Introduction
Gastric cancer is one of the most common human
gastrointestinal cancers and remains the second leading cause of
cancer-associated mortality worldwide (1,2).
Gastric cancer has the highest morbidity and mortality rate amongst
the digestive system-derived cancers (3,4).
Previous studies have demonstrated that apoptosis resistance of
gastric cancer is inevitable as the cancer progresses (5,6).
Tumor resistance to apoptosis is currently the greatest challenge
in gastric cancer therapy as it often leads to tumor metastasis
(7,8). Previous study has indicated that
targeted therapies for advanced gastric cancer are effective
(9). However, the treatment of
patients with gastric cancer is particularly challenging for those
with apoptotic resistance and tumor metastasis. Therefore, the
discovery of more effective therapeutic targets in gastric cancer
is essential.
Long non-coding RNAs (lncRNAs) are endogenous
cellular non-coding RNA molecules >200 nucleotides in length
that regulate gene expression and may function tumor cell survival
(10). Various lncRNAs have also
been associated with human cancer cell migration and metastasis
(11,12). lncRNA-maternally expressed gene 3
(MEG3) overexpression has been demonstrated to in part contribute
to the growth inhibition of epithelial ovarian cancer cells
(13). lncRNA-MEG3 has drawn
increasing attention due to its ability to act as a tumor
suppressor in several cancers (14–16).
However, the effect of lncRNA-MEG3 in gastric cancer is not yet
fully understood.
In the present study, the role of lncRNA-MEG3 in
gastric cancer cells in vitro and in vivo was
investigated. It has been reported that epithelial-to-mesenchymal
transition (EMT) could mediate gastric cancer progression (17,18).
EMT is recognized as an important mechanism for cancer metastasis;
it relies on epithelial cell morphology changes from epithelial
cobblestone phenotype to elongated fibroblast phenotype (17). The process of EMT involves the
disassembly of cell-cell junctions, actin cytoskeleton
reorganization and enhancement of cell motility and invasion
(18). Zinc-finger E-box binding
homeobox 1 (ZEB1) is expressed at the invasive front of carcinomas
where it affects gene expression to induce EMT, which upregulates
expression of vimentin and downregulates expression of E-cadherin,
which is a key event for EMT and metastasis (19). Furthermore, inhibiting the EMT
process can induce tumor cell apoptosis and autophagy in human
gastric cancer (20), and ZEB1 has
been suggested to be an important inducer of the EMT and a promoter
of tumor metastasis (21).
Therefore, the effects of lncRNA-MEG3 on EMT processes in gastric
cancer cells were analyzed. The role of lncRNA-MEG3 on the
apoptotic resistance of gastric cancer cells induced by the
chemotherapy drug cisplatin was also investigated.
Materials and methods
Cell lines and cell culture
Gastric cancer cell lines HGC-27 (catalog no.
BNCC338546) and BGC-823 (catalog no. BNCC337689), as well as the
normal gastric cell line GES-1 (catalog no. BNCC337970), were all
purchased from BeNa Culture Collection (Beijing, China; http://www.bncc.org.cn). All cells were cultured in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified chamber with 5% CO2.
lncRNA-MEG3 transfection
HGC-27 cells (1×106 cells/well) were
seeded on 6-well plates and were incubated at 37°C for a 24 h prior
to transfection. Cells were subsequently transfected at 37°C for 30
min with 100 µM pcDNA3.1-MEG3 overexpression vector (Thermo Fisher
Scientific, Inc.) or pcDNA3.1 empty vector control using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions. At
48 h post-transfection, efficiency was validated by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
ZEB1 transfection
The human ZEB gene (GenBank: BC107781.1) was
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China) and
amplified by polymerase chain reaction (PCR). Primers were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.) and had
the following sequences: ZEB, forward, 5′-GGGGACAAGCGAGAGGATCAT-3′
and reverse, 5′-TTTGAGGAACAACTTGAATAA-3′; β-actin, forward,
5′-CGGAGTCAACGGATTTGGTC-3′ and reverse, 5′-AGCCTTCTCCATGGTCGTGA-3′.
The thermocycling conditions were as follows: Initial denaturation
94°C for 30 sec; followed by followed by 25 cycles of 94°C for 30
sec, 59°C for 30 sec, 72°C for 2 min and a final elongation step at
72°C for 10 min. HGC-27 cells (1×105 cells/well in a
6-well plate) were transfected at 37°C for 30 min, following
construction of a ZEB1 pcDNA3.1 overexpression plasmid (pZEB; 100
nM; Invitrogen; Thermo Fisher Scientific, Inc.) and Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions was used as the transfection reagent.
The pcDNA3.1 empty vector was used as a transfection negative
control group. HGC-27 cells that were stably infected with pZEB
were selected using 2 µg/µl puromycin (Invitrogen; Thermo Fisher
Scientific, Inc.) 48 h post-infection and subsequently transfected
with lncRNA-MEG3, as aforementioned.
RT-qPCR
Total RNA was extracted from tumor cells
(1×105 cells/well) by using RNAeasy Mini kit (Qiagen
Sciences, Inc., Gaithersburg, MD, USA). cDNA was synthesized at
42°C for 50 min using High Capacity cDNA Reverse Transcription kit
(cat. no. 4368814; Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturer's instrument. lncRNA-MEG3 expression was
detected by qPCR using an ABI7300 Thermocycler (Applied Biosystems;
Thermo Fisher Scientific, Inc.), with β-actin as an endogenous
control (Life Technologies; Thermo Fisher Scientific, Inc.)
(22). Primers were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.) and the sequences were
as follows: lncRNA-MEG3, forward, 5′-ACATGAGGATCACCCATGT-3′
and reverse, 5′-CATGGGTGATCCTCATGT-3′; β-actin,
5′-CGGAGTCAACGGATTTGGTC-3′ and reverse, 5′-AGCCTTCTCCATGGTCGTGA-3′.
Initial denaturation was performed at 94°C for 2 min, which was
followed by 45 cycles at 95°C for 30 sec, 57.2°C for 30 sec and
72°C for 10 min. The reaction mixture (20 µl) contained 50 ng of
genomic DNA, 200 µM dNTPs, 2.5 units TaqDNA polymerase
(Takara Biotechnology Co., Ltd., Dalian, China), 200 µM primer
sequences and SYBR-Green qPCR Master Mix (5 µl; Invitrogen; Thermo
Fisher Scientific, Inc.). Relative mRNA expression was calculated
using 2−ΔΔCq (23);
relative gene expression levels were normalized to β-actin.
MTT assay
The lncRNA-MEG3-transfected HGC-27 cells
(1×103) were seeded into 96-well plates for 48 h at 37°C
in triplicate for each condition. Following incubation, 20 µl MTT
(5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in PBS
solution was added to each well and the plate was incubated for 4
h. The medium was subsequently removed and 100 µl of dimethyl
sulfoxide was added into the wells to dissolve the purple formazan
crystals. Cell proliferation was determined by optical density
using a microplate reader (Molecular Devices, LLC, Sunnyvale, CA,
USA) at wavelength of 490 nm.
Cell growth assay
The lncRNA-MEG3-transfected HGC-27
(1×104) were seeded in six-well plates and cultured in
RPMI-1640 medium at 37°C for 14 days. Medium was subsequently
removed and the cells were fixed with 100% methanol for 20 min at
37°C and stained with 0.1% (w/v) crystal violet for 5 min at 37°C
(Sigma-Aldrich; Merck KGaA). Cell colonies (>500 cells) were
counted in at least three fields under an Olympus BX51 light
microscope (Olympus Corporation, Tokyo, Japan) and counted with
Image-Pro Plus 5.0 software (Media Cybernetics, Inc., Rockville,
MD, USA).
Apoptosis assay
The transfected HGC-27 cells (1×106) were
seeded into 6-well plates for 12 h at 37°C in a humidified
incubator with 5% CO2. Cells were subsequently incubated
with cisplatin (5.0 mg/ml) for 24 h at 37°C. Cells were removed,
collected and washed with PBS three times. Subsequently, cells were
stained with fluorescein isothiocyanate (FITC)-conjugated Annexin V
and propidium iodide using the Annexin V-FITC Apoptosis Detection
kit (BD Biosciences, San Jose, CA, USA), according to
manufacturer's instructions. A flow cytometer (BD Biosciences) was
used to analyze the percentage of apoptotic HGC-27 cells.
Western blot analysis
HGC-27, BGC-823 GES-1 and lncRNA-MEG3 transfected
cells were homogenized in radioimmunoprecipitation assay lysis
buffer containing protease inhibitor (both from Sigma-Aldrich;
Merck KGaA) and centrifuged at 8,000 × g for 10 min at 4°C. A BCA
protein assay kit (Thermo Fisher Scientific, Inc.) was used to
measure protein concentration. A total of 30 µg protein was
separated by 12% SDS-PAGE, as described previously (24). Proteins were transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA), blocked in 5% skimmed milk for 1 h at 37°C and were
subsequently incubated with the following primary antibodies for 12
h at 4°C: Anti-B cell lymphoma-2 (Bcl-2; 1:1,000; cat. no. ab32124;
Abcam, Cambridge, UK), Bcl-2-like protein 2 (Bcl-w; 1:1,000; cat.
ab38629; Abcam), Bcl-associated X protein (Bax; 1:1,000; cat. no.
ab182733); Abcam), Bcl-2 associated agonist of cell death (Bad;
1:1,000; cat. no. ab90435; Abcam), E-cadherin (1:1,000; cat. no.
ab1416; Abcam), vimentin (1:1,000; cat. no. ab92547; Abcam),
fibronectin (1:1,000; cat. no. ab2413; Abcam) and β-actin (1:1,000;
cat. no. ab8227; Abcam). Membranes were then incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
monoclonal secondary antibody (1:2,000; cat. no. 6721; Abcam) or
rabbit anti-mouse IgG monoclonal secondary antibody (1:2,000; cat.
no. 6728; Abcam) for 2 h at 37°C. Protein were visualized using the
SuperSignal West Pico Chemiluminescent Substrate Trial kit (Pierce;
Thermo Fisher Scientific, Inc.). Images were obtained using the
ChemiDoc XRS system with Quantity One software (version 4.0;
Bio-Rad Laboratories, Inc., Hercules, CA, USA), and protein
expression was analyzed using BandScan 5.0 software (Glyko, Inc.,
Novato, CA, USA).
Cell migration and invasion assay
The lncRNA-MEG3-transfected HGC-27 cells were
cultured in Matrigel-coated and -uncoated Transwell inserts (8 µm
pore size; Merck KGaA) for the invasion and migration assays,
respectively. HGC-27 cells (1×104 cells/well) with 150
µl serum free DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) was
placed into the upper chamber and DMEM with 5% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc.) was placed in the
lower chamber. Following 24 h incubation at 37°C, HGC-27 cells in
the upper chamber were removed with a cotton swab and those in the
lower chamber were fixed in 4% paraformaldehyde for 15 min at 37°C,
stained with 0.1% crystal violet dye (Sigma-Aldrich; Merck KGaA)
for 20 min at 37°C and counted at three randomly selected views
using an Olympus BX51 light microscope (Olympus Corporation, Tokyo,
Japan).
Animal study
The present study was approved by the Ethics
Committee of Xi'an Jiao Tong University. A total of 40,
6–8-week-old female C57BL/6 mice (30–35 g) were used (Shanghai SLAC
Laboratory Animals Co., Ltd., Shanghai, China). All mice were
housed in a temperature-controlled environment (23±2°C), 50±5%
humidity, normal atmosphere with a 12-h light/dark cycle and
provided free access to food and water. The lncRNA-MEG3- or
lncRNA-vector control transfected HGC-27 cells (1×107)
were inoculated one time on a single side of the posterior flank
(n=10/group). The tumor volumes were calculated according to the
following formula: Length × width2 × 0.52. On day 28,
mice were euthanized with diethyl ether and 1.5% pentobarbital
sodium (100 mg/kg) tail vein injection, and the tumors were
isolated. The largest tumor size was ~2,000 mm3. Mice
were sacrificed when tumor diameter reached 16 mm.
Immunohistochemical (IHC)
analysis
Gastric tumor xenografts were fixed using 10%
formaldehyde for 2 h at room temperature, embedded in paraffin and
sectioned (4 µm). The sections were deparaffinized in xylene for 15
min at room temperature, rehydrated through graded ethanol
concentrations and endogenous peroxidase activity was blocked with
3% hydrogen peroxide for 10 min at room temperature, as previously
described (25). Tumor sections
were incubated with specific primary antibodies against vascular
endothelial growth factor (VEGF; 1:1,000; cat. no. ab69479; Abcam)
and Bcl-2 (1:1,000; cat. no. ab32124; Abcam) for 12 h at 4°C. Tumor
tissues were then incubated with HRP-conjugated rabbit anti-mouse
IgG monoclonal secondary antibody (1:10,000; cat. no. 6278; Abcam).
A Ventana Benchmark Automated Staining system (Ventana Medical
Systems, Inc., Tucson, AZ, USA) was used to analyze protein
expression in tumor tissues. The staining results were
semi-quantified by the percentage of positively stained cells as
examined using an Olympus BX51 light microscope (magnification,
×400; Olympus Corporation).
Statistical analysis
Data was expressed as the mean ± standard deviation,
and each experiment was performed at least three times. All data
were analyzed by SPSS 19.0 software (IBM Corp., Armonk, NY, USA)
and GraphPad Prism 5.0 (GraphPad Software, Inc. La Jolla, CA, USA)
using Student's t-tests or one-way analysis of variance followed by
Tukey's multiple comparison post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
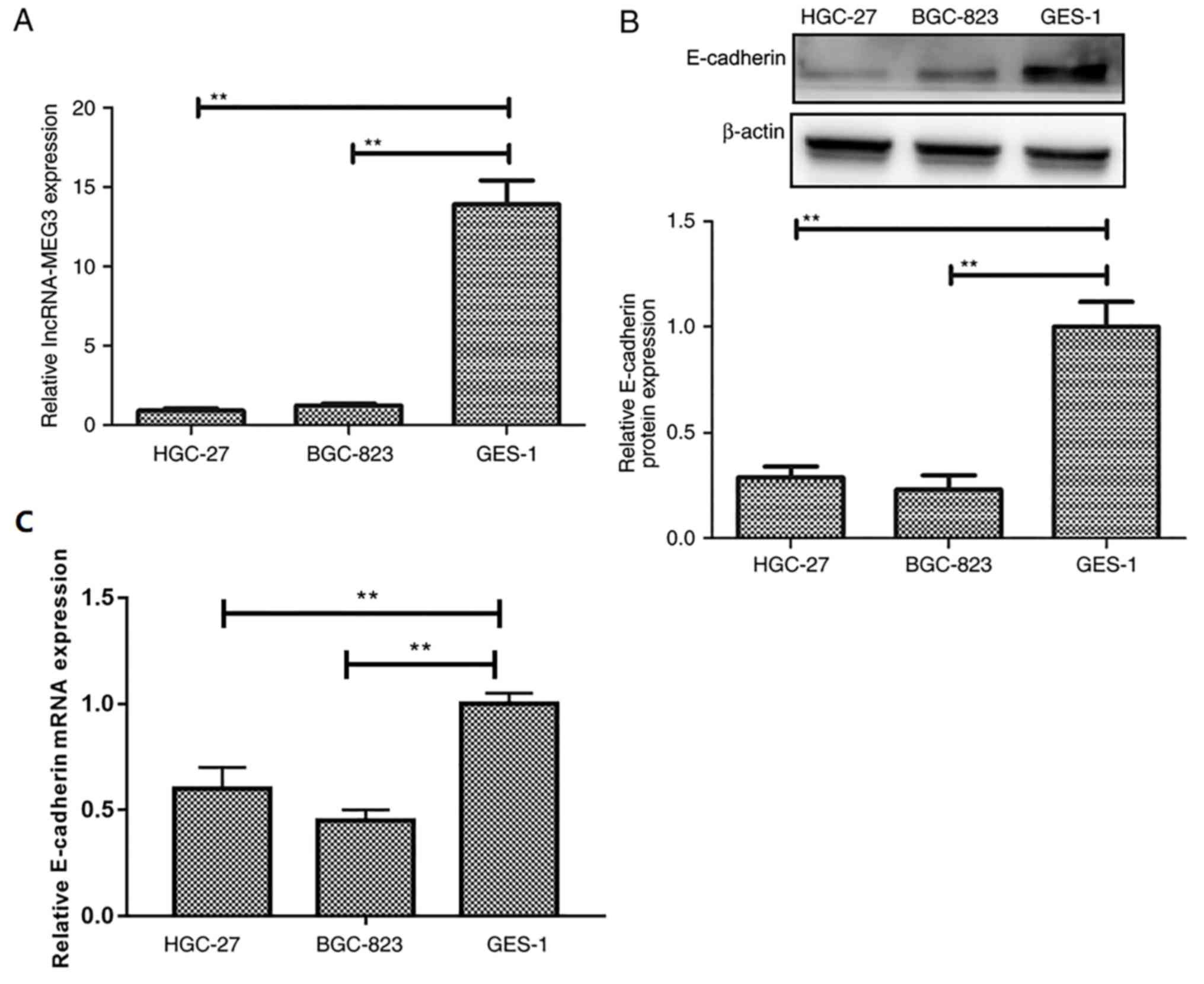
lncRNA-MEG3 expression levels are
decreased in gastric cancer cell lines
To identify the role of lncRNA-MEG3 in gastric
cancer, the expression of lncRNA-MEG3 in gastric cancer cells was
detected by RT-qPCR. lncRNA-MEG3 expression levels were
significantly lower in gastric cancer cell lines HGC-27 and BGC-823
compared with expression in the GES-1 normal gastric cell line
(Fig. 1A). E-cadherin expression
levels were significantly decreased in gastric cancer cell lines
HGC-27 and BGC-823 compared to the normal gastric cell line GES-1
(Fig. 1B and C), which suggested
that lncRNA-MEG3 may be associated with gastric cancer
progression.
lncRNA-MEG3 transfection upregulates
E-cadherin expression in gastric cancer cells
To investigate the role of lncRNA-MEG3 on E-cadherin
expression, E-cadherin gene and protein expression in HGC-27 cells
was detected following lncRNA-MEG3 transfection. lncRNA-MEG3
expression was significantly increased in HGC-27 cells following
overexpression vector transfection, compared with cells transfected
with empty vector (Fig. 2A).
Further, E-cadherin expression levels were significantly increased
in HGC-27 gastric cancer cells following transfection of
lncRNA-MEG3 (Fig. 2B), which
indicated that lncRNA-MEG3 transfection may upregulate E-cadherin
expression in gastric cancer cells.
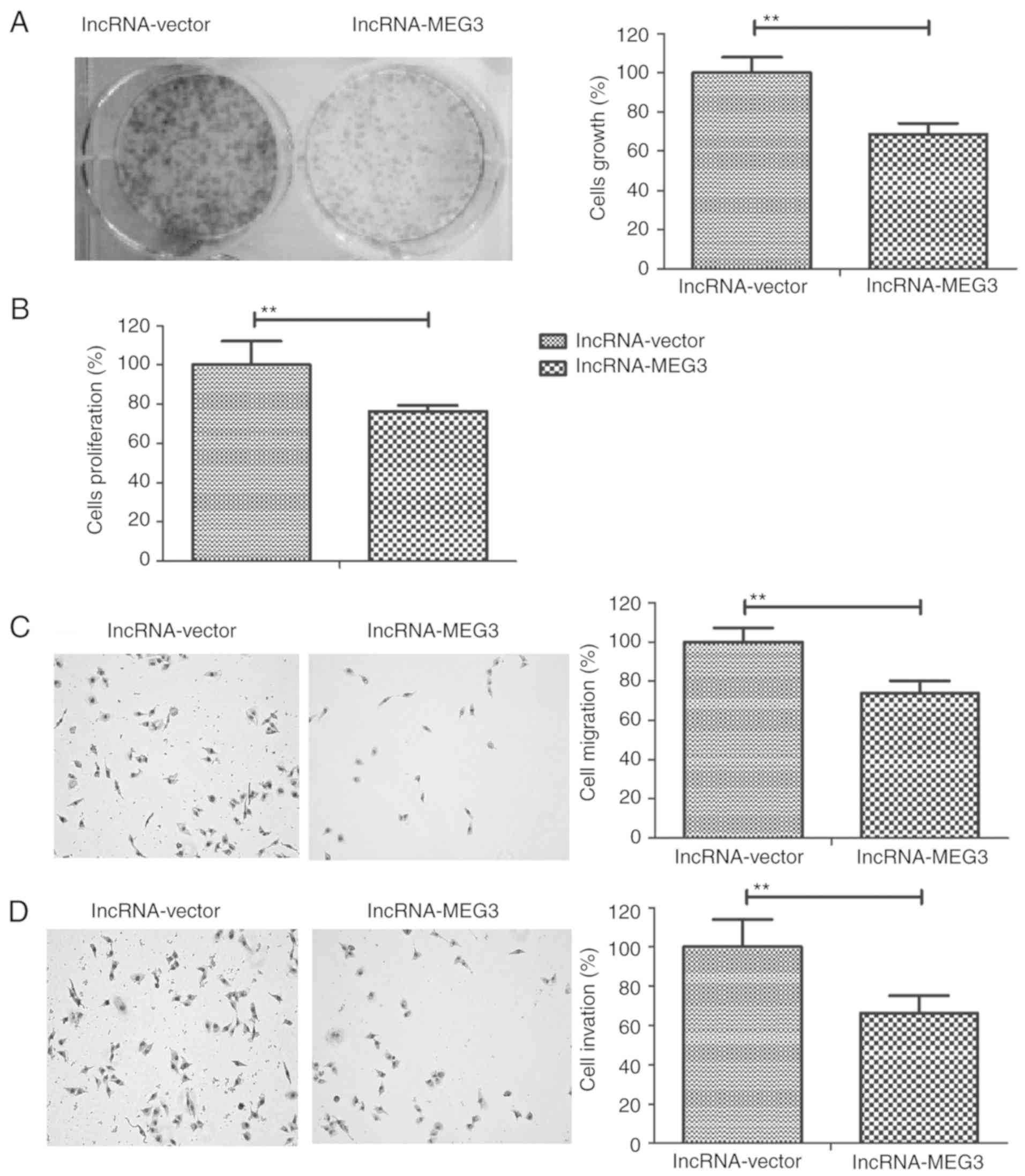
lncRNA-MEG3 inhibits gastric cancer
cell growth, migration and invasion
To investigate the role of lncRNA-MEG3 in gastric
cancer progression, the growth, proliferation, invasion and
migration of HGC-27 cells were measured. The results revealed that
lncRNA-MEG3 transfection decreased HGC-27 cell growth and
proliferation compared to the control group (Fig. 3A and B, respectively). Results from
the migration and invasion assays demonstrated that lncRNA-MEG3
transfection significantly inhibited the migratory and invasive
ability of HGC-27 cells compared with the control group(Fig. 3C and D, respectively). These
results indicated that lncRNA-MEG3 transfection may inhibit gastric
cancer cell growth, proliferation, migration and invasion.
lncRNA-MEG3 promotes apoptosis of
gastric cancer cells
The role of lncRNA-MEG3 in apoptosis of gastric
cancer cells was subsequently investigated. Flow cytometry analysis
revealed that lncRNA-MEG3 transfection further promoted the
apoptotic rates (early + late stage apoptosis) of gastric cancer
compared with the control group (Fig.
4A). Western blot analysis demonstrated that lncRNA-MEG3
transfection downregulated the expression anti-apoptotic proteins
Bcl-2 and Bcl-w (Fig. 4B) and
upregulated the expression of pro-apoptotic proteins caspase-3 and
caspase-9 in gastric cancer cells (Fig. 4C). These results suggested that
lncRNA-MEG3 may promote the apoptosis of gastric cancer cells.
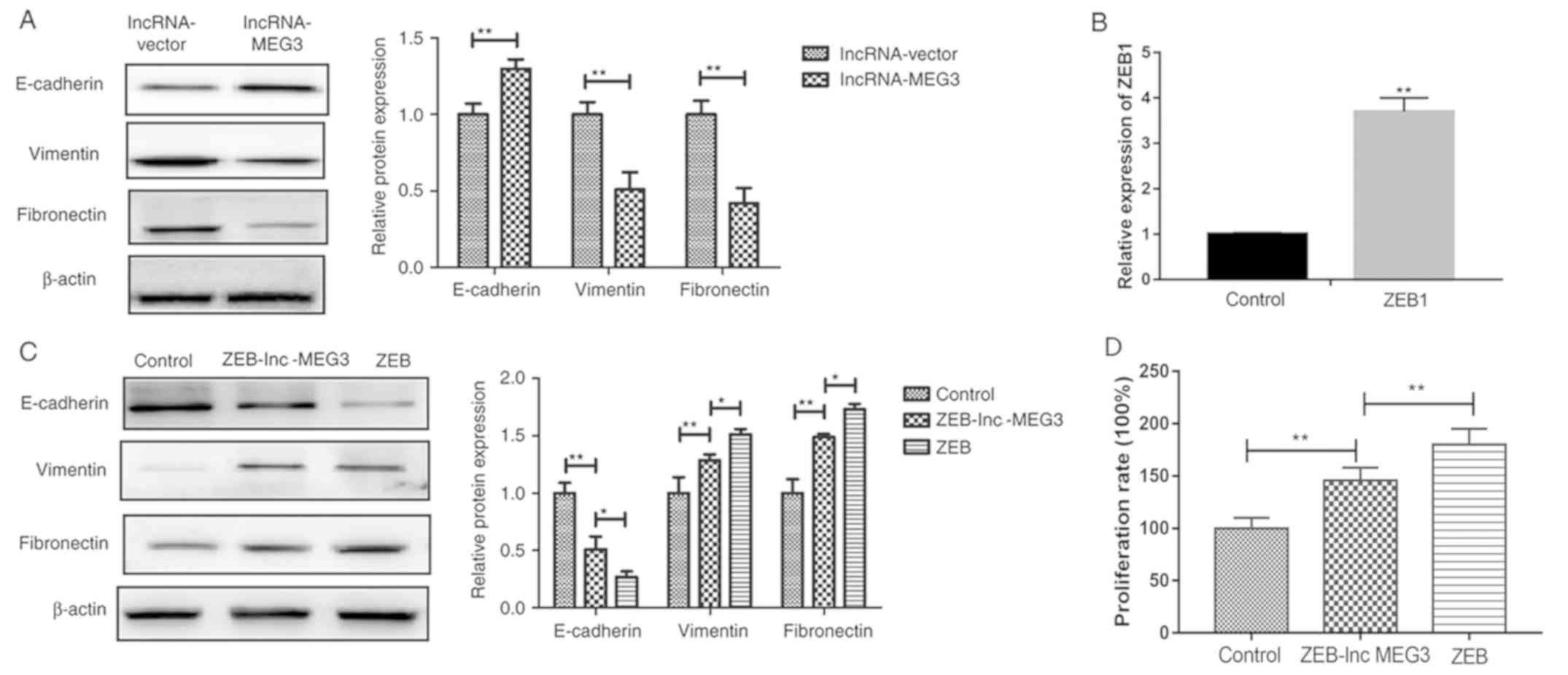
lncRNA-MEG3 inhibits gastric cancer
cells growth via EMT regulation
EMT is involved in the progression of cancer
metastasis (26). To understand
the molecular mechanism by which lncRNA-MEG3 suppressed gastric
cancer growth, EMT marker expression was detected in HGC-27 cells
following lncRNA-MEG3 transfection. lncRNA-MEG3 transfection
significantly increased epithelial marker E-cadherin expression and
significantly inhibited mesenchymal marker vimentin and fibronectin
expression in HGC-27 cells (Fig.
5A). Successful establishment of stable ZEB overexpression
cells was verified by RT-PCR (Fig.
5B). In addition, co-transfection of lncRNA-MEG3 into the ZEB
overexpressing cells upregulated epithelial marker E-cadherin
expression levels and downregulated mesenchymal markers Vimentin
and Fibronectin expression levels and inhibited gastric cancer cell
proliferation compared with ZEB-only transfection group (Fig. 5C and D). These results indicate
that lncRNA-MEG3 may inhibit gastric cancer cell growth via
regulation of EMT.
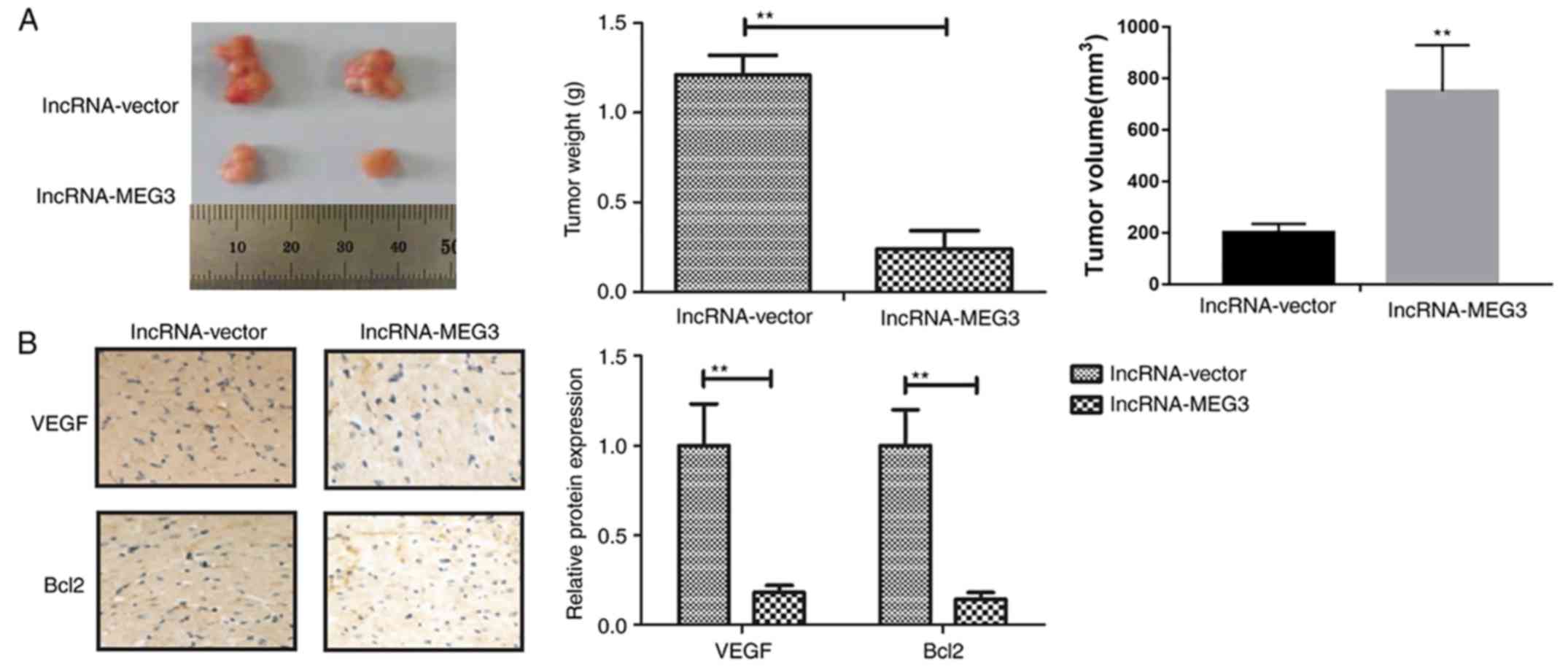
lncRNA-MEG3 inhibits gastric tumor
growth in vivo
To determine whether endogenous expression of
lncRNA-MEG3 was able to affect gastric tumor growth in vivo,
HGC-27 cells stably transfected with lncRNA-MEG3 or empty vectors
were subcutaneously injected into female mice. Transfection with
lncRNA-MEG3 markedly inhibited tumor growth compared to empty
vector group mice following a 28-day inoculation (Fig. 6A). Immunohistochemical analysis
revealed that lncRNA-MEG3 transfection suppressed tumor growth
in vivo, predominantly by decreasing VEGF and Bcl-2
expression (Fig. 6B). These
results suggested that lncRNA-MEG3 expression may inhibit gastric
tumor growth in vivo.
Discussion
Emerging evidence has demonstrated that lncRNAs are
involved in various biological processes, including cell
differentiation, growth, apoptosis and cancer metastasis (27–29).
In recent years, several lncRNAs have been demonstrated to be
associated with gastric tumor growth and metastasis (30,31).
Additionally, lncRNA-MEG3 may act as an inhibitor to regulate
gastric cancer progression (32,33).
In the present study, the role of lncRNA-MEG3 in gastric cancer
cells was analyzed. The results demonstrated that lncRNA-MEG3
transfection promoted the apoptosis and markedly inhibited the
growth and metastasis of gastric cancer cells. lncRNA-MEG3 may also
inhibit gastric cancer cell growth through the regulation of
EMT.
Numerous studies have revealed the emerging
significance of lncRNAs in various human tumors. lncRNAs serves
crucial roles in tumor invasion and metastasis through the
regulation of tumor suppressor and oncogenic pathways. The link
between lncRNAs and tumors is gaining increasing attention in
oncology. A previous study reported that lncRNA-MEG3 upregulation
and downregulation of antisense non-coding RNA at the INK4 locus
may be a clinically relevant strategy in the treatment of
gallbladder cancer (34). The
present study demonstrated that lncRNA-MEG3 transfection
significantly inhibited gastric cancer cell growth, migration and
invasion.
Tumor resistance to apoptosis in human cancer has a
crucial role in metastasis. A previous study indicated that
lncRNA-MEG3 upregulation induced renal cell carcinoma cell
apoptosis by activating the mitochondrial pathway (35). In the present study, lncRNA-MEG3
promoted the apoptosis of gastric cancer cells. lncRNA-MEG3 can
also inhibit the proliferation of cervical carcinoma cells through
the induction of cell cycle arrest and apoptosis (36). It may also inhibit non-small cell
lung cancer cell proliferation and promote apoptosis through the
regulation of p53 expression (37). The present study demonstrated that
lncRNA-MEG3 transfection increased the expression pro-apoptotic
proteins caspase-3 and caspase-9 in gastric carcinoma cells.
Additionally, lncRNA-MEG3 transfection inhibited the expression
anti-apoptotic proteins Bcl-2 and Bcl-w. Thus, the results
indicated that lncRNA-MEG3 transfection reduced the apoptotic
resistance of gastric carcinoma cells.
Several reports have demonstrated that EMT has an
essential role in the regulation of gastric carcinoma cell growth,
proliferation and apoptosis (38,39).
A previous report indicated that EMT in gastric cancer cells can be
induced through the activation of the interleukin-6/signal
transducer and activator of transcription 3 signaling pathway
(40). In the current study,
lncRNA-MEG3 transfection upregulated the expression of the
epithelial marker E-cadherin and downregulated the expression of
mesenchymal markers vimentin and fibronectin in gastric cancer
cells. EMT induction prevented the lncRNA-MEG3
transfection-inhibited growth of gastric cancer cells. The role of
lncRNA-MEG3 in suppressing gastric carcinoma cell proliferation,
migration and invasion was primarily investigated; however, the
present study did not analyze EMT-associated signaling
pathways.
In conclusion, the potential involvement of
lncRNA-MEG3 in gastric cancer was explored. The results suggest
that lncRNA-MEG3 may inhibit the growth of gastric cancer cells
through the regulation of EMT. However, further study is required
to identify other potential mechanisms of lncRNA-MEG3 involvement
in gastric cancer progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
current study are not publicly available due to further research
being performed, but are available from the corresponding author on
reasonable request.
Authors' contributions
JJ and SZ designed the study. JJ and SZ performed
the experiments. SZ analyzed the data.
Ethics approval and consent to
participate
The protocol was approved by The Ethics Committee of
Xi'an Jiao Tong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leake PA, Cardoso R, Seevaratnam R,
Lourenco L, Helyer L, Mahar A, Law C and Coburn NG: A systematic
review of the accuracy and indications for diagnostic laparoscopy
prior to curative-intent resection of gastric cancer. Gastric
Cancer. 15 (Suppl 1):S38–S47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Afuwape OO, Irabor DO, Ladipo JK and
Ayandipo B: A review of the current profile of gastric cancer
presentation in the university college hospital Ibadan, a tertiary
health care institution in the tropics. J Gastrointest Cancer.
43:177–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pimenta-Melo AR, Monteiro-Soares M,
Libânio D and Dinis-Ribeiro M: Missing rate for gastric cancer
during upper gastrointestinal endoscopy: A systematic review and
meta-analysis. Eur J Gastroenterol Hepatol. 28:1041–1049. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Veisani Y and Delpisheh A: Survival rate
of gastric cancer in Iran; A systematic review and meta-analysis.
Gastroenterol Hepatol Bed Bench. 9:78–86. 2016.PubMed/NCBI
|
|
5
|
Xia JT, Chen LZ, Jian WH, Wang KB, Yang
YZ, He WL, He YL, Chen D and Li W: MicroRNA-362 induces cell
proliferation and apoptosis resistance in gastric cancer by
activation of NF-κB signaling. J Transl Med. 12:332014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Shi Y, Li X, Du R, Luo G, Xia L,
Du W, Chen B, Zhai H, Wu K and Fan D: Proteasome inhibitor MG132
reverses multidrug resistance of gastric cancer through enhancing
apoptosis and inhibiting P-gp. Cancer Biol Ther. 7:540–546. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dittmar Y, Altendorf-Hofmann A, Rauchfuss
F, Götz M, Scheuerlein H, Jandt K and Settmacher U: Resection of
liver metastases is beneficial in patients with gastric cancer:
Report on 15 cases and review of literature. Gastric Cancer.
15:131–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roberts P, Seevaratnam R, Cardoso R, Law
C, Helyer L and Coburn N: Systematic review of
pancreaticoduodenectomy for locally advanced gastric cancer.
Gastric Cancer. 15 (Suppl 1):S108–S115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li K and Li J: Current molecular targeted
therapy in advanced gastric cancer: A comprehensive review of
therapeutic mechanism, clinical trials, and practical application.
Gastroenterol Res Pract. 2016:41056152016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong XD, Ren X, Cai MY, Yang JW, Liu X
and Yang JM: Long non-coding RNAs: An emerging powerhouse in the
battle between life and death of tumor cells. Drug Resist Updat.
26:28–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu M, Sun W, Liu Y and Dong X: The role
of lncRNA MALAT1 in bone metastasis in patients with non-small cell
lung cancer. Oncol Rep. 36:1679–1685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Z, Wang R, Zhang T and Dong X:
Hypoxia/lncRNA-AK123072/EGFR pathway induced metastasis and
invasion in gastric cancer. Int J Clin Exp Med. 8:19954–19968.
2015.PubMed/NCBI
|
|
13
|
Li J, Zhou D, Wang Z, Tan L, Zhou Y, Li J
and Sheng X: Reversal effect of 5-aza-2-deoxycytidine on the
maternally expressed gene 3 promoter hypermethylation and its
inhibitory effect on the proliferation of epithelial ovarian cancer
cells. Zhonghua Zhong Liu Za Zhi. 37:324–329. 2015.(In Chinese).
PubMed/NCBI
|
|
14
|
Kruer TL, Dougherty SM, Reynolds L, Long
E, de Silva T, Lockwood WW and Clem BF: Expression of the lncRNA
maternally expressed gene 3 (MEG3) contributes to the control of
lung cancer cell proliferation by the Rb pathway. PLoS One.
11:e01663632016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lv D, Sun R, Yu Q and Zhang X: The long
non-coding RNA maternally expressed gene 3 activates p53 and is
downregulated in esophageal squamous cell cancer. Tumour Biol. Oct
24–2016.(Epub ahead of print). View Article : Google Scholar
|
|
16
|
Yan-Hua L, Xiang-Lei L, Hong L and
Jian-Jun W: Long noncoding ribonucleic acids maternally expressed
gene 3 inhibits lung cancer tumor progression through
downregulation of MYC. Indian J Cancer. 52 (Suppl 3):E190–E193.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y,
Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, et
al: Hypoxia stimulates the EMT of gastric cancer cells through
autocrine TGFβ signaling. PLoS One. 8:e623102013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Liu L, Wang Y, Zhao G, Xie R, Liu
C, Xiao X, Wu K, Nie Y, Zhang H and Fan D: KLF8 involves in
TGF-beta-induced EMT and promotes invasion and migration in gastric
cancer cells. J Cancer Res Clin Oncol. 139:1033–1042. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zha L, Zhang J, Tang W, Zhang N, He M, Guo
Y and Wang Z: HMGA2 elicits EMT by activating the Wnt/β-catenin
pathway in gastric cancer. Dig Dis Sci. 58:724–733. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weng J, Xiao J, Mi Y, Fang X, Sun Y, Li S,
Qin Z, Li X, Liu T, Zhao S, et al: PCDHGA9 acts as a tumor
suppressor to induce tumor cell apoptosis and autophagy and inhibit
the EMT process in human gastric cancer. Cell Death Dis. 9:272018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Zhang N, Wang Y, Xu M, Liu N, Pang
X, Cao J, Ma N, Pang H, Liu L and Zhang H: Zinc finger E-box
binding homeobox 1 promotes invasion and bone metastasis of small
cell lung cancer in vitro and in vivo. Cancer Sci. 103:1420–1428.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao S, Wang J and Xiao N: MicroRNAs as
noninvasive biomarkers in bladder cancer detection: A diagnostic
meta-analysis based on qRT-PCR data. Int J Biol Markers.
31:e276–e285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
Kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernandez-Pol S, Ma L, Ohgami RS and Arber
DA: Immunohistochemistry for p53 is a useful tool to identify cases
of acute myeloid leukemia with myelodysplasia-related changes that
are TP53 mutated, have complex karyotype, and have poor prognosis.
Mod Pathol. 30:382–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loboda A, Nebozhyn MV, Watters JW, Buser
CA, Shaw PM, Huang PS, Van't Veer L, Tollenaar RA, Jackson DB,
Agrawal D, et al: EMT is the dominant program in human colon
cancer. BMC Med Genomics. 4:92011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pei Z, Du X, Song Y, Fan L, Li F, Gao Y,
Wu R, Chen Y, Li W, Zhou H, et al: Down-regulation of lncRNA CASC2
promotes cell proliferation and metastasis of bladder cancer by
activation of the Wnt/β-catenin signaling pathway. Oncotarget.
8:18145–18153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao MX, Jiang YP, Tang YL and Liang XH:
The crosstalk between lncRNA and microRNA in cancer metastasis:
Orchestrating the epithelial-mesenchymal plasticity. Oncotarget.
8:12472–12483. 2017.PubMed/NCBI
|
|
29
|
Zheng Y, Song D, Xiao K, Yang C, Ding Y,
Deng W and Tong S: LncRNA GAS5 contributes to lymphatic metastasis
in colorectal cancer. Oncotarget. 7:83727–83734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Zhang L, Zhang Y and Zhou F:
Increased expression of LncRNA BANCR is associated with clinical
progression and poor prognosis in gastric cancer. Biomed
Pharmacother. 72:109–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hang Q, Sun R, Jiang C and Li Y: Notch 1
promotes cisplatin-resistant gastric cancer formation by
upregulating lncRNA AK022798 expression. Anticancer Drugs.
26:632–640. 2015.PubMed/NCBI
|
|
32
|
Zamani M, Sadeghizadeh M, Behmanesh M and
Najafi F: Dendrosomal curcumin increases expression of the long
non-coding RNA gene MEG3 via up-regulation of epi-miRs in
hepatocellular cancer. Phytomedicine. 22:961–967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peng W, Si S, Zhang Q, Li C, Zhao F, Wang
F, Yu J and Ma R: Long non-coding RNA MEG3 functions as a competing
endogenous RNA to regulate gastric cancer progression. J Exp Clin
Cancer Res. 34:792015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu B, Shen ED, Liao MM, Hu YB, Wu K, Yang
P, Zhou L and Chen WD: Expression and mechanisms of long non-coding
RNA genes MEG3 and ANRIL in gallbladder cancer. Tumour Biol.
37:9875–9886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang M, Huang T, Luo G, Huang C, Xiao XY,
Wang L, Jiang GS and Zeng FQ: Long non-coding RNA MEG3 induces
renal cell carcinoma cells apoptosis by activating the
mitochondrial pathway. J Huazhong Univ Sci Technolog Med Sci.
35:541–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qin R, Chen Z, Ding Y, Hao J, Hu J and Guo
F: Long non-coding RNA MEG3 inhibits the proliferation of cervical
carcinoma cells through the induction of cell cycle arrest and
apoptosis. Neoplasma. 60:486–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei S, Wang L, Zhang L, Li B, Li Z, Zhang
Q, Wang J, Chen L, Sun G, Li Q, et al: ZNF143 enhances metastasis
of gastric cancer by promoting the process of EMT through PI3K/AKT
signaling pathway. Tumour Biol. 37:12813–12821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng S, Zheng Z, Feng L, Yang L, Chen Z,
Lin Y, Gao Y and Chen Y: Proton pump inhibitor pantoprazole
inhibits the proliferation, self-renewal and chemoresistance of
gastric cancer stem cells via the EMT/β-catenin pathways. Oncology
Rep. 36:3207–3214. 2016. View Article : Google Scholar
|
|
40
|
Chen G, Tang N, Wang C, Xiao L, Yu M, Zhao
L, Cai H, Han L, Xie C and Zhang Y: TNF-α-inducing protein
ofi induces epithelial-mesenchymal transition (EMT) in
gastric cancer cells through activation of IL-6/STAT3 signaling
pathway. Biochem Biophys Res Commun. 484:311–317. 2017. View Article : Google Scholar : PubMed/NCBI
|