Introduction
Abnormal invasion and migration of vascular smooth
muscle cells (VSMCs) leads to the thickening of the arterial
intima, a common pathological basis for the development of
cardiovascular disease (1). VSMCs
have substantial plasticity, allowing the cells to quickly adapt to
changes in the surrounding environment (2). Changes in vascular smooth muscle
phenotype play an important role in cardiovascular disease,
including atherosclerosis and restenosis after percutaneous
coronary angioplasty (3). Previous
studies have suggested that VSMCs have both systolic and synthetic
phenotypes, and that the normal arterial wall is mainly composed of
contractile VSMCs, which are present in adult blood vessels and are
accompanied by high protein expression (for example, smooth muscle
tendon-actin was found to be highly expressed). This previous
research also found that VSMCs play an important role in
maintaining vascular tone and homeostasis, and have a low potential
for proliferation and migration (4,5). By
contrast, in pathological states, the VSMCs found in the intima are
mainly synthetic, exhibiting reduced expression of
contraction-related proteins. However, VSMCs have great potential
for proliferation and migration in pathological states (6). Therefore, exploring the molecular
mechanisms of the phenotypic transformation of VSMCs may provide a
theoretical basis for the development and treatment of vascular
diseases.
Previous studies have focused on the molecular and
biological mechanisms of VSMC phenotype transformation, including
investigating microRNAs and transcription factors (7,8).
GC-binding factor 2 (GCF2) is a transcriptional repressors that
binds to gene promoters rich in GC and inhibits transcription
(9). As a transcriptional
repressor, the main function of GCF2 is to inhibit or downregulate
the transcription of target genes. The amino acid residues at
positions 429–528 of the peptide chain are important for the direct
binding of GCF2 to GC sequences in the promoter of genes, including
endothelial growth factor receptor (EGFR) (10). In a number of signal transduction
pathways, GCF2 is a protein that binds to related proteins, such as
dishevelled protein, and is involved in signal transduction, cell
proliferation, apoptosis and cell cycle regulation (11,12).
There are a number of cell cycle regulatory proteins involved in
maintaining genomic integrity and stability in normal cells.
However, aberrant cell cycle regulation leads to abnormal gene
expression levels, which in turn leads to uncontrolled
proliferation and apoptosis, ultimately leading to tumor growth
(13,14). At the same time, dysplasia of VSMCs
is regulated by multiple pathways, including the PI3K/AKT signaling
pathway (15,16).
The PI3K/AKT signal transduction pathway is an
important signaling pathway for survival in vivo (17). PI3K is a class of phosphorylated
inositol phospholipid 3 hydroxyl kinase with lipid and protein
kinase activities. AKT is an important downstream target in the
PI3K signal transduction pathway. AKT has serine/threonine kinase
activity, which is activated by the phosphorylation of AKT by PI3K,
further activating other downstream factors (18).
At present, the role of GCF2 in the proliferation of
VSMCs is not well documented. In the present study, small
interfering RNA (siRNA) technology was used to reduce the
expression of GCF2 and investigate the role of GCF2 in vascular
smooth muscle function. Therefore, this present study provides
mechanistic insight to facilitate the development of therapies for
the prevention, diagnosis and treatment of cardiovascular
disease.
Materials and methods
Isolation and culture of VSMCs from
the C57/BL6-mouse aorta
The adult male C57/BL6 mice (n=6; 8–10 weeks of age;
weight, 18–22 g) used in this study were obtained from the Shanghai
Laboratory Animal Co., Ltd. The animals were housed with food and
water available ad libitum and kept at a controlled room
temperature (22±2°C) and humidity (60–80%) under a 12/12 h
light/dark cycle. C57/BL6 mice were weighed and anesthetized by
intraperitoneal injection of 10% chloral hydrate (300 mg/kg), and
peritonitis was not observed in any of the mice. After anesthesia,
a continuous flow of CO2 was maintained using a flow
meter unit for 3–5 min at the flow rate of 2 l/min for sacrifice.
The mice were soaked in 75% ethanol for 2 min at room temperature
for disinfection and affixed to a wooden board. The mouse thoracic
cavity and abdominal cavity were opened, the heart removed and the
whole thoracic aorta and abdominal aorta was fully exposed along
the aorta. A 5 ml syringe was inserted through the left ventricle
and the aorta was flushed with PBS buffer, removed and placed in a
Petri dish filled with PBS buffer. The aortic intima and adventitia
were cut into approximately 1–2 mm2 sections with
ophthalmic scissors. Sections were uniformly arranged at a density
of 4–7 pieces/cm2 in culture plates and 5 ml DMEM
(Corning, Inc.) containing 100 U/ml penicillin, 100 µg/ml
streptomycin and 5 ml FBS (Gibco; Thermo Fisher Scientific, Inc.)
was added. The sections were cultured in an incubator at 37°C with
5% CO2, the medium was changed every 3 days. After 8
days, the tissue sections were removed with sterile forceps. The
cells were transferred to another culture dish, and allowed to grow
for 10 days, until they covered an area of 25 cm2. Then,
the cells were subcultured four times. Subsequently, the cells were
digested with trypsin and collected. For experiments, cells at
passage number 4–10 were used.
Immunofluorescence staining
Cells, at a density of 2×105 cells/well,
were seeded in a 24-well culture plate with built-in cover slips.
After 24 h, when the cells adhered naturally and reached a
confluence of 80%, the culture medium was discarded and the cover
slips were removed. The cover slips were washed three times for 3
min with PBS and then fixed for 30 min with 40 g/l paraformaldehyde
at room temperature and washed three times for 3 min with PBS. The
cells were incubated for 30 min with 3 g/l of Triton-X-100, washed
with PBS three times for 3 min and blocked with 250 µl FBS at room
temperature for 30 min. The cells were incubated overnight with
goat anti-mouse α-SM actin (1:100; cat. no. a5228; Sigma-Aldrich;
Merck KGaA) at 4°C, washed with TBS three times for 15 min,
incubated with a rabbit anti-sheep secondary antibody conjugated to
tetraethyl rhodamine isothiocyanate (1:100; cat. no. sc-215957;
Santa Cruz Biotechnology, Inc) at room temperature for 1 h and
washed with PBS for three times for 3 min. DAPI (1 µg/ml) was added
to the coverslips and incubated while being protected from light
for 10 min at room temperature to stain the nuclei. Images were
captured using a fluorescent microscope (magnification, ×400).
Transfections and IGF-1 treatment
Cells, at a density of 2×105 cells/well,
were seeded in 24-well culture plate and 500 µl of antibiotic-free
medium was added to each well. A confluency of 80% was reached
prior to transfection. For each well, a reaction containing 1 µl
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) was
diluted with 50 µl Opti-MEM™ Reduced Serum Medium (Gibco; Thermo
Fisher Scientific, Inc.) was prepared, gently mixed and incubated
for 5 min at room temperature. A second reaction, containing 2 µl
GCF2-siRNA (Shanghai GenePharma Co., Ltd.) diluted with 50 µl of
Opti-MEM™ Reduced Serum Medium was prepared, mixed gently and
incubated for 5 min at room temperature. The diluted
Lipofectamine® 2000 was gently mixed with the diluted
GCF2-siRNA and allowed to stand at room temperature for a further
20 min to form a GCF2-siRNA-transfection reagent mixture. The
GCF2-siRNA-transfection reagent mixture was added to the culture
medium containing the cells, mixed and placed in an incubator at
37°C with 5% CO2 for 48 h. All experiments were
performed according to the manufacturer's protocol (Thermo Fisher
Scientific, Inc.). The sequences of the siRNAs used in the present
study were as follows: GCF2-siRNA sense,
5′-GGAAAUCAAGGACUCUCUAGCAGAA-3′ and antisense,
5′-UUCUGCUAGAGAGUCCUUGAUUUCC-3′; negative control-siRNA (siNC)
sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. In addition, to further investigate
the mechanism of GCF2 on VSMCs, cells were treated with IGF-1 (120
ng/ml) for 48 h. Subsequently, cell viability, migration and
invasion were assessed. The cells were divided into six groups: i)
control, normal cell group; ii) siNC, cells transfected with siNC;
iii) siGCF2, cells transfected with siGCF2; iv) IGF-1, cells
treated with 120 ng/ml IGF-1; v) siNC + IGF-1, cells transfected
with siNC and treated with 120 ng/ml IGF-1; and vi) siGCF2 + IGF-1,
cells transfected with siGCF2 and treated with 120 ng/ml IGF-1.
Cell Counting Kit-8 (CCK-8)
Transfected cells, at a density of 3×103
cells/well, were seeded into 96-well plates and cultured for 24, 48
or 72 h in an incubator with 5% CO2 at 37°C. CCK-8
(Nanjing Jiancheng Bioengineering Institute) and serum-free DMEM at
a ratio of 1:10 were mixed and 100 µl was added to each well and
incubated at 37°C with 5% CO2 for 1 h. A microplate
reader (Bio-Rad Laboratories, Inc.) was used to determine the
optical density of each well at an absorbance of 450 nm. Cell
viability was determined using the CCK-8 Kit according to the
manufacturer's protocol.
Wound healing assay
After the VSMCs had been transfected for 48 h, a
straight gap was created using a 200 µl sterile tip in the middle
of the well. The cells were washed twice with DMEM to smooth the
edges of the scratch and remove floating cells. After incubation at
37°C for 0 or 48 h, the cells were observed under a florescent
microscope (Keyence Corporation; magnification, ×100) and the
distance of migration was determined. Images were captured using
Image-Pro Plus Analysis software (version 6.0; Media Cybernetics,
Inc.).
Transwell
Transwell chambers with 8.0 µm pores (Corning, Inc.)
were placed on a 24-well plate with a 50 µl layer of Matrigel (BD
Biosciences). The Matrigel was diluted 1:8, coated on the upper
chamber of the bottom membrane of the Transwell chamber and placed
in an incubator at 37°C for 30 min to polymerize the Matrigel into
a gel. A cell suspension, at a density of 1×105
cells/200 µl, was added to the upper layer of the Transwell chamber
and 600 µl of 20% FBS was added to the lower chamber. After
incubation for 48 h at 37°C, cells that had not invaded the
Transwell chamber were gently removed with a cotton swab. The
chamber was air-dried and fixed in 4% paraformaldehyde for 15 min
and stained with 0.1% crystal violet for 20 min. Cells from five
randomly selected fields were observed under a florescent
microscope (magnification, ×200) and counted.
Apoptosis
VSMCs, at a density of 2×105 cells/well,
were seeded in 6-well plates. After the cells had been treated, the
supernatant was collected in a 15 ml centrifuge tube and the
culture flask was gently washed once using 2 ml PBS. The cells were
digested with 1 ml trypsin without EDTA, gently shaken, and the
trypsin was aspirated immediately. The mixture was left at room
temperature for 1 min, after which DMEM containing 10% FBS was
added to terminate the digestion. The cells were centrifuged at
1,000 × g for 3 min at 4°C and the supernatant was removed. The
cells were washed twice with pre-cooled PBS and resuspended in 1X
Annexin V binding buffer. According to the Annexin-V-FITC apoptosis
kit (BioVision, Inc.), cells were collected and stained with
Annexin V-FITC and propidium iodide (PI) for 15 min at room
temperature. Cells were counted by flow cytometry (FACS Calibur™;
Becton, Dickinson and Company) and analyzed using FlowJo (version
10.0; FlowJo, LLC). Flow cytometry scatter diagrams showed that
living cells were in the lower left quadrant, necrotic cells were
in the left upper quadrant, advanced apoptotic cells were in the
upper right quadrant, and early apoptotic cells were in the lower
right quadrant.
Flow cytometry cell cycle
analysis
Cell cycle analysis was performed using
flow-cytometry. For fixation, 5×105 cells were incubated
with 70% ice-cold ethanol at −20°C overnight. The next day, the
fixed cells were centrifuged at 1,200 × g for 1 min at 4°C and
washed twice with PBS. The cells were resuspended in 200 µl RNaseA
(1 mg/ml) for 10 min at 37°C, 300 µl PI (BioVision, Inc.) was
added, and the cells were incubated at room temperature for 20 min
to stain the DNA. The cells were protected from light during the
staining procedure. The cells were analyzed for cellular DNA
content using Mod Fit LT software V2.0 (Becton Dickinson and
Company) with a FACScan flow cytometer (Becton, Dickinson, and
Company).
Reverse transcription-quantitative
(RT-q)PCR
RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. For reverse transcription, 1 µg RNA was to
generate cDNA according to the SuperScript III CellsDirect cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.). The reaction
conditions were as follows: 42°C for 15 min and 80°C for 15 sec.
SYBR Green PCR Master Mix (Roche Diagnostics) was used to conduct
the qPCR experiments using Opticon RT-PCR Detection System (ABI
7500; Life Technologies; Thermo Fisher Scientific, Inc.). The qPCR
conditions were as follows: 95°C for 10 min, followed by 40 cycles
of 94°C for 15 sec, 60°C for 1 min and 60°C for 1 min. The
expression levels of the genes were analyzed using the
2−ΔΔCq method (19).
GAPDH expression was used for normalization. The primer sequences
of GCF2, Bcl-2, Bax, Cleaved caspase-3, cyclin E, CDK2, P21 and
GAPDH are listed in Table I.
 | Table I.Primers for reverse
transcription-quantitative PCR. |
Table I.
Primers for reverse
transcription-quantitative PCR.
| Genes | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| GCF2 |
TGAAAGGGAAAAACACGCC |
TCATTTTTCACCTCCACTTCAC |
| Bcl-2 |
CGACTTTGCAGAGATGTGCA |
ATGCCGGTTCAGGTACTCAG |
| Cleaved
caspase-3 |
AGCAGCTTTGTGTGTGTGATTAA |
AGTTTCGGCTTTCCAGTCAGAC |
| Cyclin E |
GTTACAGATGGCGCTTGCTC |
AGCCAGGACACAATGGTCAGCAGT |
| CDK2 |
TTGGAGTCCCTGTCCGAACT |
CGGGTCACCATTTCAGCAAAG |
| p21 |
CAAAGTGTGCCGTTGTCTCTT |
TCAAAGTTCCACCGTTCTCG |
| GAPDH |
CATCACCATCTTCCAGGAGGG |
TGACCTTGCCCACAGCCTTG |
Western blot analysis
Total proteins were extracted using RIPA lysis
buffer (Cell Signaling Technology, Inc.). A bicinchoninic acid
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.) was used
to determine protein concentration. Concentration were adjusted to
6 µg/µl using 1X loading buffer and diethyl pyrocarbonate-treated
water. Samples (30 µg/lane) were separated using 10% SDS-PAGE gels,
which were then transferred onto a PVDF membrane. After blocking in
5% non-fat milk in PBS-0.1% Tween-20 for 1 h at room temperature,
the membrane was probed with primary antibodies overnight at 4°C.
Membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies, including goat
anti-mouse (1:2,000; cat. no. sc-516102; Santa Cruz Biotechnology,
Inc.) and goat anti-rabbit (1:2,000; cat. no. sc-2357; Santa Cruz
Biotechnology, Inc.) at room temperature for 2 h. The EZ-ECL kit
(Biological Industries) was used to visualize the protein bands and
densitometric analysis was carried out using ImageJ (version 5.0;
National Institutes of Health). The primary antibodies utilized
included anti-GAPDH (mouse; 1:1,000; cat. no. LS-B1625; LifeSpan
BioSciences, Inc.), anti-cleaved caspase-3 (rabbit; 1:1,000; cat.
no. ab13847; Abcam), anti-Bax (rabbit; 1:1,000; cat. no. ab32503;
Abcam), anti-Bcl-2 (rabbit; 1:1,000; cat. no. ab32124; Abcam),
anti-CDK2 (rabbit; 1:1,000; cat. no. ab32147; Abcam), anti-P21
(rabbit; 1:1,000; cat. no. ab109520; Abcam), anti-AKT (rabbit;
1:1,000; cat. no. ab8805; Abcam), anti-p-AKT (rabbit; 1:1,000; cat.
no. ab38449; Abcam), anti-p-PI3K (rabbit; 1:1,000; cat. no.
ab182651; Abcam) and anti-PI3K (rabbit; 1:1,000; cat. no. 4257;
Cell Signaling Technology, Inc.).
Statistical analysis
All data are expressed as the mean ± SEM. The
differences between the experimental groups were compared by
Student's t-test and one-way ANOVA followed by Dunnett's post hoc
test. Data analysis was performed using the statistical software
Prism 6 (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference. All experiments
were independently repeated three times.
Results
Identification of VSMCs by
immunofluorescence staining and analysis of cell viability of
following treatment with siGCF2
To explore the effect of GCF2 on migration and
invasion, primary VSMCs were isolated from the mouse aorta and
siGCF2 was used to reduce the expression of GCF2. The cells were
stained for α-SM actin and immunofluorescence analysis was used to
determine whether cell function was restored. Immunofluorescence
analysis showed α-SM actin staining in the cytoplasm, and that the
morphology and the nuclear outline were clear. α-SM actin had a
positive expression, indicating that the cells were VSMCs (Fig. 1A). GCF2 expression was successfully
reduced by transfection with siGCF2 at the mRNA level (Fig. 1B). Cell viability was inhibited by
transfection with siGCF2 at 48 and 72 h (Fig. 1C).
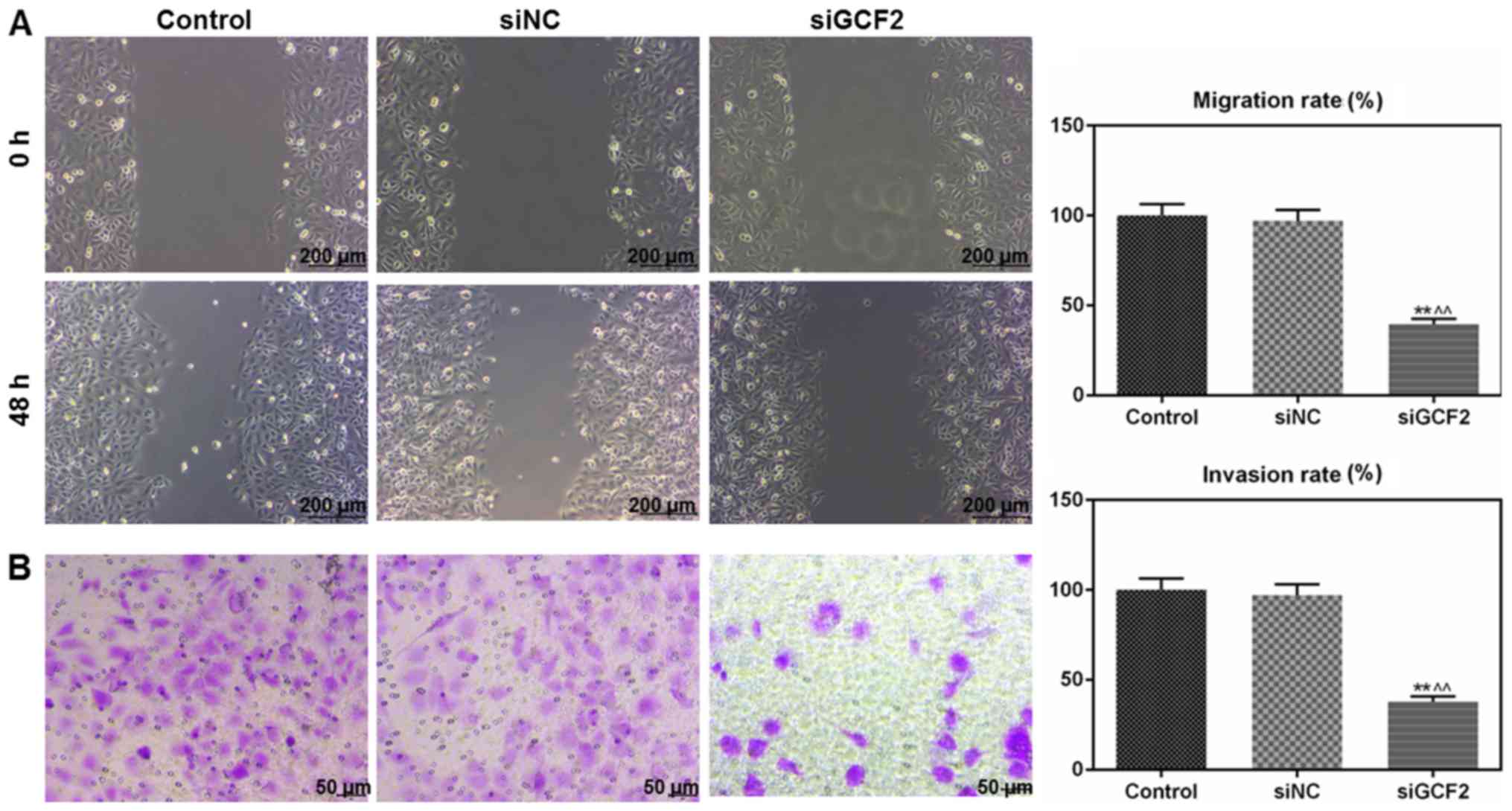
Migration and invasion are inhibited
by siGCF2
Wound healing and Transwell assays were used to
determine the migration and invasion ability of cells to
investigate the function of GCF2 in VSMCs. Migration (Fig. 2A) and invasion (Fig. 2B) were inhibited by siGCF2 at 48 h
post transfection.
Apoptosis is increased following
transfection with siGCF2
Apoptosis is a physiological process that is
important for cell survival. In this present study, apoptosis was
analyzed by flow cytometry. The level of apoptosis was found to be
higher in cells transfected with siGCF2 compared to control cells
and cells transfected with a non-targeting siRNA control (siNC)
(Fig. 3A). Additionally, the mRNA
and protein expression levels of apoptosis-related factors were
determined by RT-qPCR and western blot analysis. The expression of
Bcl-2 was downregulated, while the expression of Bax and cleaved
caspase-3 was upregulated in cells transfected with siGCF2 at the
mRNA (Fig. 3B) and/or protein
level (Fig. 3C and D).
Cell cycle arrest is induced by
siGCF2
Cell cycle analysis was conducted using flow
cytometry. The results showed that cell cycle arrest in the
G0/G1 phase of the cell cycle was induced by
siGCF2 (Fig. 4A). Additionally,
the mRNA and protein expression levels of cell cycle regulators
were determined by RT-qPCR and western blot analysis. The levels of
cyclin E and CDK2 were higher in control cells and those
transfected with siNC than in cells transfected with siGCF2. By
contrast, the expression of p21 was lower in control cells and
those transfected with siNC than in cells transfected with in
siGCF2 at the mRNA (Fig. 4B) and
protein level (Fig. 4C and D).
Phosphorylation of PI3K and AKT is
downregulated by siGCF2
As the PI3K/AKT signaling pathway is important in
the development of VSMCs, western blot analysis was used to
determine the protein levels of phosphorylated PI3K and AKT. The
levels of phosphorylated PI3K and AKT were reduced by siGCF2
(Fig. 5).
Inhibitory effects of siGCF2 on cell
viability, migration and invasion were reversed by insulin-like
growth factor 1 (IGF-1)
In order to investigate the role of the PI3K/AKT
signaling pathway in VSMCs, cells were exposed to IGF-1 (120
ng/ml), which is a specific agonist of AKT, in combination with
siRNA. The levels of cell viability, migration and invasion were
analyzed by CCK-8, wound healing and Transwell assays,
respectively. The results indicated that cell viability (Fig. 6A), migration (Fig. 6B) and invasion (Fig. 6C) were reduced in cells exposed to
IGF-1 in combination with siGCF2. Representative images of
migration and invasion are shown in Fig. 6D and E, respectively.
Discussion
GCF2, also known as leucine-rich repeat
flightless-interacting protein, is an inhibitory transcription
factor that can bind GC in the gene promoter sequences of
platelet-derived growth factor A, EGFR and tumor necrosis factor α
to inhibit transcription (9,20,21).
In this present study, it was found that invasion and migration
were inhibited in cells transfected with siGCF2, while apoptosis
and cell cycle arrest in the G0/G1 phase were
induced by siGCF2 in VSMCs. These effects may be associated with
the PI3K/AKT signaling pathway.
In order to study the molecular basis of the
abnormal proliferation of VSMCs, VSMCs were stripped from the
aortas of mice. To confirm whether VSMCs from mice could be used as
model, cells were stained for α-SM actin, which has an important
role in maintaining vascular tone and homeostasis. The results
showed that α-SM actin was positively expressed, suggesting that
VSMCs were successfully obtained and could be used in subsequent
experiments.
RNA interference technology is an effective means to
study gene function, and can be used to efficiently and
specifically reduce the expression of target genes in vitro
(22). siGCF2 sequences targeting
GCF2 were designed and transfected into VSMCs. The results showed
that siGCF2 effectively downregulated GCF2 mRNA expression in
VSMCs, therefore facilitating the investigation of the effect of
GCF2 on the migration and invasion of VSMCs.
It is thought that the phenotypic transition of
VSMCs is an important pathophysiological basis for neointimal
hyperplasia after vascular injury, and that this can ultimately
lead to stenosis of the lumen (23). In a pathological case, a synthetic
phenotype characterized by proliferation and migration is exhibited
(24). A previous study
demonstrated that smooth muscle cell proliferation is inhibited by
GCF2 (20). In the present study,
it was found that cell viability, migration and invasion are
inhibited by siGCF2. Therefore, GCF2 may enhance the proliferation
of VSMCs.
Proliferation and apoptosis are often linked
(25); apoptosis plays an
important role in maintaining the balance between the production of
new cells and cell death in tissues (26). In the present study, the analysis
of apoptosis showed that the level of apoptosis and the levels of
cleaved caspase 3 and Bax were significantly increased. By
contrast, the expression level of the anti-apoptotic protein Bcl-2
was downregulated after GCF2 knockdown. These findings indicated
that GCF2 knockdown may inhibit the proliferation of VSMCs by
regulating the expression of apoptosis-associated proteins. In the
present study, the mechanism by which siGCF2 promotes apoptosis was
not explored further. The expression levels of forkhead box protein
O3, which is a key regulator of the PI3K/Akt signaling pathway, and
the pro-apoptotic protein Bcl-2-like protein 11, were not
investigated in this present study. These factors will be assessed
in future studies.
Abnormal cell proliferation reflects misregulation
of the cell cycle (27). The
results of the present study showed that downregulation of GCF2
expression caused VSMC proliferation to be blocked, suggesting
misregulation of the cell cycle. The proportion of VSMCs in the
G0/G1 phase of the cell cycle was
significantly higher in siGCF2 group than in the control group,
indicating that downregulation of GCF2 expression caused cell cycle
arrest in the G1 phase. The cell cycle is regulated by
cyclins and CDKs; together they form cyclin-CDK complexes that
regulate cell cycle transitions and DNA synthesis. Cyclin E-CDK2 is
important during the G1 to S phase transition (28,29).
p21 is a CDK inhibitor with broad kinase inhibitory activity. p21
has an inhibitory effect on proliferation (30). In the present study, it was found
that the downregulation of GCF2 expression led to a significant
reduction in the expression of cyclin E and CDK2, while p21
expression was significantly increased. Based on the findings of
the present study, it is speculated that the inhibitory effect of
GCF2 downregulation on the proliferation of VSMCs could be a result
of changes to the regulation of the cell cycle.
The PI3K/AKT signaling pathway is important in VSMC
proliferation and migration (15,31).
Previous studies have reported that PI3K/AKT activation is
important in promoting the migration of cultured VSMCs; however,
this effect is increased by AKT depletion (32,33).
Another study also found that PI3K/AKT inhibition blocked
serum-stimulated VSMC lamellipodia formation (15). In the present study, the
phosphorylation levels of PI3K and AKT were found to be reduced by
siGCF2. IGF-1 reversed the inhibitory effect of siGCF2 on
viability, migration and invasion in VSMCs. These results suggested
that the inhibition of VSMC proliferation observed following the
transfection of cells with siGCF2 may be mediated by the PI3K/AKT
signaling pathway.
The effect of GCF2 downregulation on VSMC
proliferation, migration and invasion is only supported by in
vitro experiments in the present study. Therefore, such an
effect should be further investigated by conducting in vivo
experiments. Additionally, the underlying mechanism of the
regulation of VSMCs by siGCF2 is still unclear and requires further
investigation.
In conclusion, a novel role for GCF2 in VSMC
proliferation in vitro has been identified. The inhibition
of VSMC proliferation and migration by siGCF2 was mediated by the
PI3K/AKT signaling pathway. The findings of the present study
suggest that GCF2 may be used as a novel therapeutic agent for
vascular restenosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data sets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YM and YR contributed to the concept and design of
the present study. JG was involved in data acquisition, analysis
and interpretation. YM and YR drafted and critically revised the
article for important intellectual content. All authors gave their
approval for publication of the article. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All procedures for animal care were approved by the
Animal Management Committee of Qingdao University. All animal
experiments were performed in compliance with the Guidelines for
Proper Conduct of Animal Experiments, established by the Science
Council.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang D, Uhrin P, Mocan A, Waltenberger B,
Breuss JM, Tewari D, Mihaly-Bison J, Huminiecki Ł, Starzyński RR,
Tzvetkov NT, et al: Vascular smooth muscle cell proliferation as a
therapeutic target. Part 1: Molecular targets and pathways.
Biotechnol Adv. 36:1586–1607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alexander MR and Owens GK: Epigenetic
control of smooth muscle cell differentiation and phenotypic
switching in vascular development and disease. Annu Rev Physiol.
74:13–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Francis DJ, Parish CR, McGarry M, Santiago
FS, Lowe HC, Brown KJ, Bingley JA, Hayward IP, Cowden WB, Campbell
JH, et al: Blockade of vascular smooth muscle cell proliferation
and intimal thickening after balloon injury by the sulfated
oligosaccharide PI-88: Phosphomannopentaose sulfate directly binds
FGF-2, blocks cellular signaling, and inhibits proliferation. Circ
Res. 92:e70–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabbiani G, Schmid E, Winter S, Chaponnier
C, de Ckhastonay C, Vandekerckhove J, Weber K and Franke WW:
Vascular smooth muscle cells differ from other smooth muscle cells:
Predominance of vimentin filaments and a specific alpha-type actin.
Proc Natl Acad Sci USA. 78:298–302. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayashi K, Takahashi M, Nishida W, Yoshida
K, Ohkawa Y, Kitabatake A, Aoki J, Arai H and Sobue K: Phenotypic
modulation of vascular smooth muscle cells induced by unsaturated
lysophosphatidic acids. Circ Res. 89:251–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Finney AC and Orr AW: Guidance molecules
in vascular smooth muscle. Front Physiol. 9:13112018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Low EL, Baker AH and Bradshaw AC: TGFβ,
smooth muscle cells and coronary artery disease: A review. Cell
Signal. 53:90–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rikiyama T, Curtis J, Oikawa M, Zimonjic
DB, Popescu N, Murphy BA, Wilson MA and Johnson AC: GCF2:
Expression and molecular analysis of repression. Biochim Biophys
Acta. 1629:15–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohtsuka H, Oikawa M, Ariake K, Rikiyama T,
Motoi F, Katayose Y, Unno M and Johnson AC: GC-binding factor 2
interacts with dishevelled and regulates Wnt signaling pathways in
human carcinoma cell lines. Int J Cancer. 129:1599–1610. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reed AL, Yamazaki H, Kaufman JD,
Rubinstein Y, Murphy B and Johnson AC: Molecular cloning and
characterization of a transcription regulator with homology to
GC-binding factor. J Biol Chem. 273:21594–21602. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li JP, Cao NX, Jiang RT, He SJ, Huang TM,
Wu B, Chen DF, Ma P, Chen L, Zhou SF, et al: Knockdown of
GCF2/LRRFIP1 by RNAi causes cell growth inhibition and increased
apoptosis in human hepatoma HepG2 cells. Asian Pac J Cancer Prev.
15:2753–2758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chilakamarthi U, Koteshwar D, Jinka S,
Vamsi Krishna N, Sridharan K, Nagesh N and Giribabu L: Novel
amphiphillic G-quadruplex binding synthetic derivative of TMPyP4
and its effect on cancer cell proliferation and apoptosis
induction. Biochemistry. 57:6514–6527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abbastabar M, Kheyrollah M, Azizian K,
Bagherlou N, Tehrani SS, Maniati M and Karimian A: Multiple
functions of p27 in cell cycle, apoptosis, epigenetic modification
and transcriptional regulation for the control of cell growth: A
double-edged sword protein. DNA Repair (Amst). 69:63–72. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang H, Yang S, Luo Y, Zhang C, Rao Y, Liu
R, Feng Y and Yu J: Notoginsenoside R1 inhibits vascular smooth
muscle cell proliferation, migration and neointimal hyperplasia
through PI3K/Akt signaling. Sci Rep. 8:75952018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li XG and Wang YB: SRPK1 gene silencing
promotes vascular smooth muscle cell proliferation and vascular
remodeling via inhibition of the PI3K/Akt signaling pathway in a
rat model of intracranial aneurysms. CNS Neurosci Ther. 25:233–244.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Very N, Vercoutter-Edouart AS, Lefebvre T,
Hardiville S and El Yazidi-Belkoura I: Cross-Dysregulation of
O-GlcNAcylation and PI3K/AKT/mTOR Axis in human chronic diseases.
Front Endocrinol (Lausanne). 9:6022018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mi XJ, Hou JG, Wang Z, Han Y, Ren S, Hu
JN, Chen C and Li W: The protective effects of maltol on
cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/Akt
and p53 signaling pathways. Sci Rep. 8:159222018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khachigian LM, Santiago FS, Rafty LA, Chan
OL, Delbridge GJ, Bobik A, Collins T and Johnson AC: GC factor 2
represses platelet-derived growth factor A-chain gene transcription
and is itself induced by arterial injury. Circ Res. 84:1258–1267.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suriano AR, Sanford AN, Kim N, Oh M,
Kennedy S, Henderson MJ, Dietzmann K and Sullivan KE: GCF2/LRRFIP1
represses tumor necrosis factor alpha expression. Mol Cell Biol.
25:9073–9081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song B, Liu X, Wang Q, Zhang R, Yang T,
Han Z and Xu Y: Adenovirus-mediated shRNA interference against
HSV-1 replication in vitro. J Neurovirol. 22:799–807. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iyemere VP, Proudfoot D, Weissberg PL and
Shanahan CM: Vascular smooth muscle cell phenotypic plasticity and
the regulation of vascular calcification. J Intern Med.
260:192–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang MJ, Zhou Y, Chen L, Wang YQ, Wang X,
Pi Y, Gao CY, Li JC and Zhang LL: An overview of potential
molecular mechanisms involved in VSMC phenotypic modulation.
Histochem Cell Biol. 145:119–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang YQ, Chen C, Chen Z, Xu Y, Wang Y,
Xiao BK, Chen SM and Tao ZZ: Indole-3-carbinol inhibits cell
proliferation and induces apoptosis in Hep-2 laryngeal cancer
cells. Oncol Rep. 30:227–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bennetts PS and Pierce JD: Apoptosis:
Understanding programmed cell death for the CRNA. AANA J.
78:237–245. 2010.PubMed/NCBI
|
|
27
|
Shibley IA Jr, Gavigan MD and Pennington
SN: Ethanol's effect on tissue polyamines and ornithine
decarboxylase activity: A concise review. Alcohol Clin Exp Res.
19:209–215. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin SL, Chang DC, Ying SY, Leu D and Wu
DT: MicroRNA miR-302 inhibits the tumorigenecity of human
pluripotent stem cells by coordinate suppression of the CDK2 and
CDK4/6 cell cycle pathways. Cancer Res. 70:9473–9482. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Narasimha AM, Kaulich M, Shapiro GS, Choi
YJ, Sicinski P and Dowdy SF: Cyclin D activates the Rb tumor
suppressor by mono-phosphorylation. Elife. 32014.
|
|
30
|
Karimian A, Ahmadi Y and Yousefi B:
Multiple functions of p21 in cell cycle, apoptosis and
transcriptional regulation after DNA damage. DNA Repair (Amst).
42:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye G, Fu Q, Jiang L and Li Z: Vascular
smooth muscle cells activate PI3K/Akt pathway to attenuate
myocardial ischemia/reperfusion-induced apoptosis and autophagy by
secreting bFGF. Biomed Pharmacother. 107:1779–1785. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Chen L, Zhao Z, Wu Y, Zhong J,
Wen G, Cao R, Zu X and Liu J: HMGA1 mediated high-glucose-induced
vascular smooth muscle cell proliferation in diabetes mellitus:
Association between PI3K/Akt signaling and HMGA1 expression. DNA
Cell Biol. 37:389–397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Q, Cao Y, Luo Q, Wang P, Shi P, Song
C, E M, Ren J, Fu B and Sun H: The transient receptor potential
vanilloid-3 regulates hypoxia-mediated pulmonary artery smooth
muscle cells proliferation via PI3K/AKT signaling pathway. Cell
Prolif. 51:e124362018. View Article : Google Scholar : PubMed/NCBI
|