Introduction
Anorectal malformations (ARMs) are frequently
encountered anomalies that represent an important component of
pediatric surgery practice. The incidence rate ranges from 1:1,500
to 1:5,000 live births (1). There
also appears to be a wide spectrum of ARM epidemiology ranging from
isolated cloacal, supralevator and infralevator anal atresia (with
and without fistula) to ectopic anus malformation, and have
variable clinical presentations ranging from mild forms that may
require only minor surgical interventions to more complicated cases
that require management with multi-staged operations (2,3).
Despite the continuous progression of ARM surgical treatments,
certain patients still have anal dysfunctions following surgery,
which seriously affects their quality of life (4). The cause of ARM is unknown, although
arrest of the descent of the urorectal septum towards the cloacal
membrane between the 4th and 8th weeks of gestation was previously
considered the basic event leading to ARM (5). Since the molecular deteminants during
blastogenesis are overlapping for many body systems, and these
elements are closely related in timing and spacing, thus defects in
blastogenesis often involve two or more progenitor fields (6). Several studies have reported on the
mechanisms of enteric nervous system (ENS) development in embryos,
and P2Y receptors have been revealed to primarily regulate muscle
relaxation (7–10). Mulè et al (11), revealed that the P2Y receptor
antagonist suramin inhibited ATP-induced muscle relaxation,
confirming that P2Y receptors participate in muscle relaxation. P2Y
receptor deficiency could lead to dysfunction in intestinal
relaxation, resulting in intestinal spasticity (12). Our previous study indicated that
purinergic receptor P2Y2 (P2Y2) may be one of the basic factors
leading to ENS dysplasia at the end of the rectum of fetal rats
with ARM in day 21 embryos (13).
Another study demonstrated that P2Y2 is involved in the direct
regulation of ENS, smooth muscle contractility and control of
intestinal peristalsis (14).
Hu antigen D (HuD) is considered to be expressed
specifically in neurons, while the other member of the Hu protein
family, HuR, is ubiquitously expressed (15). Hu proteins have three RNA
recognition motifs through which they associate with mRNAs bearing
specific sequences that are often AU- and U-rich. HuD binds to and
stabilizes the 3′-untranslated region of target mRNAs, including
p21, tau and GAP-43 mRNAs (15).
HuD regulates the expression of neuron-specific genes and serves
important roles in the growth, development and differentiation of
neurons. It is a necessary protein for the formation and
regeneration of nervous processes (16). It also modulates target mRNA
translation. HuD demonstrates aberrant expression in diseased
intestinal canals of children with Hischsprung's disease, which
indicates that HuD has a close relationship with the development of
the ENS (17). Our previous study
revealed that the HuD protein is aberrantly expressed in the nerve
plexuses of the intestinal wall of the terminal rectum of ARM
embryonic 20-day rats, which suggests that HuD may participate in
the development and maturation of the ENS in ARM embryonic rats
(18). However, a previous study
investigating different tissues unexpectedly observed HuD
expression in ENS with ARMs (19);
it was not clear whether P2Y2 and HuD continued to participate in
the development of the ENS prior to the emergence of ARMs. To
provide insights into the pattern of P2Y2 expression and the
possible role of HuD during ENS development, the present study
examined the expression of P2Y2 and HuD in normal and ARMs model
rat embryos at 17, 19 and 21 days.
Materials and methods
Animal model
A total of 120 Sprague Dawley (SD) rats at 10–12
weeks of age (210–260 g) were obtained from the Experimental Animal
Center at the Daping Hospital of the Third Military Medical
University (Chongqing, China). Ethical approval was obtained from
the Zunyi Medical College Animal Ethics Committe (no. 20150820014)
prior to the commencement of the study. Mating was performed at
night with male and female rats at a 3 to 1 ratio. In the early
morning, vaginal smears were obtained from female rats; if sperm or
vaginal suppositories were discovered under a light microscope, it
was recorded as embryonic day zero (E0). A total of 40 mated
pregnant SD rats were randomly divided into the following two
groups: Ethylenethiourea (ETU)-treated (n=30) and normal groups
(n=10). The animals were maintained in a temperature-controlled
environment (20–24°C), a humidity of 50–70% and a 12-h light/dark
cycle. Solid laboratory chow and water were available ad
libitum. In the ETU-treated group, 30 pregnant rats were
administered a single dose of 125 mg/kg of 1% ETU (CAS no.
03940-I00G; Sigma-Aldrich; Merck KGaA) by oral gavage on E10 to
induce the ARM model (ARM group). Rats in the normal group were
treated the same as the ETU group, however, they received
corresponding doses of ETU-free saline on E10 instead of ETU.
Embryos were harvested by cesarean delivery at E17, E19 and E21.
The presence of ARMs was determined by light microscope. Then, the
embryos were divided into normal, ETU and ARM groups.
Tissue handling
The pregnant rats were anesthetized with chloral
hydrate by intraperitoneal injection (0.3 ml/100 g body weight;
Sinopharm Chemical Reagent Co., Ltd.). The uterine horn was incised
through the abdominal wall. Embryos were obtained by cesarean
section on E17, E19 and E21. Subsequently, pregnant rats were
sacrificed with an excessive dose of sodium pentobarbital. The
embryos were then divided into the normal, ETU (therapeutic rats
that exhibited no deformities) and ARM groups. One-third of the
embryos, based on their weight, were fixed in 4% paraformaldehyde
for 12 to 24 h. Then the embryos of all age groups were dehydrated
and embedded in paraffin. Immunohistochemical staining was
performed on serial and sagittal sections of up to 4-µm thickness.
The presence of ARMs was determined under a light microscope. Under
a magnifying glass, the distal rectum of the remaining two-thirds
of the embryos was dissected and removed from the surrounding
tissues. The distal rectum was immediately frozen in liquid
nitrogen for western blot analysis and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Hematoxylin and eosin (H&E)
staining
The paraffin-embedded terminal rectum tissues were
consecutively cut to a thickness of 3 µm. Following conventional
H&E staining, the structure of the distal rectum, and the
number and morphology of the intermuscular and submucosal nervous
plexus of the distal rectum were observed under an optical
microscope.
Immunohistochemistry
Immunohistochemical staining was performed as
previously described (9,13). Sections were incubated overnight at
4°C with the primary rabbit anti-mouse P2Y2 (1:125; cat. no.
sc-518091) and mouse anti-rat HuD (1:80; cat. no. sc-28299; both
from Santa Cruz Biotechnology, Inc.) polyclonal antibodies.
Following primary antibody incubation, the sections were washed and
incubated with biotinylated goat anti-rabbit secondary antibody A
(cat. no. A0456; dilution 2 µg/l; ZSGB-BIO; OriGene Technologies,
Inc.) for 20 min at room temperature. Following incubation, the
sections were incubated with reagent B (ZSGB-BIO; OriGene
Technologies, Inc.) for 30 min at room temperature.
Immunoreactivity was visualized under an ordinary light microscope
following the addition of 3′3-diaminobenzidine (ZSGB-BIO; OriGene
Technologies, Inc.) and the chromogenic degree was observed to
subsequently adjust the incubation time. Sections were stained with
hematoxylin for 3 sec at room temperature. Negative controls
received PBS instead of primary antibodies. Human cerebral cortex
tissues from tissue bank (cat. no. 201324; Research Center for
Medical & Biology Tissue Bank, Zunyi Medical University) were
used for the P2Y2 positive control, while human breast tissue (cat.
no. 201324; Research Center for Medical & Biology Tissue Bank;
Zunyi Medical University) was used for the HuD positive control.
All sections were photographed using an optical microscope
(magnification, ×400). The target images were collected and
compiled with the Leica QWin image analysis system. The integral
optical density was calculated using Image Pro Plus 6 image
analysis software (Media Cybernetics, Inc.).
Protein preparation and western blot
analysis
Protein preparation was performed as previously
described (20). Total protein was
extracted from the distal rectum collected from the normal, ETU and
ARM groups on days E17, E19 and E21, and frozen at −80°C. The
Enhanced Bicinchoninic Acid Protein Assay kit (Beyotime Institute
of Biotechnology) was used for protein quantification according to
the manufacturer's protocol. Protein extracts (50 µg/lane) were
mixed with 5X SDS-PAGE (Beyotime Institute of Biotechnology)
loading buffer (40 µl), heated at 90°C for 5 min, transferred to
polyvinylidene fluoride membranes and blocked with 5% fat-free milk
in Tris-buffered saline for 3 h at room temperature. Membranes were
incubated with primary rabbit anti-mouse antibodies against P2Y2
(1:400; cat. no. sc-518091; Santa Cruz Biotechnology, Inc.) and
primary mouse anti-rat antibodies against HuD (1:200; cat. no.
sc-28299; Santa Cruz Biotechnology, Inc.), and β-actin (1:400;
mouse monoclonal; cat. no. 610154; Wuhan Boster Biological
Technology, Ltd.). The membranes were subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies anti-mouse
(1:4,000; cat. no. sc-516102; Santa Cruz Biotechnology, Inc.) or
anti-rabbit (1:6,000; cat. no. sc-2357; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. The membranes were developed
using an ECL substrate kit (cat. no. tf-2012215; Pierce; Thermo
Fisher Scientific, Inc.) and densitometric values were analyzed
using the Enhance Chemiluminescence Plus detection system (EMD
Millipore). Protein levels were normalized to β-actin.
RNA isolation and RT-qPCR
Total RNA was isolated from the rat distal rectum of
the normal, ETU and ARM groups using RNAiso Plus (TRIzol; Takara
Biotechnology Co., Ltd.), following the manufacturer's
instructions. The A260/A280 OD value of the total RNA ranged from
1.8 to 2.0. The extracted RNA was diluted to a concentration of 1
µg/µl, and its equivalents were stored at −80°C. Single-strand cDNA
was reverse transcribed with the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd.). Primers used for RT-qPCR included the
following: P2Y2 forward 5′-TGCTCTACTTTGTCACCACCA-3′ and reverse,
5′-CTTGTCATCCCGTCAATGG-3′; HuD forward, 5′-ACCAGGCTCAAAGATTCAGG-3′
and reverse, 5′-CTTGTCATCCCGTCAATGG-3′; and β-actin (used as an
endogenous control) forward, 5′-GGAGATTACTGCCCTGGCTCCTA-3′ and
reverse, 5′-GACTCATCGTACTCCTGCTTGCTG-3′. RT-qPCR was conducted with
SYBR Premix Ex Tap (cat. no. DRR081S; Takara Biotechnology Co.,
Ltd.) on a 7900HT fast real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) under the following thermocycling
conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec and 60°C for 60 sec. The results of qPCR were
analyzed with LightCycler 1.5 System software (Roche Diagnostics
GmbH). Melting curves were generated during the dissociation
process to determine the specificity of the amplification. Relative
levels of gene expression were determined as ΔCq=(Cq gene)-(Cq
reference), and the fold change in gene expression was calculated
using the 2−∆∆Cq method (21).
Statistical analysis
SPSS version 16.0 (SPSS Inc.) was used for
statistical analyses. The data are consistent with normal
distribution, with homogeneity of variance and one-way analysis of
variance and Tukey's post hoc test used to analyze the levels of
P2Y2 and HuD expression between the normal, ETU and ARM model rat
groups. All results were expressed as the mean ± standard
deviation, and P<0.05 was considered to indicate a statistically
significant difference.
Results
General observation
In the present study, no anomalies were identified
in the 142 normal model rat embryos examined. A total of 104
ETU-treated fetal rats were normal and 146 ARM model rat embryos
were obtained from the 256 ETU-treated rat embryos. All rats
produced from ETU-treated embryos exhibited a short or no tail, and
6 perished during the Cesarean section. Spinal bifida and/or
meningocele were also observed externally. The incidence of ARMs
(manifesting as rectourethral fistula or common cloaca) in
ETU-treated embryos from E17 to E21 was 57.3%. The anal opening
position was normal in the control and ETU groups, and the anal
opening was a closed blind end in the ARM group (Table I).
 | Table I.Distribution of embryos in the
various age and treatment groups. |
Table I.
Distribution of embryos in the
various age and treatment groups.
|
| Normal | ETU | ARM |
|---|
|
|
|
|
|
|---|
| E(d)/group | H&E | IHC | WB | PCR | H&E | IHC | WB | PCR | H&E | IHC | WB | PCR |
|---|
| 17 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| 19 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| 21 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Total | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
H&E staining
At E17, the nerve plexus count in the submucousal
and intermuscular clusters was not different between the three
groups (Fig. 1). On E19, the nerve
plexus count in the ARM group was greater than that at E17
(P<0.05; Fig. 1). On E21, the
nerve plexus count in the ETU group was significantly increased
compared with that at E19 (P<0.01; Fig. 1). There was decreased development
of the distal rectum nerve plexus count in the E19 and E21 ARM
groups compared with the normal and ETU groups. Within the ARM
group, the nerve plexus count was significantly different between
the E17 and E19 embryos (P<0.01; Fig. 1).
Immunohistochemical results
P2Y2 and HuD were mainly expressed in the distal
submucosa and intermuscular plexus of the fetal rats, and were
primarily expressed in the cytoplasm. There was a small amount of
HuD expression in the nucleus. As the embryo developed, the color
corresponding to protein expression changed from yellowish to
yellowish brown to yellow brown; the range of expression also
increased. At E21, the expression of P2Y2 strong-positive rate was
50% in the normal group, 40% in the ETU group, and 10% in the ARM
group; the expression of HuD strong-positive rate was 40% in the
normal group, 40% in the ETU group, 10% in the ARM group. The nerve
plexus in the ARM group was small compared with the normal and ETU
groups (Tables II and III).
 | Table II.Expression of P2Y2-positive rate at
the distal rectum in the three groups. |
Table II.
Expression of P2Y2-positive rate at
the distal rectum in the three groups.
|
| Normal group/ETU
group/ARM group (n=10) |
|
|
|---|
|
|
|
|
|
|---|
| E(d) | − | + | ++ | +++ | Total | Negative rate
(%) | Strong-positive
rate (%) |
|---|
| 17 | 2/3/3 | 8/7/7 | 0/0/0 | 0/0/0 | 10/10/10 | 20/30/30 | 0/0/0 |
| 19 | 0/0/3 | 7/6/6 | 3/4/1 | 0/0/0 | 10/10/10 | 0/0/30 | 0/0/0 |
| 21 | 0/0/1 | 2/3/7 | 3/3/1 | 5/4/1 | 10/10/10 | 0/0/10 | 50/40/10 |
 | Table III.Expression of HuD positive rate at
the distal rectum in the three groups. |
Table III.
Expression of HuD positive rate at
the distal rectum in the three groups.
|
| Normal group/ETU
group/ARM group (n=10) |
|
|
|---|
|
|
|
|
|
|---|
| E(d) | − | + | ++ | +++ | Total | Negative rate
(%) | Strong-positive
rate (%) |
|---|
| 17 |
2/3/4 | 8/7/6 | 0/0/0 | 0/0/0 | 10/10/10 | 20/30/40 | 0/0/0 |
| 19 | 0/0/2 | 8/8/6 | 2/2/2 | 0/0/0 | 10/10/10 |
0/0/20 | 0/0/0 |
| 21 | 0/0/0 | 1/2/7 | 5/4/2 | 4/4/1 | 10/10/10 |
0/0/0 | 40/40/10 |
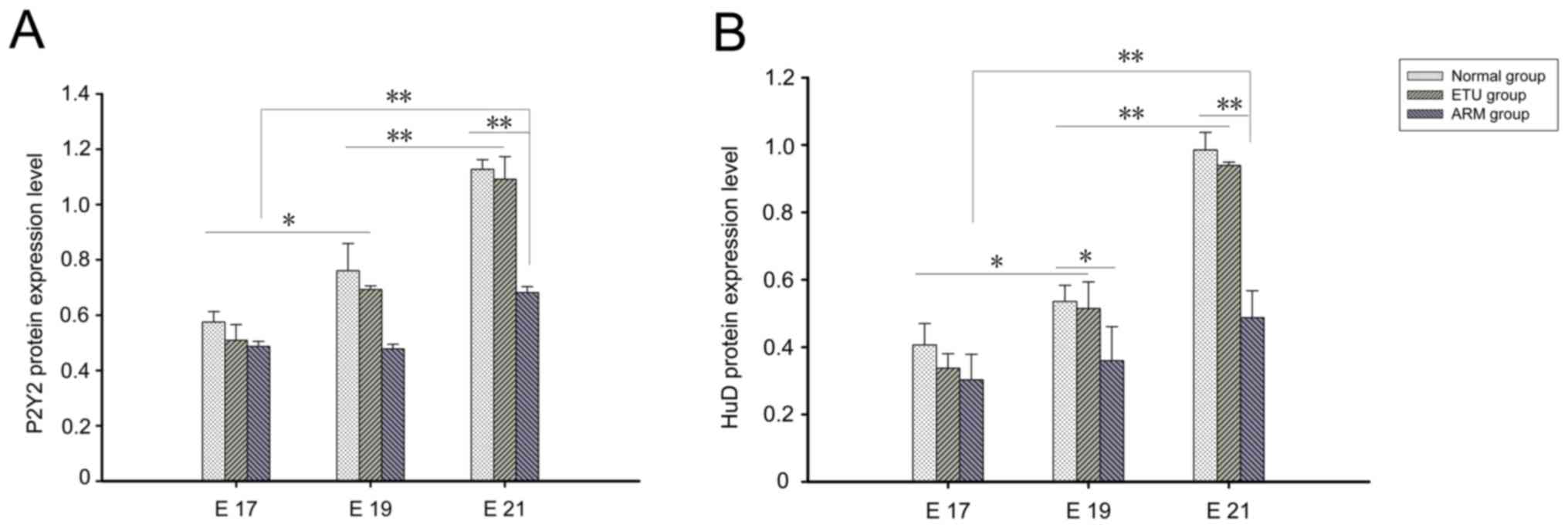
P2Y2 protein quantitative
analysis
On E17, a small amount of P2Y2 protein with
yellowish staining was observed at the distal rectum of the three
groups. Respectively 8, 7, and 7 cases in the normal, ETU and ARM
groups were observed with weak-positive staining. The expression of
P2Y2 was low, and there was no significant difference between the
three groups (P>0.05). At E19, the normal and ETU groups were
stained yellowish, while some areas appeared yellowish brown.
Respectively 7, 6, and 6 cases in the normal, ETU and ARM groups
were observed with weak-positive staining; and 3, 4, 1 cases in the
normal, ETU and ARM groups, respectively, were observed with
positive staining. The degree of staining increased in intensity
when compared with the staining in E17, however the expression was
still low; there was no difference between the three groups. At
E21, the distal rectum in the normal and ETU groups appeared
yellowish brown or dark brown, with a marked increase in intensity;
yellow staining could be clearly observed in the intermuscular and
mucous membrane. Respectively 2, 3, and 7 cases in the normal, ETU
and ARM groups were observed with weak-positive staining; 3, 3, and
1 in the normal, ETU and ARM groups respectively, were observed
with positive staining; and 5, 4, and 1 in the normal, ETU and ARM
groups, respectively, were observed with strong-positive staining.
There was a significant difference between the E21 and E19 embryos
(P<0.01). The expression reported in the ARM group was
significantly lower than that of the normal group (P<0.01).
There was no significant difference between the E17 and E19 ARM
groups, while a significant increase in expression was observed in
the E21 ARM group when compared with the E17 ARM group (P<0.01;
Figs. 2A and 3).
HuD protein quantitative analysis
At E17, respectively 8, 7, and 6 cases in the
normal, ETU and ARM groups were observed with weak-positive
staining, a small amount of HuD protein with yellowish staining was
observed at the distal rectum and there was no significant
difference between the three groups (P>0.05). At E19,
respectively 8, 8, and 6 cases in the normal, ETU and ARM groups
were observed with weak-positive staining; and 2, 2, and 2 in the
normal, ETU and ARM groups, respectively, were observed with
positive staining. The normal and ETU groups were stained yellowish
and brown in some areas, which was more pronounced than at E17
(P<0.01). At E21, respectively 1, 2, and 7 case(s) in the
normal, ETU and ARM groups were observed with weak-positive
staining; 5, 4, and 2 in the normal, ETU and ARM groups,
respectively, were observed with positive staining; and 4, 4, and 1
in the normal, ETU and ARM groups, respectively, were observed with
strong-positive staining. The distal rectum was stained yellowish
brown or dark brown in the normal and ETU groups, with a marked
increase in expression. The yellow dye could be clearly observed
between the muscles and the mucosa, and there was a significant
difference between the E21 and E19 embryos (P<0.01). The HuD
expression level in the ARM group was significantly decreased when
compared with the normal group (P<0.01); the expression
increased with the development of the embryo in the ARM group.
There was no significant difference between the E17 and the E19 ARM
groups; however, the expression level of the E21 ARM group was
significantly higher than the E17 ARM group (P<0.01; Figs. 2B and 4).
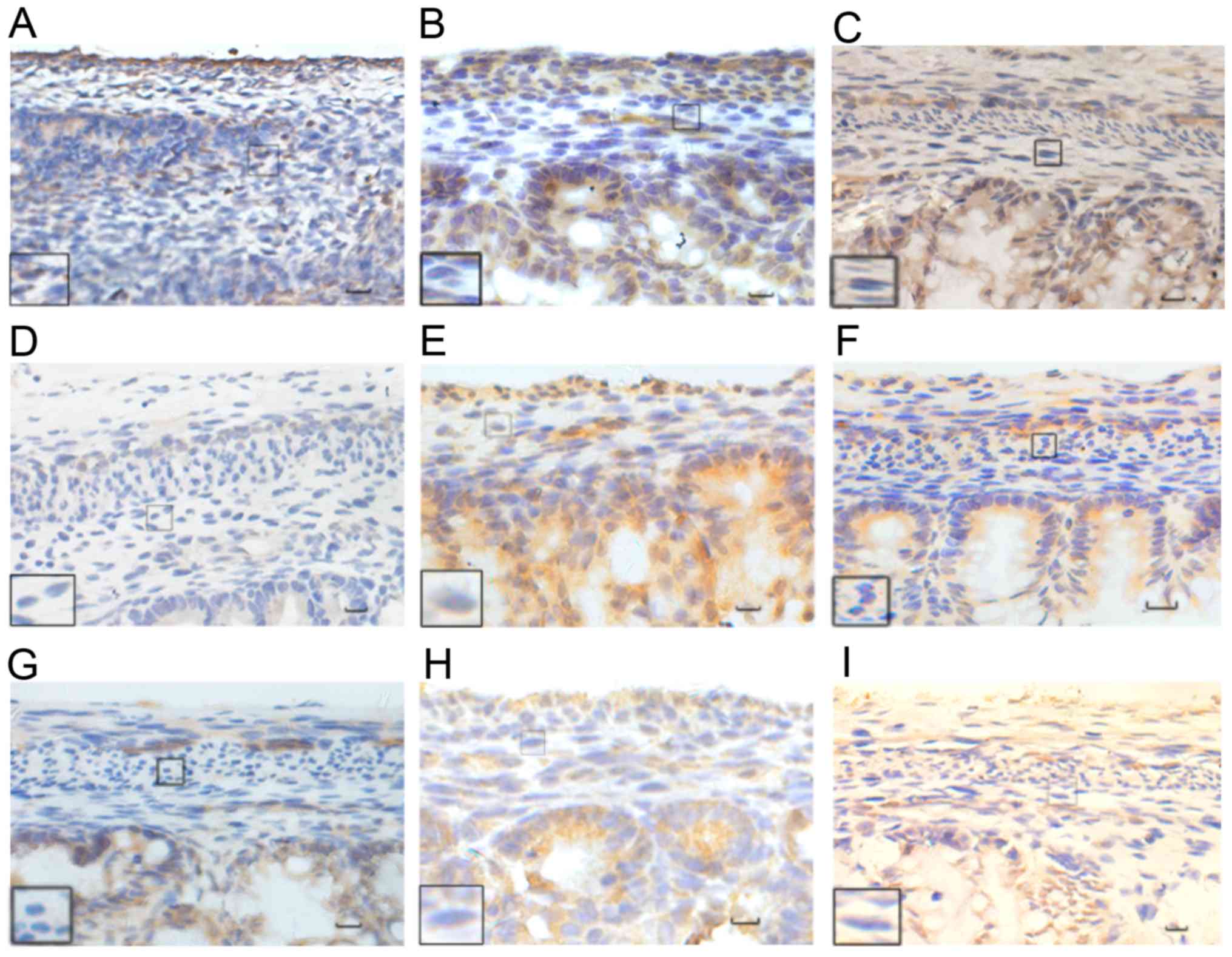 | Figure 4.Immunohistochemical analysis of HuD
protein. The normal group at (A) E17, (B) E19, and (C) E21. The ETU
group at (D) E17, (E) E19, (F) E21. The ARM group at (G) E17, (H)
E19, (I) E21. HuD was mainly expressed in the cytoplasm, with a
small amount observed in the nucleus, and in the distal submucosa
and intermuscular plexus of the fetal rat. The color of the
expressed proteins changed from yellowish to yellowish brown to
yellow brown as the embryo developed, with an increase in the range
of expression also observed. Black rectangles reveal images at a
higher magnification. Scale bar, 25 µm; magnification, ×400. HuD,
Hu antigen D; ETU, ethylenethiourea. ARM, anorectal
malformations. |
Western blot analysis
The expression levels of the P2Y2 and HuD proteins
were evaluated by western blot analysis during ENS development in
the normal, ETU and ARM group model rats. P2Y2 and HuD bands were
normalized to the corresponding β-actin band. The expression of
P2Y2 and HuD proteins increased with time in the three groups.
Significantly decreased HuD expression was detected in the distal
rectum of the ARM group at E19 and E21 when compared with the other
groups (P<0.05); in addition, the decreased expression of P2Y2
protein was detected in the distal rectum of ARM model rats at E21
when compared with the other groups (P<0.05; Fig. 5).
RT-qPCR analysis
The expression levels of P2Y2 and HuD mRNA were
normalized to β-actin in the same specimen. The results obtained
were consistent with those of western blotting; the expression
levels of P2Y2 and HuD mRNA increased with time in the normal and
ARM groups. Subsequently, the expression levels of P2Y2 mRNA in the
distal rectum of the ARM model rats at E21 were significantly lower
than those of the other groups (P<0.05), while the levels of HuD
protein in the distal rectum of the ARM group at E19 and E21 were
significantly lower when compared with the other groups (P<0.05;
Fig. 6).
Discussion
ARMs represent a wide spectrum of defects and are
some of the most common digestive tract anomalies encountered in
pediatric surgery. In the past, many surgical techniques to repair
ARM have been described. These included endorectal dissection
(1–3), an anterior perineal approach to a
rectourethral fistula (4), and
many different types of anoplasties. However, children with ARMs
often have more complications at the postoperative stage, including
constipation, diarrhea and feces incontinence, which seriously
affect patient quality of life following surgery (4). At present, the key to addressing this
disease is to devise a treatment method to reduce postoperative
complications. A previous study has revealed that ARMs are often
accompanied by ENS dysplasia (22). When the ENS is absent
(aganglionosis) or defective, children may develop constipation,
vomiting, abdominal pain and growth failure, and some may even
perish (23,24). However, whether P2Y2 and HuD have
parallel developments with the ENS of ARMs remains to be clarified
(25,26). The present study was designed to
investigate the potential role of P2Y2 and HuD during ENS
development by examining the expression patterns of P2Y2 and HuD
mRNA and protein in normal and ARM model rats at different
embryonic developmental stages.
P2Y receptors (P2YRs) are typical 7-channel
trans-membrane receptors and are heterotrimeric G protein-coupled
(27,28). Activation of P2Y2R by ATP or UTP
can induce the phosphorylation of growth factor receptors (29,30),
which increases the activities of the mitogen-activated protein
kinases, extracellular signal-regulated kinase-1/2 and the
associated adhesion focal tyrosine kinase; these go on to regulate
the ENS or directly regulate the contractility of intestinal smooth
muscle to control intestinal peristalsis (31,32).
In our previous study, the glial cell factor, glial cell-derived
neurotrophic factor and its signal receptor RET had an effect on
the development of the ENS (10).
Wullaert et al (32) used
upstream agonists and inhibitors to control the purine receptor. It
was revealed that ATP could promote axonal growth through the P2Y2
receptor (33). In addition,
Arthur et al (12) also
demonstrated that ATP activates the G protein-coupled receptor
following activation P2Y2, which acts on the regionalization and
association of tyrosine receptor A and P2Y2 receptors, promoting
the growth, differentiation and migration of neurons. In addition,
the endogenous ATP, which is used for the first time in the human
intestinal tract, acts on the P2Y receptor located in the
intestinal smooth muscle and regulates intestinal relaxation; this
effect can be blocked by P2YR (34). By combining the aforementioned
results with our own previous research, it appears that there is a
difference between the P2Y2 receptor and the distal end of the
rectum in ARMs and normal fetal rats. It was suggested that P2Y2
may partly be related to the development of the intestinal nervous
system.
HuD has been revealed to promote neuronal
differentiation and axonal outgrowth in neurons in culture and
in vivo (15). HuD can be
used as a supplement to P2Y2 for the accurate counting of
intestinal neurons and analysis of neuron types. In a study that
used an anti-Hu antibody, anti-PGP9.5 and anti-neuron specific
enolase for triple staining in cultured intestinal neurons, HuD
protein was markedly expressed on all of the neurons in the
intramuscular clusters of the small intestine, indicating that HuD
has distinct specificity (35).
The expression of HuD in Hirschsprung disease was significantly
lower than in the normal segment (36,37).
However, there are few reports of HuD in ARM-associated
diseases.
The present experimental study revealed that at E17,
normal fetal rats had completely ruptured the anal membrane to form
the anus, while the ETU malformation group did not form an anus,
had developed multiple deformities and the development of the nerve
plexus was poor. P2Y2 and HuD were mainly expressed in the distal
submucosa and intermuscular plexus of the fetal rat and were
primarily expressed in the cytoplasm. The expression of P2Y2 and
HuD in the rectum was lower, and there was no difference in the
three groups at E17. These results indicated that there was a
relative imbalance in expression between the normal and ARM model
embryos during ENS development. While ETU can cause ARMs in
embryos, it had no marked effect on the expression of P2Y2 and HuD.
At E21, the expression levels of P2Y2 and HuD in the ETU group were
significantly lower than the ARM group, suggesting that P2Y2 and
HuD may be responsible for the abnormal development of the ENS. The
changes in these two microenvironmental factors hindered the
development and migration of the intestinal nervous system. The
expression of P2Y2 and HuD from E17 to E19 changed more slowly than
that observed between E19 to E21. It has been suggested that E19 is
the critical period of ENS formation.
In conclusion, the results from the present study,
and those of previous studies, suggested that the downregulation
pattern of P2Y2 and HuD may be important for ENS development in the
anorectum of fetal rats with ETU-induced ARMs. Since many signaling
molecules have been revealed to be expressed and function
differently during different phases of ENS development, the present
study was unable to determine whether P2Y2 and HuD were the primary
factors that led to ENS anomalies. Further studies are required to
define the two signaling molecules that are involved in regulating
ENS formation during embryonic development and to clarify the
specific roles of molecular mechanisms mediating the maldevelopment
of the ENS, and thus help to improve our understanding of
postoperative defecation disorder and patient quality of life.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Fund of China (grant no. 81650029).
Availability data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and BC performed the majority of experiments,
contributed equally to the work, authored or reviewed drafts of the
paper, and approved the final draft. ZJ performed the experiments,
authored or reviewed drafts of the paper, and approved the final
draft. MG, CT, YM, and YQ analyzed the data, authored or reviewed
drafts of the paper, and approved the final draft. YL conceived and
designed the experiments, contributed the
reagents/materials/analysis tools, authored or reviewed drafts of
the paper, and approved the final draft and all authors agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Ethical approval was obtained from the Zunyi Medical
College Animal Ethics Committee (no. 20150820014). Animal care and
handling procedures for ex vivo studies performed in China
were as per the protocol approved by the Institutional Animal Care
and Use Committee of Zunyi Medical College. Animal handling
procedures for in vivo studies conducted in China were
approved by the Institutional Animal Care and Use Local
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peña A, Guardino K, Tovilla JM, Levitt MA,
Rodriguez G and Torres R: Bowel management for fecal incontinence
in patients with anorectal malformations. J Pediatr Surg.
33:133–137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levitt MA and Peña A: Outcomes from the
correction of anorectal malformations. Curr Opin Pediatr.
17:394–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sonnino RE, Reinberg O, Bensoussan AL,
Laberge JM and Blanchard H: Gracilis muscle transposition for anal
incontinence in children: Long-term follow-up. J Pediatr Surg.
26:1219–1223. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai Y, Yuan Z and Wang W, Zhao Y, Wang H
and Wang W: Quality of life for children with fecal incontinence
after surgically corrected anorectal malformation. J Pediatr Surg.
35:462–464. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rintala RJ and Lindahl H: Is normal bowel
function possible after repair of intermediate and high anorectal
malformations? J Pediatr Surg. 30:491–494. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Froster UG, Wallner SJ, Reusche E,
Schwinger E and Rehder H: VACTERL with hydrocephalus and branchial
arch defects: Prenatal, clinical, and autopsy findings in two
brothers. Am J Med Genet. 62:169–172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levin MD: The pathological physiology of
the anorectal defects, from the new concept to the new treatment.
Eksp Klin Gastroenterol. 11:38–48. 2013.(In Russian).
|
|
8
|
Li L, Li Z, Wang LY and Xiao FD: Anorectal
anomaly: Neuropathological changes in the sacral spinal cord. J
Pediatr Surg. 28:880–885. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan Z, Bai Y, Zhang Z, Ji S, Li Z and
Wang W: Neural electrophysiological studies on the external anal
sphincter in children with anorectal malformation. J Pediatr Surg.
35:1052–1057. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernández-Fraga X, Azpiroz F and
Malagelada JR: Significance of pelvic floor muscles in anal
incontinence. Gastroenterology. 123:1441–1450. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mulè F, Naccari D and Serio R: Evidence
for the presence of P2Y and P2X receptors
with different functions in mouse stomach. Eur J Pharmacol.
513:135–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arthur DB, Akassoglou K and Insel PA: P2Y2
receptor activates nerve growth actor/TrkA signaling to enhance
neuronal differentiation. Proc Natl Acad Sci USA. 102:19138–19143.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Kong M, Jin Z, Gao M, Qu Y and
Zheng Z: Expression of the P2Y2 receptor in the terminal rectum of
fetal rats with anorectal malformation. Int J Clin Exp Med.
8:1669–1676. 2015.PubMed/NCBI
|
|
14
|
Nasser Y, Fernandez E, Keenan CM, Ho W,
Oland LD, Tibbles LA, Schemann M, MacNaughton W, Anne R and Sharkey
KA: Role of enteric glia in intestinal physiology: Effects of the
gliotoxin fluorocitrate on motor and secretory function. Am J
Physiol Gastrointest Liver Physiol. 291:G912–G927. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hinman MN and Lou H: Diverse molecular
functions of Hu proteins. Cell Mol Life Sci. 65:3168–3181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bolognani F, Merhege MA, Twiss J and
Perrone-Bizzozero NI: Dendritic localization of the RNA-binding
protein HuD in hippocampal neurous: Association with polysomes and
upregulation during contextual learning. Neurosci Lett.
371:152–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deschenes-Furry J, Belanger G,
Perrone-Bizzozero N and Jasmin BJ: Post-transcriptional regulation
of acetylcholinesterase mRNAs in nerve growth factor-treated PC12
cells by the RNA-binding protein HuD. J Biol Chem. 278:5710–5717.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong M, Wu Y and Liu Y: The impact of HuD
protein on the intestinal nervous system in the terminal rectum of
animal models of congenital anorectal malformation. Mol Med Rep.
16:4797–4802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdelmohsen K, Hutchison ER, Lee EK,
Kuwano Y, Kim MM, Masuda K, Srikantan S, Subaran SS, Marasa BS,
Mattson MP and Gorospe M: miR-375 inhibits differentiation of
neurites by lowering HuD levels. Mol Cell Biol. 30:4197–4210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mandhan P, Quan QB, Beasley S and Sullivan
M: Sonic hedgehog, BMP4 and Hox genes in the development of
anorectal malformations in ethylenethiourea-exposed fetal rats. J
Pediatr Surg. 41:2041–2045. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Young HM: Functional development of the
enteric nervous system-from migration to motility.
Neurogastroenterol Motil. 20 (Suppl 1):S20–S31. 2008. View Article : Google Scholar
|
|
23
|
Lake JI and Heuckeroth RO: Enteric nervous
system development: Migration, differentiation, and disease. Am J
Physiol Gastrointest Liver Physiol. 305:G1–G24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kapur RP, Yost C and Palmiter RD: A
transgenic model for studying development of the enteric nervous
system in normal and aganglionic mice. Development. 116:167–175.
1992.PubMed/NCBI
|
|
25
|
Fu M, Lui VC, Sham MH, Cheung AN and Tam
PK: HOXB5 expression is spatially and temporarily regulated in
human embryonic gut during neural crest cell colonization and
differentiation of enteric neuroblasts. Dev Dyn. 228:1–10. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu M, Tam PK, Sham MH and Lui VC:
Embryonic development of the ganglion plexuses and the concentric
layer structure of human gut: A topographical study. Anat Embryol
(Berl). 208:33–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang Y, Liu M and Gershon MD: Netrins and
DCC in the guidance of migrating neural crest-derived cells in the
developing bowel and pancreas. Dev Biol. 258:364–384. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flores RV, Hernández-Pérez MG, Aquino E,
Garrad RC, Weisman GA and Gonzalez FA: Agonist-induced
phosphorylation and desensitization of the P2Y2 nucleotide
receptor. Mol Cell Biochem. 280:35–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandenburg LO, Jansen S, Wruck CJ, Lucius
R and Pufe T: Antimicrobial peptide rCRAMP induced glial cell
activation through P2Y receptor signalling pathways. Mol Immunol.
47:1905–1913. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kong Q, Peterson TS, Baker O, Stanley E,
Camden J, Seye CI, Erb L, Simonyi A, Wood WG, Sun GY and Weisman
GA: Interleukin-1beta enhances nucleotide-induced and
α-secretase-dependent amyloid precursor protein processing in rat
primary cortical neurons via up-regulation of the P2Y(2) receptor.
J Neurochem. 109:1300–1310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Degagné E, Grbic DM, Dupuis AA, Lavoie EG,
Langlois C, Jain N, Weisman GA, Sévigny J and Gendron FP: P2Y2
receptor transcription is increased by NF-kappa B and stimulates
cyclooxygenase-2 expression and PGE2 released by intestinal
epithelial cells. J Immunol. 183:4521–4529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wullaert A, Bonnet MC and Pasparakis M:
NF-κB in the regulation of epithelial homeostasis and inflammation.
Cell Res. 21:146–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agresti C, Meomartini ME, Amadio S,
Ambrosini E, Volonté C, Aloisi F and Visentin S: ATP regulates
oligodendrocyte progenitor migration, proliferation,
differentiation: Involvement of etabotropic P2 receptor. Brain Res
Brain Res Rev. 48:157–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weisman GA, Camden JM, Peterson TS, Ajit
DV, Woods LT and Erb L: P2 receptors for extracellular nucleotides
in the central nervous system: Role of P2X7 and P2Y2
Receptor Interactions in neuroinflammation. Mol Neurobiol.
46:96–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hayashi S, Yano M, Igarashi M, Okano HJ
and Okano H: Alternative role of HuD splicing variants in neuronal
differentiation. J Neurosci Res. 93:399–409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Evangelisti C, Bianco F, Pradella LM,
Puliti A, Goldoni A, Sbrana I, Rossi M, Vargiolu M, Seri M, Romeo
G, et al: Apolipoprotein B is a new target of the GDNF/RET and
ET-3/EDNRB signalling pathways. Neurogastroenterol Motil.
24:e497–e508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang X, Wen G, Tuo B, Zhang F, Wan H, He
J, Yang S and Dong H: Molecular mechanisms of calcium signaling in
the modulation of small intestinal ion transports and bicarbonate
secretion. Oncotarget. 9:3727–3740. 2017.PubMed/NCBI
|