Introduction
Breast cancer is the most common diagnosed
malignancy in women and the leading cause of malignancy-associated
mortality in women worldwide, with an estimated 270,000 new cases
and 70,000 mortalities recorded in China in 2015 (1). The majority of mortalities of
patients with breast cancer are due to cancer metastasis, and
studies have reported that breast cancer invasion and metastasis
are closely associated with multiple signalling pathways. A study
has suggested that STAT3, a mediator in the Janus kinase (JAK)/STAT
signalling pathway, has an important role in promoting metastasis
in breast cancer (2). Cell
proliferation and tumour metastasis are associated with disorders
of cell signal transduction pathways, which makes it important to
focus on identifying novel antitumor drugs targeting the abnormal
signalling systems in tumour cells.
The current study explored the effects of
saikosaponin b2 (SSb2) on the metastasis of breast cancer by
studying the JAK/STAT signalling pathway. STAT3 is a member of the
STAT family and its abnormal activation has an important role in
invasion and metastasis of various types of tumour (3,4).
Studies have demonstrated that activated STAT3 increases the
transcriptional activity of the matrix metallopeptidase 2 (MMP2)
promoter region and upregulates MMP2 protein expression (5).
Bupleurum is used as a herbal therapy that is
considered to relieve liver qi stagnation in traditional Chinese
herbal medicine (6).
Bupleurum has various properties, including
immunoregulation, protection of the liver and gall bladder, and
antifever, anti-inflammatory, sedative, analgesic, antiviral and
antitumor effects (7–9). Pharmacological analysis suggests that
the pharmacological effects of Bupleurum are mediated by
saikosaponins, which are the major active compounds in
Bupleurum. Saikosaponins are triterpenoids, which are
subdivided into saikosaponin a, b1, b2, c and d (10). Saikosaponin a (SSa), Saikosaponin c
(SSc) and Saikosaponin d (SSd) exert anti-inflammatory effects
(11–13), and SSa and SSd inhibit cancer cell
proliferation and invasion (14–16).
SSa induces apoptosis in MCF-7 cells and the process may be
regulated by the Bcl-2 family and dependent on p53/p21 signalling
pathway (14). SSd inhibits NF-κB
and STAT3 phosphorylation in acetaminophen-induced hepatotoxicity
in mice (17). SSa is an epimer of
SSd that further inhibits NF-κB activation in
lipopolysaccharide-induced RAW264.7 cells (12). SSb2 can be extracted from
Bupleurum spp. roots (Radix Bupleuri). SSb2 is another
active compound present in saponin; however, there is limited
research on the effects of SSb2. The aim of the current study was
to elucidate the pharmacological effects and molecular mechanisms
of SSb2 in inhibiting breast cancer cell proliferation and
migration.
Materials and methods
Cell culture and plasmid
transfection
MCF-7 human breast cancer cells were obtained from
the Department of Pathophysiology, Basic Medical College of Wuhan
University (Wuhan, China). Cells were maintained in DMEM (HyClone;
GE Healthcare Life Sciences) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and antibiotics (100 U/ml
penicillin and 100 U/ml streptomycin) at 37°C in a humidified
incubator supplemented with 5% CO2. To inhibit
expression of pSTAT3 and VASP, MCF-7 cells were treated with 15 µM
NSC74859 for 24 h before western blot analysis. A total of
1.5×105 cells were plated in 2 ml growth medium without
antibiotics in 6-well plates. A total of 10 µl Lipofectamine™ 2000
and 4 µg pCGN-HAM-STAT3 or pCGN-HAM-vector diluted in 50 µl
Opti-MEMI Reduced Serum medium were mixed gently and incubated for
20 min at room temperature. pCGN-HAM-STAT3 was previously
constructed in our laboratory with primer, STAT3, forward,
5′-AATCTCGAGGGATGGCCCAATGGAATCAGCTA-3′, and reverse,
5′-AATGTCGACTCACATGGGGGAGGTAGCGC-3′. The resultant mixture was
added to 1.5×105 cells and 100 µl Opti-MEMI medium was
added to a blank control groups. Cells were incubated for 48 h
prior to analysis of transgene expression. MCF-7 cells were also
treated with IL-6 (10 ng/ml, 37°C) for 30 min before western blot
analysis to explore the effect of IL-6-induced STAT3
phosphorylation on VASP protein expression.
Human breast cancer samples
A total of 16 human breast cancer and matched
adjacent tissues were collected between July 2016 to May 2017 at
Zhongnan Hospital of Wuhan University (Wuhan, China). Prior to the
surgery, patients signed informed consent forms for the present
study, which included consent for the use of their tissue for
research. The inclusion criteria were: i) Adults aged 18–60; ii)
primary invasive tumors; iii) no metastasis; iv) no previous
therapy prior to surgical resection. The exclusion criteria were:
i) 18> age >60; ii) male breast cancer; iii) with metastasis;
iv) recurrent lesions. The study was endorsed by the Ethics
Committee of the Zhongnan Hospital of Wuhan University and was
conducted in accordance with the principles of the Declaration of
Helsinki.
Reverse transcription-quantitative PCR
(RT-qPCR) assays
Total RNA was extracted from breast cancer and
matched normal tissues using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and was used for first-strand cDNA
synthesis using the RevertAid™ kit (Fermentas; Thermo Fisher
Scientific, Inc.). For reverse transcription, RNA strand was
incubated with 1 µl RNAase inhibition 2 µl 10 mM dNTP mix at 37°C
for 5 mins. cDNA was synthetized with reverse transcriptase at 42°C
for 60 mins and 72°C for 10 mins. PCR amplification of cDNA was
conducted with 10 µl of 2X SYBR Master mix (Toyobo Life Science)
using the following primers: STAT3, forward
5′-GCACTTGTAATGGCGTCTTCA-3′ and reverse
5′-TCTAGCTGTTCTGCCTCACCT-3′; vasodilator-stimulated phosphoprotein
(VASP), forward 5′-AAAGTCAGCAAGCAGGAGGA-3′ and reverse
5′-ATTCATCCTTGGGGGTTTTC-3′; and internal control GAPDH, forward
5′-TGATGACATCAAGAAGGTGGTGAAG-3′ and reverse
5′-TCCTTGGAGGCCATGTGGGCCAT-3′. Cycling conditions were 95°C for 10
sec, 65°C for 20 sec and 72°C for 15 sec (40 cycles).
Cell proliferation assays
MTT assays (Amresco, LLC) were performed to
determine proliferation. Cells were seeded in 96-well plates at
2×103 cells/well and incubated. Cells were treated with
various SSb2 concentrations (0, 0.1, 0.2, 0.5, 1, 2, 5, 10, 20 and
50 µM) for 48 h. Following treatment with 100 µl MTT (5 mg/ml),
cells were incubated for further 4 h at 37°C. Medium was discarded
and 150 µl DMSO was added (Sigma-Aldrich; Merck KGaA). Absorbance
at 490 nm was determined using an ELISA plate reader
(Infinite® 200 PRO; Tecan Group, Ltd.). The viability of
SSb2-treated cells was calculated by comparing the absorbance of
each group with DMSO-treated cells, which were arbitrarily assigned
100%.
Cell migration assay
The effect of SSb2 on breast cancer cell migration
was measured using wound-healing assays. Cells were plated at
1×105 cells/well in 6-well plates and allowed to grow to
60–70% confluence. Cells were serum-starved for 12 h, washed twice
with PBS and supplied with DMEM supplemented with 10% FBS and
various concentrations of SSb2 (0.2, 1 and 5 µM). Wounds were
created by scratching across the cell monolayer and cells within
the same fields of view were captured at 0, 24 and 48 h under a
light microscope (Olympus, Japan). The cell migration area was
measured between cut regions using Image J software (V1.8.0;
National Institutes of Health) and normalized to control cells.
Colony formation assay
Cells treated with varying concentrations of SSb2
(0.2, 2, 10, 20 and 50 µM) for 48 h were plated at 200 cells/dish
and incubated at 37°C in a 5% CO2 incubator. Following
treatment with different concentrations of SSb2, the cells were
incubated for two additional weeks and the clones stained with
crystal violet (0.5%, m/v) for 30 mins at room temperature. Cells
were washed with PBS (4×5 min), clones were counted and images were
captured. Clone formation rate (%)=(number of clones/number of
inoculated cells) ×100.
Western blot analysis
Following washes with PBS, cells were incubated in
ice-cold RIPA assay buffer (20 mM Tris-HCl pH 7.5, 1 mM
phenyl-methylsulfonylfluoride, 150 mM NaCl, 10 µg/ml leupeptin, 10
µg/ml aprotinin, 0.25% deoxycholate, 1.5 mM MgCl2, 1 mM
egtazic acid, 10 mM NaF, 1% Triton X-100 and 10 mM pervanadate).
After 15 min on the ice, mixtures were centrifuged (15,000 × g, 15
min) to extract proteins. A Coomassie Brilliant Blue assay was used
to determine the protein concentration in the supernatant. Lysates
were mixed with 5X sample buffer containing 2-mercaptoethanol and
boiled for 5 min prior to loading onto 12% gels and separation by
SDS-PAGE. Proteins were then transferred to nitrocellulose
membranes over 2 h. Membranes were incubated at 4°C overnight with
antibodies, following a blocking with 5% (w/v) BSA in Tris-buffer
for 1.5 h at room temperature. Anti-VASP (1:1,000; cat. no.
sc-376226), anti-STAT3 (1:2,000; cat. no. sc-293151), anti-phospho
(p)STAT3 (1:2,000; cat. no. sc-56747), anti-c-myc (1:1,000; cat.
no. sc-53854), anti-cyclin D1 (1:1,000; cat. no. sc-450), anti-MMP2
(1:1,000; cat. no. sc-13594), anti-MMP9 (1:1,000; cat. no.
sc-21733) and anti-GAPDH (1:2,000; cat. no. sc-66163) primary
antibodies (Santa Cruz Biotechnology, Inc.) were diluted in 1X TBS
containing 0.1% Tween 20 with 5% BSA. Following three washes with
TBS containing 0.1% Tween 20, blots were incubated with horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat: 516102;
Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. An ECL
reagent kit (Axygen; Corning Inc., Corning, NY, USA) was used to
detect protein bands. Band Scan (version 5.0, Glyko lnc.) was used
to quantitatively analyze each band for statistical comparison.
Haematoxylin and eosin (H&E)
staining
H&E staining was used to assess the
hepatotoxicity and nephrotoxicity in Kunming mice treated with
SSb2. Kunming mice (n=12, 8-weeks old, 18–24 g) were maintained
under sterile conditions and fed with sterile feed and water at
18°C and a 12-h light/dark cycle. The mice were obtained from
Animal Biosafety Level 3 Laboratory of Wuhan University and
randomly divided into 2 groups with 6 females in each. Mice in the
experimental group were administered SSb2 (30 mg/kg) daily by
intraperitoneal injection for 1 month and the control group was
administered same volume of saline. The experimental mice were kept
at a constant 20°C with 50% humidity and with regular clean food
and water. Fresh liver and kidney tissues were obtained from the
mice and tissues were cut into small pieces, fixed with 4%
paraformaldehyde for 24 h at room temperature, embedded in paraffin
and stained with H&E. The slices were stained with hematoxylin
for 10 min and eosin for 30 sec at 60°C. Paraffin sections were
observed under a light microscope (magnification, ×400). All
experiments followed the Guidelines for Animal Experimentation of
the Wuhan University (Wuhan, China) and protocols were approved by
the Ethics Committee for Animal Experimentation.
Statistical analysis
Data are presented as the mean ± SEM representing
multiple experiments. Student's t-tests were performed to compare
data from two groups, and differences between three or more groups
were analysed using one-way ANOVA analysis. Tukey's honestly
significant difference procedure was used for post hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
STAT3 and VASP mRNA is increased in
human breast cancer tissues
To detect STAT3 and VASP mRNA expression in breast
cancer tissues, RT-qPCR analysis was performed. As presented in
Fig. 1, STAT3 and VASP mRNA
expression in cancer tissues were significantly increased compared
with the adjacent normal tissues (P<0.05).
SSb2 inhibits proliferation, migration
and colony numbers of MCF-7 breast cancer cells
To study the effect of SSb2 on MCF-7 proliferation,
cells were treated with SSb2 (0.1, 0.2, 0.5, 1, 2, 5, 10, 20 and 50
µM) for 48 h. Following treatment, MTT assays were performed to
measure cell proliferation. As presented in Fig. 2A, the cell proliferation rate was
significant inhibited compared with the control group in a
dose-dependent manner (P<0.05). Western blot analysis also was
performed following cell treatment with 5 µM SSb2 for 48 h. As
presented in Fig. 2B, treatment
with 5 µM SSb2 significantly reduced the expression of c-myc and
cyclin D1, which are related to cell proliferation in the STAT3
signalling pathway, compared with the control (P<0.05).
To observe the effect of SSb2 on the migration of
breast cancer cells, cells were treated with SSb2 (0.2, 1 and 5 µM)
for 24 and 48 h. Following treatment, wound-healing assays were
performed to evaluate MCF-7 migration. As presented in Fig. 2C, following treatment with SSb2,
cell migration rates decreased along with the increased
concentration of SSb2 at 24 or 48 h in a dose-dependent manner.
To determine whether SSb2 affects the clonal
formation ability of MCF-7 breast cancer cells, colony formation
assays were performed. Varying concentrations of SSb2 (0.2, 2, 10,
20 and 50 µM) were used to treat MCF-7 cells for 48 h. As presented
in Fig. 2D, the colony formation
ability was significantly suppressed using 2, 10, 20 or 50 µM SSb2
compared with the control (P<0.05) in a dose-dependent
manner.
SSb2 affects pSTAT3, STAT3 and VASP
protein levels
To study effects of SSb2 on pSTAT3, STAT3 and VASP
protein levels in MCF-7 cells, western blot analysis was performed
following cell treatment with SSb2 (0.2, 0.5 and 1 µM) for 48 h. As
presented in Fig. 3, treatment
with 0.2, 0.5 and 1 µM SSb2 significantly reduced pSTAT3 in MCF-7
cells compared with the control (P<0.05). SSb2 at 1 µM
significantly reduced STAT3 expression compared with the control
(P<0.05), while 0.2 and 0.5 µM SSb2 had no significant effect on
STAT3 expression. Analysis of the pSTAT3/STAT3 ratio demonstrated
that STAT3 phosphorylation was reduced by 0.5 and 1 µM SSb2. In
addition, 0.5 and 1 µM of SSb2 significantly reduced VASP
expression in MCF-7 cells compared with the untreated control
(P<0.05).
Inhibition of STAT3 phosphorylation
affects VASP expression
To investigate whether SSb2 affected VASP protein
expression by inhibiting STAT3 phosphorylation, cells were treated
with NSC74859 (15 µM) a specific inhibitor of STAT3 phosphorylation
and pSTAT3, STAT3 and VASP levels were determined by western blot.
As presented in Fig. 4, pSTAT3 and
VASP levels in the NSC74859-treated cells were significantly
decreased compared with the control (P<0.05), while STAT3 levels
exhibited no significant changes.
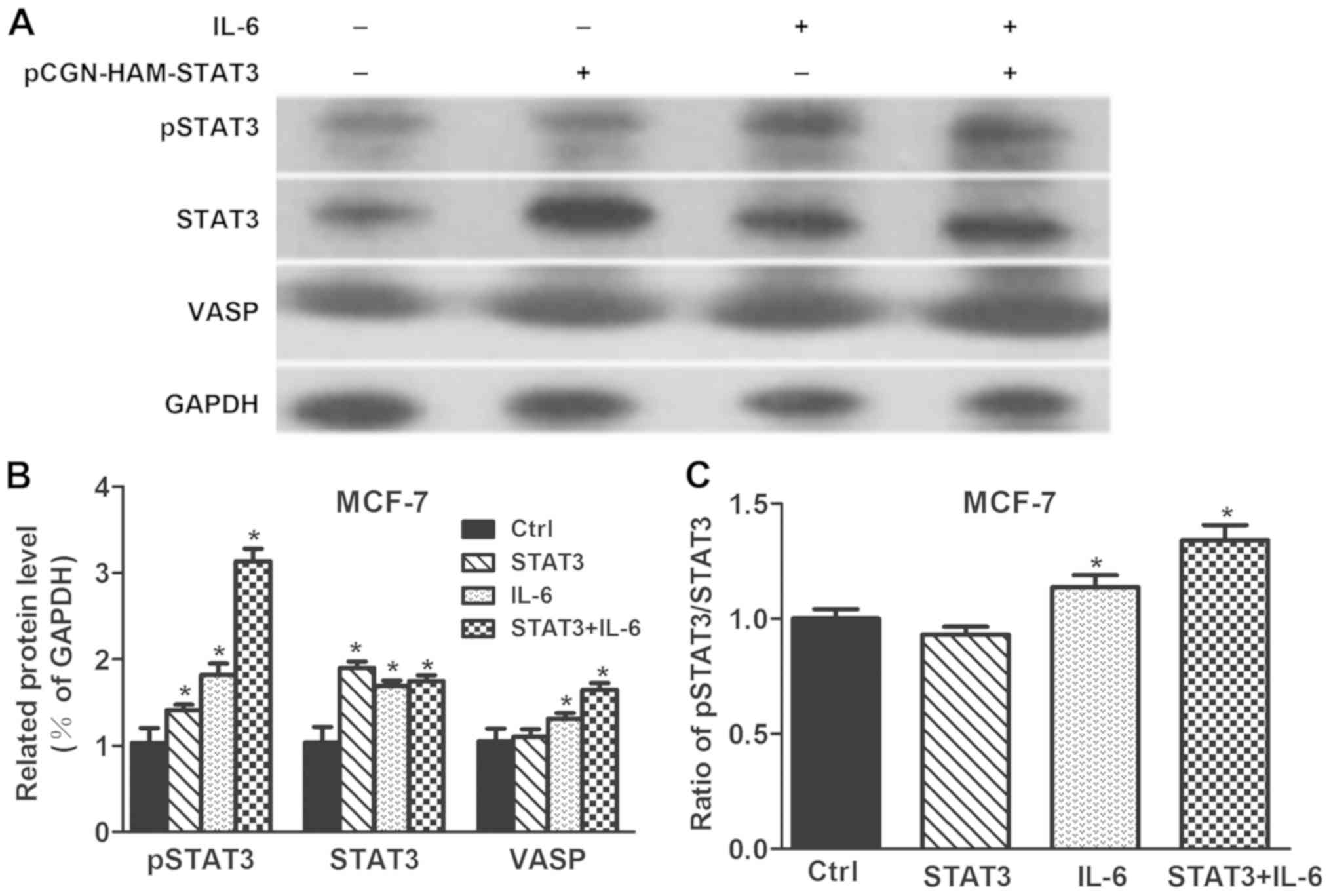
STAT3 phosphorylation induced by
interleukin-6 (IL-6) affects VASP levels
To observe whether STAT3 phosphorylation affects
VASP expression, STAT3, pSTAT3 and VASP levels were determined in
STAT3 overexpressing cells or following IL-6 treatment of MCF-7
cells. As presented in Fig. 5,
STAT3 and pSTAT3 levels in STAT3 overexpressing MCF-7 cells were
significantly increased compared to the control (P<0.05).
Stimulation with IL-6 significantly increased STAT3, pSTAT3 and
VASP levels compared with the control (P<0.05). Treatment of
STAT3 overexpressing cells with IL-6 significantly increased STAT3,
pSTAT3 and VASP levels compared with the control (P<0.05). In
conclusion, VASP level could be only elevated when STAT3 was
phosphorylated by IL-6. Thereby, STAT3 phosphorylation induced by
IL-6 could affect VASP levels.
SSb2 affects MMP2 and MMP9
expression
To detect the effect of SSb2 on MMP2 and MMP9
expression, MCF-7 cells were treated with 5 µM SSb2 for 48 h and
expression was detected by western blot analysis. As presented in
Fig. 6, MMP2 and MMP9 expression
was significantly decreased in SSb2-treated cells compared with the
control group.
SSb2 exerts no liver or kidney
toxicity in Kunming mice
To observe whether SSb2 treatment induces liver or
kidney damage, an experimental group of Kunming mice was injected
intraperitoneally with 30 mg/kg/day SSb2 for 30 days. Liver and
kidney tissues from mice of the experimental and the untreated
control groups were H&E stained. As presented in Fig. 7, no hepatocellular degeneration or
necrosis was observed in the experimental group compared with the
control group and no inflammatory cells were detected to have
infiltrated the hepatic lobules or the portal area (Fig. 7A and B). There was no significant
increase in the number of cells in the glomerular capillary loop
and no neutrophils and lymphocyte invasion in the experimental
compared with the control mice (Fig.
7C and D).
Discussion
Incidence and mortality rates of patients with
breast cancer remain high, ranking second to cervical cancer in
developing countries (18). Cancer
cell metastasis became the leading cause of death in patients with
breast cancer. Saikosaponins are small molecule compounds extracted
from the medicinal Bupleurum plants. Studies have revealed
that SSd effectively downregulates tumour necrosis
factor-α-mediated NF-κB signalling pathway to inhibit proliferation
and invasion of cancer cells (16). SSd inhibits the NF-κB/STAT3
signalling pathway, protecting against acetaminophen-induced liver
injury (17). Another study
demonstrated that SSb2 hinders hepatitis C virus entry into cells
through affecting the E2 protein in the virus, which may useful for
anti-hepatitis C virus treatment (19). The current study examined the
mechanisms of action of SSb2 on breast cancer cells and explored
whether the antitumor effect of SSb2 in breast cancer was
associated with the JAK/STAT signalling pathway.
The JAK/STAT signalling pathway is involved in cell
proliferation, differentiation, apoptosis, immune regulation and
other biological processes. The STAT family contains seven protein
members, including STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and
STAT6. Of these, STAT3 is closely associated with tumorigenesis and
has received increasing attention. STAT3 has an important role in
cellular signal transduction (20). It transmits signals from outside
the cell to the nucleus and induces the transcription of target
genes. Epidermal growth factor activates the STAT3 signalling
pathway in prostate cancer cells and enhances the transcriptional
activity of its downstream targets. Small interfering RNA targeting
STAT3 reduces the ability of cell migration (21). Another study reported that STAT3
inhibition significantly reduces proliferation of prostate cancer
cells (22,23). In breast cancer, compared with
paracancerous tissues, STAT3 expression is markedly increased
(3), which is consistent with the
results obtained in the current study evaluating protein expression
in cancer and adjacent tissues of patients with breast cancer.
VASP, an important member of the Ena/VASP family, is
an actin-regulating protein in mammalian cells. Within the cells,
VASP is mainly located in regions associated with cell adhesion and
movement with key roles in the regulation of tumour cell migration
and metastasis (24,25). VASP expression is regulated by
upstream factors to affect cell metastasis (26). From the perspective of drug
intervention, identifying effective VASP-targeting drugs to inhibit
cell transfer may provide novel research directions.
MMP 2 and MMP 9 are members of the MMP family that
contribute to cancer progression through extracellular matrix
degradation, allowing cancer cells to migrate away from primary
tumours and metastasise. Particularly, MMP2 is capable of degrading
the most abundant type IV collagen in basement membranes.
Degradation of the basement membrane is a key step in the
progression of most cancer metastases (27). In aggressive breast tumours, MMP2
and MMP9 are highly expressed and associated with poor prognosis
(28). In addition, various
studies have reported that MMP2 and MMP9 expression is associated
with multiple signalling pathways. Formononetin flavonoids inhibit
migration and invasion of breast cancer cells by reducing MMP2 and
MMP9 expression via the PI3K/AKT signalling pathway (29). Furthermore, studies have revealed
that activation of the STAT3 signalling pathway is involved in the
upregulation of MMP2 and MMP9 (30,31).
Based on these findings, effects of SSb2 on MMP2 and MMP9
expression were evaluated.
To explore the effects of SSb2 on proliferation and
migration of breast cancer cells, MTT assays were performed at
varying SSb2 concentrations. Treatment of MCF-7 cells with SSb2 for
48 h significantly decreased proliferation rates in MCF-7 cells
compared with untreated cells. It also demonstrated that SSb2
significantly reduced protein expression of c-myc and cyclin D1,
which are associated with cell proliferation via the STAT3
signalling pathway, compared with the control group. The effects of
varying SSb2 concentrations on the migration of MCF-7 cells at
different times were investigated using wound-healing experiments.
The results suggested that with increasing SSb2 migration rates
gradually declined compared with the control group. In a colony
formation assay, SSb2 further suppressed colony numbers of MCF-7
cells. The experimental results illustrated that SSb2 suppressed
proliferation and migration of breast cancer cells.
To explore the mechanism by which SSb2 inhibited
proliferation and migration of breast cancer cells, western
blotting was performed to evaluate pSTAT3, STAT3, VASP, MMP2 and
MMP9 protein levels. The results demonstrated that SSb2 treatment
decreased STAT3 phosphorylation and VASP levels. In subsequent
experiments, following the inhibition of STAT3 phosphorylation,
pSTAT3 levels decreased significantly and VASP protein expression
was reduced compared with the control. The treatment with IL-6,
which induces STAT3 activation in MCF-7 cells, led to increased
pSTAT3 and VASP protein levels. This suggested that STAT3
phosphorylation in MCF-7 cells may positively regulate VASP
expression and that SSb2 inhibited VASP expression by inhibiting
STAT3 phosphorylation. In addition, the current study demonstrated
that MMP2 and MMP9 expression was inhibited in MCF-7 cells
following treatment with 5 µM SSb2 for 48 h.
In summary, the current study suggested that SSb2
inhibited proliferation and migration of MCF-7 cells by reducing
the activation of STAT3, and inhibiting VASP, MMP2 and MMP9 protein
expression. The results further indicated that SSb2 may reduce the
migration of breast cancer cells. A limitation of our study is that
only the MCF-7 cell line was investigated, and that the results
require validation in other breast cancer cell lines. The current
study provided a novel direction for molecular-targeted therapy for
patients with breast cancer.
Acknowledgements
Not applicable.
Funding
This study received support from the National
Natural Science Foundation of China (grant nos. 81472765 and
81572943).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QM and FG conceived and designed the study,
performed the experiments, analysed the data and drafted the
manuscript. XH, KL and NJ performed the analysis of data and
drafted the manuscript. YG, LD and WS were involved in recruiting
patients and collecting patients' samples. XX, YH and WP performed
the experiments. JZ and LW supported the funding of study, designed
the study and modified the manuscript.
Ethics approval and consent to
participate
Written informed consent was provided by each
patient recruited and the present study was approved by the local
Human Ethics Committee of Zhongnan Hospital of Wuhan
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee HT, Xue J, Chou PC, Zhou A, Yang P,
Conrad CA, Aldape KD, Priebe W, Patterson C, Sawaya R, et al: Stat3
orchestrates interaction between endothelial and tumor cells and
inhibition of Stat3 suppresses brain metastasis of breast cancer
cells. Oncotarget. 6:10016–10029. 2015.PubMed/NCBI
|
|
3
|
Berclaz G, Altermatt HJ, Rohrbach V,
Siragusa A, Dreher E and Smith PD: EGFR dependent expression of
STAT3 (but not STAT1) in breast cancer. Int J Oncol. 19:1155–1160.
2001.PubMed/NCBI
|
|
4
|
Wang T, Yuan J, Zhang J, Tian R, Ji W,
Zhou Y, Yang Y, Song W, Zhang F and Niu R: Anxa2 binds to STAT3 and
promotes epithelial to mesenchymal transition in breast cancer
cells. Oncotarget. 6:30975–30992. 2015.PubMed/NCBI
|
|
5
|
Xuan X, Li S, Lou X, Zheng X, Li Y, Wang
F, Gao Y, Zhang H, He H and Zeng Q: Stat3 promotes invasion of
esophageal squamous cell carcinoma through up-regulation of MMP2.
Mol Biol Rep. 42:907–915. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Law BY, Mo JF and Wong VK: Autophagic
effects of Chaihu (dried roots of Bupleurum Chinense DC or
Bupleurum scorzoneraefolium WILD). Chin Med. 9:212014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi FH, Wang ZX, Cai PP, Zhao L, Gao JJ,
Kokudo N, Li AY, Han JQ and Tang W: Traditional Chinese medicine
and related active compounds: A review of their role on hepatitis B
virus infection. Drug Discov Ther. 7:212–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah AS and Alagawadi KR:
Anti-inflammatory, analgesic and antipyretic properties of
Thespesia populnea Soland ex. Correa seed extracts and its
fractions in animal models. J Ethnopharmacol. 137:1504–1509. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong VK, Li T, Law BY, Ma ED, Yip NC,
Michelangeli F, Law CK, Zhang MM, Lam KY, Chan PL and Liu L:
Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell
death in apoptosis-defective cells. Cell Death Dis. 4:e7202013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang YY, Tang YZ, Fan CL, Luo HT, Guo PR
and Chen JX: Identification and determination of the saikosaponins
in Radix bupleuri by accelerated solvent extraction combined with
rapid-resolution LC-MS. J Sep Sci. 33:1933–1945. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sai J, Zhao Y, Shan W, Qu B, Zhang Y,
Cheng J, Qu H and Wang Q: Development of an enzyme-linked
immunosorbent assay and immunoaffinity column chromatography for
saikosaponin d using an anti-saikosaponin d monoclonal antibody.
Planta Med. 82:432–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ,
Liao M and Chen JX: Saikosaponin a and its epimer saikosaponin d
exhibit anti-inflammatory activity by suppressing activation of
NF-κB signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Y, Bao Y, Wang S, Li T, Chang X, Yang G
and Meng X: Anti-inflammation effects and potential mechanism of
saikosaponins by regulating nicotinate and nicotinamide metabolism
and arachidonic acid metabolism. Inflammation. 39:1453–1461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen JC, Chang NW, Chung JG and Chen KC:
Saikosaponin-A induces apoptotic mechanism in human breast
MDA-MB-231 and MCF-7 cancer cells. Am J Chin Med. 31:363–377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim BM and Hong SH: Sequential caspase-2
and caspase-8 activation is essential for saikosaponin a-induced
apoptosis of human colon carcinoma cell lines. Apoptosis.
16:184–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong VK, Zhang MM, Zhou H, Lam KY, Chan
PL, Law CK, Yue PY and Liu L: Saikosaponin-d enhances the
anticancer potency of TNF-α via overcoming its undesirable response
of activating NF-Kappa B signalling in cancer cells. Evid Based
Complement Alternat Med. 2013:7452952013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu A, Tanaka N, Sun L, Guo B, Kim JH,
Krausz KW, Fang Z, Jiang C, Yang J and Gonzalez FJ: Saikosaponin d
protects against acetaminophen-induced hepatotoxicity by inhibiting
NF-κB and STAT3 signaling. Chem Biol Interact. 223:80–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin LT, Chung CY, Hsu WC, Chang SP, Hung
TC, Shields J, Russell RS, Lin CC, Li CF, Yen MH, et al:
Saikosaponin b2 is a naturally occurring terpenoid that efficiently
inhibits hepatitis C virus entry. J Hepatol. 62:541–548. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wieczorek M, Ginter T, Brand P, Heinzel T
and Krämer OH: Acetylation modulates the STAT signaling code.
Cytokine Growth Factor Rev. 23:293–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rössle C, Carpentier YA, Richelle M,
Dahlan W, D'Attellis NP, Fürst P and Elwyn DH: Medium-chain
triglycerides induce alterations in carnitine metabolism. Am J
Physiol. 258:E944–E947. 1990.PubMed/NCBI
|
|
22
|
Ni Z, Lou W, Leman ES and Gao AC:
Inhibition of constitutively activated Stat3 signaling pathway
suppresses growth of prostate cancer cells. Cancer Res.
60:1225–1228. 2000.PubMed/NCBI
|
|
23
|
Gao L, Zhang L, Hu J, Li F, Shao Y, Zhao
D, Kalvakolanu DV, Kopecko DJ, Zhao X and Xu DQ: Down-regulation of
signal transducer and activator of transcription 3 expression using
vector-based small interfering RNAs suppresses growth of human
prostate tumor in vivo. Clin Cancer Res. 11:6333–6341. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lacayo CI, Pincus Z, VanDuijn MM, Wilson
CA, Fletcher DA, Gertler FB, Mogilner A and Theriot JA: Emergence
of large-scale cell morphology and movement from local actin
filament growth dynamics. PLoS Biol. 5:e2332007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chhabra ES and Higgs HN: The many faces of
actin: Matching assembly factors with cellular structures. Nat Cell
Biol. 9:1110–1121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bear JE and Gertler FB: Ena/VASP: Towards
resolving a pointed controversy at the barbed end. J Cell Sci.
122:1947–1953. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
28
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: A
mini-review. Med Sci Monit. 15:RA32–RA40. 2009.PubMed/NCBI
|
|
29
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F,
Sawaya R and Huang S: Stat3 activation regulates the expression of
matrix metalloproteinase-2 and tumor invasion and metastasis.
Oncogene. 23:3550–3560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dechow TN, Pedranzini L, Leitch A, Leslie
K, Gerald WL, Linkov I and Bromberg JF: Requirement of matrix
metalloproteinase-9 for the transformation of human mammary
epithelial cells by Stat3-C. Proc Natl Acad Sci USA.
101:10602–10607. 2004. View Article : Google Scholar : PubMed/NCBI
|