Introduction
Increasing evidence has revealed that stem cells can
express neural cell markers and improve neurological function when
transplanted into animal models of spinal cord injury (SCI)
(1,2). In particular, mesenchymal stem cells
(MSCs) have emerged as one of the most promising types of stem
cells due to favorable ethical considerations and improved safety
(3). Notably, ectomesenchymal stem
cells (EMSCs), which are derived from the cranial neural crest and
isolated from the nasal septum, are capable of self-renewal,
possess the potential for multi-directional differentiation, and
have a strong propensity to differentiate into neurons, and osseous
and glial cells (4). A previous
study from our laboratory demonstrated that EMSCs transplanted into
a rat model of SCI can reduce the functional deficits associated
with SCI and promote histological reconstruction in the injured
spinal cords of rats and behavioral recovery from an SCI (5). Since EMSCs can be easily isolated
from the nasal septa of adult donors without invasive surgery,
EMSCs represent a promising type of stem cell for the treatment of
SCI (6). However, the use of EMSCs
in tissue regeneration has been limited, due to their low
proliferation rate, limited lifespan and the progressive loss of
their stem cell properties during in vitro expansion
(7,8).
Transglutaminase type 2 (TG2) is a unique member of
the transglutaminase family of Ca2+-dependent
crosslinking enzymes (9,10). An increasing body of evidence from
the past decade has revealed that TG2 has multiple and complex
activities at the cell surface and within the extracellular matrix
(ECM). In particular, TG2 has a crucial role in the regulation of
cell-ECM interactions and in outside-in signaling via several types
of transmembrane receptor (11).
In addition, a number of studies reported that TG2 has important
enzymatic and non-enzymatic functions, including the modulation of
cell interactions with the ECM and soluble growth factors via
non-covalent interactions with, and regulation of, integrins and
growth factor receptors (12,13).
Furthermore, TG2 can also act as an adhesion receptor for
fibronectin (FN) and laminin (LN) at the cell surface, including
vascular smooth muscle cells, connective tissue fibroblasts,
osteoblasts, neurons and astrocytes (11). To the best of our knowledge, TG2 is
involved in numerous processes, including cellular differentiation,
apoptosis, adhesion and matrix assembly (14,15).
In particular, it has been demonstrated that TG2 is associated with
cell differentiation in neurons and astrocytes, and that it is
crucial for neuronal differentiation in human neuroblastoma SH-SY5Y
cells (16). Although biochemical
and biological responses induced by TG2 on primary cultured cells
or established cell lines have been extensively studied (17,18),
little is currently known regarding TG2 effects on EMSCs
undifferentiated cells.
To determine whether TG2 expression could improve
EMSC proliferation and neurogenesis, the present study investigated
the biological mechanism involved in the effects of TG2 on the
proliferation, migration and differentiation of EMSCs isolated from
the nasal septum of rats. The therapeutic effects of transplanting
TG2-transfected EMSCs in rats following SCI were subsequently
investigated.
Materials and methods
Isolation and expansion of rat
EMSCs
EMSCs were isolated following the protocol described
in our previous study (10).
Briefly, two female Sprague-Dawley (SD) rats (weight, 120 g;
provided by Jiangsu University Animal Center) were sacrificed by
intraperitoneal injection of pentobarbital sodium overdose (200
mg/kg). Subsequently, nasal septum mucosa tissue samples were
collected from the lower third of the rat nasal septum (4). These mucous membranes were gently
minced into pieces (0.5–1 mm3). and cultured in
Dulbecco's modified Eagle's medium/nutrient mixture F12 (Hyclone;
GE Healthcare Life Sciences) containing 10% fetal bovine serum
(FBS; GIBCO; Thermo Fisher Scientific, Inc.) and
penicillin-streptomycin (100 U/ml) and placed at 37°C in a
humidified incubator containing 5% CO2. After seven
days, medium was replaced and non-adherent cells were removed.
Spindle-shaped adherent EMSCs were expanded and purified via three
passages following the initial seeding. Medium was then replaced
and cells were observed with an inverted phase contrast microscope
every three days. After 14 days, spindle-shaped adherent EMSCs were
harvested using 0.25% trypsin and subcultured until they reach
80–90% confluence. EMSCs (passage 3) were identified by detecting
the expression of nestin (rabbit polyclonal, ab27952, Abcam),
vimentin (mouse monoclonal; cat. no. BM0135; Wuhan Boster
Biological Technology, Ltd.; 1:400), CD133 (rabbit polyclonal; cat.
no. BA3993-2; Wuhan Boster Biological Technology, Ltd.; 1:400) and
CD44 (rabbit polyclonal; cat. no. A00052; Wuhan Boster Biological
Technology, Ltd.; 1:400) by immunofluorescence staining according
to the protocol described in the following section.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde (PFA) for
30 min at 4°C and then washed three times with PBS and treated with
PBS containing 0.3% Triton X-100 and 3% bovine serum albumin
(Beijing Solarbio Science & Technology Co., Ltd.) for 20 min at
room temperature. Following another round of washing with PBS,
cells were incubated with the aforementioned primary antibodies
against nestin, vimentin, CD133 and CD44 at 37°C for 3 h. Cells
were washed three times with PBS and were incubated with the
corresponding secondary antibodies, including Alexa Fluor
488/Cy3-conjugated goat anti-mouse/rabbit immunoglobulin (Ig)G
Cy3-labeled goat anti-mouse/rabbit IgG (1:200; cat. no. C5838/C2821
Sigma-Aldrich; Merck KGaA) and Alexa Fluor 488-conjugated goat
anti-mouse IgG (1:200; cat. no. SAB4600388; Sigma-Aldrich; Merck
KGaA) at 37°C for 2 h. The nuclei were counterstained with DAPI
(0.5 µg/ml; Sigma-Aldrich; Merck KGaA) at room temperature for 10
min and cells were observed under an immunofluorescence microscope
(magnification, ×100 or ×200).
Hematoxylin and eosin staining
After 8 weeks, the rats were sacrificed by deep
anesthetization with an intraperitoneal injection of phenobarbital
sodium (200 mg/kg), and fresh spine cord were fixed in 4%
paraformaldehyde overnight at 4°C. After dehydration in a graded
series of ethanol solutions, the tissue was embedded in paraffin.
Serial longitudinal tissue sections were prepared and subjected to
staining and observation as follows. The spine cord specimens were
cut into 10-µm sections, which were stained with hematoxylin and
eosin at room temperature for 30 min to evaluate the repair
effects.
Construction of recombinant
adenoviruses
The following primers were designed to synthesize
the TG2 gene (2061 base pairs): TG2, forward
5′-CTAGCTAGCGCCACCatggccgaggagctgaacct-3′ and rTG2, reverse,
5′-GGAATTCttaggccgggccgatgatga-3′. A recombinant rat TG2(rTG2)
adenovirus shuttle plasmid was constructed by Nanjing Genscript
Bioengineering Technology and Services Co., Ltd. and confirmed by
sequencing. Briefly, TG2 genes were inserted into a
pAdShuttle-IRES-hrGFP2-TG2 vector to prepare shuttle vectors, and
the pAdShuttle-IRES-hrGFP2 vector was set as a parallel group.
Subsequently, 293A cells (2×106 cells/well; American
Type Culture Collection) were seeded in 6-cm2 dishes in
DMEM supplemented with 10% FBS and placed at 37°C in a humidified
incubator containing 5% CO2 overnight. The medium was
replaced before transfection. The cells were transfected at 80–90%
confluency with pacAd5-9.2-100 (2×108 TU/ml) and the
pAdShuttle-IRES-hrGFP2-TG2 (4×108 TU/ml) shuttle plasmid
using Lipofectamine 2000ä (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Following 12
days of transfection, recombinant adenoviruses harboring the GFP
positive cells were selected and TG2 (Ad-TG2-GFP) gene were
harvested. All recombinant adenoviruses were amplified in 293A
cells and purified using double cesium chloride density gradient
ultracentrifugation (4°C, 12,000 g, 2.5 h). Titers of the
adenoviral stocks were determined using a plaque assay in 293A
cells. EMSCs (2×105) at passage three were infected with
Ad-TG2-GFP at a multiplicity of infection (MOI) of 10 pfu/cell
following standard infection procedures (19), and the adenovirus with no insert
was used as the control (Ad-EMSCs). After 24 h, TG2-GFP-EMSCs
(TG2-EMSCs) and Ad-EMSCs were harvested, and TG2-GFP and GFP gene
expression was detected using a fluorescence microscope.
Western blot analysis
Protein was detected by western blotting. Briefly,
cells were lysed with RIPA buffer (Beijing Solarbio Science &
Technology Co., Ltd.) at 0°C for 30 min containing
phenylmethylsulfonyl fluoride, a phosphatase inhibitor (Beijing
Solarbio Science & Technology Co., Ltd.) cocktail and EDTA
(Beijing Solarbio Science & Technology Co., Ltd.). The protein
concentrations were determined using a BCA kit (Thermo Fisher
Scientific, Inc.). Protein (20 µg) from each sample was loaded into
8% polyacrylamide gels, separated by SDS-PAGE, and transferred onto
a polyvinylidene difluoride (PVDF) membrane (EMD Millipore) by
electrophoresis. Then, membranes were blocked with 3% skimmed dried
milk (dissolved in TBS + Tween-20 (TBST; 20 mM Tris-HCl, 150 mM
NaCl pH 7.5 and 0.1% Tween-20) at 4°C for 8 h and incubated with
primary antibodies, including anti-TG2 (1:200; sc-48387, Santa Cruz
Biotechnology, Inc.), anti-β III Tubulin (1:300; cat. no. ab1827;
Abcam), anti-GAP-43 (l:500; cat. no. ab16053; Abcam; 1:300),
anti-MAP2 (1:500; cat. no. ab11267; Abcam), anti-NF-200 (1:300;
NF-200; cat. no. sc-32729; Santa Cruz Biotechnology, Inc.),
anti-BDNF (1:200; cat. no. PB0013; Wuhan Boster Biological
Technology, Ltd.), anti-NGF (1:500; cat. no. BA0611-2; Wuhan Boster
Biological Technology, Ltd.), anti-FN (1:500; cat. no. BA1772;
Wuhan Boster Biological Technology, Ltd.) and anti-LN (1:500; cat.
no. BM4921; Wuhan Boster Biological Technology, Ltd.) at 4°C for 12
h. Then, membranes were washed with TBS + Tween-20 (TBST; 20 mM
Tris-HCl, 150 mM NaCl pH 7.5 and 0.1% Tween-20) and incubated with
horseradish peroxidase-conjugated secondary antibodies (IgG;
1:10,000; cat. no. ZB-2301; OriGene Technologies, Inc.) or
HRP-conjugated goat anti-mouse IgG (1:10,000; cat. no. ZB-2305;
OriGene Technologies, Inc.) for 1 h at 37°C. Following washing with
TBST, bands were detected using enhanced chemiluminescence
substrate (Wuhan Boster Biological Technology, Ltd.). β-actin
(1:400; cat. no. BM3873; Wuhan Boster Biological Technology, Ltd.)
served as the protein loading control. The protein bands were
scanned with Typhoon 9400 Variable Mode Imager (GE Healthcare Life
Sciences). Each experiment was repeated at least three times for
statistical analysis.
TG2-EMSCs proliferation in vitro
In the present study, Ki-67 immunofluorescence and
MTT assay were used to evaluate cell proliferation. TG2-EMSCs,
Ad-EMSCs and EMSCs were cultured in 24-well plates at a density of
1×104 cells/well and stained by immunofluorescence for
Ki-67 (rabbit polyclonal; cat. no. ab15580; Abcam; 1:400) according
to the aforementioned procedure; DAPI was used to counterstain the
nuclei. Images were captured by fluorescence microscopy
(magnification, ×200; Eclipse Ti; Nikon Corporation) and the
percentage of Ki-67-positive cells were analyzed and calculated
with ImageJ software (version 1.51k, National Institutes of
Health). The experiment was performed in triplicate, and five
visual fields were calculated in each well.
The MTT assay (Beijing Solarbio Science &
Technology Co., Ltd.) was conducted to quantify the proliferative
capacity of EMSCs according to the manufacturer's instructions.
Briefly, cells were harvested, seeded into 96-well plates at a
density of 1×104 cells/well and cultured for 1, 3, 5 and
7 days. MTT solution (20 µl) was then added to each well and
incubated at 37°C for 2 h. Absorbance was read by a HTS 7000 Plus
Bio Assay reader (PerkinElmer, Inc.) at 490 nm. To yield the
cumulative doubling levels, the population doubling level of each
passage was calculated and then added to the population doubling
levels of the previous passages.
Neural differentiation in vitro
The neuronal differentiation assay was performed
following our previous work (4).
Briefly, cells were seeded into 24- or 6-well plates at a density
of 1×104 or 2×105 cells/well with neurogenic
medium consisting of 10% FBS (GE Healthcare Life Sciences), 2% B27
(Gibco; Thermo Fisher Scientific, Inc.), 1 µg/ml ATRA (Merck KGaA),
50 ng/ml sonic hedgehog (PeproTech, Inc.) and 50 ng/ml NT-3
(PeproTech, Inc.) and placed at 37°C in a humidified incubator
containing 5% CO2 for two weeks; the medium was replaced
every three days. These differentiated cells were fixed with 4% PFA
for immunofluorescence staining at 4°C for 12 h, digested using a
0.25% trypsin solution, and washed several times with PBS according
to the aforementioned protocol. The primary antibodies were
purchased from Chemicon International; Thermo Fisher Scientific,
Inc. and comprised of mouse anti-β III Tubulin (Tuj-1; rabbit
polyclonal; cat. no. ab1827; Abcam; 1:300), mouse anti-growth
associated protein-43 (GAP-43; rabbit polyclonal; cat. no. ab16053;
Abcam; 1:300), mouse anti-microtubule-associated protein 2 (MAP2;
mouse monoclonal; cat. no. ab11267; Abcam; 1:300) and mouse
anti-actin (mouse monoclonal; cat. no. BM5422; Wuhan Boster
Biological Technology, Ltd.; 1:400).
Neural differentiation in vivo
Adult female SD rats weighing 200 g were used in the
present study and all animal procedures were approved by the
Jiangsu University School of Medicine and Gaochun People's Hospital
Animal Experiment Committee. Animals were anaesthetized with sodium
pentobarbital (50 mg/kg, intraperitoneal injection) and were
subjected to a dorsal laminectomy at T10 to expose the cervical
spinal cord (20). Briefly, the
dura mater was opened and a spinal resection at the T10 level was
performed using iridotomy scissors. After hemostasis, transplanted
cells were prepared at a density of 2×105 cells/µl and 5
µl of the cell suspension was poured into a fibrin gel in order to
prevent cell loss. The fibrin gel containing EMSCs and the fibrin
gel without cells (control group) were immediately implanted into a
hemisected cavity. Following surgery, rats received extensive care
including intramuscular injection of penicillin (50,000 U/kg/day)
for 3 days and manual emiction twice daily. A total of 18 animals
(n=6 in each of the Ad-EMSCs, EMSCs and TG2-EMSC groups) were
sacrificed at 4 weeks post-treatment for western blotting and
immunofluorescent staining. Firstly, at 4 weeks after SCI (20), three animals in each group were
sacrificed with sodium pentobarbital (200 mg/kg). The spinal cord
was carefully isolated, fixed overnight in 4% PFA at 4°C, and
dehydrated with saturated sucrose (21). Serial longitudinal sections were
cut with a cryostat and subjected to staining. Immunofluorescence
staining was performed on 15-µm spinal cord sections incubated with
mouse anti-NF-200 (1:300; NF-200; cat. no. sc-32729; Santa Cruz
Biotechnology, Inc.) and anti-Tuj-1 (1:300; Tuj-1; cat. no. ab1827;
Abcam) monoclonal antibodies overnight at 4°C, and with
Cy3-conjugated rabbit anti-mouse IgG (1:200; cat. no. sc-358916;
Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. Images were
captured with a Nikon fluorescence microscope. Secondly, western
blotting was performed to analyze NF-200 and MAP2 expression in
rats following SCI. Briefly, at the end of the experiment, three
animals per group were sacrificed with intraperitoneal injections
of an overdose of pentobarbital sodium (200 mg/kg) and 1-cm-long
spinal cord segments containing the lesion center were isolated.
For western blotting, the spinal cords were removed and homogenized
in SDS sample buffer containing a protease inhibitor cocktail.
Protein samples were separated on an 8% SDS-polyacrylamide gel and
transferred onto nitrocellulose membranes according to the
aforementioned protocol.
Behavioral assessment
A total of 18 rats (n=6 in each of the Ad-EMSCs,
EMSC and TG2-EMSC groups) were allowed to survive for eight weeks
and were subjected to behavioral assessments. Behavioral tests
[Grid-walk and Basso, Beattie, and Bresnahan (BBB) score] were
performed on the same set of animals used for the functional
recovery analysis (22,23). Animals were pre-trained for 7 days
prior to surgery to walk on a grid runway (20×120 cm with 5×5 cm
holes) to receive a food reward. A Grid-walk test was performed 8
weeks post-surgery. At the time of the test, rats were allowed to
pass through the grid runway 4 times, which was recorded with a
digital camera. The number of paw placement errors (when the hind
paw passes through the hole and the knee joint is visible under the
grid) and the average number of errors per run and per animal were
then calculated. The BBB score was used to evaluate the recovery of
neurological function following SCI. Animals were pre-acclimatized
to the open field for 7 days prior to surgery. Each animal was
given scores on a weekly basis by two observers who were blinded to
treatment allocation.
Statistical analysis
All experiments were performed at least twice. Data
are expressed as the mean ± standard deviation. All data were
analyzed using SPSS 22.0 statistical software (IBM Corp.). One-way
analysis of variance followed by Tukey's post-hoc test was used for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
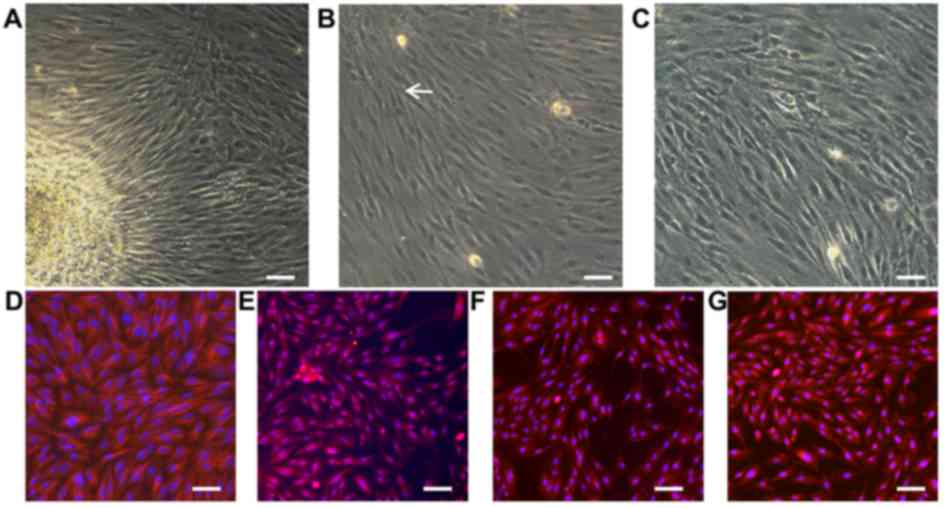
Morphological characterization and
identification of EMSCs
As presented in Fig.
1A, EMSCs migrated out from the lamina propria. After three
days, typical spindle-like shaped cells adhered to the surface and
formed colonies, which was observed with a high proliferation rate
(Fig. 1B). The cell distribution
was found to be uniform with a polar shape after fusion and
swirling growth (Fig. 1C).
Furthermore, EMSCs typically expressed different antigenic markers.
Our previous work revealed that the surface markers vimentin, s100,
CD44 and nestin can be important antigenic markers for EMSCs
(4). To evaluate the cell
stemness, antibodies against neural crest and mesenchymal stem cell
markers, including vimentin (Fig.
1D), s100 (Fig. 1E), CD44
(Fig. 1F) and nestin (Fig. 1G), were therefore used for
immunofluorescent staining. The results demonstrated that EMSCs
were positive for those markers, which was consistent with the
results from previous reports (24,25).
These results suggested that high-purity EMSCs may be harvested
simply and efficiently through adherent culture.
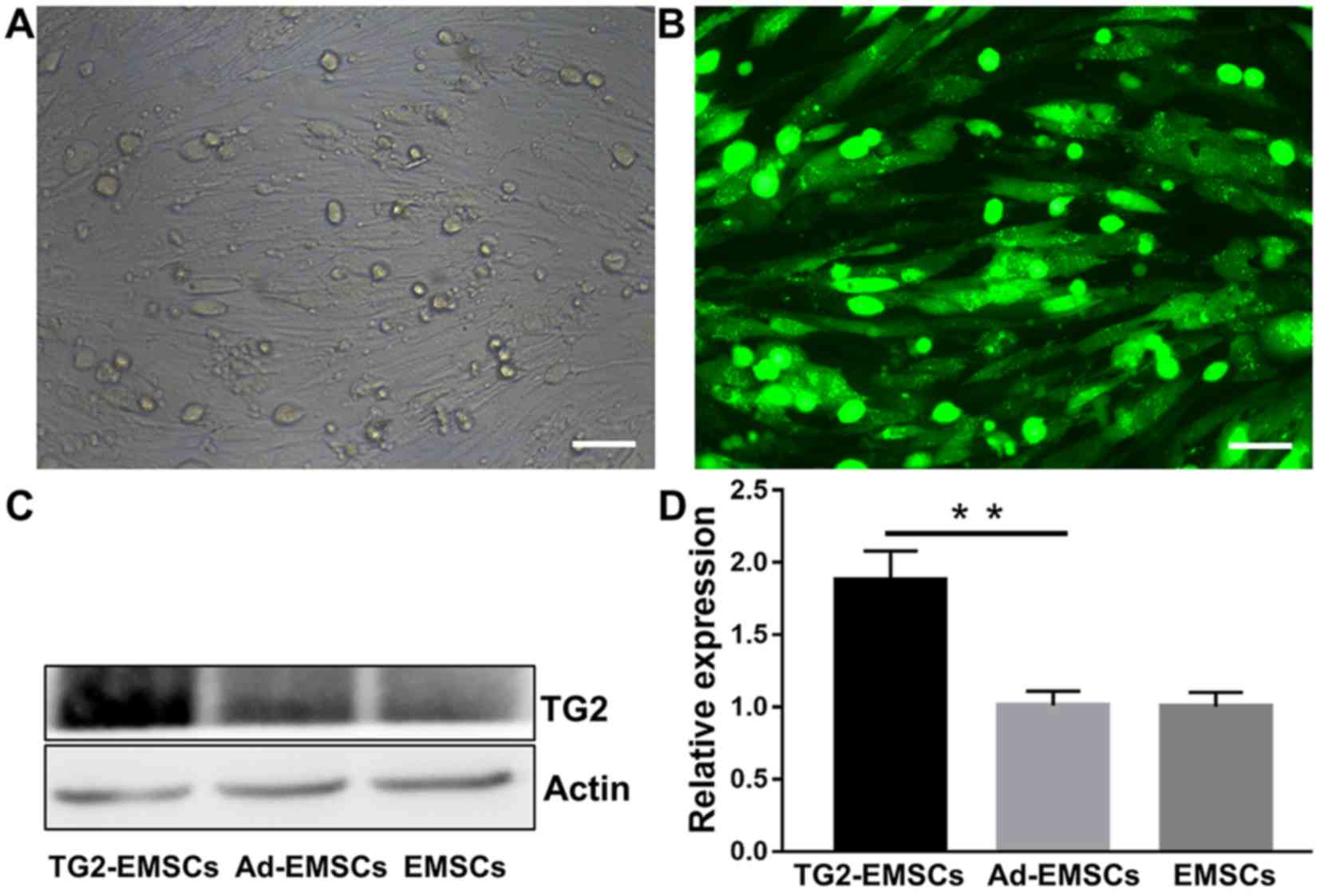
Transfection efficiency of adenovirus
vector with TG2 in EMSCs
To evaluate the efficiency of TG2 transfection, TG2
expression was evaluated by immunofluorescence and western
blotting. As presented in Fig. 2A,
following a 48-h transfection with the adenovirus, EMSCs in the
transfected group displayed GFP fluorescence, and an abundant
grit-like TG2 (9,10) was observed around the visible
nucleus under fluorescence microscope (Fig. 2B). This indicated that the TG2
adenovirus had been successfully transfected into EMSCs without any
observable cytopathic effects. Western blotting was employed to
determine protein expression levels. As presented in Fig. 2C and D, TG2 expression was
significantly increased following transfection of adenoviral vector
with TG2 in EMSCs, whereas EMSCs and cells transfected with
adenoviral vector without TG2 only expressed only a small amount of
TG2. These results indicated that cell transfection with a TG2
adenoviral vector significantly increased TG2 expression in
EMSCs.
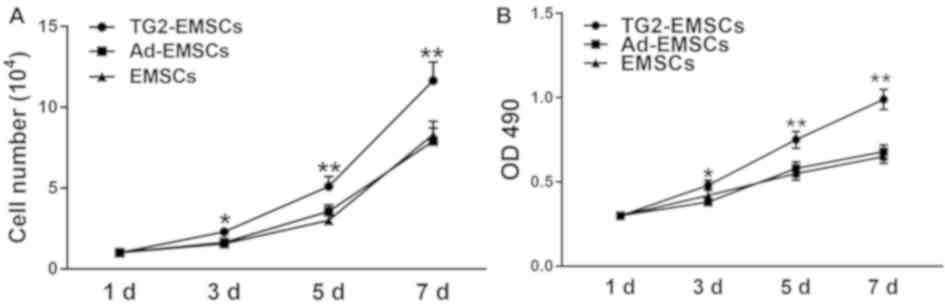
Proliferative abilities of
TG2-EMSCs
Results from Ki-67 immunofluorescent staining
demonstrated that the majority of TG2-EMSCs exhibited a high
nuclear expression of Ki-67 (Fig. 3A
and B). Conversely, Ki-67 expression in the EMSCs and Ad-EMSCs
groups was significantly weaker. Furthermore, the results from the
MTT assay and the cell count further confirmed that TG2-EMSCs
exhibited a better proliferative capacity than the control cells
(Fig. 4A and B). Collectively,
these data demonstrated that TG2-overexpressing EMSCs may have
higher expansive and proliferative capacities than control
cells.
Differences in neurogenic
differentiation in vitro
Following induction, morphological changes of the
differentiated cells were observed by inverted phase contrast
microscopy. As shown in Fig. 5A,
certain cells exhibited neuron-like phenotypes (white arrow). In
particular, the cytoplasm in TG2-EMSCs formed a contracted
multipolar shape and presented with neuronal morphology, including
a small cell body and some long extensions reminiscent of neurons.
Conversely, few cells displayed morphological changes in the
control groups, which indicated failure of induction. In addition,
as presented in Fig. 5A, neuronal
markers, including MAP2, GAP-43 and Tuj-1 were used to identify the
differentiation of neuronal cells. These neuronal cells expressed
myelination-related molecules, such as MAP2, GAP-43 and Tuj-1, more
strongly than did the ad-EMSCs and EMSCs. In addition, western
blotting demonstrated that MAP2, GAP-43 and Tuj-1expression levels
were significantly increased in TG2-EMSCs (Fig. 5B and C) compared with the control
group. Furthermore, significantly higher percentages of TG2-EMSCs
were differentiated into GAP-43+, MAP2+ and
Tuj-1+ neuron-like cells compared with the control group
(Fig. 5D).
 | Figure 5.Cells were induced to differentiate
into neuronal-like cells following two weeks of culture in
neurogenic differentiation media. (A) Representative images of
GAP-43, MAP2 and Tuj-1 staining in cells following induction. (B)
Representative western blotting images. (C) Western blotting
quantification (n=4). (D) Quantitative analysis of cells positive
for GAP-43, MAP2 and Tuj-1. Data represent the mean ± standard
error of the mean of samples analyzed in triplicate. **P<0.01,
as indicated. Scale bar, 50 µm. Ad, adenovirus; EMSCs,
ectomesenchymal stem cells; GAP-43, growth associated protein-43;
MAP2, microtubule-associated protein 2; TG2, transglutaminase type
2; Tuj-1, β III tubulin. |
Involvement of ECM and neurotrophic
factors in the differentiation of EMSCs
ECM is an important component of the cellular
microenvironment and is involved in MSC differentiation (10). In the present study, western
blotting was used to examine the effects of TG2 adenovirus
transfection on ECM and neurotrophic factors in EMSCs. The results
demonstrated that transfection with TG2 adenovirus promoted ECM
deposition, including LN and FN, on EMSCs. According to western
blotting results, TG2 adenovirus transfection increased the protein
levels of neurotrophic factors including nerve growth factor (NGF)
and brain derived neurotrophic factor (BDNF) in differentiated
EMSCs (Fig. 6).
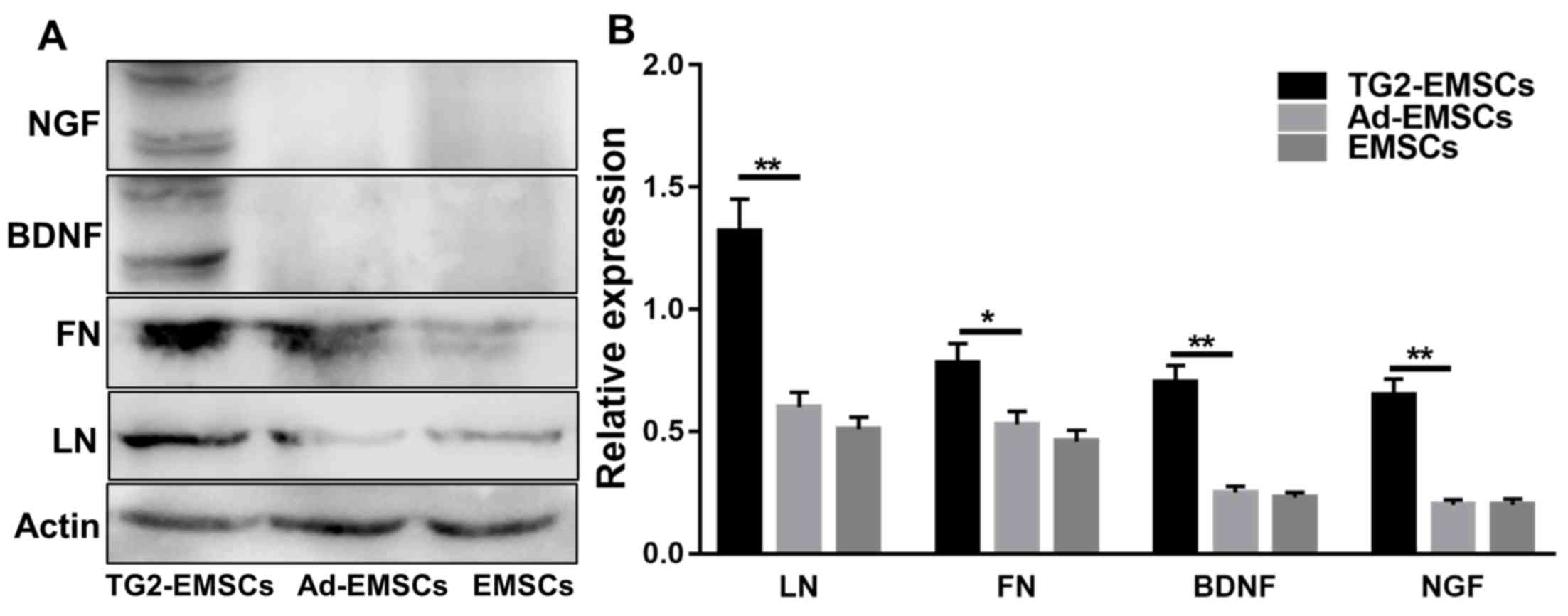 | Figure 6.Effects of TG2 adenovirus
transfection on the ECM and neurotrophic factors in EMSCs following
neural induction. (A) Western blotting of LN, FN, BDNF and NGF
expression levels (B) Western blotting quantification. Data
represent the mean ± standard deviation of measurements made from
three replicates. *P<0.05 and **P<0.01, as indicated. Ad,
adenovirus; BDNF, brain-derived neurotrophic factor; EMSCs,
ectomesenchymal stem cells; FN, fibronectin; LN, laminin; NGF,
nerve growth factor; TG2, transglutaminase type 2. |
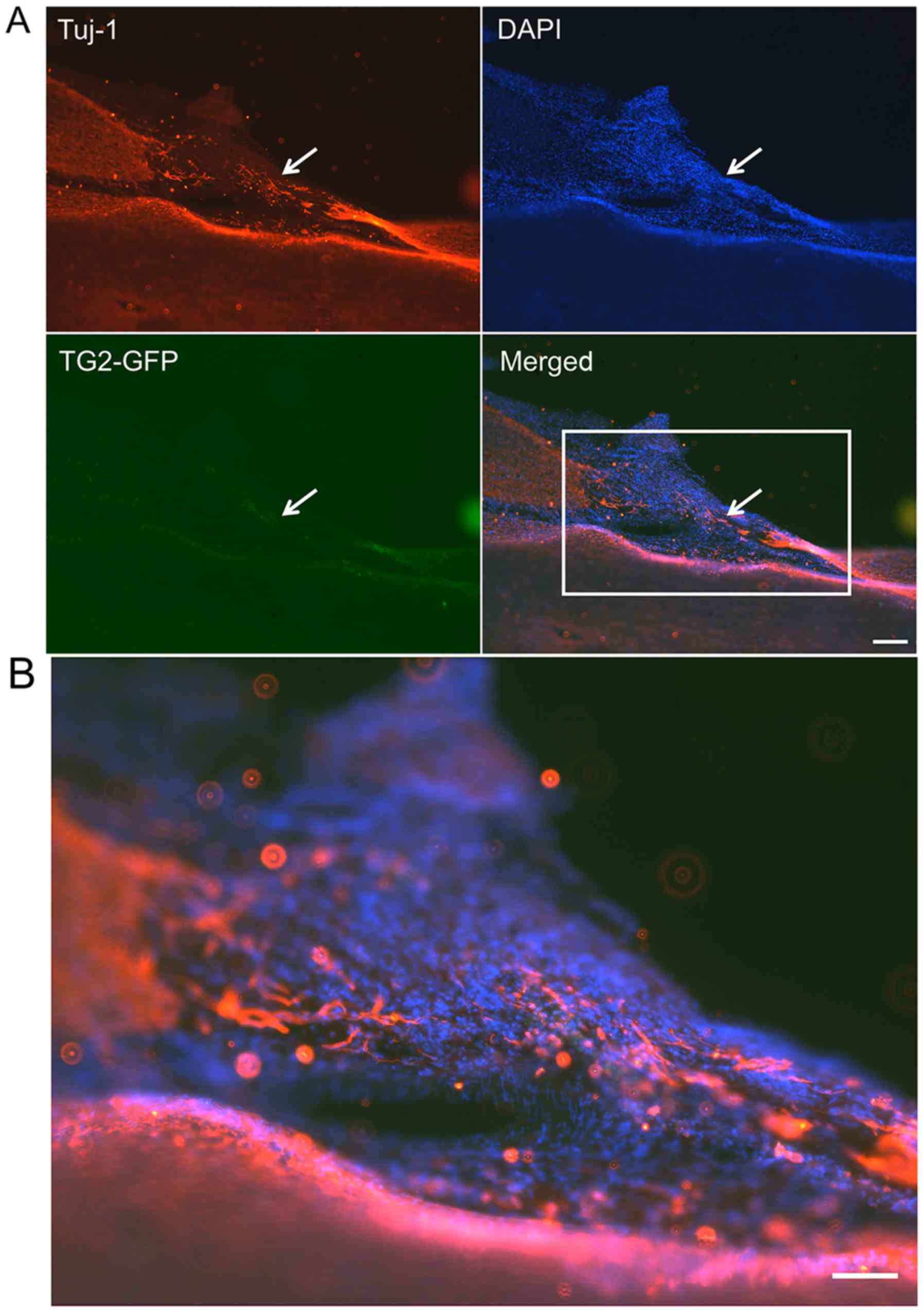
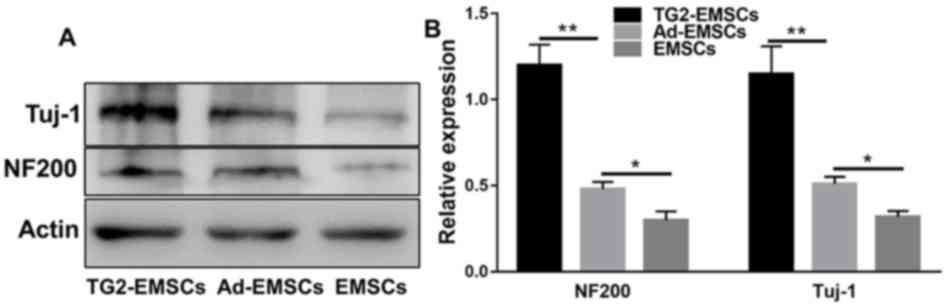
Axon marker expression and neurogenic
differentiation of TG2-EMSCs in vivo
These experiments aimed to assess the effect of TG2
overexpression on the differentiation of transplanted EMSCs in a
SCI lesion in rats. Two weeks following transplantation, the
TG2-GFP-EMSCs were dispersed in the lesion sites, as observed by
immunofluorescent microscopy. The results demonstrated that
NF-200+ cells (Fig. 7)
and Tuj-1+ cells (Fig.
8) were co-localized with GFP+EMSCs (green) in the SCI lesion
of rats that were transplanted with TG2-EMSCs. These findings
indicated that TG2-EMSCs may differentiate into neurons in
vivo.
TG2-EMSCs transplantation promotes
rats functional recovery following SCI
BBB and Grid-walk analyses were performed to
determine the effect of TG2-EMSCs transplantation in rats on
functional recovery following SCI. One week following injury, the
average BBB score of the three groups was 2.33±0.35. At 4 weeks
following TG2-EMSCs transplantation, there was no significant
difference in BBB scores between groups. However, rats transplanted
with TG2-EMSCs presented a significant improvement in the BBB score
during continued recovery compared with the control group (Fig. 9A). In addition, rats were assessed
by a Grid-walk test 8 weeks following transplantation. Compared
with the EMSCs- or Ad-EMSCs-transplanted groups, rats that were
transplanted with TG2-EMSCs exhibited a reduced number of footprint
errors (Fig. 9B).
NF-200 and Tuj-1 expression and EMSC
survival
As presented in Fig.
10, western blotting demonstrated that the NF-200 and Tuj-1
axonal markers were expressed in the lesion. At eight weeks
post-operation, NF-200 and Tuj-1 expression significantly higher in
the TG2-EMSC group compared with the control group. These results
suggested that TG2-EMSCs may enhance axonal regeneration.
TG2-EMSC transplantation increases
tissue sparing in rats following SCI
To determine whether TG2-EMSC transplantation can
increase tissue retention, the gross morphology of the injured
spinal cord was examined at 8 weeks following transplantation. As
presented in Fig. 11, the results
indicated that TG2-EMSCs transplantation markedly decreased the
lesion volume compared with the control groups.
Discussion
Central nervous system (CNS) damage invariably leads
to severe dysfunction. This is due to permanent nerve tissue damage
caused by the neuron's inability to regenerate effectively
(26). At present, the efficacy of
available treatments remains largely unsatisfactory. However,
thanks to advances in stem cell transplantation therapies, the
situation is gradually improving as increasing evidence suggests
that the treatment of traumatic nervous system damage is possible
(27–29). Notably, the type of cell chosen is
critical for the therapy efficacy. The present study used EMSCs
because they present stem cell characteristics, including nestin,
vimentin, S100 and CD133 expression, which may increase neuronal
survival rate and axon length. In cases of injured CNS, EMSC
transplantation has been reported to improve functional recovery
following traumatic SCI (25,30).
Previous studies from our laboratory demonstrated that EMSCs can
significantly promote the transformation of oligodendrocyte
precursor cells into oligodendrocytes, and to stimulate
oligodendrocyte processes and mature growth in vitro
(4,25,31).
Furthermore, EMSCs are a source of numerous trophic molecules,
including NGF, NT3 and BDNF, which provide essential support to the
CNS following injury, and may therefore serve a crucial role in CNS
regeneration (24,25,31).
These findings suggest that EMSCs may constitute a valuable cell
source for the treatment of neurological diseases.
EMSCs are pluripotent adult stem cells derived from
cranial neural crest that have a strong tendency to differentiate
into neurons (4,6,25,31).
Vanella et al (32)
reported that TG2 is increased during neuronal differentiation in
human MSCs, which suggests that TG2 could serve a key role in the
biochemical pathway involved in the differentiation of human MSCs
in neural cells and that TG2 may be a part of downstream events
associated with the neural differentiation of MSCs. In the present
study, EMSCs overexpressing TG2 were designed to examine the
effects of endogenous TG2 on neural cell proliferation and
differentiation. The results demonstrated that high levels of TG2
were detected by western blotting without altering cell morphology.
In addition, the results from immunofluorescence staining revealed
a higher percentage of Ki-67+ cells in the TG2-EMSC
group compared with control groups, which were confirmed by the
results of the MTT assay and cell count. Furthermore, the
EMSC-induced differentiation into neurons was more efficient in the
TG2-EMSC group than in the control groups. For instance, EMSCs
revealed neuron-shaped and polygonal morphology, which was further
confirmed by GAP-43, Tuj-1 and MAP2 antigenic markers (33,34).
In addition, western blotting results indicated that the expression
levels of GAP-43, Tuj-1 and MAP2 were significantly increased in
TG2-EMSCs in vitro.
TG2 is well known to interact non-covalently with
the ubiquitous and abundant ECM in vitro (35,36).
Previous studies demonstrated the ability of cell-surface TG2 to
bind to soluble proteins of the ECM, including FN and LN, and to
promote ECM deposition (37,38).
The present study demonstrated that the expression levels of FN and
LN were significantly higher in the TG2-EMSC group compared with
the control groups. We therefore hypothesized that deposited ECM
may promote EMSCs proliferation by providing strong biological
stimuli, including arginine-glycine-aspartic acid (RGD) sequences
contained in the ECM (39).
However, numerous studies have reported that EMSCs isolated and
cultured in vitro can secrete various neurotrophic factors,
including NGF, NT-3 and BDNF, which serve crucial roles in neural
cell growth, proliferation and differentiation (40,41).
However, these soluble neurotrophic factors are rapidly cleared
in vivo and in vitro (42), which might explain the weaker cell
proliferation and differentiation rates observed in the control
groups. Conversely, more neurotrophins were detected in the
TG2-EMSC group compared with the control groups. These interesting
results are reminiscent of the soluble neurotrophic factors
cross-linked by TG2. Increasing evidence suggests that TG2 could
cross-link cytokines without losing biological activity (10,14).
Taken together, the results from the present study demonstrated
that TG2 overexpression promoted EMSC proliferation and enhanced
EMSC differentiation into neurons by facilitating ECM deposition
and cross-linking endogenous neurotrophic factors.
Although the safety of cell-based gene therapy has
not been firmly established, TG2-EMSCs did not cause tumor
formation within eight weeks following transplantation in rats.
Conversely, TG2-EMSCs-transplanted rats presented more nerve fibers
than rats from the control groups. NF-200 and Tuj-1 are considered
to be markers of axons. The present study revealed that the
TG2-EMSCs-transplanted group had higher NF-200 and Tuj-1 expression
compared with control groups. These neuron-associated proteins may
be derived from the differentiation of endogenous neural stem
cells, and TG2-EMSCs may form a microenvironment that facilitates
nerve regeneration at the site of SCI. Another possible source of
nerve fibres was via replenishment of the degenerated nerve fibers
by the neurons directly differentiated from TG2-EMSCs. The results
from immunofluorescence staining demonstrated that TG2-EMSCs could
survive for four weeks and presented neuron-like phenotypes and
morphologies following SCI.
By promoting the expression of neurotrophins and ECM
(FN and LN), TG2-EMSCs may improve the neuroregeneration
microenvironment in spinal cord lesions and enhance functional
recovery. Increasing evidence reports that neurotrophins can
significantly promote neurogenesis and contribute to
neuroplasticity and functional recovery following SCI. Stable
neurotrophic effects may therefore partially explain the axonal
regeneration and functional recovery observed in the present study.
The Grid-walk score assesses voluntary movements controlled by
dorsal descending tracts. Improvements in hindlimb performance are
important, as the four-point increase in the BBB score reveals
(43). In the present study, the
BBB and Grid-walk scores of rats from the TG2-EMSC group exhibited
a significant improvement compared with rats in the control groups.
Previous studies demonstrated that EMSC treatment can significantly
improve functional recovery rats following SCI (4,6,25,31).
In the present study, the TG2-EMSC group had more trophic factors,
ECM, and axon regeneration. The results revealed that TG2-EMSCs may
enhance the functional recovery of rats following SCI. Recent
studies reported that TG2 is closely associated with NF-κB
signaling pathway (44,45). Since TG2 might enhance EMSC quality
and promote SCI repair by activating the NF-κB signaling pathway,
future investigations are required to provide novel information
about the therapeutic potential of TG2 in SCI.
In conclusion, the present study demonstrated that
EMSCs overexpressing TG2 may promote functional recovery following
SCI through complex processes. Further investigation is needed to
fully understand the underlying mechanisms of TG2-associated
protection against SCI.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grants no. 81571830 and 81501077), the
Jiangsu Provincial Development Fund Project (Clinical Application
of 3D Printing Technology in Complex Pelvic Fractures; grant no.
YKK16228) and the Clinical Access Development Fund of Jiangsu
University School of Medicine (Digital Study of Anatomical
Reconstruction of the Clavicular Canal of the Tibial Ligament;
grant nos. JLY20180040 and JLY20160185).
Availability of data and materials
All data generated or analyzed during the present
study were included in this published article.
Authors' contributions
WS and ZZ conceived and designed the study. DL, SB
and ZX performed the experiments. YQ and DW wrote the manuscript
and contributed to the analysis or interpretation of the data. All
authors have read and approved the final manuscript and agreed to
be accountable for all aspects of the research in ensuring that the
accuracy and integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal procedures were approved by the Jiangsu
University School of Medicine and Gaochun People's Hospital Animal
Experiment Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin MC, Medress ZA, Azad TD, Doulames VM
and Veeravagu A: Stem cell therapies for acute spinal cord injury
in humans: A review. Neurosurgical Focus. 46:E102019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Konig N, Trolle C, Kapuralin K, Adameyko
I, Mitrecic D, Aldskogius H, Shortland PJ and Kozlova EN: Murine
neural crest stem cells and embryonic stem cell-derived neuron
precursors survive and differentiate after transplantation in a
model of dorsal root avulsion. J Tissue Eng Regen Med. 11:129–137.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richardson SM, Kalamegam G, Pushparaj PN,
Matta C, Memic A, Khademhosseini A, Mobasheri R, Poletti FL,
Hoyland JA and Mobasheri A: Mesenchymal stem cells in regenerative
medicine: Focus on articular cartilage and intervertebral disc
regeneration. Methods. 99:69–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Z, He Q, Deng W, Chen Q, Hu X, Gong
A, Cao X, Yu J and Xu X: Nasal ectomesenchymal stem cells:
Multi-lineage differentiation and transformation effects on fibrin
gels. Biomaterials. 49:57–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Chen Q, Zhang Z, Zheng Y, Sun X,
Cao X, Gong A, Cui Y, He Q and Jiang P: Fibrin scaffolds containing
ectomesenchymal stem cells enhance behavioral and histological
improvement in a rat model of spinal cord injury. Cells Tissues
Organs. 198:35–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Gao X, Zou H, Liu J and Zhang Z:
Rat nasal respiratory mucosa-derived ectomesenchymal stem cells
differentiate into schwann-like cells promoting the differentiation
of PC12 cells and forming myelin in vitro. Stem Cells Int.
2015:3289572015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdallah B and Kassem M: Human mesenchymal
stem cells: From basic biology to clinical applications. Gene Ther.
15:109–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sahni V and Kessler JA: Stem cell
therapies for spinal cord injury. Nat Rev Neurol. 6:363–372. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Badarau E, Collighan RJ and Griffin M:
Recent advances in the development of tissue transglutaminase (TG2)
inhibitors. Amino Acids. 44:119–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belkin AM: Extracellular TG2: Emerging
functions and regulation. FEBS J. 278:4704–4716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zemskov EA, Janiak A, Hang J, Waghray A
and Belkin AM: The role of tissue transglutaminase in cell-matrix
interactions. Front Biosci. 11:1057–1076. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collighan RJ and Griffin M:
Transglutaminase 2 cross-linking of matrix proteins: Biological
significance and medical applications. Amino Acids. 36:659–670.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanchan K, Fuxreiter M and Fésüs L:
Physiological, pathological, and structural implications of
non-enzymatic protein-protein interactions of the multifunctional
human transglutaminase 2. Cell Mol Life Sci. 72:3009–3035. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lorand L and Graham RM: Transglutaminases:
Crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell
Biol. 4:140–156. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soluri MF, Boccafoschi F, Cotella D, Moro
L, Forestieri G, Autiero I, Cavallo L, Oliva R, Griffin M, Wang Z,
et al: Mapping the minimum domain of the fibronectin binding site
on transglutaminase 2 (TG2) and its importance in mediating
signaling, adhesion, and migration in TG2-expressing cells. FASEB
J. 33:2327–2342. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tucholski J: TG2 protects neuroblastoma
cells against DNA-damage-induced stress, suppresses p53 activation.
Amino Acids. 39:523–532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Strien ME, Drukarch B, Bol JG, van der
Valk P, van Horssen J, Gerritsen WH, Breve JJ and van Dam AM:
Appearance of tissue transglutaminase in astrocytes in multiple
sclerosis lesions: A role in cell adhesion and migration? Brain
Pathol. 21:44–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pitolli C, Pietroni V, Marekov L,
Terrinoni A, Yamanishi K, Mazzanti C, Melino G and Candi E:
Characterization of TG2 and TG1-TG2 double knock-out mouse
epidermis. Amino Acids. 49:635–642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ginn SL, Amaya AK, Alexander IE, Edelstein
M and Abedi MR: Gene therapy clinical trials worldwide to 2017: An
update. J Gene Med. 20:e30152018. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu Y, Zhao J, Wang Y and Gao Z: Silencing
ephrinB3 improves functional recovery following spinal cord injury.
Mol Med Rep. 9:1761–1766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ribault A, Loinard C, Flamant S, Lim S and
Tamarat R: Exosomes derived from human mesenchymal stromal cells
promote wound healing in a mouse model of radiation-induced injury.
Cytotherapy. 20:e2–e3. 2018. View Article : Google Scholar
|
|
22
|
Yuan X, Wu Q, Wang P, Jing Y, Yao H, Tang
Y, Han R, He W, Li Z, Zhang H and Xiu R: Intraspinal administration
of interleukin-7 promotes neuronal apoptosis and limits functional
recovery through JAK/STAT5 pathway following spinal cord injury.
Biochem Biophys Res Commun. 514:1023–1029. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caglar YS, Demirel A, Dogan I, Huseynov R,
Eroglu U, Ozgural O, Cansiz C, Bahadir B, Kilinc MC and Al-Beyati
ESM: Effect of riluzole on spinal cord regeneration with
hemisection method before injury. World Neurosurg. 114:e247–e253.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Q, Zhang Z, Liu J, He Q, Zhou Y, Shao
G, Sun X, Cao X, Gong A and Jiang P: A fibrin matrix promotes the
differentiation of EMSCs isolated from nasal respiratory mucosa to
myelinating phenotypical schwann-like cells. Mol Cells. 38:221–228.
2015.PubMed/NCBI
|
|
25
|
Deng W, Shao F, He Q, Wang Q, Shi W, Yu Q,
Cao X, Feng C, Bi S, Chen J, et al: EMSCs Build an All-in-One niche
via cell-cell lipid raft assembly for promoted neuronal but
suppressed astroglial differentiation of neural stem cells. Adv
Mater. 31:e18068612019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Egawa N, Lok J, Washida K and Arai K:
Mechanisms of axonal damage and repair after central nervous system
injury. Transl Stroke Res. 8:14–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Wu H, Chen G, Huang X, Shan Y, Shi
H, Zhang Q and Zheng Y: Genetically engineered recombinant
adenovirus expressing interleukin-2 for hepatocellular carcinoma
therapy. Mol Med Rep. 17:300–306. 2018.PubMed/NCBI
|
|
28
|
Sandner B, Ciatipis M, Motsch M, Soljanik
I, Weidner N and Blesch A: Limited functional effects of subacute
syngeneic bone marrow stromal cell transplantation after rat spinal
cord contusion injury. Cell Transplant. 25:125–139. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Guo W, Xiong M, Zhang S, Han H, Chen
J, Mao D, Yu H and Zeng Y: Erythropoietin facilitates the
recruitment of bone marrow mesenchymal stem cells to sites of
spinal cord injury. Exp Ther Med. 13:1806–1812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ibarretxe G, Crende O, Aurrekoetxea M,
García-Murga V, Etxaniz J and Unda F: Neural crest stem cells from
dental tissues: A new hope for dental and neural regeneration. Stem
Cells Int. 2012:1035032012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Li Z, Deng W, He Q, Wang Q, Shi
W, Chen Q, Yang W, Spector M, Gong A, et al: Ectoderm mesenchymal
stem cells promote differentiation and maturation of
oligodendrocyte precursor cells. Biochem Biophys Res Commun.
480:727–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vanella L, Raciti G, Barbagallo I,
Bonfanti R, Abraham N and Campisi A: Tissue transglutaminase
expression during neural differentiation of human mesenchymal stem
cells. CNS Neurol Disord Drug Targets. 14:24–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hung CC, Lin CH, Chang H, Wang CY, Lin SH,
Hsu PC, Sun YY, Lin TN, Shie FS, Kao LS, et al: Astrocytic GAP43
induced by the TLR4/NF-κB/STAT3 axis attenuates
astrogliosis-mediated microglial activation and neurotoxicity. J
Neurosci. 36:2027–2043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Locatelli F, Corti S, Donadoni C, Guglieri
M, Capra F, Strazzer S, Salani S, Del Bo R, Fortunato F, Bordoni A
and Comi GP: Neuronal differentiation of murine bone marrow Thy-1-
and Sca-1-positive cells. J Hematother Stem Cell Res. 12:727–734.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stephens P, Grenard P, Aeschlimann P,
Langley M, Blain E, Errington R, Kipling D, Thomas D and
Aeschlimann D: Crosslinking and G-protein functions of
transglutaminase 2 contribute differentially to fibroblast wound
healing responses. J Cell Sci. 117:3389–3403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nelea V, Nakano Y and Kaartinen MT: Size
distribution and molecular associations of plasma fibronectin and
fibronectin crosslinked by transglutaminase 2. Protein J.
27:223–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chau DY, Brown SV, Mather ML, Hutter V,
Tint NL, Dua HS, Rose FR and Ghaemmaghami AM: Tissue
transglutaminase (TG-2) modified amniotic membrane: A novel
scaffold for biomedical applications. Biomed Mater. 7:0450112012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pavlyukov MS, Antipova NV, Balashova MV
and Shakhparonov MI: Detection of transglutaminase 2 conformational
changes in living cell. Biochem Biophys Res Commun. 421:773–779.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Li X, Jing Z, Kawazoe N and Chen G:
Induction of chondrogenic differentiation of human mesenchymal stem
cells by biomimetic gold nanoparticles with tunable RGD density.
Adv Healthc Mater. 62017.doi: 10.1002/adhm.201700317.
|
|
40
|
Pollock K, Dahlenburg H, Nelson H, Fink
KD, Cary W, Hendrix K, Annett G, Torrest A, Deng P, Gutierrez J, et
al: Human mesenchymal stem cells genetically engineered to
overexpress brain-derived neurotrophic factor improve outcomes in
huntington's disease mouse models. Mol Ther. 24:965–977. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barde YA, Edgar D and Thoenen H:
Purification of a new neurotrophic factor from mammalian brain.
EMBO J. 1:549–553. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jia Y, Wu D, Zhang R, Shuang W, Sun J, Hao
H, An Q and Liu Q: Bone marrow-derived mesenchymal stem cells
expressing the Shh transgene promotes functional recovery after
spinal cord injury in rats. Neurosci Lett. 573:46–51. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mifflin KA, Frieser E, Benson C, Baker G
and Kerr BJ: Voluntary wheel running differentially affects disease
outcomes in male and female mice with experimental autoimmune
encephalomyelitis. J Neuroimmunol. 305:135–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jambrovics K, Uray IP, Keressztesy Z,
Keillor JW, Fésüs L and Balajthy Z: Transglutaminase 2 programs
differentiating acute promyelocytic leukemia cells in all-trans
retinoic acid treatment to inflammatory stage through NF-kB
activation. Haematologica. 104:505–515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Feola J, Barton A, Akbar A, Keillor J and
Johnson GVW: Transglutaminase 2 modulation of NF-κB signaling in
astrocytes is independent of its ability to mediate astrocytic
viability in ischemic injury. Brain Res. 1668:1–11. 2017.
View Article : Google Scholar : PubMed/NCBI
|