Introduction
Acute pancreatitis (AP) is a common acute clinical
disease, which, if left untreated may be fatal (1). AP can also induce organ failure with
systemic inflammatory response syndrome (2,3), and
its pathogenesis is not completely understood (2). Accelerated or excessive secretion of
pancreatic juices caused by gallstones or overeating, if not
excreted in time, will induce the activation of inactive pancreatic
enzymes and produce a digestive effect on the autologous pancreas
and the surrounding tissues, leading to various effects on the body
(4). When AP occurs, the body
releases a variety of cytokines and inflammatory mediators such as
interleukin (IL)-1, IL-6, IL-8 and tumor necrosis factor (TNF)-α in
response to the stimuli, leading directly or indirectly to the
inflammatory cascade, stimulating organs and eventually causing
acute respiratory distress syndrome (5). Therefore, it is necessary to find a
drug that can effectively control the progress of such a
disease.
Curcumin is a phenolic pigment extracted from the
rhizome of turmeric (6) and
possesses anti-inflammatory, antioxidant and anticancer effects
(7). Anchi et al (8) demonstrated that sustained-release
curcumin decreased the serum amylase and lipase levels, and
inflammatory cytokines in cerulein-induced acute pancreatitis. Zhu
et al (9) demonstrated that
curcumin may protect the kidney from acute renal injury in an AP
animal model (9). Therefore,
curcumin may be a potential therapeutic option for the treatment of
AP; however, the underlying mechanisms of the protective effects of
curcumin are not completely understood.
Therefore, the role of curcumin in animal and cell
models of AP was investigated. Additionally, the underlying
mechanism was also examined to improve our understanding of the
therapeutic effects of curcumin. The present study indicated the
potential of curcumin in the treatment of AP.
Materials and methods
Establishment of an animal model of AP
and sample collection
The present study was approved by The Ethical Board
of Qilu Hospital of Shandong University. A total of 48 12 week-old
female Sprague Dawley rats (200–220 g) were purchased from The
Model Animal Research Center of Nanjing University. Rats were fed
in a climate-controlled room (20±1°C at 50% humidity) under a 12/12
h light/dark cycle and supplied with clean water and food daily.
All rats were randomly divided into three groups (Sham, AP and
CUR), with 16 rats in each group. For the AP (acute pancreatitis
alone) and CUR (acute pancreatitis + curcumin) groups, rats were
fasted for 12 h before the surgery and were anesthetized by
intraperitoneal injection of 4% chloral hydrate at room
temperature. A laparotomy in the midline of abdomen was performed.
At the level of the hepatic hilum, the biliopancreatic duct was
blocked with a vascular clip and cannulated transduodenally with a
catheter. A solution of 5% sodium taurocholate (Sigma-Aldrich;
Merck KGaA; 1 ml/kg of bodyweight) was slowly injected into the
biliopancreatic duct. For animals in the Sham group, their
biliopancreatic ducts were injected with an equal volume of sterile
physiological saline. Animals in the CUR group were acute
pancreatitic rats receiving an intraperitoneal injection of
curcumin solution (Sigma-Aldrich; Merck KGaA; 200 mg/kg of body
weight) dissolved in DMSO immediately after surgery. Subsequently,
rats were fed in the climate-controlled environment 4 h after the
surgery. At 12, 24 and 36 h after surgery, animals were sacrificed
by CO2 suffocation separately. Blood, ascites and
pancreatic tissues were collected. The weights of the pancreatic
tissues were recorded immediately. The ascites were gathered from
the abdominal cavity with a syringe and transferred to tubes to
immediately measure the volume of ascites.
Assessment of amylase activity and
arterial blood gas (ABG)
The partial pressure (Pa)O2 and
PaCO2, and the serum amylase activity, were measured by
taking blood samples from the abdominal aorta. Samples were
centrifuged at 3,000 × g for 5 min at 4°C. The supernatant was
collected and stored at −80°C. The amylase activity of the serum
and AR42J cells was determined by the G7-PNP method (amylase
activity assay kit; MAK009-1KT; Sigma-Aldrich; Merck KGaA) using a
7600 automatic biochemical analyzer (Hitachi, Ltd.). The
PaO2 and PaCO2 in serum were analyzed by an
ABL80 automatic blood gas analyzer (Radiometer Medical ApS).
Hematoxylin and eosin staining
Pancreatic tissues were fixed with 4% formaldehyde
at room temperature for 2 h after sampling. Tissues were cut into 1
cm ×1 cm ×1 cm cubes and embedded in paraffin and further cut into
sections (thickness, 5 µm) and mounted onto slides. The sections
were dewaxed as follows: i) 5 min in xylene I; ii) 5 min in xylene
II; iii) 5 min in absolute ethanol I; 5 min in absolute ethanol II;
iv) 5 min in 95% ethanol; v) 2 min in 90% ethanol; vi) 2 min in 80%
ethanol; vii) 2 min in 70% ethanol; and viii) 2 min in distilled
water. The sections were stained with hematoxylin and eosin as
follows: i) Stained with hematoxylin for 10 min; ii) washed with
distilled water for 1 min; iii) placed in 1% acidic alcohol
differentiation for 5 sec; iv) washed with distilled water for 1
min; v) placed in 0.2% ammonia for 30 sec; vi) washed with
distilled water for 1 min; vii) stained with eosin for 5 min; and
viii) washed with distilled water for 30 sec. All steps were
performed at room temperature. The sections were then sealed with
neutral balsam and observed under a light microscope
(magnification, ×100 and ×200).
Cell culture and modeling
The AR42J rat acinar cell line was purchased from
the Type Culture Collection of the Chinese Academy of Sciences, and
cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.).
Cerulein (CR; cat. no. C9026) and lipopolysaccharide (LPS; cat. no.
L2630) were both purchased from Sigma-Aldrich (Merck KGaA) and were
used to mimic AP in the AR42J cells. The cells were cultured in
flasks at 37°C with 5% CO2 and passaged every 2–3 days.
To optimize the AP cell modeling, the cells were treated as
follows: i) Medium only; ii) 0.5 nM CR and 0.1 µg/ml LPS; iii) 0.5
µg/ml LPS; iv) 1.0 µg/ml LPS; v) 0.5 nM CR and 0.1 µg/ml LPS; v)
0.5 nM CR and 0.5 µg/ml LPS; and vi) 0.5 nM CR and 1.0 µg/ml LPS,
and termed the Blank, CR, LPS 0.1, LPS 0.5, LPS 1, CR + LPS 0.1 and
CR + LPS 0.5 and CR + LPS 1 groups, respectively. To investigate
the role of curcumin, the cells were treated with either: i) Medium
only; ii) 0.5 nM CR with 1.0 µg/ml LPS; iii) 2.5 mg/l curcumin; iv)
5 mg/l curcumin; v) 10 mg/l curcumin; vi) 2.5 mg/l curcumin, 0.5 nM
CR and 1.0 µg/ml LPS; vii) 5 mg/l curcumin, 0.5 nM CR and 1.0 µg/ml
LPS; and viii) 10 mg/l curcumin, 0.5 nM CR and 1.0 µg/ml LPS, and
were termed the Blank, CR + LPS, CUR 2.5, CUR 5, CUR 10, CUR 2.5 +
CR + LPS, CUR 5 + CR + LPS and CUR 10 + CR + LPS groups,
respectively.
Measurement of IL-6, TNF-α and
C-reactive protein (CRP) levels
The rat IL-6 ELISA kit (cat. no. RAB0311-1KT) rat
TNF-α ELISA kit (cat. no. RAB0480-1KT) and rat CRP ELISA kit (cat.
no. RAB0097-1KT) were all purchased from Sigma-Aldrich (Merck KGaA)
and were used to measure the levels of IL-6, TNF-α and CRP,
respectively, in the serum of rats or in AR42J cells.
Cell Counting Kit 8 (CCK-8) assay
Cell viability was tested using a CCK-8 assay
(MedChemExpress). A total of 4×104 AR42J cells/well were
plated in a 96-well plate in RPMI-1640 medium. The cells were
incubated in a CO2 incubator at 37°C for 24 h, after
which time the cells were further mixed with 10 µl CCK-8 solution,
according to the manufacturer's protocol, and further incubated in
the incubator for 60 min. The optical density (OD) values of wells
were read at 450 nm using a Multiskan™ microplate reader (Thermo
Fisher Scientific, Inc.).
Western blotting
AR42J cells were lysed in RIPA buffer (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol (Thermo Fisher Scientific, Inc.) (10), and centrifuged at 4°C at 16,000 × g
for 30 min. The protein concentration was measured using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). The proteins (20 µg/lane) were resolved using a 10%
SDS-PAGE for 100 min at 100 V. Subsequently, the proteins were
transferred to a PVDF membrane for 20 min at 80 V, followed by
washing the membrane PBS with 0.2% Tween-20 (PBST) three times, and
blocking with 5% non-fat milk for 1 h at room temperature. The
membranes were incubated with the following primary antibodies: i)
Anti-p38 (phospho T180 + Y182) antibody (cat. no. ab4822); ii)
anti-p38 antibody (cat. no. ab170099); or iii) anti-GAPDH antibody
(cat. no. ab9485), all at a 1:500 dilution at 4°C in a shaker
overnight. The membranes were washed three times with PBST and
incubated with a immunoglobulin G H+L-horseradish
peroxidase-conjugated secondary antibody (cat. no. ab6721; 1:2,000)
for 1 h at room temperature. All antibodies used for western
blotting were purchased from Abcam. To visualize the signals, 150
µl Pierce™ enhanced chemiluminescence western blotting substrate
(Thermo Fisher Scientific, Inc.) was added for 1 min. Membranes
were visualized using a ChemiDoc MP (Bio-Rad Laboratories, Inc.).
The gray levels of the blots were measured using ImageJ (version
1.42; National Institutes of Health). GAPDH served as an internal
control.
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments. Differences between groups were analyzed
using one-way ANOVA followed by Bonferroni's post hoc test.
Analysis was performed using SPSS version 16.0 (SPSS, Inc.).
P<0.05 was considered to indicate a statistically
significant.
Results
Curcumin reduces the severity of
AP
To determine whether the severity of AP was
attenuated by curcumin, alterations of several hallmarks
representing the severity of AP, as well as the pathology of the
pancreas from the rats, were examined. The volume of ascites in the
AP group was significantly larger compared with the Sham group 12,
24 and 36 h after the surgery (Fig.
1A; P<0.01). The ascites volume in the curcumin group was
decreased compared with the AP group at 12 and 24 h after the
surgery (P<0.05), although there was no significant difference
between the curcumin and AP groups at 36 h after surgery (Fig. 1A).
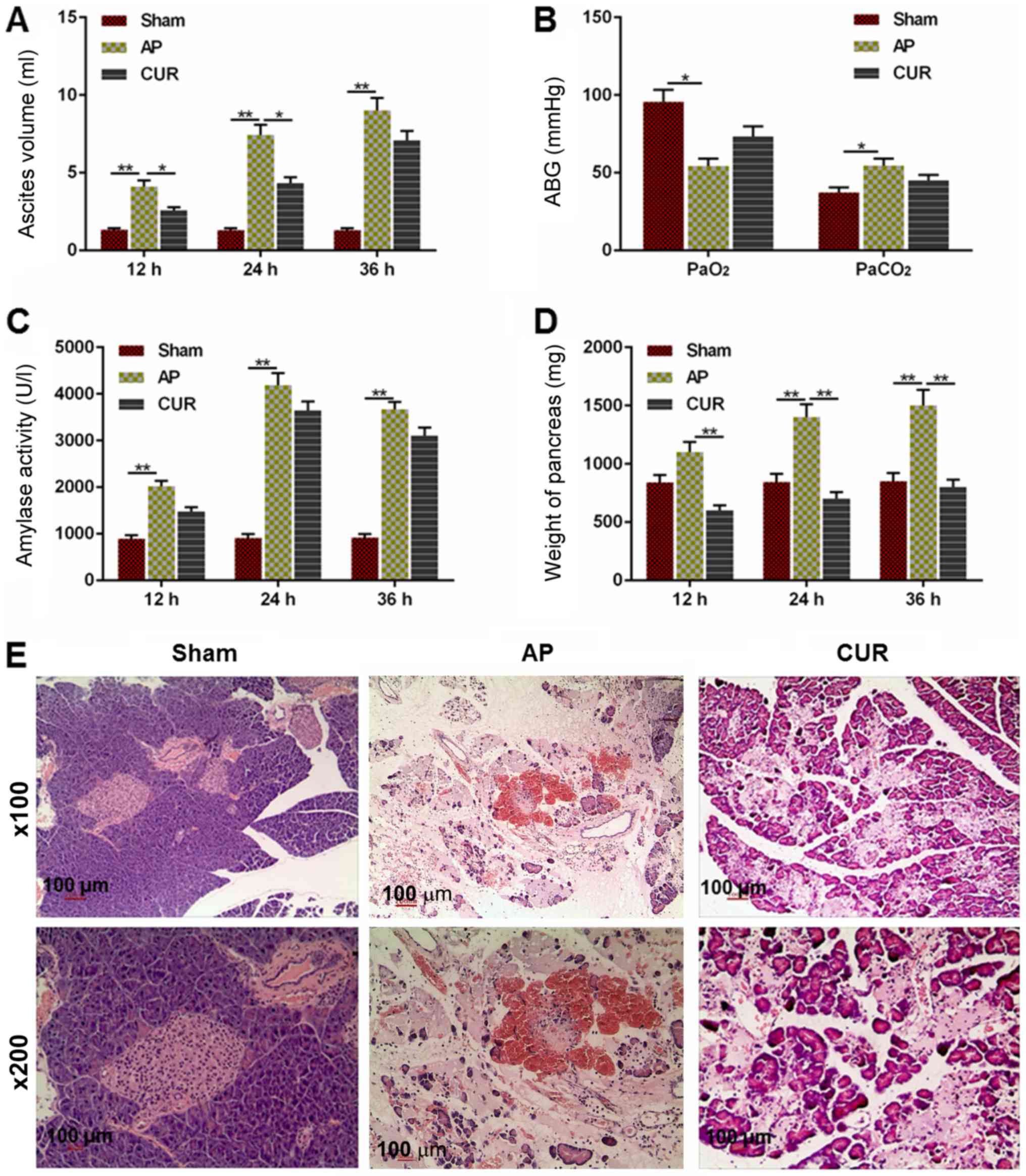 | Figure 1.Extent of AP-induced damage in the
Sham, AP and CUR groups. (A) Ascites volume in each group 12, 24
and 36 h after surgery. (B) ABG level (PaO2 and
PaCO2) in each group. (C) Amylase activity in each group
at 12, 24 and 36 h after surgery. (D) Weight of the pancreas in
each group at 12, 24 and 36 h after surgery. (E) Hematoxylin and
eosin staining of pancreatic tissue in each group at ×100 and ×200
magnification. *P<0.05, **P<0.01. Sham, control; AP, acute
pancreatitis; CUR, curcumin; ABG, arterial blood gas; Pa, partial
pressure. |
The PaO2 level in the AP group was lower
compared with the Sham group (P<0.05) and the PaCO2
in the AP group was elevated compared to the Sham group (Fig. 1B; P<0.05). However, there was no
significant difference between the curcumin and AP groups in terms
of PaO2 or PaCO2 level (Fig. 1B).
Similarly, the amylase in the AP group was
significantly higher compared with Sham group at 12, 24 and 36 h
after surgery (P<0.01); however, there was no significant
difference between the curcumin and AP groups (Fig. 1C). The weight of the pancreas in
the AP group was significantly heavier in the Sham group at 24 and
36 h after surgery (P<0.01), and the weight was significantly
lighter in the curcumin group compared with the AP group at all
time points (P<0.01; Fig. 1D).
As presented in Fig. 1E, the
hematoxylin and eosin staining demonstrated that there was no
observable necrosis of acinar cells in the Sham group, which had
clear lobular and interstitial structures, with no notable
infiltration of inflammatory cells apart from a few edematous
acinar cells (Fig. 1E). The acinar
cells in the AP group were swollen and flaky with a disordered
lobular structure, and a notably larger number of inflammatory
cells. There was also a large amount of inflammatory exudation in
the pancreas tissue in the widened and ruptured interstitial space
and microvessels, with a large number of overflowing erythrocytes
(Fig. 1E). The acinar cells in the
curcumin group were edematous, but the pancreas also displayed a
widened and edematous interstitial space. Necrosis of acinar cells,
infiltration of a large number of inflammatory cells, inflammatory
exudation between the lobes and microvascular rupture, as well as
the effusion of erythrocytes, were also seen in the pancreas
tissue; however, these pathological changes were notably reduced
compared with the AP group (Fig.
1E). Taken together, the results demonstrated that curcumin may
reduce the severity of AP.
Curcumin reduces the inflammatory
response in an animal model of AP
To determine whether the inflammatory response was
affected by curcumin, the levels of IL-6, CRP and TNF-α were
measured by ELISA. The levels of IL-6 and TNF-α in the AP group
were significantly higher compared with the Sham group, whereas the
TNF-α level in the curcumin group was significantly lower compared
with the AP group (all P<0.01; Fig.
2A). However, there was no significant difference in the levels
of IL-6 between the AP and curcumin groups (Fig. 2A). Furthermore, CRP levels in the
AP group were significantly higher compared with both the Sham and
curcumin groups (P<0.01; Fig.
2B), indicating that the inflammatory response was reduced due
to the treatment with curcumin in the AP model.
Curcumin reduces cell viability and
downregulates amylase activity in AR42J cells
To further determine the effect of curcumin on AP,
an AR42J cell line based model was established by treating with
cells with CR and LPS. The cell viability and amylase activity were
measured in addition to the levels of IL-6 and TNF-α. Cell
viability was decreased when the cells were treated with 20 mg/l
curcumin, compared to the cells without treatment (Fig. 3A; P<0.01). Furthermore, amylase
activity in the CR group was increased compared with the blank
group, and the blank group had reduced amylase activity compared to
the CR + LPS 0.1, CR + LPS 0.5 and CR + LPS 1 groups (Fig. 3B; P<0.01). Based on the results
presented in Fig. 3B, 1 µg/ml LPS
alone had no significant effects on amylase activity. Therefore, 1
µg/ml LPS was used in combination with CR for the establishment of
an AP cell model. Amylase activity in the Blank group was
significantly decreased compared with the CR + LPS group
(P<0.01), and the CR + LPS group had elevated levels of amylase
activity compared to the CUR 2.5 + CR + LPS (P<0.05), CUR 5 + CR
+ LPS (P<0.01) and CUR 10 + CR + LPS groups (P<0.01; Fig. 3C). The levels of IL-6 and TNF-α in
cells treated with CR + LPS were significantly increased compared
with the Blank group, although they did not differ significantly
from those in the CR + LPS, CUR 2.5 + CR + LPS, CUR 5 + CR + LPS or
CUR 10 + CR + LPS groups (Fig. 3D and
E; P<0.01). Based on these results, curcumin may attenuate
AP in AR42J cells, but did not affect the inflammatory
response.
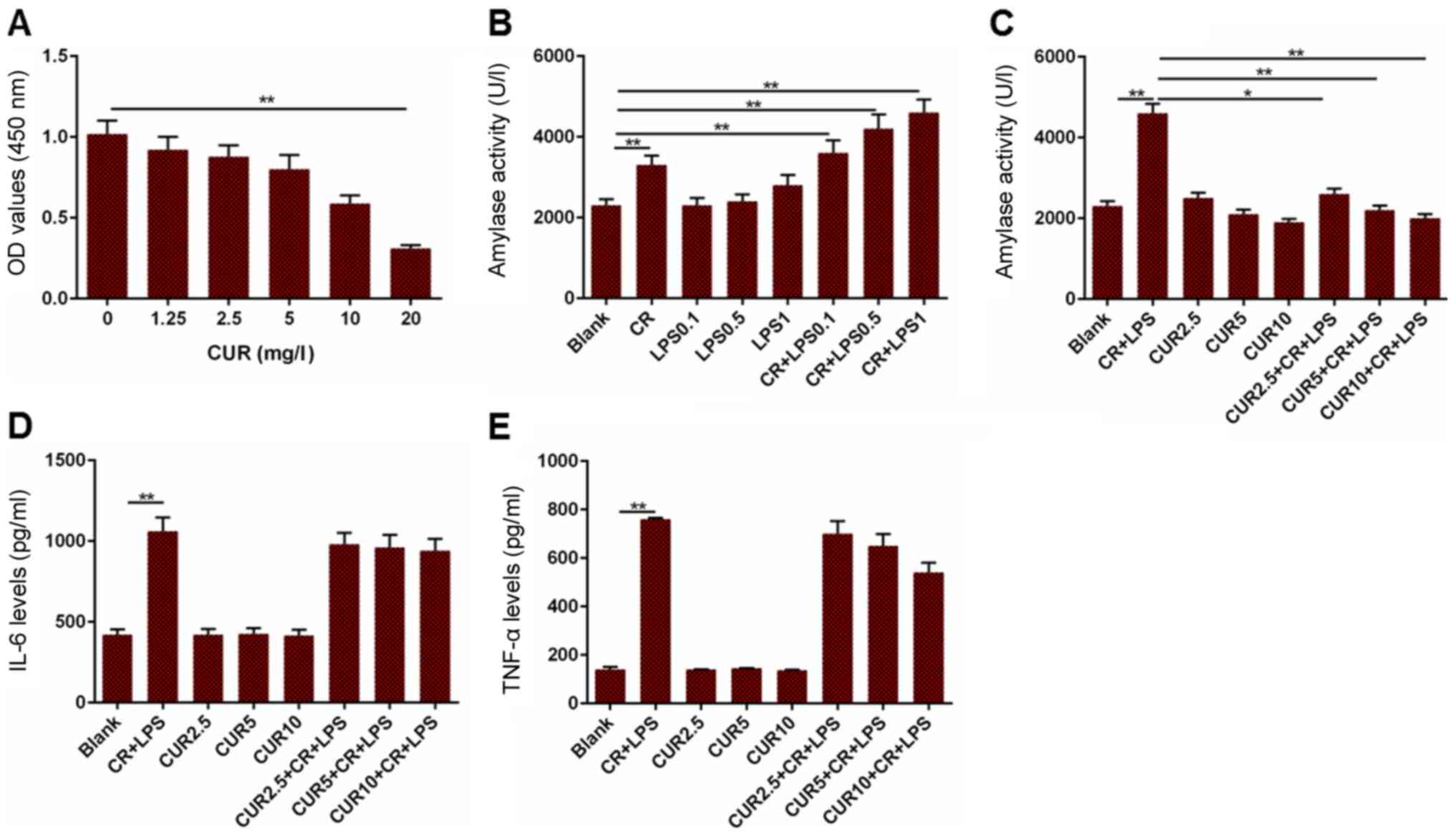 | Figure 3.Measurement of cell viability, amylase
activity, and levels of IL-6 and TNF-α in a cell model of AP. (A)
OD values in cells treated with different concentrations of CUR.
(B) Amylase activity in cells treated with different combinations
CR and LPS to establish a cell model of AP. (C) Amylase activity in
the cell model of AP treated with different concentrations of CUR.
(D) IL-6 levels in the cell model of AP treated with different
concentrations of CUR. (E) TNF-α levels in the cell model of AP
treated with different concentrations of CUR. *P<0.05,
**P<0.01. IL-6, interleukin 6; TNF-α, tumor necrosis factor-α;
OD, optical density; CUR, curcumin; CR, cerulein; LPS,
lipopolysaccharide; AP, acute pancreatitis. |
Curcumin downregulates the
phosphorylation of p38 in AR42J cells
Activation of p38 in AR42J was measured to determine
whether the mitogen-activated protein kinase (MAPK) signaling
pathway was involved in curcumin-mediated apoptosis. The ratio of
phosphorylated (p)-p38/total (t)-p38 in the Blank group was
significantly lower compared with CR + LPS group, and the CR + LPS
group had a significantly higher p-p38/t-p38 ratio compared with
the CUR 10 + CR + LPS group (P<0.01; Fig. 4). The change in the ratio suggests
that the MAPK signaling pathway may be deactivated following
treatment with curcumin in the AP cell model.
Discussion
In the present study, curcumin treatment reduced the
severity of AP and the inflammatory response to AP in an AP animal
model. Furthermore, the viability and amylase activity were also
reduced in an acinar cell line when treated with curcumin.
Therefore, deactivation of p38, the key molecule in the MAPK
signaling pathway, may underlie the beneficial effect of curcumin
on AP.
Ascites volume in the AP animal model was decreased
when treated with curcumin at 12 and 24 h after surgery, and the
weight of the pancreas was additionally reduced at 12, 24 and 36 h
after surgery. In addition, pathologically, the degree of
pancreatic injury was notably reduced when treated with curcumin.
Curcumin treatment did not alter the ABG or amylase activity in the
animal model of AP. Dugernier et al (11) demonstrated that the concentration
of pro-inflammatory factors in ascites was significantly higher
compared with that in the lymph fluid and plasma, and this may
underlie injury to the pancreas. Furthermore, the formation of
ascites was closely associated with the prognosis of patients with
AP (11). Therefore, the
measurement of ascites may reflect the severity of AP indirectly.
ABG is additionally closely associated with the prognosis of
patients with AP (12), thus
PaO2 and PaCO2 were considered important for
assessing the severity of AP. Blood amylase activity is the first
physiological indicator to be altered in acute pancreatitis, and
may directly indicate the severity and therapeutic effect of AP
(13). In the process of AP, the
liquid in the pancreatic tissue is increased, which is an important
hallmark of pathological changes in the pancreatic tissue (14). This buildup of fluid ultimately
leads to edema of the cells (14).
Therefore, the pancreatic weight is measured as an indication of
the liquid contained in the pancreatic tissue (14). Based on the results of the present
study, curcumin may somehow promote the metabolism or removal of
fluid from the pancreatic tissue, resulting in a reduction of the
ascites volume and pancreas weight, thereby preventing further
damage to the pancreatic cells. However, curcumin may not regulate
the activity of amylase and ABG in vivo.
TNF-α is secreted by activated macrophages and
lymphocytes, and is a critical pro-inflammatory cytokine in the
body (15,16). It serves a role in initiating the
development of AP by inducing the expression of IL-1, IL-6, IL-8
and other inflammatory markers, leading directly or indirectly to
the uncontrolled release of the inflammatory mediators (5). IL-6 promotes the upregulation of
neutrophil function and regulates the secretion of cytokines,
adhesion molecules and inflammatory mediators such as nitric oxide,
which are associated with the severity of AP (5,17).
CRP is a sensitive indicator of infectious and non-infectious
inflammation (18). Normally, CRP
serum levels are relatively low; however, when the body is
experiences trauma or during an inflammatory response, CRP is an
early indicator, upregulated during the earliest stages of the
inflammatory response, and thus it is often used as an indicator
for the detection of an inflammatory response in the body (18). In the AP animal model, TNF-α and
CRP levels were downregulated by curcumin, whereas IL-6 was not.
Zhong (19) demonstrated that
curcumin had a protective effect in a rat model of severe AP,
resulting in reduced TNF-α levels. Gulcubuk et al (20) demonstrated that curcumin markedly
reduced serum TNF-α and IL-6 levels in the late phase of AP, but
did not prevent injury to the pancreatic tissue. Fisic et al
(21) demonstrated that CRP was
significantly increased and may be a valuable prognostic factor of
the severity and systemic complications of AP. Based on these
studies, curcumin may suppress the inflammatory response during the
initial stages of inflammation in AP, to some extent.
Furthermore, curcumin decreased cell viability and
downregulated amylase activity in the AP cell model. However, the
levels of IL-6 and TNF-α were not significantly affected in this
model. Bimonte et al (22)
demonstrated that curcumin inhibited tumor growth in a mouse model
of human pancreatic cancer (22).
These results suggest the possibility that curcumin may decrease
the cell viability of acinar cells in pancreatic tissues. There are
numerous in vitro models of AP in acinar cells, the most
frequently used of which was used in the present study (23–25),
and it was demonstrated that 0.5 nM CR in combination with 1 µg/ml
LPS resulted in the largest increase in amylase activity, a
critical hallmark of AP. Based on the results of the present study,
2.5, 5 and 10 mg/l curcumin (below the concentration that
significantly deceased the cell viability) all significantly
decreased amylase activity in the AP cell model compared with the
control. Yu et al (26)
additionally demonstrated that pretreatment with curcumin reduced
the amylase activity in AP rats as well as the levels of IL-6 and
TNF-α. This suggests the possibility that other factors may exist
in pancreatic tissue that affect the activity of curcumin in AP.
Therefore, the mechanism underlying the activity of curcumin in
vitro compared with in vivo in models of AP require
further study.
Phosphorylation of p38 was when cells were treated
with curcumin in the AP cell model, suggesting that the MAPK
signaling pathway was deactivated by curcumin. The MAPK signaling
pathway is a ubiquitous signaling pathway in eukaryotic cells and
includes proteins that belong to the serine/threonine protein
kinase family. This family consists of three primary types of
proteins: p38 MAPK, c-Jun N-terminal kinase (JNK) and extracellular
regulated protein kinases (ERKs) (27). p38 MAPK is a stress-responsive MAPK
and is a pivotal protein during intracellular signal transduction
(28). p38 MAPK participates in
the regulation of various physiological and pathophysiological
processes, including cell differentiation, proliferation, migration
and apoptosis (28). Inflammatory
factors such as IL-1β and TNF-α activate the p38 MAPK signaling
pathway by inducing activation of macrophages to promote apoptosis
of tissue cells (29,30). Phosphorylation of p38 MAPK promotes
the activation of NF-κB and its translocation to the nuclear
domain, thereby stimulating the synthesis of inflammatory factors
such as TNF-α (29,31). However, it stills remains to be
determined which specific regulatory pathway is affected by
curcumin treatment, and whether the same pathway is affected both
in vitro and in vivo.
In conclusion curcumin may lower the severity of the
inflammatory response via the MAPK signal pathway, to some extent,
in in vitro and in vivo models of AP. Further studies
are required to elucidate the specific underlying mechanisms
regulated by curcumin treatment. However, the present study
highlighted the therapeutic potential of curcumin for treating
AP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and CB conceived and designed the study. KW, RW
and JW acquired and interpreted the data. KW and JW drafted the
manuscript and critically revised and added important intellectual
content. The final version of the manuscript has been read and
approved by all of the authors. All authors agree to be accountable
for all aspects of the work, ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The present study was approved by The Ethical Board
of Qilu Hospital of Shandong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Golay V and Roychowdhary A: Acute
pancreatitis in chronic kidney disease-a common but often
misunderstood combination. Ren Fail. 34:1338–1340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cavestro GM, Leandro G, Di Leo M, Zuppardo
RA, Morrow OB, Notaristefano C, Rossi G, Testoni SG, Mazzoleni G,
Alessandri M, et al: A single-centre prospective, cohort study of
the natural history of acute pancreatitis. Dig Liver Dis.
47:205–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okuturlar Y, Soylu A, Dogan H, Cakmak S,
Kirac Utku I, Oztosun B, Akarsu C, Ocak Serin S, Avci A, Kones O,
et al: Mean platelet volume in patients with biliary and
non-biliary acute pancreatitis. Int J Clin Exp Pathol. 8:2051–2056.
2015.PubMed/NCBI
|
|
4
|
Albulushi A, Siddiqi A, Alqarshoubi I,
Aladawi M, Alkhadhouri G and Farhan H: Pattern of acute
pancreatitis in a tertiary care center in oman. Oman Med J.
29:358–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dabrowski A, Osada J, Dabrowska MI,
Wereszczynska-Siemiatkowska U and Siemiatkowski A: Increased
expression of the intercellular adhesion molecule-1 (ICAM-1) on
peripheral blood neutrophils in acute pancreatitis. Adv Med Sci.
59:102–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen SQ, Zhang Y, Xiang JJ and Xiong CL:
Protective effect of curcumin against liver warm
ischemia/reperfusion injury in rat model is associated with
regulation of heat shock protein and antioxidant enzymes. World J
Gastroenterol. 13:1953–1961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jurenka JS: Anti-inflammatory properties
of curcumin, a major constituent of Curcuma longa: A review of
preclinical and clinical research. Altern Med Rev. 14:141–153.
2009.PubMed/NCBI
|
|
8
|
Anchi P, Khurana A, Swain D, Samanthula G
and Godugu C: Sustained-release curcumin microparticles for
effective prophylactic treatment of exocrine dysfunction of
pancreas: A preclinical study on cerulein-induced acute
pancreatitis. J Pharm Sci. 107:2869–2882. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu S, Zhang C, Weng Q and Ye B: Curcumin
protects against acute renal injury by suppressing JAK2/STAT3
pathway in severe acute pancreatitis in rats. Exp Ther Med.
14:1669–1674. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhai KF, Duan H, Khan GJ, Xu H, Han FK,
Cao WG, Gao GZ, Shan LL and Wei ZJ: Salicin from alangium chinense
ameliorates rheumatoid arthritis by modulating the Nrf2-HO-1-ROS
pathways. J Agric Food Chem. 66:6073–6082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dugernier TL, Laterre PF, Wittebole X,
Roeseler J, Latinne D, Reynaert MS and Pugin J:
Compartmentalization of the inflammatory response during acute
pancreatitis: Correlation with local and systemic complications. Am
J Respir Crit Care Med. 168:148–157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polyzogopoulou E, Bikas C, Danikas D,
Koutras A, Kalfarentzos F and Gogos CA: Baseline hypoxemia as a
prognostic marker for pulmonary complications and outcome in
patients with acute pancreatitis. Dig Dis Sci. 49:150–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cöl C, Dinler K, Hasdemir AO and Bugdayci
G: The effect of an intraperitoneal injection of melatonin on serum
amylase levels in acute pancreatitis. JOP. 10:306–309.
2009.PubMed/NCBI
|
|
14
|
Bonior J, Warzecha Z, Ceranowicz P,
Gajdosz R, Pierzchalski P, Kot M, Leja-Szpak A, Nawrot-Porąbka K,
Link-Lenczowski P, Pędziwiatr M, et al: Capsaicin-sensitive sensory
nerves are necessary for the protective effect of ghrelin in
cerulein-induced acute pancreatitis in rats. Int J Mol Sci.
18(pii): E14022017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bishehsari F, Sharma A, Stello K, Toth C,
O'Connell MR, Evans AC, LaRusch J, Muddana V, Papachristou GI and
Whitcomb DC: TNF-alpha gene (TNFA) variants increase risk for
multi-organ dysfunction syndrome (MODS) in acute pancreatitis.
Pancreatology. 12:113–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malleo G, Mazzon E, Siriwardena AK and
Cuzzocrea S: Role of tumor necrosis factor-alpha in acute
pancreatitis: From biological basis to clinical evidence. Shock.
28:130–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Culić O, Eraković V, Cepelak I, Barisić K,
Brajsa K, Ferencić Z, Galović R, Glojnarić I, Manojlović Z, Munić
V, et al: Azithromycin modulates neutrophil function and
circulating inflammatory mediators in healthy human subjects. Eur J
Pharmacol. 450:277–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bezmarevic M, Mirkovic D, Soldatovic I,
Stamenkovic D, Mitrovic N, Perisic N, Marjanovic I, Mickovic S and
Karanikolas M: Correlation between procalcitonin and
intra-abdominal pressure and their role in prediction of the
severity of acute pancreatitis. Pancreatology. 12:337–343. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong K: Curcumin Mediates a Protective
effect via TLR-4/NF-κB signaling pathway in rat model of severe
acute pancreatitis. Cell Biochem Biophys. 73:175–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gulcubuk A, Altunatmaz K, Sonmez K,
Haktanir-Yatkin D, Uzun H, Gurel A and Aydin S: Effects of curcumin
on tumour necrosis factor-alpha and interleukin-6 in the late phase
of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin
Med. 53:49–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fisic E, Poropat G, Bilic-Zulle L, Licul
V, Milic S and Stimac D: The role of IL-6, 8, and 10, sTNFr, CRP,
and pancreatic elastase in the prediction of systemic complications
in patients with acute pancreatitis. Gastroenterol Res Pract.
2013:2826452013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bimonte S, Barbieri A, Palma G, Luciano A,
Rea D and Arra C: Curcumin inhibits tumor growth and angiogenesis
in an orthotopic mouse model of human pancreatic cancer. Biomed Res
Int. 2013:8104232013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin T, Fu Q, Pan YF, Liu CJ, Wang YZ, Hu
MX, Tang Q and Zhang HW: Expressions of miR-22 and miR-135a in
acute pancreatitis. J Huazhong Univ Sci Technolog Med Sci.
34:225–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel K, Durgampudi C, Noel P, Trivedi RN,
de Oliveira C and Singh VP: Fatty acid ethyl esters are less toxic
than their parent fatty acids generated during acute pancreatitis.
Am J Pathol. 186:874–884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Talukdar R, Sareen A, Zhu H, Yuan Z, Dixit
A, Cheema H, George J, Barlass U, Sah R, Garg SK, et al: Release of
cathepsin b in cytosol causes cell death in acute pancreatitis.
Gastroenterology. 151:747–758.e5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu S, Wang M, Guo X and Qin R: Curcumin
attenuates inflammation in a severe acute pancreatitis animal model
by regulating TRAF1/ASK1 signaling. Med Sci Monit. 24:2280–2286.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Samuel I, Zaheer A and Fisher RA: In vitro
evidence for role of ERK, p38, and JNK in exocrine pancreatic
cytokine production. J Gastrointest Surg. 10:1376–1383. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen P, Huang L, Zhang Y, Qiao M and Yuan
Y: SiRNA-mediated PIAS1 silencing promotes inflammatory response
and leads to injury of cerulein-stimulated pancreatic acinar cells
via regulation of the P38MAPK signaling pathway. Int J Mol Med.
26:619–626. 2010.PubMed/NCBI
|
|
29
|
Kim D and Haynes CL: The role of p38 MAPK
in neutrophil functions: Single cell chemotaxis and surface marker
expression. Analyst. 138:6826–6833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HA, Kim KJ, Seo KH, Lee HK and Im SY:
PTEN/MAPK pathways play a key role in platelet-activating
factor-induced experimental pulmonary tumor metastasis. FEBS Lett.
586:4296–4302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SJ, Kim WJ and Moon SK: Role of the
p38 MAPK signaling pathway in mediating interleukin-28A-induced
migration of UMUC-3 cells. Int J Mol Med. 30:945–952. 2012.
View Article : Google Scholar : PubMed/NCBI
|


















