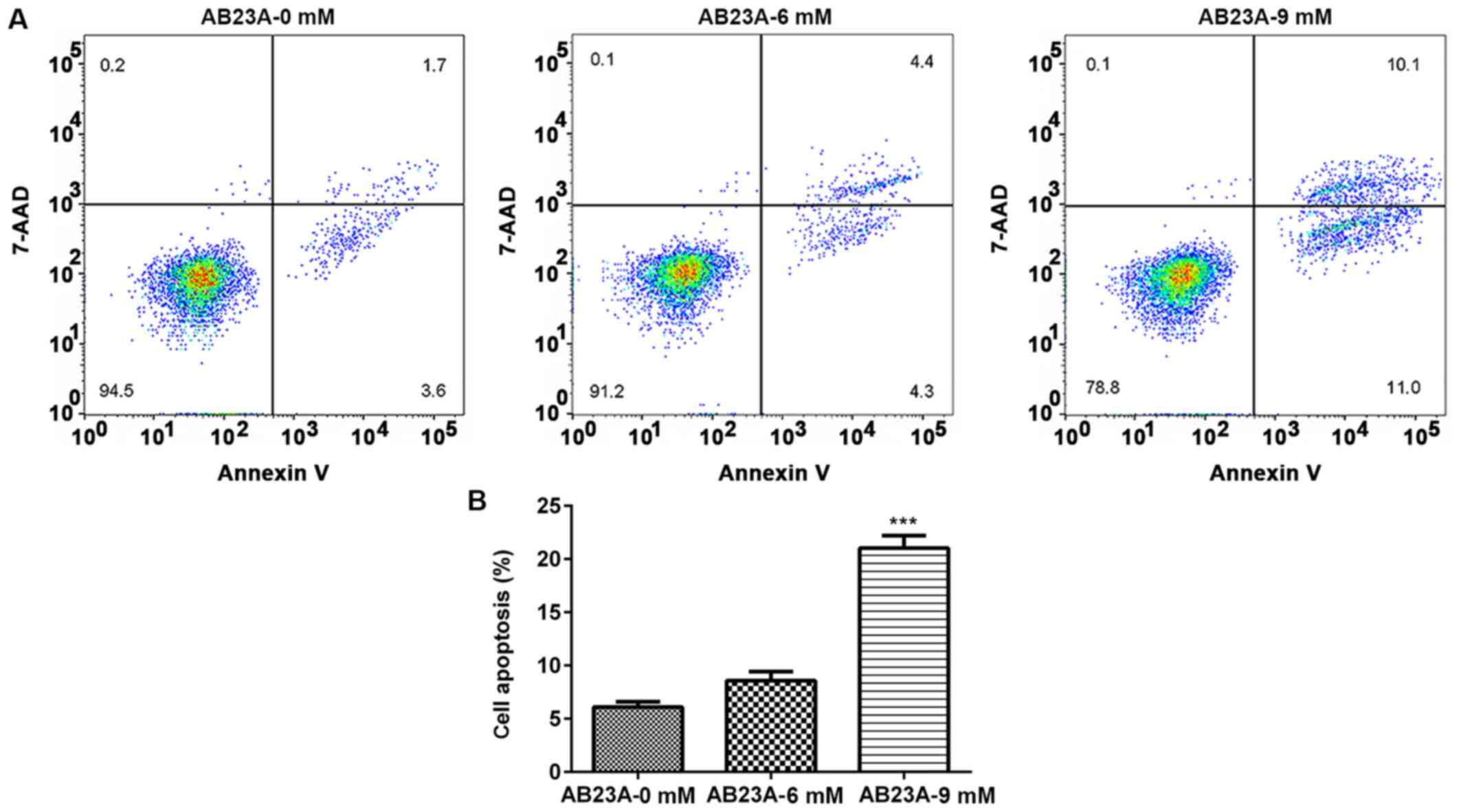
AB23A induces A549 cell apoptosis
After the cells were treated with different
concentrations of AB23A (6 and 9 mM) for 24 h, the percentage of
apoptotic cells was detected using flow cytometry. Treatment with 9
mM AB23A induced a significant increase in the percent of apoptotic
cells (P<0.001; Fig. 3). To
clarify this effect, the expression levels of the
apoptosis-associated proteins Bax and Bcl-2 were detected using
western blotting analysis. The levels of Bcl-2 expression were
markedly downregulated and those of Bax expression were
significantly elevated following treatment with various
concentrations of AB23A (6 and 9 mM) for 24 h (P<0.05; Fig. 4). These results suggested that
AB23A induces apoptosis of NSCLC cells.
AB23A suppresses A549 cell migration
and invasion
The migration and invasion abilities of A549 cells
are important for NSCLC metastasis; therefore, the impact of AB23A
on the abilities of the A549 cells was assessed using a wound
healing assay and Transwell assay. The mobility and invasion of the
A549 cells were significantly reduced following treatment with
AB23A (6 and 9 mM) for 24 and 48 h (P<0.05; Figs. 5 and 6).
AB23A reduces the phosphorylation
levels of PI3K, AKT and mTOR in A549 cells
To investigate the mechanism of the apoptosis
induced by AB23A, western blot analysis was performed to detect
proteins in the PI3K/AKT/mTOR signaling pathways. As presented in
Fig. 7, upon treatment with AB23A
(6 and 9 mM) for 24 and 48 h, the phosphorylation levels of PI3K,
AKT and mTOR were remarkably reduced in a dose-and time-dependent
manner, however, the levels of PI3K, AKT and mTOR were not
significantly affected.
Discussion
The present study demonstrates, for the first time
to the best of our knowledge, the therapeutic effects of AB23A on
NSCLC cells. The majority of patients with NSCLC are treated with
chemotherapy drugs, including platinum-based drugs; however, the
side-effects of chemotherapy drugs reduced the survival rate of
patients. Toxicity, drug resistance and a high risk of death have
been observed in the clinic as side-effects of chemotherapy drugs
used to treat NSCLC (14).
Resistance of NSCLC to chemotherapy drugs has become a difficult
issue to overcome. In such a severe situation, the natural products
used in traditional Chinese medicine exhibit clear advantages,
including few side-effects. Therefore, it is imperative to develop
novel chemotherapy drugs to overcome refractory chemotherapy
resistance and the side-effects of the current chemotherapeutic
drugs.
Traditional Chinese medicine has the advantages of
low cost, wide safety range, low toxicity, broad spectrum and
numerous targets. Therefore, a number of researchers have
investigated the rich resources of Chinese medicinal herbs
(15). A previous study
demonstrated that AB23A can inhibit nitric oxide production without
cytotoxic effects (16). AB23A is
commonly used for treatment of a number of diseases, including
diabetes, pyelonephritis, inflammation and cancer (17,18).
The results of the present study revealed that the growth rate was
reduced to 50% when the A549 cells were treated with 9 mM AB23A for
24 h, which indicates that AB23A has an inhibitory effect on cell
vitality. In addition, in the present study, the inhibitory effect
of AB23A on the activity of normal human lung epithelial cells
(BEAS-2B) is not obvious, which indicates the low toxicity of AB23A
to human body. However, it cannot be excluded that a high dose of
AB23A will cause cytotoxicity to normal cells. Additionally,
although the A549 and BEAS-2B growth rate reduced a little between
12 and 48 h in untreated cells (AB23A 0 mM), there was no
significant difference and the survival rate of the cells was all
>95%. The present study hypothesized that the change in cell
viability may be due to the slight effect of the DMSO solution on
A549 cells. These all requires further research.
A previous study demonstrated that AB23A inhibits
the proliferation of human breast cells through inducing apoptosis
(19). AB23A also induces
apoptosis, blocked the G1 phase, and inhibits migration and
invasion of ovarian cancer cells (20). The apoptosis-associated genes Bax
and Bcl-2 serve a vital role in the initiation and maintenance of
apoptosis (21). Interference with
cell cycle progression is one of the characteristics of numerous
anticancer drugs. AB23A has been demonstrated to induce cell cycle
arrest in the G1 phase in human colon cancer and ovarian cancer
cell lines (20,22). The results of the present study
indicate that AB23A markedly induces cell cycle arrest in the G0/G1
phase in A549 cells. In addition, the results of the western
blotting and flow cytometric analyses demonstrate that AB23A
induces apoptosis and elevates the protein levels of Bax/Bcl-2 in a
dose-dependence manner. Therefore, analysis of apoptosis and the
cell cycle may be vital for developing AB23A as a potential
anticarcinogen.
Activation of migration, invasion and metastasis is
a crucial characteristic of malignancy, which is one of the
hallmark capabilities of cancer (23). The high morbidity and mortality
rates of patients with NSCLC are closely associated with the
alterations observed in the biological behavior of lung cancer
cells. Due to the high metastasis rate of cancer cells, NSCLC is
difficult to eradicate. Therefore, inhibition of the biological
behavior of NSCLC cells is a promising treatment for lung cancer.
Wound healing and Transwell assays have been widely used to assess
cancer cells metastasis and invasiveness in vitro (24,25).
In the present study, the results revealed that AB23A may markedly
suppress the migration and invasion of A549 cells in a
concentration-dependent manner following treatment for 24 and 48 h.
Additionally, cell apoptosis was evaluated following treatment for
24 h, and apoptosis significantly increased in a
concentration-dependent manner. When detecting the invasion and
migration of A549 cells, it was observed that the cytotoxicity of
AB23A on A549 cells was effected by inducing apoptosis and reducing
cell growth, invasion and migration. Determining the underlying
mechanisms requires further study.
The PI3K/AKT/mTOR pathway serves critical roles in
diverse cellular processes, from proliferation, transcription,
migration or survival to cell death or apoptosis, which are
involved in cancer (26). Out of
the molecules associated with this pathway, AKT is an important
regulator of cellular activities that can be activated by PI3K and
that phosphorylate a number of key pro-cancer factors to promote
cell viability and inhibit apoptosis. mTOR belongs to the
PI3K-related kinase family and is a vital kinase for regulating
cell proliferation, apoptosis and viability (27). AKT is localized upstream of mTOR
and the suppression of AKT phosphorylation can result in an
significant reduction in phosphorylation of downstream mTOR
(28). It is well known that mTOR
serves an important role in regulating autophagy in mammalian
signaling (29) and mTOR signaling
is very important for cellular growth in response to a number of
physiological conditions through regulating downstream effectors to
control the translation and transcription of a variety of proteins
(30). These key signaling
molecules from this pathway serve a vital role in regulating cell
survival and apoptosis. Drugs that target the PI3K/Akt/mTOR
signaling pathway have the potential to inhibit survival pathways
and induce apoptosis in cancer cells (16). Previous studies have reported that
apigenin could inhibit the growth and proliferation, promote
apoptotic cell death, induce cell cycle arrest via the
PI3K/Akt/mTOR signaling pathway (31,32).
To further clarify the possible mechanism by which AB23A induces
apoptosis of NSCLC cells, the impact of AB23A on the expression
levels of PI3K, AKT and mTOR was investigated in the present study.
Upregulation of Akt phosphorylation by the activation of PI3K and
mTOR can integrate upstream activating signals through the PI3K/AKT
pathway in return to result in phosphorylation (33). In the present study, AB23A induced
apoptosis and inhibited the viability of the A549 cells via
inhibition of the PI3K/AKT/mTOR signal pathway. Furthermore,
alterations in the expression of phosphorylated PI3K/AKT/mTOR
proteins and alterations in the cell phenotype in response to the
AB23A stimulus were investigated. The results demonstrated that
following treatment with AB23A (6 and 9 mM) for 24 and 48 h, the
protein levels of p-PI3K, p-AKT and p-mTOR were significantly
reduced in the A549 cells, but there was no significant difference
in the levels of PI3K, AKT and mTOR. In conclusion, treatment of
NSCLC cells with AB23A has an anti-tumor effect, reduces cell
viability and induces apoptosis via the activation of the
PI3K/AKT/mTOR signaling pathway. The results reveal that AB23A
could be an effective anticancer drug for the treatment of
NSCLC.
In conclusion, the present study demonstrated that
the anticancer activity of AB23A in A549 cells is mediated by the
induction of apoptosis, interference with the cell cycle and
suppression of cell activities, accompanied by inhibition of the
PI3K/AKT/mTOR pathway in vitro. However, further studies are
required to elucidate the underlying mechanism of action of AB23A
and its anticancer activity in vivo also requires further
evaluation. The results of this study may provide a novel
therapeutic strategy for the treatment of human NSCLC.
Acknowledgements
We thank Vice President Zheng Xinhua of Pingdingshan
College Medical College and Director Wang Hao of Pingdingshan
Second People's Hospital for their guidance in the design of the
scheme and the experimental operation.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YL designed the experiments and drafted the
manuscript. YL, XCX and LYM performed the experiments. YL, YW and
YML analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaudhary UB and Haldas JR: Long-term
complications of chemotherapy for germ cell tumours. Drugs.
63:1565–1577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brouwers EE, Huitema AD, Beijnen JH and
Schellens JH: Long-term platinum retention after treatment with
cisplatin and oxaliplatin. BMC Clin Pharmacol. 8:72008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang YT, Huang DM, Chueh SC, Teng CM and
Guh JH: Alisol B acetate, a triterpene from Alismatis rhizoma,
induces Bax nuclear translocation and apoptosis in human
hormone-resistant prostate cancer PC-3 cells. Cancer Lett.
231:270–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oral O, Akkoc Y, Bayraktar O and Gozuacik
D: Physiological and pathological significance of the molecular
cross-talk between autophagy and apoptosis. Histol Histopathol.
31:479–498. 2016.PubMed/NCBI
|
|
6
|
Crowell JA: The chemopreventive agent
development research program in the Division of Cancer Prevention
of the US National Cancer Institute: An overview. Eur J Cancer.
41:1889–1910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mann J: Natural products in cancer
chemotherapy: Past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee AY, Park JY, Chun JM, Moon BC, Kang
BK, Seo YB, Shin HK and Kim HK: Optimization of extraction
condition for alisol B and alisol B acetate in alismatis rhizoma
using response surface methodology. J Liq Chromatogr Relat Technol.
36:513–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Law BY, Wang M, Ma DL, Al-Mousa F,
Michelangeli F, Cheng SH, Ng MH, To KF, Mok AY, Ko RY, et al:
Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic
reticulum Ca(2+) ATPase pump, induces autophagy, endoplasmic
reticulum stress, and apoptosis. Mol Cancer Ther. 9:718–730. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu W, Li T, Qiu JF, Wu SS, Huang MQ, Lin
LG, Zhang QW, Chen XP and Lu JJ: Anti-proliferative activities of
terpenoids isolated from Alisma orientalis and their
structure-activity relationships. Anticancer Agents Med Chem.
15:228–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang ZY, Zhang XM, Zhang FX, Liu N, Zhao
F, Zhou J and Chen JJ: A New triterpene and anti-hepatitis B virus
active compounds from Alisma orientalis. Planta Med. 72:951–954.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin HG, Jin Q, Ryun Kim A, Choi H, Lee JH,
Kim YS, Lee DG and Woo ER: A new triterpenoid from Alisma orientale
and their antibacterial effect. Arch Pharm Res. 35:1919–1926. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Feng L, Ma L, Chen H, Tan X, Hou
X, Song J, Cui L, Liu D, Chen J, et al: Alisol a 24-acetate and
alisol B 23-acetate induced autophagy mediates apoptosis and
nephrotoxicity in human renal proximal tubular cells. Front
Pharmacol. 8:1722017.PubMed/NCBI
|
|
14
|
Xiong Y, Huang BY and Yin JY:
Pharmacogenomics of platinum-based chemotherapy in non-small cell
lung cancer: Focusing on DNA repair systems. Med Oncol. 34:482017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Yang C, Li C, Zhao Q, Liu L, Fang
X and Chen XY: Recent advances in biosynthesis of bioactive
compounds in traditional Chinese medicinal plants. Sci Bull
(Beijing). 61:3–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu W, Li X, Lin N, Zhang X, Huang X, Wu T,
Tai Y, Chen S, Wu CH, Huang M and Wu S: Pharmacokinetics and tissue
distribution of five major triterpenoids after oral administration
of Rhizoma Alismatis extract to rats using ultra high-performance
liquid chromatography-tandem mass spectrometry. J Pharm Biomed
Anal. 146:314–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Li XY, Lin N, Zhao WL, Huang XQ,
Chen Y, Huang MQ, Xu W and Wu SS: Diuretic activity of compatible
triterpene components of alismatis rhizoma. Molecules. 22(pii):
E14592017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuda H, Kageura T, Toguchida I,
Murakami T, Kishi A and Yoshikawa M: Effects of sesquiterpenes and
triterpenes from the rhizome of Alisma orientale on nitric oxide
production in lipopolysaccharide-activated macrophages: Absolute
stereostructures of alismaketones-B 23-acetate and -C 23-acetate.
Bioorg Med Chem Lett. 9:3081–3086. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang A, Sheng Y and Zou M:
Antiproliferative activity of Alisol B in MDA-MB-231 cells is
mediated by apoptosis, dysregulation of mitochondrial functions,
cell cycle arrest and generation of reactive oxygen species. Biomed
Pharmacother. 87:110–1107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang LL, Xu YL, Tang ZH, Xu XH, Chen X,
Li T, Ding CY, Huang MQ, Chen XP, Wang YT, et al: Effects of alisol
B 23-acetate on ovarian cancer cells: G1 phase cell cycle arrest,
apoptosis, migration and invasion inhibition. Phytomedicine.
23:800–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Melo-Lima S, Lopes MC and Mollinedo F:
ERK1/2 acts as a switch between necrotic and apoptotic cell death
in ether phospholipid edelfosine-treated glioblastoma cells.
Pharmacol Res. 95-96:2–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Y, Li ETS and Wang M: Alisol B
23-acetate induces autophagic-dependent apoptosis in human colon
cancer cells via ROS generation and JNK activation. Oncotarget.
8:70239–70249. 2017.PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujimura K, Choi S, Wyse M, Strnadel J,
Wright T and Klemke R: Eukaryotic translation initiation factor 5A
(EIF5A) regulates pancreatic cancer metastasis by modulating RhoA
and Rho-associated kinase (ROCK) protein expression levels. J Biol
Chem. 290:29907–29919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gutschner T, Hämmerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morgan TM, Koreckij TD and Corey E:
Targeted therapy for advanced prostate cancer: Inhibition of the
PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 9:237–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin KA and Blenis J: Coordinate
regulation of translation by the PI 3-kinase and mTOR pathways. Adv
Cancer Res. 86:1–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Surviladze Z, Sterk RT, DeHaro SA and
Ozbun MA: Cellular entry of human papillomavirus type 16 involves
activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway
and inhibition of autophagy. J Virol. 87:2508–2517. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Foster KG and Fingar DC: Mammalian target
of rapamycin (mTOR): Conducting the cellular signaling symphony. J
Biol Chem. 285:14071–14077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu W, Sun H, Zha W, Cui W, Xu L, Min Q and
Wu J: Apigenin attenuates adriamycin-induced cardiomyocyte
apoptosis via the PI3K/AKT/mTOR pathway. Evid Based Complement
Alternat Med. 2017:25906762017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang J, Pi C and Wang G: Inhibition of
PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy
in hepatocellular carcinoma cells. Biomed Pharmacother.
103:699–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|