Introduction
Osteoporosis (OP), which is a type of body-wide
metabolic osteopathy, is characterized by osteopenia, skeletal
fragility, and microarchitectural deterioration. The main cause of
OP is the imbalance between bone formation and resorption (1). The chronic pain, malformation and
pathological fracture induced by OP severely affect the quality of
life of the elderly, postmenopausal or estrogen-deficient women,
and it even leads to death (2,3).
With the increase of the aging population, OP has gradually
attracted the attention of many countries and society. It has been
reported that the balance of bone metabolism is maintained by
osteoclasts and osteoblasts, and the weakness of osteoblast
proliferation is one of the most important pathogenesis of OP
(4,5). Therefore, enhancing the proliferation
and differentiation of osteoblasts is vital to develop OP treatment
strategies. To date, the main treatments of OP were based on drug
therapies, including calcitonin, and bisphosphonates which
functioned as bone resorption inhibitors by blocking the function
of osteoclasts (6). Estrogens
could directly regulate the expression of bone formation-related
genes by binding to estrogen receptor (ER) in osteoblasts (4,7).
However, a long-term use of these agents has been associated with
severe side effects (8,9). For instance, a long term use of
bisphosphonates in clinical practice could lead to gastrointestinal
intolerance, osteonecrosis of the jaw, atypical femur fractures,
oesophageal cancer, atrial fibrillation and chronic musculoskeletal
pain (10). In addition, although
estrogen (E2) therapy was believed to be the most effective
treatment for postmenopausal osteoporosis (PMOP) via the notch
signaling pathway (11), recent
research has indicated that the prolonged use of E2 could increase
the risk of endometrial and breast cancer (12,13).
Hence, it is necessary to identify safer and more effective natural
compounds for OP treatment.
Bone formation contains a complex series of events
including osteoblastic differentiation. In the process of
osteoblastic differentiation, bone morphogenetic protein-2 (BMP-2),
a member of the BMP subfamily, is responsible for the production of
bone-specific matrix proteins (14). BMP-2 participates in osteoblastic
differentiation by activating osteoblast-associated transcriptional
factors such as Osterix, runt-related transcription factor 2
(Runx2) and osteocalcin (15,16).
Notably, numerous studies have indicated that protein kinase A
(PKA) plays a crucial role in osteoblast differentiation and could
regulate several osteoblast-specific transcriptional factors
(17,18). However, the functional role of PKA
still requires further exploration.
Pinoresinol (PINO), a high-value plant-derived
lignan, is the third most abundant phenolic compound in virgin
olive oil (VOO) (19). PINO was
demonstrated to be able to notably inhibit the proliferative
ability of human leukemia cells (20), and attenuate colon cancer
progression by inducing tumor cell cycle arrest and apoptosis
(21). Furthermore, a previous
study indicated that the phenolic compounds of VOO could promote
osteoblast proliferation, however, the signaling pathways involved
in this treatment effect of VOO are still unclear (22). PINO is a phytoestrogen and has a
chemical structure that is similar to that of E2, therefore, it was
speculated that PINO could have similar functional effects as
estrogen on OP. The aim of the present study was to investigate the
effects of PINO on osteoblastic proliferation and differentiation
and the underlying mechanism.
Materials and methods
Cell culture and viability
detection
The mouse osteoblast MC3T3-E1 cell line (cat. no.
CRL-2595; ATCC) was cultured in α-Minimum Essential Medium (α-MEM,
cat. no. A1049001; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; cat. no. 30-2020; ATCC), 1%
penicillin and 1% streptomycin at 37°C in the presence of 5%
CO2. The medium was refreshed every 3 days. The
morphology of MC3T3-E1 cells was observed using an inverted
microscope (Nikon Eclipse TC 100; Nikon Corporation).
MC3T3-E1 cell viability was examined using Cell
Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Inc.).
Briefly, MC3T3-E1 cells were embedded in 96-well plates at a cell
density of 4×103 cells/well for 24 h at 37°C with 5%
CO2. Then, the cells were treated with 0,
10−7, 10−6, 10−5, 10−4,
10−3, 10−2, 0.1, 1 and 100 µg/l PINO (CAS no.
487-36-5; Sigma-Aldrich; Merck KGaA) concentrations. The treatment
of 0.01 µmol/l E2 served as a positive control. After 24 and 48 h
of incubation, 10 µl CCK-8 reagent was added to each well and
cultured for another 1 h. Absorbance at 450 nm was examined using a
microplate reader (Bio-Rad Laboratories, Inc.).
Cell migration assay
The logarithmic phase of MC3T3-E1 cells was
trypsinized and collected by centrifugation at 3,000 × g for 5 min.
The cells were randomly divided into five groups and incubated in
96-well plates (3×104 cells/well) at 37°C with 5%
CO2, including a control group (untreated cells), PINO
group (cells were treated with 0.1 µg/l PINO for 2 days) and E2
group. The treated MC3T3-E1 cells were plated in 6-cm culture
dishes and maintained in the presence of 5% CO2 at 37°C.
After cell confluence reached >90%, a cell-free line was created
by a 200 µl sterile pipette tip. At 48 h after the scratch, wound
healing images were captured on a light microscope and the
migration rates were calculated based on the changes of the width
of wound closure.
Real-time quantitative polymerase
chain reaction (RT-qPCR)
Total RNA from MC3T3-E1 cells was extracted using
TRIzol reagent (Thermo Fisher Scientific, Inc.). Total extract (1
µg) was reversely-transcribed by iScript™ reverse transcription
(Bio-Rad Laboratories, Inc.). RT-qPCR was performed using the SYBR
Green real-time PCR Master mix (Toyobo Co., Ltd.) on an ABI PRISM
7000 Sequence 10 Detection System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Reaction mixture (20 µl) consisted of
each primer (10 µM), 10 µl SYBR fluorescent dye, 2 µl cDNA and
RNase-Free dH2O. The basic protocol used for the RT-PCR
analysis was an initial denaturation at 95°C for 10 min, followed
by 45 cycles at 95°C for 15 sec, 56°C for 30 sec and elongation at
72°C for 2 min. The mRNA expression levels were normalized against
that of GAPDH, and the levels of the transcripts were quantified
using the 2−ΔΔCq method (23). All primers are listed in Table I.
 | Table I.Primers for reverse
transcription-quantitative PCR. |
Table I.
Primers for reverse
transcription-quantitative PCR.
| Gene name | Primer
sequences |
|---|
| Col-I | F:
5′-GAGCGGAGTACTGGATCG-3′ |
|
| R:
5′-GCTTCTTTTCCTTGGGGTT-3′ |
| ALP | F:
5′-GATCATTCCCACGTTTTCACATT-3′ |
|
| R:
5′-TTCACCGTCCACCACCTTGT-3′ |
| OPN | F: 5′-
GCCACAAGTTTCACAGCCAC-3′ |
|
| R:
5′-AAAATGCAGTGGCCGTTTGC-3′ |
| Runx2 | F:
5′-AGCGGCAGAATGGATGAGTC-3′ |
|
| R:
5′-ACCAGACAACACCTTTGACG-3 |
| BMP-2 | F:
5′-TGAGCAAAGTGCTTGCACAC-3′ |
|
| R:
5′-AGCCCCCTGGAAGGGATTAT-3′ |
| GAPDH | F:
5′-AGGTCGGTGTGAACGGATTTG-3′ |
|
| R:
5′-GGGGTCGTTGATGGCAACA-3 |
Western blotting
The treated MC3T3-E1 cells were lysed in RIPA lysis
buffer (Beyotime Institute of Biotechnology). The protein
concentrations were quantified by BCA Protein Assay reagent
(Pierce; Thermo Fisher Scientific, Inc.). Total protein (20 µg) was
run on 12% sodium dodecyl sulfate-polyacrylamide gel and
electro-transferred to polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc.), which were then blocked with 5% non-fat dry
milk in Tris-buffered saline, followed by the incubation with the
primary antibodies anti-collagen type I (Col-I; 1:5,000; 130 kDa;
cat. no. ab34710), anti-ALP (1:500; 39 kDa, cat. no. ab83259),
anti-osteopontin (OPN; 1:1,000; 66 kDa, cat. no. ab8448),
anti-Runx2 (1:1,000; 57 kDa; cat. no. ab23981), and anti-BMP-2
(1:1,000; 45 kDa; cat. no. ab14933; all from Abcam), anti-PKA
(1:1,000; 42 kDa; cat. no. 4782), anti-p-cAMP response
element-binding protein (CREB; 1:1,000; 43 kDa; cat. no. 9198) and
anti-CREB (1:1,000; 43 kDa; cat. no. 9197; all from Cell Signaling
Technology (CST)) overnight at 4°C. Following the primary
antibodies, the membranes were incubated with goat anti-rabbit
HRP-conjugated secondary antibodies (cat. no. ab205718; Abcam) at
4°C for 1 h. Protein bands were detected with an enhanced
chemiluminescence detection system (EMD Millipore), and GAPDH
(1:1,000; 36 kDa; cat. no. ab8245; Abcam) was used as the internal
control.
Alkaline phosphatase (ALP) activity
assay
The cells from all the groups were embedded into
24-well plates and maintained in 5% CO2 at 37°C for 2
days. The cells were washed 2 times with phosphate-buffered saline
(PBS) and lysed in 0.2% Triton X-100. Then, cell lysates were
subjected to a 5-min centrifugation at 10,000 × g. The supernatant
was mixed with p-nitrophenyl phosphate (pNPP) (Sigma-Aldrich; Merck
KGaA) and maintained at 37°C for 15 min. NaOH (2 mol/l) was used
for terminating the reaction. ALP activity at 405 nm was examined
under a microplate reader.
Mineralization detection
Alizarin red staining was used to determine the
mineralization level of MC3T3-E1 cells. Firstly, Alizarin red S
could selectively bind to calcium and produce a dark red stain.
Each group of cells (2×105) was respectively cultured in
24-well plates for 14 days and the medium was changed every 3 days.
After wiping off the medium, the cells were then stained with 40 mM
Alizarin red S (pH 4.2; Sigma-Aldrich; Merck KGaA) for 15 min with
gentle agitation, and the images of calcified matrices were
captured by light microscope.
Enzyme-linked immunosorbent assay
(ELISA)
All treated MC3T3-E1 cells were resuspended by cell
extraction buffer (Thermo Fisher Scientific, Inc.) containing 1 mM
PMSF and protease inhibitor cocktail. Total cAMP levels in the
supernatant of each group of cells were assessed by commercially
available cAMP ELISA kits (cat. no. STA-501; Cell Biolabs, Inc.).
In this assay, these plates were coated overnight with specific
antibodies to cAMP (cat. no. ab134901; 1:1,000; Abcam), and the
following morning, the plates were washed and blocked for 2 h. The
prepared standard liquid and diluted supernatant (supernatant:
diluent, 1:4) were added to an ELISA plate. After being washed by
PBS, the streptavidin-horseradish peroxidase-conjugated (HRP)
antibody-labeled secondary cAMP (cat. no. ab205719; Abcam) was
added to each well at room temperature for 1 h of incubation. After
an additional washing step, tetramethylbenzidine substrate solution
(Clinical Science Products, Inc.) was added to produce a substrate
solution. The enzymatic activities were then quantified by
detecting the optical density at 450 nm with a microplate
reader.
Pathway inhibition
To further explore whether the cAMP/PKA signaling
pathway participated in the functional effects of PINO on
osteoblast proliferation and differentiation, H89, an effective
inhibitor of PKA, was used for co-treatment with PINO. The effects
on the MC3T3-E1 cells were assessed by CCK-8 kit and wound healing
assay.
Statistical analysis
Data are expressed as the mean ± SD, and statistical
analysis among three and more groups was performed by one-way
analysis of variance (ANOVA) followed by Tukey's post hoc test. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
PINO treatment enhances cell viability
and migratory capacity of MC3T3-E1 cells
The morphology of MC3T3-E1 cells was observed under
a light microscope, and as revealed in Fig. 1A, the MC3T3-E1 cells were cuboidal
or fusiform. After treatment with various concentrations of PINO,
it was revealed (Fig. 1B) that low
concentrations of PINO had a positive effect on MC3T3-E1 cell
viability, and PINO treatment at 0.1 µg/l for 48 h had the most
significant effect on cell viability. Hence, PINO at a
concentration of 0.1 µg/l was selected for the following
experiments. To further investigate the functional effects of PINO
on the MC3T3-E1 cells, the migratory capacity was assessed by wound
healing assay. As revealed in Fig.
1C, E2 was set as the positive control, and the migration rate
in the E2 group was greater than that in the control group
(P<0.01). PINO treatment had similar effects to E2 on MC3T3-E1
cells and it significantly enhanced the migratory ability.
Collectively, the results revealed that PINO had similar effects to
E2 on osteoblastic viability and migration.
 | Figure 1.PINO treatment enhances cell viability
and migratory capacity of MC3T3-E1 cells. (A) The cellular
morphology of MC3T3-E1 cells was observed under a light microscope
(magnification, ×100 and ×200). (B) MC3T3-E1 cells were treated
with various concentrations of PINO (0, 10−7,
10−6, 10−5, 10−4, 10−3,
10−2, 0.1, 1 and 100 µg/l). E2 treatment at the
concentration of 0.01 µmol/l served as the positive control.
Following incubation for 24 and 48 h, the changes of cell viability
with PINO treatment were detected by CCK-8 kit. *P<0.05,
**P<0.01 vs. 0 µg/l PINO group (24 h); #P<0.05,
##P<0.01 vs. 0 µg/l PINO group (48 h). (C) The
changes in the migration rates of MC3T3-E1 cells, with treatment of
0.1 µg/l PINO or 0.01 µmol/l E2. Each value was represented by the
mean ± SEM (n=3). **P<0.01 vs. the control group;
##P<0.01 vs. the PINO group. PINO, pinoresinol; E2,
estrogen. |
PINO treatment promotes the expression
of differentiation-associated factors in MC3T3-E1 cells
For the assessment of osteoblastic differentiation,
the expression levels of several osteoblast-specific genes (Col-I,
ALP, OPN, Runx2, and BMP-2) were detected in MC3T3-E1 cells. As one
of the first-line drugs for OP treatment, E2 significantly
increased the mRNA and protein levels of these osteoblastic
differentiation-related genes (P<0.01, Fig. 2). In addition, the present results
revealed that PINO had similar effects to E2. Although the effects
of PINO on inducing Col-I, ALP, OPN, Runx2 and BMP-2 expression
were not as strong as those of E2, the mRNA and protein levels of
these genes were also significantly upregulated under PINO
treatment. Therefore, the results indicated that PINO had the
ability to promote osteoblastic differentiation.
 | Figure 2.PINO treatment promotes the
expression of differentiation-associated factors in MC3T3-E1 cells.
(A) In order to assess the effects of PINO treatment on the
differentiation of MC3T3-E1 cells, the mRNA levels of several
osteoblast-specific factors (Col-I, ALP, OPN, Runx2, and BMP-2)
were detected by RT-qPCR. (B) The protein levels of these genes
were detected by western blotting. Each value was represented by
the mean ± SEM (n=3). GAPDH was used as an internal control.
*P<0.05 and **P<0.01 vs. the control group. PINO,
pinoresinol; Col-I, collagen type I; ALP, alkaline phosphatase;
OPN, osteopontin; Runx2, runt-related transcription factor 2;
BMP-2, bone morphogenetic protein-2. |
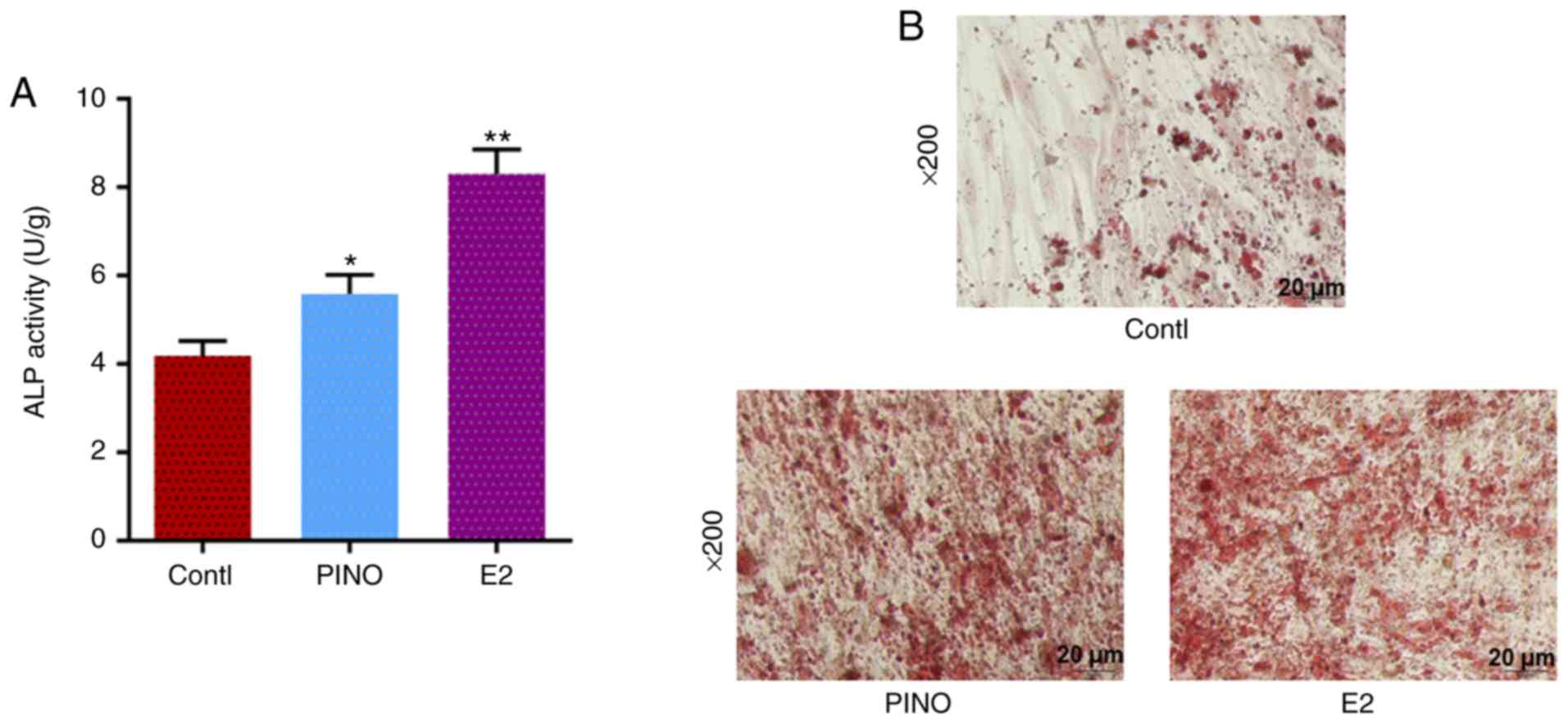
PINO stimulates ALP activity and
mineralization in MC3T3-E1 cells
ALP activity and mineralization were also detected
to further determine MC3T3-E1 cell differentiation. After
incubation with α-MEM in the presence of PINO and E2, pNPP was used
as a substrate for ALP activity detection. In comparison to the
control group, the ALP activity in the E2 group was the highest and
the PINO group also had a significant increase (P<0.05), as
revealed in Fig. 3A. After
Alizarin red staining, the red region in the PINO and E2 groups
were denser than in the control group (Fig. 3B), indicating that both PINO and E2
contributed to the mineralization of MC3T3-E1 cells. Collectively,
our findings indicated that PINO treatment could promote the
differentiation of osteoblastic MC3T3-E1 cells.
The cAMP/PKA signaling pathway
participates in the promotive effects of PINO on osteoblastic
differentiation
As aforementioned, the cAMP/PKA signaling pathway
was involved in the regulation of multiple osteoblast-specific
transcriptional factors. The protein levels of cAMP, PKA,
phosphorylated and unphosphorylated CREB were assessed to further
study the molecular mechanism of the effect of PINO on MC3T3-E1
cells. As revealed in Fig. 4A, a
significantly increased cAMP level was revealed in the PINO and E2
groups, compared to the control group (P<0.01), and the cAMP
level in the PINO group was slightly lower than that in the E2
group. Similarly, the protein level of PKA was also notably
upregulated under the treatment of PINO or E2 (P<0.01). PINO had
limited effect on unphosphorylated CREB expression, however, the
phosphorylation levels of CREB were markedly increased (P<0.01,
Fig. 4B). Moreover, the effects of
PINO on cAMP/PKA signals were not as strong as E2. Collectively,
the promoting effect of PINO on osteoblastic differentiation may
partially rely on the activation of cAMP/PKA signals.
H89 co-treatment reverses the positive
effects of PINO on the viability and migration of MC3T3-E1
cells
The H89 solution was used as an inhibitor of the
cAMP/PKA signaling pathway to further verify the role of cAMP/PKA
in the functional effects of PINO on MC3T3-E1 cells. In comparison
to the control, H89 treatment alone significantly decreased cell
viability (P<0.01, Fig. 5A).
When cells were co-treated with H89, the positive effects of PINO
on MC3T3-E1 cell viability were also abolished (P<0.01). In
addition, H89 treatment alone could significantly decrease the
migration rate by less than half of the control group. Furthermore,
H89 co-treatment could also significantly limit the capacity of
PINO in enhancing the migration of MC3T3-E1 cells (P<0.01,
Fig. 5B and C). Therefore, the
present results indicated that the functional effects of PINO on
osteoblastic differentiation and proliferation may be attributed to
the activation of cAMP/PKA signals.
Discussion
It is recognized that osteoblasts occupy a
fundamental place in bone formation and remodeling, therefore,
enhancing osteoblastic proliferation and differentiation can
radically improve OP treatment (24,25).
E2, one of the first-line drugs for OP treatment, could effectively
enhance osteoblastic proliferation and differentiation, however,
the side effects of a long-term use of E2 make it imperative to
identify safer and more effective drugs. In the present study, E2
treatment was set as the positive control to assess whether PINO
treatment had the ability to promote osteoblastic proliferation and
differentiation in MC3T3-E1 cells. Although a previous study
revealed that PINO did not affect cell proliferation of osteoblasts
(22), the result was not in
conflict with our study due to the differences in the concentration
of PINO. The present results indicated that only PINO at a
concentration of 1×10−2 µg/l could induce a slight
upregulation of MC3T3-E1 cell viability, and promote cell
migration. In addition, PINO had a positive effect on osteoblastic
differentiation by inducing the expression of multiple
differentiation-associated genes. Moreover, the involvement of
cAMP/PKA signaling pathway played a vital role in the molecular
mechanism of the effect of PINO on osteoblastic proliferation and
differentiation. Although the positive effects of PINO on
osteoblastic proliferation and differentiation were not as strong
as those of E2, the present results indicated that PINO has the
potential to replace E2 in terms of OP treatment.
Osteoblastic differentiation is a complex process
mediated by a variety of regulators such as Col-I, ALP, OPN, Runx2,
and BMP-2. In the present study, when treating with PINO, a
significant increase of these differentiation-associated genes was
revealed in MC3T3-E1 cells. The Col-I protein is a fundamental
component of the bone extracellular matrix and it is responsible
for connecting the cell surface integrins with other extracellular
matrix proteins (26). ALP can
hydrolyze organic phosphate and pyrophosphate, subsequently
promoting bone mineral formation and mineralization (27). In addition, ALP and Col-I both are
recognized as the earliest biomarkers of osteoblastic
differentiation (28,29). A previous study indicated that the
treatment of fermented red ginseng extract (FRGE) could enhance the
ALP level and Col-I expression, ultimately improving bone formation
and MC3T3-E1 cell differentiation (30). Therefore, the increased ALP
activity, mineralization and Col-I in the present results indicated
that PINO treatment could promote osteoblastic MC3T3-E1 cell
differentiation. OPN is a type of acidic protein secreted by
osteoblasts and plays a crucial role in biomineralization and bone
remodeling (31). As another
specific marker of bone formation, increased OPN expression in the
present study also indicated that PINO treatment contributed to the
improvement of osteoblastic differentiation. It is recognized that
BMPs can induce undifferentiated mesenchymal cells to gather and
differentiate to osteoblasts and chondrocytes, finally promoting
osteogenic activity (32). In
addition, BMP-2 expression induced the activated downstream
transcriptional factors (Runx2 and Osterix) to bind to the promoter
regions of ALP and OPN (33).
Aucubin, an iridoid glucoside separated from multiple Chinese
herbs, was reported to inhibit titanium particle-induced osteoblast
apoptosis and promote bone formation by activating the BMP-2/Runx2
pathway (34). Collectively, the
promoting effect of PINO treatment on osteoblastic differentiation
and osteogenesis may rely on the regulation of BMP-2/Runx2
pathway.
In the cAMP/PKA signaling pathway, PKA functions as
a second messenger to mediate the various functions of cAMP in
mammalian cells. A previous study demonstrated that PKA played an
essential role in the process of the promotion of osteoblastic
differentiation (35). In
addition, another study demonstrated that cAMP could promote
drosophila mothers against decapentaplegic protein (SMAD)-mediated
BMP-2/Runx2 signaling via the PKA pathway (36). Hence, the potential relationship
between the cAMP/PKA and BMP-2/Runx2 signaling pathways was
surmised based on this research. Recent research revealed that
linarin (LIN), a natural flavonoid compound in Flos Chrysanthemi
Indici (FCI), could enhance the expression of BMP-2/Runx2
signals via the activation of the cAMP/PKA signaling pathway.
However, when the cells were treated with H89, the positive effects
of LIN were effectively abrogated (37). This research was basically
consistent with the present results. After co-treatment with a PKA
inhibitor (H89), the increased cAMP, PKA and phosphorylated CREB
expression levels in the PINO group were nearly decreased to the
control levels (data not shown). Furthermore, PINO was also
reported to be able to inhibit cell viability and promote apoptosis
of human leukemia cell line (HL60) and colorectal cancer (CRC) cell
lines through the upregulation of the CDK inhibitor
p21Waf1/Cip1 or ATM-p53 cascade (20,21).
However, there are many differences in the gene expression,
metabolism and other cellular morphological and phenotypic profiles
between HL60 or CRC cells and MC3T3-E1 cells due to cell
heterogeneity. This does not signify that PINO could also
upregulate the p21 and ATM-p53 cascade, and subsequently, inhibit
cell proliferation and differentiation in MC3T3-E1 cells. In the
present study, the data demonstrated a promoting effect of PINO on
osteoblast proliferation and differentiation. Collectively, the
present study indicated that PINO-induced osteoblastic
differentiation was largely attributed to the regulation of the
cAMP/PKA signaling pathway.
There were some limitations in our study. The
duration of PINO treatment was too short to completely reflect the
effects of PINO on the growth and differentiation of osteoblasts.
Moreover, the effects of PINO on the ER pathway require further
investigation.
In summary, the present study revealed that PINO had
a similar function to E2 in OP treatment. PINO induced osteoblast
differentiation and mineralization by regulating BMP-2/Runx2
signaling via activation of the cAMP/PKA pathway. Collectively,
PINO may be considered as an alternative to E2 in OP treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Qiqihar
Science and Technology Plan Project (grant no. SFZD-2015037).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XJ and WC substantially contributed to the
conception and design of the study. FS, JX, HG, WS, HS and WX
performed the data acquisition, data analysis and interpretation.
XJ and WC drafted the study and critically revised it for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tang D, Ju C, Liu Y, Xu F, Wang Z and Wang
D: Therapeutic effect of icariin combined with stem cells on
postmenopausal osteoporosis in rats. J Bone Miner Metab.
36:180–188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krege JH and Wan X: Teriparatide and the
risk of nonvertebral fractures in women with postmenopausal
osteoporosis. Bone. 50:161–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hannafon F and Cadogan MP: Recognition and
treatment of postmenopausal osteoporosis. J Gerontol Nurs.
40:10–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ming LG, Lv X, Ma XN, Ge BF, Zhen P, Song
P, Zhou J, Ma HP, Xian CJ and Chen KM: The prenyl group contributes
to activities of phytoestrogen 8-prenynaringenin in enhancing bone
formation and inhibiting bone resorption in vitro. Endocrinology.
154:1202–1214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ke K, Li Q, Yang X, Xie Z, Wang Y, Shi J,
Chi L, Xu W, Hu L and Shi H: Asperosaponin VI promotes bone marrow
stromal cell osteogenic differentiation through the PI3K/AKT
signaling pathway in an osteoporosis model. Sci Rep. 6:352332016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antebi B, Pelled G and Gazit D: Stem cell
therapy for osteoporosis. Curr Osteoporos Rep. 12:41–47. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vico L and Vanacker JM: Sex hormones and
their receptors in bone homeostasis: Insights from genetically
modified mouse models. Osteoporos Int. 21:365–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reginster JY, Pelousse F and Bruyere O:
Safety concerns with the long-term management of osteoporosis.
Expert Opin Drug Saf. 12:507–522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cosman F: Long-term treatment strategies
for postmenopausal osteoporosis. Curr Opin Rheumatol. 30:420–426.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lewiecki EM: Safety of long-term
bisphosphonate therapy for the management of osteoporosis. Drugs.
71:791–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan JZ, Yang L, Meng GL, Lin YS, Wei BY,
Fan J, Hu HM, Liu YW, Chen S, Zhang JK, et al: Estrogen improves
the proliferation and differentiation of hBMSCs derived from
postmenopausal osteoporosis through notch signaling pathway. Mol
Cell Biochem. 392:85–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burkman RT: Hormone replacement therapy.
Current controversies. Minerva Ginecol. 55:107–116. 2003.PubMed/NCBI
|
|
13
|
Komm BS, Morgenstern D, A Yamamoto L and
Jenkins SN: The safety and tolerability profile of therapies for
the prevention and treatment of osteoporosis in postmenopausal
women. Expert Rev Clin Pharmacol. 8:769–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canalis E, Economides AN and Gazzerro E:
Bone morphogenetic proteins, their antagonists and the skeleton.
Endocr Rev. 24:218–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Phimphilai M, Zhao Z, Boules H, Roca H and
Franceschi RT: BMP signaling is required for RUNX2-dependent
induction of the osteoblast phenotype. J Bone Miner Res.
21:637–646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang B, Lin X, Tan J, She X, Liu Y and
Kuang H: Root bark of Sambucus Williamsii Hance promotes rat
femoral fracture healing by the BMP-2/Runx2 signaling pathway. J
Ethnopharmacol. 191:107–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He S, Choi YH, Choi JK, Yeo CY, Chun C and
Lee KY: Protein kinase A regulates the osteogenic activity of
Osterix. J Cell Biochem. 115:1808–1815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Jeong HM, Choi YH, Kim JH, Choi JK,
Yeo CY, Jeong HG, Jeong TC, Chun C and Lee KY: Protein kinase a
phosphorylates Dlx3 and regulates the function of Dlx3 during
osteoblast differentiation. J Cell Biochem. 115:2004–2011.
2014.PubMed/NCBI
|
|
19
|
Owen RW, Mier W, Giacosa A, Hull WE,
Spiegelhalder B and Bartsch H: Identification of lignans as major
components in the phenolic fraction of olive oil. Clin Chem.
46:976–988. 2000.PubMed/NCBI
|
|
20
|
Sepporta MV, Mazza T, Morozzi G and
Fabiani R: Pinoresinol inhibits proliferation and induces
differentiation on human HL60 leukemia cells. Nutr Cancer.
65:1208–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fini L, Hotchkiss E, Fogliano V, Graziani
G, Romano M, De Vol EB, Qin H, Selgrad M, Boland CR and
Ricciardiello L: Chemopreventive properties of pinoresinol-rich
olive oil involve a selective activation of the ATM-p53 cascade in
colon cancer cell lines. Carcinogenesis. 29:139–146. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
García-Martínez O, De Luna-Bertos E,
Ramos-Torrecillas J, Ruiz C, Milia E, Lorenzo ML, Jimenez B,
Sánchez-Ortiz A and Rivas A: Phenolic compounds in extra virgin
olive oil stimulate human osteoblastic cell proliferation. PLoS
One. 11:e01500452016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo Z, Liu M, Sun L and Rui F: Icariin
recovers the osteogenic differentiation and bone formation of bone
marrow stromal cells from a rat model of estrogen
deficiency-induced osteoporosis. Mol Med Rep. 12:382–388. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu C, Bao N, Chen S and Zhao J: Dioscin
enhances osteoblastic cell differentiation and proliferation by
inhibiting cell autophagy via the ASPP2/NF-κβ pathway. Mol Med Rep.
16:4922–4926. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li B, Hu RY, Sun L, Luo R, Lu KH and Tian
XB: Potential role of andrographolide in the proliferation of
osteoblasts mediated by the ERK signaling pathway. Biomed
Pharmacother. 83:1335–1344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma XN, Ma CX, Shi WG, Zhou J, Ma HP, Gao
YH, Xian CJ and Chen KM: Primary cilium is required for the
stimulating effect of icaritin on osteogenic differentiation and
mineralization of osteoblasts in vitro. J Endocrinol Invest.
40:357–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shao X, Cao X, Song G, Zhao Y and Shi B:
Metformin rescues the MG63 osteoblasts against the effect of high
glucose on proliferation. J Diabetes Res. 2014:4539402014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fusaro M, Crepaldi G, Maggi S, D'Angelo A,
Calo L, Miozzo D, Fornasieri A and Gallieni M: Bleeding, vertebral
fractures and vascular calcifications in patients treated with
warfarin: Hope for lower risks with alternative therapies. Curr
Vasc Pharmacol. 9:763–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siddiqi MZ, Siddiqi MH, Kim YJ, Jin Y, Huq
MA and Yang DC: Effect of fermented red ginseng extract enriched in
ginsenoside Rg3 on the differentiation and mineralization of
preosteoblastic MC3T3-E1 cells. J Med Food. 18:542–548. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi ST, Kim JH, Kang EJ, Lee SW, Park MC,
Park YB and Lee SK: Osteopontin might be involved in bone
remodelling rather than in inflammation in ankylosing spondylitis.
Rheumatology (Oxford). 47:1775–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SH, Park Y, Song M, Srikanth S, Kim S,
Kang MK, Gwack Y, Park NH, Kim RH and Shin KH: Orai1 mediates
osteogenic differentiation via BMP signaling pathway in bone marrow
mesenchymal stem cells. Biochem Biophys Res Commun. 473:1309–1314.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishimura R, Hata K, Matsubara T,
Wakabayashi M and Yoneda T: Regulation of bone and cartilage
development by network between BMP signalling and transcription
factors. J Biochem. 151:247–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu Z, Xie Q, Huang Y, Zhang S and Chen Y:
Aucubin suppresses Titanium particles-mediated apoptosis of
MC3T3-E1 cells and facilitates osteogenesis by affecting the
BMP2/Smads/RunX2 signaling pathway. Mol Med Rep. 18:2561–2570.
2018.PubMed/NCBI
|
|
35
|
Lo KW, Kan HM, Ashe KM and Laurencin CT:
The small molecule PKA-specific cyclic AMP analogue as an inducer
of osteoblast-like cells differentiation and mineralization. J
Tissue Eng Regen Med. 6:40–48. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohta Y, Nakagawa K, Imai Y, Katagiri T,
Koike T and Takaoka K: Cyclic AMP enhances Smad-mediated BMP
signaling through PKA-CREB pathway. J Bone Miner Metab. 26:478–484.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Hao L, Wu J, Zhang J and Su J:
Linarin promotes osteogenic differentiation by activating the
BMP-2/RUNX2 pathway via protein kinase A signaling. Int J Mol Med.
37:901–910. 2016. View Article : Google Scholar : PubMed/NCBI
|