Introduction
Osteosarcoma (OS) is a primary bone malignancy that
mainly occurs in young adults and adolescents (1). In spite of recent developments in
combinational chemotherapy and surgical techniques, the long-term
survival of OS patients remains unsatisfactory, with metastatic and
recurrent disease observed (2).
Thus, there is an urgent need for exploring the pathogenesis of OS,
and identifying novel and efficient biomarkers for predicting the
prognosis of OS patients.
MicroRNAs (miRNAs) are a type of highly conserved
noncoding RNAs with a length of 18–25 nucleotides, which regulate
gene expression by binding to the 3′-untranslated regions (UTRs) of
their target mRNA in order to repress transcription or induce mRNA
degradation (3,4). Currently, growing evidence suggests
that the involvement of miRNAs in multiple cellular processes,
including cell apoptosis, proliferation and metastasis (5,6).
Dysregulated miRNAs may contribute to the development of numerous
types of human cancer, including OS. For instance, an evident
increase in miR-378 was reported in OS tissues and cells, while
miR-378 overexpression promoted the proliferation of OS cells by
targeting Krüppel-like factor 9 (7). Liu et al (8) reported decreased expression of
miR-935 in OS tissues, and restoration of this expression evidently
restricted cell proliferation and invasion. In addition, the study
by Yang et al (9)
demonstrated that miR-183 suppressed the LDL receptor-related
protein 6/Wnt/β-catenin signaling and, thereby, inhibited MG63 cell
growth, migration and invasion in vivo and in vitro.
Furthermore, Dong et al (10) reported that miR-223 was markedly
decreased in the serum of OS patients, suggesting that miR-223 may
serve as a potential diagnostic and prognostic biomarker of OS.
The aberrant expression of miR-330-5p has been
reported in certain types of cancer, including glioblastoma
(11) and pancreatic cancer
(12). A study by Wang et
al (13) revealed that high
miR-330-5p expression was correlated with worse prognosis in
patients with breast cancer. It was also reported that miR-330-5p
was significantly decreased in cutaneous malignant melanoma (CMM)
tissues, and forced expression of miR-330-5p suppressed CMM cell
proliferation and invasion (14).
In addition, Wei et al (15) demonstrated that miR-330-5p
functioned as an oncogene in non-small cell lung cancer (NSCLC)
through activating the mitogen-activated protein
kinase/extracellular signal-regulated kinase (ERK) signaling
pathway. Nevertheless, the biological function and clinical value
of miR-330-5p in OS remain to be investigated.
In the present study, the expression levels of
miR-330-5p in OS tissues and cell lines were investigated, and the
correlation between miR-330-5p expression and the
clinicopathological characteristics of patients was then analyzed.
The study also investigated the effects of miR-330-5p expression on
the proliferation, invasion, apoptosis and cell cycle distribution
of OS cells. In addition, the regulatory mechanisms of miR-330-5p
on OS cells, as well as the potential relationship between
miR-330-5p and proto-oncogene survivin (also known as baculoviral
IAP repeat-containing protein 5) were investigated. The study
findings provide novel insights into the role of miR-330-5p in the
development of OS.
Materials and methods
Patients and samples
A total of 63 surgically resected OS tissue
specimens were acquired from patients with OS at the Department of
Traumatic Orthopaedics at Anhui Provincial Hospital, Anhui Medical
University (Hefei, China) between January 2012 and December 2016.
The patients were assigned into two groups according to the
presence or absence of metastasis, as determined by radiology. The
clinicopathological data of the patients are shown in Table I. The clinical stage of the
patients was classified according to the Tumor Node Metastasis
(TNM) Classification of Malignant Tumors (Sixth edition) from the
Union for International Cancer Control (10). In addition, 20 osteochondroma (a
benign bone lesion) tumor tissue samples from amputees were
selected and served as the Control group; the Control group
included 9 males and 11 females, whose ages ranged between 10 and
57 with a mean age of 25.93±10.57. The present study was approved
by the Institutional Ethics Review Board of Anhui Provincial
Hospital, Anhui Medical University, and informed consent was
obtained from adult participant or from the legal guardians of
participants <18 years old prior to participation in this study.
The samples were immediately snap-frozen in liquid nitrogen and
stored at −80°C until further use.
 | Table I.Association between miR-330-5p
expression and clinicopathological features of osteosarcoma
patients. |
Table I.
Association between miR-330-5p
expression and clinicopathological features of osteosarcoma
patients.
|
|
| miR-330-5p
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | No. of cases | High (n=25) | Low (n=38) | P-value |
|---|
| Sex |
|
|
| 0.961 |
| Male | 33 | 13 | 20 |
|
|
Female | 30 | 12 | 18 |
|
| Age (years) |
|
|
| 0.493 |
| ≥18 | 41 | 15 | 26 |
|
|
<18 | 22 | 10 | 12 |
|
| Tumor location |
|
|
| 0.065 |
|
Tibia/femur | 39 | 12 | 27 |
|
|
Other | 24 | 13 | 11 |
|
| Clinical stage |
|
|
| 0.028a |
|
IIA | 45 | 14 | 31 |
|
|
IIB/III | 18 | 11 | 7 |
|
| Tumor size
(cm) |
|
|
| 0.274 |
| ≥8 | 28 | 9 | 19 |
|
|
<8 | 35 | 16 | 19 |
|
| Distant
metastasis |
|
|
| 0.011a |
|
Present | 19 | 3 | 16 |
|
|
Absent | 44 | 22 | 22 |
|
Cell culture
The OS-derived human cell lines HOS, U2OS and MG63,
and the conditionally immortalized human fetal osteoblastic cell
line hFOB1.19 were purchased from the Shanghai Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). The 293T cell line
was obtained from the American Type Culture Collection (Manassas,
VA, USA). HOS, U2OS, MG63 and 293T cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The
hFOB1.19 cells were maintained in a 1:1 mixture of Ham's F12 medium
and DMEM supplemented with 2.5 ml glutamine (without phenol red)
and 10% FBS. All cells were incubated in a humidified atmosphere
containing 5% CO2 at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues or cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
miRNA was extracted using the mirVana RNA isolation kit (Ambion;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. RNA concentration was measured using NanoDrop ND-1000
(Thermo Fisher Scientific, Inc., Wilmington, DE, USA). RT reactions
for miRNA detection were performed using a miRNA Reverse
Transcription kit (Qiagen, Inc., Valencia, CA, USA), whereas RT
reactions for mRNA detection were conducted using the Takara
PrimeScript™ First Strand cDNA Synthesis (Takara
Biotechnology Co., Ltd., Dalian, China). Subsequently, an ABI 7500
system (Thermo Fisher Scientific, Inc.) was used to conduct qPCR
using a standard protocol described in the SYBR Green PCR kit
(Toyobo Life Science, Osaka, Japan). Briefly, the amplification
protocol was set as follows: Initial denaturation at 95°C for 5
min; followed by 30 cycles of denaturation at 94°C for 15 sec,
annealing at 55°C for 30 sec and extension at 70°C for 30 sec. U6
and GAPDH were used as the internal references and to normalize
expression data for miR-330-5p and survivin levels, respectively.
The sequences of the primers were as follows: miR-330-5p, forward
5′-TCTCTGGGCCTGTGTCTTAGGC-3′, reverse
5′-GCTATCTCAGGGCTTGTTGCTTCAGTCCTCCTGGG-3′; U6, forward
5′-TGCGGGTGCTCGCTTCGCAGC-3′, reverse 5′-CCAGTGCAGGGTCCGAGGT-3′;
survivin, forward 5′-GCACTTTCTTCGCAGTTTC-3′, reverse
5′-GTGAGGTGTGCTGTTCGAGA-3′; GAPDH, forward
5′-AGGTCGGTGTGAACGGATTTG-3′, reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
The relative expression levels were calculated using the
2−ΔΔCq method (16).
Cell transfection
miR-330-5p mimics (5′-TCTCTGGGCCTGTGTCTTAGGC-3′),
negative control mimics (mimics NC; 5′-GCCTAAGACACAGGCCCAGAGA-3′),
miR-330-5p inhibitor and inhibitor NC were purchased from
GenePharma Co., Ltd. (Shanghai, China). In addition, the coding
domain sequences of survivin mRNA were amplified by PCR and
inserted into a pcDNA3.0 overexpression vector (pcDNA-survivin;
Invitrogen; Thermo Fisher Scientific, Inc.). Cells were cultured to
80% confluence and subsequently transfected with miR-330-5p mimics
(50 nM), control mimics (50 nM), miR-330-5p inhibitor (50 nM) or
control inhibitor (50 nM) using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
a previous study (11).
pcDNA-survivin (2 µg) and the control empty plasmid pcDNA3.0 (2 µg)
was transfected into OS cells with Lipofectamine 2000, following
manufacturer's protocol. Transfected cells were incubated in a 37°C
incubator with 5% CO2 for 24, 48 or 72 h. For cell
viability assay, cell apoptosis assay and cell cycle distribution
assay, cells were harvested for further experiments after 48 h of
transfection at 37°C. Cell invasion assay and wound-healing assay
were performed after 24 h of transfection at 37°C, and images of
the stained cells were captured at the next 24 h.
Cell viability assay
For the cell viability assay, U2OS and MG63 cells
were seeded in a 96-well plate at the density of 5×103
cells per well and transfected with the miR-330-5p mimics,
miR-330-5p inhibitor or pcDNA-survivin. After 24, 48 or 72 h of
incubation, the relative viability of the cells was determined
using a Cell Counting Kit-8 (CCK-8) assay (Beyotime Institute of
Biotechnology, Jiangsu, China). Briefly, 10 µl CCK-8 solution was
added to each well and incubated at 37°C in a 5% CO2
cell incubator for 90 min. Next, the absorbance rates were measured
at 450 nm with a SpectraMax M5 reader (Molecular Devices, LLC,
Shanghai, China). All experiments were performed in triplicate.
Cell apoptosis assay
After 48 h of transfection, an Annexin V-fluorescein
isothiocyanate (FITC) Apoptosis Detection kit (Abcam, Cambridge,
UK) was used to detect cell apoptosis, according to the
manufacturer's protocol. Following harvesting and washing twice
with PBS, the cells were stained with Annexin V-FITC and propidium
iodide (PI), and incubated for 15 min at room temperature in the
dark. Cell apoptosis was subsequently detected using a FACScan flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Cell cycle distribution assay
After 48 h of transfection, U2OS and MG63 cells were
harvested, washed twice with PBS and fixed with 70% ethanol at 4°C
overnight, washed with PBS twice and incubated with 100 µl RNaseA
at 37°C in dark for 25 min. The cells were washed with PBS and
stained with PI staining solution (50 µg/ml; containing 1 mg/ml
RNase A and 0.1% Triton X-100 in PBS). Finally, the cell cycle
distribution was assessed with a BD FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA). Experiments were performed in
triplicate.
Cell invasion Transwell assays
A cell invasion assay was conducted in a 24-well
plate with Transwell chamber inserts (pore size, 8 mm; Corning
Incorporated, Corning, NY, USA). Transfected cells
(1×105 cells) were placed into the upper chamber with
Matrigel (BD Biosciences), respectively. The lower chamber was
loaded with medium supplemented with 10% FBS. Following incubation
for 48 h, the cells on the upper surface of the membrane were
removed, while the invading cells on the bottom surface of the
Transwell chambers were fixed with methanol for 20 min at room
temperature and stained with 0.1% crystal violet for 30 min. Images
of the stained cells were captured, and the number of cells was
counted under an inverted microscope (Olympus Corporation, Tokyo,
Japan). The results were averaged among three independent
experiments.
Wound-healing assay
U2OS and MG63 cells (2×105 per well) were
plated onto 6-well plates and cultured until confluence of ~90% was
reached. After 24 h of transfection, the plates were scrapped with
a 10 µl pipet tip, and the medium was replaced with fresh
serum-free medium. Initial images were acquired as a reference, and
further images of the previously photographed region were collected
after 48 h. The percentage of wound-healing rate=[(width at 0
h-width at 48 h)/width at 0 h] ×100.
Bioinformatics analysis and luciferase
reporter assay
The miRNA target prediction websites, including
PicTar (https://pictar.mdc-berlin.de),
TargetScan (http://targetscan.org) and miRDB
(http://www.mirdb.org) were used to search for the
putative targets of miR-330-5p. Luciferase complexes were
constructed by ligating oligonucleotides containing the wild-type
(wt) or mutated (mut) putative target site of the survivin
3′-untranslated region (3′UTR) into the multi-cloning site of the
pGL3 luciferase reporter vector (Promega Corporation, Madison, WI,
USA). Subsequently, 293T cells (1×105 cells/well) were
plated in a 24-well plate and co-transfected with 80 ng of
pGL3-survivin-3′-UTR (wt) or pGL3-survivin-3′-UTR (mut) and 50
nmol/l of the mimics or inhibitors (including miR-330-5p mimics,
mimics NC, miR-330-5p inhibitor and inhibitor NC) using
Lipofectamine® 2000, according to the manufacturer's
protocol. Cells were transfected for 48 h at 37°C, then luciferase
activities were measured using the Dual-Luciferase Reporter Assay
System (Promega Corporation). To correct for differences in
transfection and harvesting efficiencies, Renilla luciferase
activity was used to normalize the firefly luciferase activity.
Western blot analysis
Total protein was extracted from the cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China), and the total cellular protein
concentration was measured using a BCA assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Next, total protein samples (40 µg) were
separated by 8% SDS-PAGE and subsequently transferred to
polyvinylidene difluoride membranes (GE Healthcare, Chicago, IL,
USA) by electroblotting. Primary antibodies against survivin (cat
no. ab76424; 1:5,000; Abcam, Cambridge, MA, USA) and β-actin (cat
no. sc-58673; 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) were then probed with the proteins on the membrane overnight
at 4°C. Following further incubation with secondary antibodies (cat
no. 7076; 1:10,000; Cell Signaling Technology, Inc., Danvers, MA,
USA) at 37°C for 1 h. Protein bands were visualized with an
Enhanced Chemiluminescence kit (GE Healthcare). Finally, ImageJ
software (version 1.46; National Institutes of Health, Bethesda,
MD, USA) was used to assess the intensity of the bands of interest;
the endogenous control β-actin was used to normalize the expression
of the selected genes.
Statistical analysis
Statistical analysis was performed using the SPSS
program (version 18.0; SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Student's t-test or
one-way analysis of variance followed by Tukey's post hoc test was
used to analyze the difference among two or more than two sample
groups, respectively. Spearman's analysis was used to calculate the
correlation between the expression of miR-330-5p and survivin. The
survival rate of patients was determined using the Kaplan-Meier
method, and differences between groups were examined using the
log-rank test. P<0.05 was considered to denote a difference that
was statistically significant.
Results
Expression of miR-330-5p and its
diagnostic value in OS tissues and cells
To investigate the potential role of miR-330-5p in
OS, the expression of miR-330-5p was first determined in 63 OS
tumor and 20 benign osteochondroma (control) tissues by RT-qPCR
assay. As shown Fig. 1A, compared
with the control group, miR-330-5p was significantly downregulated
in OS tissues. Furthermore, the expression of miR-330-5p was
markedly lower in tumors with distant metastasis as compared with
that in tumors without distant metastasis (Fig. 1B), indicating that miR-330-5p
downregulation is associated with OS metastasis. To further
determine the clinical value of miR-330-5p, the patients were
divided into the high miR-330-5p expression group (n=25) and low
expression group (n=38), using the median value of miR-330-5p
expression in patients OS as a cutoff point (0.997). The results
demonstrated that low miR-330-5p expression was associated with the
clinical stage and distant metastasis, but not with age, gender,
tumor location and tumor size in the OS patients (Table I). Compared with the patients in
the high miR-330-5p expression group, patients in the low
miR-330-5p expression group had a significantly lower 5-year
overall survival rate (Fig. 1C).
Taken together, these findings indicated that miR-330-5p may serve
as an effective biomarker for the prognosis of patients with
OS.
miR-330-5p expression levels were also measured in
the HOS, U2OS and MG63 OS cell lines. In accordance with the
results in OS tissues, the expression levels of miR-330-5p were
significantly downregulated in the HOS, U2OS and MG63 cells, as
compared with those in normal hFOB1.19 cells (Fig. 1D). U2OS and MG63 cells were
selected for use in further experiments since they exhibited the
lowest expression of miR-330-5p.
Overexpression of miR-330-5p inhibits
OS cell viability, promotes cell apoptosis and induces cell cycle
arrest
The downregulation of miR-330-5p in OS tissues
suggested that miR-330-5p may function as a tumor suppressor in OS
progression. To test this hypothesis, miR-330-5p mimics or
inhibitor were transfected into U2OS and MG63 cells. Following
miR-330-5p transfection, the expression level of miR-330-5p in the
OS cell lines was significantly increased when compared with that
in the control groups (Fig. 2A).
Conversely, miR-330-5p expression levels were significantly
decreased in miR-330-5p inhibitor-transfected cells compared with
inhibitor NC-transfected cells (Fig.
2A). Next, the effect of miR-330-5p overexpression on cell
viability was examined by a CCK-8 assay. The results revealed that
overexpression of miR-330-5p significantly suppressed the viability
of U2OS and MG63 cells, as compared with that in the mimics NC
group (Fig. 2B and C).
Furthermore, the overexpression of miR-330-5p significantly
promoted the apoptosis of U2OS and MG63 cells (Fig. 2D). Cell cycle analysis was further
performed to demonstrate the underlying mechanisms by which
miR-330-5p affected the cell viability. Flow cytometric analysis
revealed that the overexpression of miR-330-5p in U2OS and MG63
cells significantly increased the number of cells at
G2/M phase (Fig. 2E and
F). Taken together, these results suggested that miR-330-5p
inhibited cell proliferation by inducing apoptosis and cell cycle
arrest at G2/M phase.
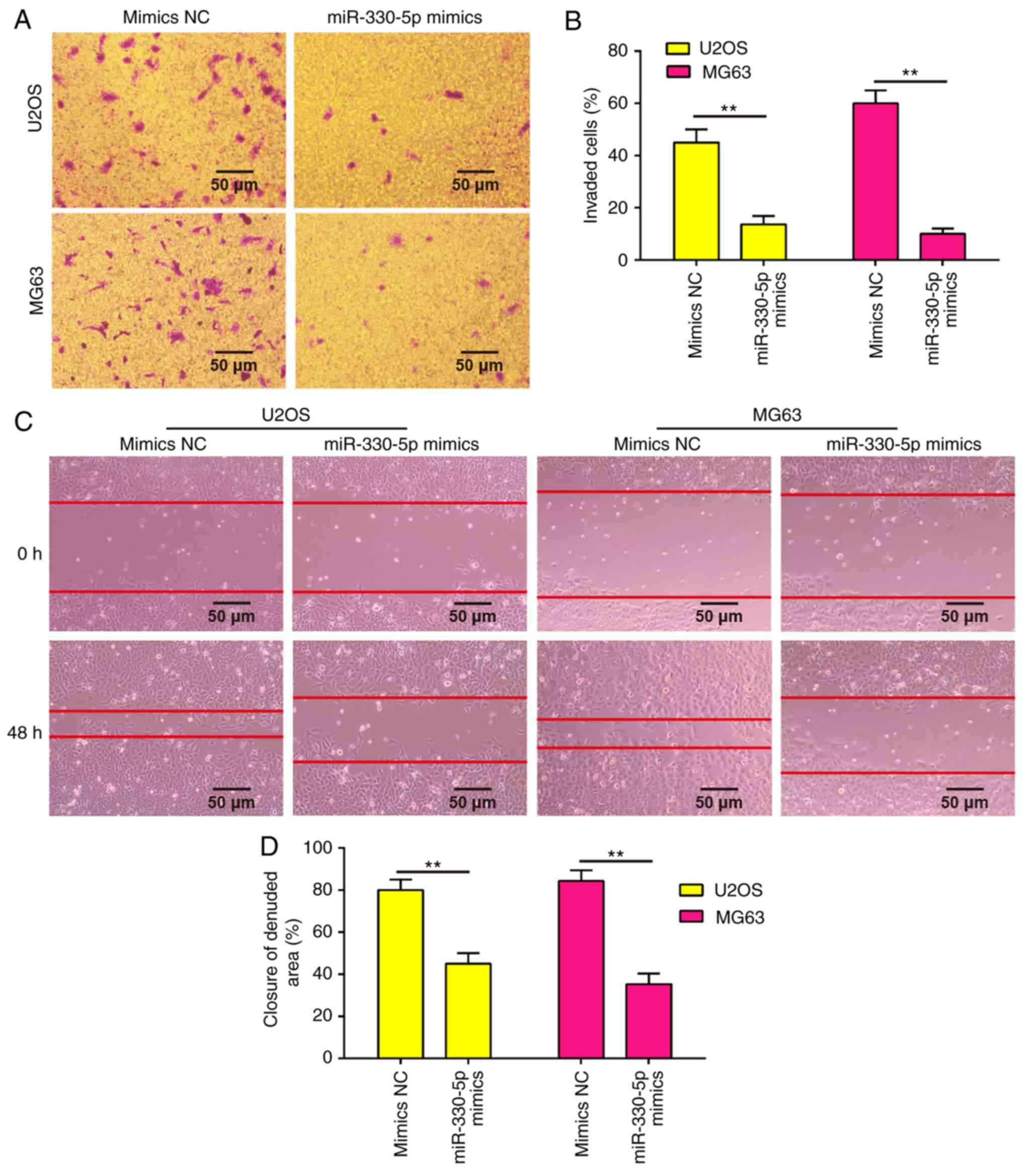
Overexpression of miR-330-5p inhibits
the invasion and migration abilities of OS cells
Since the metastatic ability of OS is a critical
factor in the poor prognosis of patients, the present study
examined whether miR-330-5p is able to modulate the metastatic
ability of OS cells using Transwell invasion and wound healing
assays. As shown in Fig. 3A and B,
overexpression of miR-330-5p in U2OS and MG63 cells significantly
decreased the cell invasion ability as compared with the mimics NC
group. Similarly, the wound healing assay demonstrated that
overexpression of miR-330-5p clearly decreased the cell migration
distance as compared with that of cells transfected with negative
controls (Fig. 3C and D).
Collectively, these data suggested that miR-330-5p suppressed the
metastatic ability of OS cells.
Survivin is a direct target of
miR-330-5p
Several studies have reported that miR-330-5p is
involved in tumor progression via targeting the 3′-UTR of its
target genes (11,14), including MUC1, ITGA5 and Pdia3
(17). To identify new potential
target genes of miR-330-5p, the databases PicTar, TargetScan and
miRBase were searched for candidate genes. Bioinformatics analysis
revealed a miR-330-5p binding site in the 3′-UTR of survivin
(Fig. 4A). In addition, it was
observed that the mRNA level of survivin was significantly
upregulated in OS tissues compared with the control group (Fig. 4B), and miR-330-5p expression was
inversely correlated with survivin expression in OS tissues
(r=−0.8696; P<0.01; Fig. 4C).
To validate whether the 3′-UTR of survivin is a functional target
of miR-330-5p, a luciferase reporter assay was performed. Compared
with the NC group, introduction of miR-330-5p mimics markedly
inhibited the relative luciferase activity of cells co-transfected
with the survivin-3′-UTR wt, while miR-330-5p inhibitor
significantly increased the luciferase activity of the wt group.
However, the luciferase activity of the reporters containing the
mut binding site exhibited no evident changes (Fig. 4D). To further investigate whether
miR-330-5p regulates survivin expression, the effect of miR-330-5p
overexpression or inhibition on survivin protein expression was
examined in OS cells. It was clearly observed that overexpression
of miR-330-5p inhibited the protein expression of survivin in the
U2OS and MG63 cells, whereas knockdown of miR-330-5p promoted
survivin expression (Fig. 4E and
F). These results demonstrated that miR-330-5p was able to
suppress the expression of the proto-oncogene survivin in OS cells
by directly targeting the survivin 3′-UTR.
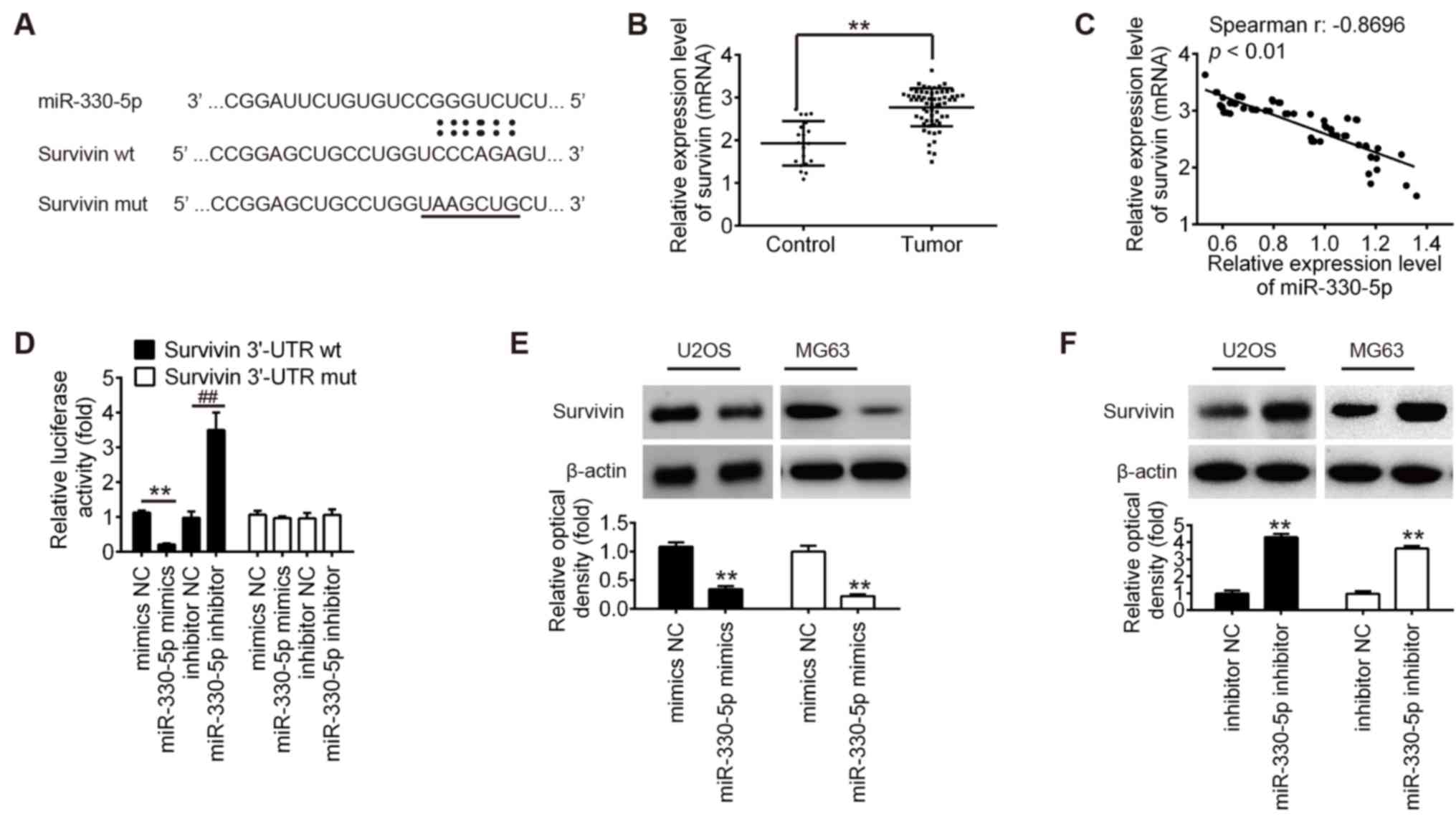 | Figure 4.Survivin is a direct target of
miR-330-5p. (A) Schematic of the survivin 3′-UTR containing the
miR-330-5p binding site. (B) Survivin expression was measured by
reverse transcription-quantitative polymerase chain reaction in OS
tissues (n=63) and osteochondroma tissues (n=20). (C) Survivin
expression was negatively associated with miR-330-5p expression in
OS tissues (r=−0.8696, P<0.01). (D) 293T cells were
co-transfected with 80 ng survivin-3′-UTR wt or mut reporter
plasmids, and with 50 nM miR-330-5p mimics, mimics NC, miR-330-5p
inhibitor or inhibitor NC for 48 h, and relative luciferase
activity was measured. (E) U2OS and (F) MG63 cells were transfected
with the 50 nM miR-330-5p mimics, mimics-NC, miR-330-5p inhibitor
or inhibitor NC for 48 h, and then survivin protein expression was
determined by western blot analysis. Data represent the mean ±
standard deviation of three independent experiments. **P<0.01.
miR, microRNA; OS, osteosarcoma; NC, negative control; 3′-UTR,
3′-untranslated region; wt, wild-type; mut, mutant. |
miR-330-5p suppresses cell growth and
induces cell apoptosis through targeting survivin
First, the overexpression efficiency of
pcDNA-survivin transfection in OS cells was determined (Fig. 5A); the expression levels of
survivin in was significantly higher compared with empty
vector-transfected cells, which indicated successful transfection
of the overexpression vector. To investigate whether miR-330-5p
exerts an antitumor effect through regulating survivin, U2OS and
MG63 cells were co-transfected with miR-330-5p mimics and
pcDNA-survivin plasmid, and cell viability, apoptosis, invasion and
migration was assessed. As shown in Fig. 5B, the transfection of cells with
survivin expression vectors significantly upregulated survivin
expression and restored the decreased survivin expression in
miR-330-5p mimics-transfected cells. CCK-8 assay and flow cytometry
assays were then conducted to determine the cell viability and
apoptosis, respectively. The results demonstrated that
overexpression of survivin significantly increased the viability
and inhibited apoptosis in miR-330-5p mimics +
pcDNA-vector-transfected cells (Fig.
5C and D). Furthermore, the inhibitory effects of miR-330-5p
overexpression on the invasion and migration abilities of cells
were reversed by survivin overexpression (Fig. 5E and F). These results suggest that
miR-330-5p functions as a tumor suppressor in OS, at least
partially, through regulating survivin expression.
Discussion
In the present study, the results indicated that
miR-330-5p was significantly downregulated in OS tissues and cell
lines, and its low expression was closely associated with the
clinical stage and overall survival of OS patients. Subsequently,
functional experiments in OS cell lines revealed that
overexpression of miR-330-5p inhibited the cell viability,
migration and invasion, promoted cell apoptosis and induced cell
cycle arrest. Mechanism research demonstrated that the underlying
mechanism of miR-330-5p was associated with targeting and
inhibiting survivin, a well-known oncogenic gene. Overall, the
current study provided an insight into the possible involvement of
the miR-330-5p/survivin axis in the development and progression of
OS.
Recent publications have demonstrated that
miR-330-5p functions as a tumor suppressor or an oncogene in the
context of different cancer types. For instance, Kong et al
(18) reported that miR-330-5p was
significantly decreased in NSCLC, while overexpression of
miR-330-5p markedly inhibited NSCLC cell growth and promoted cell
apoptosis. Lee et al (19)
also demonstrated that miR-330 functioned as a tumor suppressor and
induced the apoptosis of prostate cancer cells through targeting
E2F1. By contrast, miR-330 was found to be upregulated in
glioblastoma and to function as an oncogenic factor by enhancing
proliferation and invasion, and inhibiting apoptosis through the
activation of ERK and phosphoinositide 3-kinase/protein kinase B
pathways (20,21). However, the expression and role of
miR-330-5p in OS remain unknown. In the current study, miR-330-5p
was significantly downregulated in OS tissues, with a markedly
lower expression detected in metastatic tissues, indicating that
decreased miR-330-5p was associated with OS metastasis. The study
also identified that low miR-330-5p expression in OS patients was
significantly associated with the clinical stage and distant
metastasis, as well as poorer prognosis. These findings suggested
that miR-330-5p may serve as an effective biomarker for the
prognosis of patients with OS. Next, the role of miR-330-5p in
malignant progression of OS was examined. In OS cells,
overexpression of miR-330-5p significantly inhibited the cell
proliferation, promoted cell apoptosis and induced cell cycle
arrest at G2/M phase in vitro. It was also observed that the
migration and invasion of OS cells were suppressed by miR-330-5p
overexpression, which suggests that miR-330-5p overexpression
exerts an antitumor effect in OS.
Survivin, the most important member of the inhibitor
of apoptosis family, is known to serve important roles in tumor
cell proliferation and invasion, therapeutic resistance and poor
prognosis (22,23). For instance, a previous study
reported that survivin was apparently overexpressed in ovarian
cancer, and its overexpression induced cell proliferation and
angiogenesis (24). Wang and Ye
(25) reported that interfering
with the expression of survivin was able to inhibit the cell
proliferation, migration and invasion, and promote apoptosis in
breast cancer cells. These previous findings suggested that
survivin exerts an oncogenic role in tumorigenesis. Recently, Chen
et al (26) demonstrated
that miR-34a and miR-203 repressed the proliferation of human OS
cells by downregulating the expression of survivin. In the present
study, the results identified survivin as a direct and functional
target of miR-330-5p, and miR-330-5p was found to directly
negatively regulate survivin expression in OS cells. Furthermore,
it was demonstrated that survivin was highly expressed in OS
tissues, and a significant negative correlation was observed
between miR-330-5p and survivin expression. Notably, overexpression
of survivin partially reversed the inhibitory effects of miR-330-5p
in OS cells. These data suggest that miR-330-5p exerts its tumor
suppressive role by targeting survivin.
In conclusion, the data of the present study
revealed that miR-330-5p functions as a tumor suppressor, and
participates in the inhibition of cell proliferation and invasion,
as well as promotes apoptosis and induces cell cycle arrest in OS
cells by directly regulating the expression of survivin. These
findings indicated that miR-330-5p may be a promising therapeutic
target for the treatment of OS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HW and LL performed the experiments, contributed to
data analysis and wrote the paper. SF designed the study and
contributed to data analysis and experimental materials. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All participants provided informed consent for the
use of human specimens for clinical research. The present study was
approved by the Anhui Provincial Hospital, Anhui Medical University
(Hefei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kobayashi E, Hornicek FJ and Duan Z:
MicroRNA involvement in osteosarcoma. Sarcoma. 2012:3597392012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruland OS, Bauer H, Alvegaard T and
Smeland S: Treatment of osteosarcoma. The Scandinavian Sarcoma
Group experience. Cancer Treat Res. 152:309–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM
and Zhang GZ: Biological functions of microRNAs: A review. J
Physiol Biochem. 67:129–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sotillo E and Thomas-Tikhonenko A:
Shielding the messenger (RNA): microRNA-based anticancer therapies.
Pharmacol Ther. 131:18–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng N, Miao Z, Wang L, Liu B, Wang G and
Guo X: MiR-378 promotes the cell proliferation of osteosarcoma
through down-regulating the expression of Kruppel-like factor 9.
Biochem Cell Biol. 96:515–521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Z, Li Q, Zhao X, Cui B, Zhang L and
Wang Q: MicroRNA-935 inhibits proliferation and invasion of
osteosarcoma cells by directly targeting High mobility group box 1.
Oncol Res. Feb 22–2018. View Article : Google Scholar
|
|
9
|
Yang X, Wang L, Wang Q, Li L, Fu Y and Sun
J: MiR-183 inhibits osteosarcoma cell growth and invasion by
regulating LRP6-Wnt/β-catenin signaling pathway. Biochem Biophys
Res Commun. 496:1197–1203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong J, Liu Y, Liao W, Liu R, Shi P and
Wang L: miRNA-223 is a potential diagnostic and prognostic marker
for osteosarcoma. J Bone Oncol. 5:74–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng L, Ma J, Ji H, Liu Y and Hu W:
miR-330-5p suppresses glioblastoma cell proliferation and
invasiveness through targeting ITGA5. Biosci Rep. 37(pii):
BSR201700192017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trehoux S, Lahdaoui F, Delpu Y, Renaud F,
Leteurtre E, Torrisani J, Jonckheere N and Van Seuningen I:
Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by
targeting the MUC1 mucin in pancreatic cancer cells. Biochim
Biophys Acta. 1853:2392–2403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Chen SH, Kong P, Zhang LY, Zhang
LL, Zhang NQ and Gu H: Increased expression of miR-330-3p: A novel
independent indicator of poor prognosis in human breast cancer. Eur
Rev Med Pharmacol Sci. 22:1726–1730. 2018.PubMed/NCBI
|
|
14
|
Su BB, Zhou SW, Gan CB and Zhang XN:
MiR-330-5p regulates tyrosinase and PDIA3 expression and suppresses
cell proliferation and invasion in cutaneous malignant melanoma. J
Surg Res. 203:434–440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei CH, Wu G, Cai Q, Gao XC, Tong F, Zhou
R, Zhang RG, Dong JH, Hu Y and Dong XR: MicroRNA-330-3p promotes
cell invasion and metastasis in non-small cell lung cancer through
GRIA3 by activating MAPK/ERK signaling pathway. J Hematol Oncol.
10:1252017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim BK, Yoo HI, Choi K and Yoon SK:
miR-330-5p inhibits proliferation and migration of keratinocytes by
targeting Pdia3 expression. FEBS J. 282:4692–4702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong R, Liu W, Guo Y, Feng J, Cheng C,
Zhang X, Ma Y, Li S, Jiang J, Zhang J, et al: Inhibition of NOB1 by
microRNA-330-5p overexpression represses cell growth of non-small
cell lung cancer. Oncol Rep. 38:2572–2580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KH, Chen YL, Yeh SD, Hsiao M, Lin JT,
Goan YG and Lu PJ: MicroRNA-330 acts as tumor suppressor and
induces apoptosis of prostate cancer cells through E2F1-mediated
suppression of Akt phosphorylation. Oncogene. 28:3360–3370. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu S, Yao Y, Shang C, Xue Y, Ma J, Li Z
and Liu Y: MicroRNA-330 is an oncogenic factor in glioblastoma
cells by regulating SH3GL2 gene. PLoS One. 7:e460102012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao Y, Xue Y, Ma J, Shang C, Wang P, Liu
L, Liu W, Li Z, Qu S, Li Z and Liu Y: MiR-330-mediated regulation
of SH3GL2 expression enhances malignant behaviors of glioblastoma
stem cells by activating ERK and PI3K/AKT signaling pathways. PLoS
One. 9:e950602014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luk SU, Xue H, Cheng H, Lin D, Gout PW,
Fazli L, Collins CC, Gleave ME and Wang Y: The BIRC6 gene as a
novel target for therapy of prostate cancer: Dual targeting of
inhibitors of apoptosis. Oncotarget. 5:6896–6908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kusner LL, Ciesielski MJ, Marx A, Kaminski
HJ and Fenstermaker RA: Survivin as a potential mediator to support
autoreactive cell survival in myasthenia gravis: A human and animal
model study. PLoS One. 9:e1022312014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang HX, Chen G, Li GL and Jiang YJ:
Expression and significance of Survivin and Smac in ovarian
mucinous tumors. Zhonghua Bing Li Xue Za Zhi. 39:387–390. 2010.(In
Chinese). PubMed/NCBI
|
|
25
|
Wang H and Ye YF: Effect of survivin siRNA
on biological behaviour of breast cancer MCF7 cells. Asian Pac J
Trop Med. 8:225–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Chen XG, Hu X, Song T, Ou X and
Zhang C, Zhang W and Zhang C: MiR-34a and miR-203 Inhibit survivin
expression to control cell proliferation and survival in human
osteosarcoma cells. J Cancer. 7:1057–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|