Introduction
Steroid-induced avascular necrosis of the femoral
head (SANFH) is caused by a variety of factors, including
apoptosis, inflammation, reactive oxygen species and oxidative
stress (1). SANFH is characterized
by bone trabeculae and bone marrow necrosis (1). Cases of SANFH are increasing
worldwide and patient outcomes are poor (2). Thus, developing strategies to
effectively prevent and/or treat SANFH is of critical importance
(2).
SANFH is defined by the interruption and impairment
of blood supply to the femoral head, which triggers osteocyte and
bone marrow necrosis (3). This
leads to alterations in the structure of the femoral head, collapse
and joint function disturbance. However, the pathogenesis of SANFH
remains largely unclear (4).
Clinically, femoral head necrosis can be divided into traumatic and
non-traumatic types (4). The
prolonged administration of hormones is a risk factor for
non-traumatic femoral head necrosis (5).
Ginsenoside Rb1 is a dammarane-type triterpenoid
saponin that is predominantly found in Panax plants, such as
ginseng, Panax notoginseng and American ginseng (6). Panax plants are valuable Chinese
herbal medicines with a long history of application (7). Ginsenoside Rb1 harbors a variety of
pharmacological activities in the central nervous system,
cardiovascular system and immune system, as well as anti-tumor,
anti-hepatic ischemia-reperfusion injury, and hypoglycemic effects
(7,8). Shen et al (9) reported that Ginsenoside Rb1 reduces
fatty liver by activating adenosine monophosphate-activated protein
kinase in obese rats. Wang et al (10) reported that Ginsenoside Rb1
inhibits free fatty acidinduced oxidative stress and inflammation
in 3T3L1 adipocytes. The present study was designed to investigate
whether Ginsenoside Rb1 weakened the steroid-induced avascular
necrosis of the femoral head (SANFH) and to explore the possible
mechanisms of the above effects.
Materials and methods
Animals, reagents and in vivo
experiments
Adult male Sprague-Dawley rats (230–260 g; 6–9
weeks; n=30) were housed at 24±2°C and 55±2% humidity, with free
access to food and water, and a 12-h light/dark cycle, at the
Department of Laboratory Animal Science Affiliated to Southwest
Medical University (Luzhou, China). All procedures were approved by
the Ethical Committee of Animal Experimentation at Southwest
Medical University.
Rats were randomly divided into three groups: i)
Blank control group (sham; n=10); ii) model group (n=10); and iii)
Ginsenoside Rb1 treatment group (n=10). Rats in the model and
Ginsenoside Rb1 groups received intragluteal injections of 50 mg/kg
dexamethasone twice per week for 6 weeks. After the induction of
SANFH for 3 weeks, rats in the Ginsenoside Rb1 group received 200
mg/kg/week Ginsenoside Rb1 (Fig.
1A; Shanghai No. 1 Biochemical & Pharmaceutical Co., Ltd.)
by oral gavage for 3 weeks (11).
In the blank control and model groups, rats were given normal
saline for 3 weeks by the same method.
ELISA
Following treatment with Ginsenoside Rb1, blood
samples were collected under anesthesia (35 mg/kg of pentobarbital
sodium) and centrifugated at 12,000 × g for 10 min at 4°C. Serum
osteocalcin (OST; cat. no. H152), total cholesterol (cat. no.
A111-1-1) and the ratio of low-density lipoprotein to high-density
lipoprotein (LDL/HDL; cat. no. A113-1-1 and A112-1-1) were examined
using ELISA (all from Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer's protocol. Absorbance was measured
at 450 nm with a microplate reader (Bio-Rad Laboratories).
Histopathological examination
Following treatment with Ginsenoside Rb1, rats were
sacrificed by decapitation under anesthesia (35 mg/kg pentobarbital
sodium). Femur samples were obtained and fixed in 10% buffered
neutral formalin solution for 3–4 days at room temperature and
decalcified in 10% EDTA, 0.1 M phosphate buffer. Tissue was
dehydrated with ethanol, embedded in paraffin and cut into 4 µm
thick sections. Sections were stained with hematoxylin for 10 min
at room temperature, and rinsed with running water for 15 min.
Sections were then stained with eosin for 30 sec at room
temperature, and double distilled water was used to wash the
sections. The sections were dehydrated using 100% ethanol for 1 min
at room temperature, cleared in xylene and sealed with neutral
balsam. Sections were observed using a Zeiss Axioplan 2 light
microscope (magnification, ×10; Carl Zeiss AG).
Inflammatory factor, oxidative stress,
alkaline phosphatase (ALP), OST and caspase-3 activity
analysis
Femoral heads were washed in PBS and lysed with RIPA
buffer (Beyotime Institute of Biotechnology) on ice for 1 h. The
supernatant was collected by centrifugation at 12,000 × g for 10
min at 4°C. For caspase-3 activity, samples were incubated with
specific colorimetric peptide substrates (Ac-DEVD-pNA; Beyotime
Institute of Biotechnology) for 1 h at 37°C, and the absorbance was
measured at 405 nm with a microplate reader (Eppendorf). ALP (cat.
no. A059-2-2), OST (cat. no. H152), p65 (cat. no. H202), tumor
necrosis factor (TNF)-α (cat. no. H052), interleukin (IL)-1β (cat.
no. H002), IL-6 (cat. no. H007), malondialdehyde (MDA; cat. no.
A003-1-2), superoxide dismutase (SOD; cat. no. A001-3-2),
chloramphenicol acetyltransferase (CAT; cat. no. A007-1-1) and
glutathione peroxidase (GSH-PX; cat. no. A005-1-2) expression was
measured using ELISA kits (all from Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
protocol. Absorbance was measured at 450 nm with a microplate
reader (Eppendorf).
Western blot analysis
Femoral heads were washed in PBS and lysed with RIPA
buffer on ice for 1 h. The supernatant was collected by
centrifugation at 12,000 × g for 10 min at 4°C and protein
concentration was determined with a bicinchoninic acid protein
assay. Proteins (50 µg/lane) were separated by 8–10% SDS-PAGE and
transferred to polyvinylidene fluoride membranes. Membranes were
blocked with 5% non-fat milk in TBST for 1 h at 37°C and incubated
with anti-apoptosis regulator BAX (Bax; 1:500; cat. no. sc-20067;
Santa Cruz Biotechnology, Inc.), anti-cellular tumor antigen p53
(p53; 1:500; cat. no. sc-47698; Santa Cruz Biotechnology, Inc.),
anti-vascular endothelial growth factor (VEGF) receptor (VEGFR;
1:2000; cat. no. 2479; Cell Signaling Technology, Inc.), anti-VEGF
(1:500; cat. no. sc-7269; Santa Cruz Biotechnology, Inc.),
anti-Runt-related transcription factor 2 (RUNX2; 1:500; cat. no.
sc-390715; Santa Cruz Biotechnology, Ltd.), anti-bone morphogenetic
protein 2 (BMP2; 1:500; cat. no. sc-137087; Santa Cruz
Biotechnology, Ltd.) and anti-GAPDH (1:2,000; cat. no. sc-69778;
Santa Cruz Biotechnology, Ltd.) primary antibodies at 4°C
overnight. Following washing with TBST, the membranes were
incubated with a goat anti-rabbit IgG antibody conjugated to
horseradish peroxidase (1:5,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) for 1 h at 37°C. Membranes were visualized
using BeyoECL Moon reagent (Beyotime Institute of Biotechnology)
and analyzed with Quantity One software (version 3.0; Bio-Rad
Laboratories, Inc.).
Statistical analysis
All data were expressed as the mean ± standard
deviation and analyzed using SPSS 17.0 (SPSS, Inc.). A minimum of
three independent experiments were carried out and were analyzed by
one-way analysis of variance and Tukey's post hoc test. using SPSS
17.0. P<0.05 was considered to indicate a statistically
significant difference.
Results
Ginsenoside Rb1 improves ALP and OST
activities in the SANFH rat model
To assess the effects of Ginsenoside Rb1 on ALP and
OST activity in the SANFH rat model, ALP and OST activities were
determined using ELISA kits. Firstly, H&E staining showed that
the bone cell number was reduced in SANFH rats, compared with the
control group (Fig. 1B).
Ginsenoside Rb1 appeared to increase the number of bone cells,
compared with the untreated rat model (Fig. 1B). Furthermore, ALP (Fig. 1C) and OST (Fig. 1D) activity in the rat model was
significantly decreased, compared with the control group.
Ginsenoside Rb1 administration significantly increased ALP and OST
activity, compared with untreated model rats.
Protective effects of Ginsenoside Rb1
on avascular necrosis of the SANFH rat model
Serum OST expression was significantly decreased in
the SANFH model group, compared with the control (Fig. 2A). In addition, the total
cholesterol (Fig. 2B) and LDL/HDL
ratio (Fig. 2C) were higher in the
model group, compared with the control. Treatment with Ginsenoside
Rb1 significantly elevated serum OST expression, and reduced total
cholesterol and LDL/HDL ratio in SANFH rats.
Protective effects of Ginsenoside Rb1
on inflammation of the SANFH rat model
To assess the effects of Ginsenoside Rb1 on
inflammation in SANFH, p65, TNF-α, IL-1β and IL-6 expression was
determined using ELISA kits. p65 (Fig.
3A), TNF-α (Fig. 3B), IL-1β
(Fig. 3C) and IL-6 (Fig. 3D) expression was significantly
upregulated in SANFH model rats, compared with the control group.
Treatment with Ginsenoside Rb1 significantly reduced p65, TNF-α,
IL-1β and IL-6 expression in SANFH rats compared with model
rats.
Ginsenoside Rb1 reduces oxidative
stress in SANFH rats
Next, it was demonstrated that MDA expression was
increased (Fig. 4A), whereas SOD
(Fig. 4B), CAT (Fig. 4C) and GSH-PX (Fig. 4D) expression was decreased in the
model group, compared with the control. Consistently, treatment
with Ginsenoside Rb1 significantly reduced MDA levels, and
increased SOD, CAT and GSH-PX levels in SANFH rats, compared with
the model group.
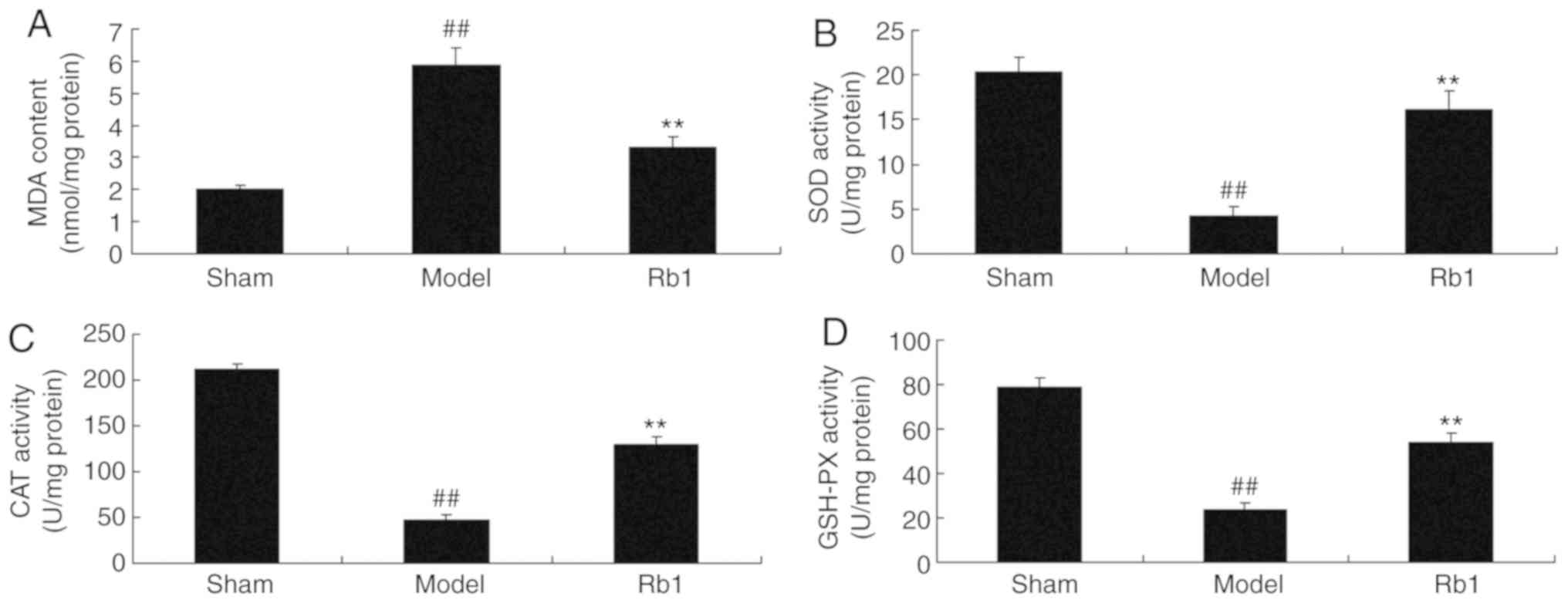 | Figure 4.Protective effects of Ginsenoside Rb1
on oxidative stress in the SANFH rat model. Protective effects of
Ginsenoside Rb1 on (A) MDA, (B) SOD, (C) CAT and (D) GSH-PX. Data
are presented as the mean ± standard deviation.
##P<0.01 vs. Sham; **P<0.01 vs. Model. SANFH,
steroid-induced avascular necrosis of the femoral head; Sham, sham
control group; Model, SANFH model group; Rb1, 200 mg/kg of
Ginsenoside Rb1 group; MDA, malondialdehyde; SOD, superoxide
dismutase; CAT, chloramphenicol acetyltransferase; GSH-PX,
glutathione peroxidase. |
Ginsenoside Rb1 reduces bone cell
death in a rat model of SANFH
The protective effects of Ginsenoside Rb1 against
apoptosis in the femoral head were also explored. Specifically, Bax
and p53 protein expression and caspase-3 activity were measured by
western blotting (Fig. 5A). It was
demonstrated that p53 (Fig. 5B),
Bax (Fig. 5C) and caspase-3
(Fig. 5D) protein expression was
significantly increased in SANFH rats, compared with the control
group. Treatment with Ginsenoside Rb1 significantly reduced the
induction of Bax, p53 and caspase-3 protein expression in SANFH
rats.
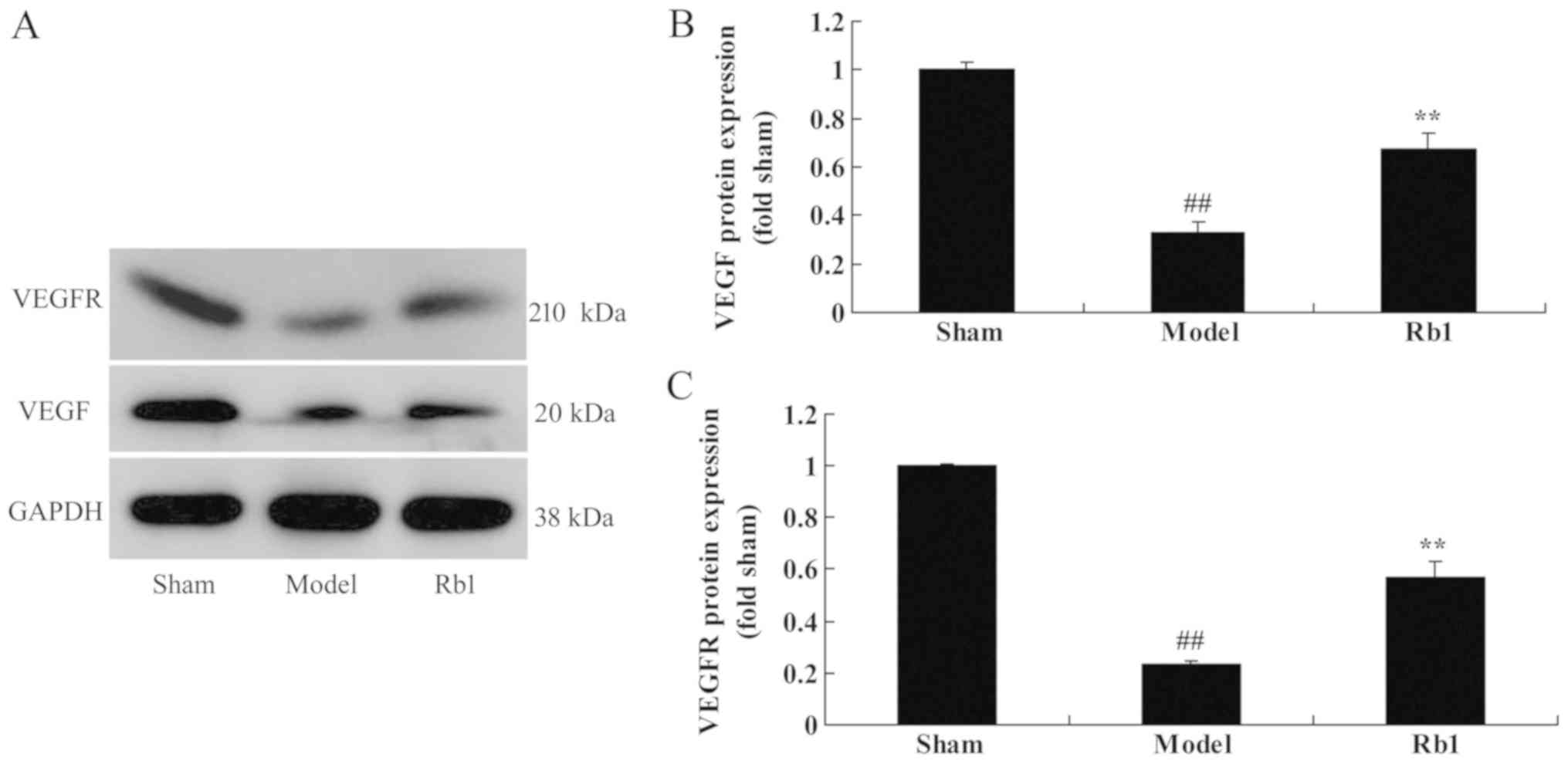
Ginsenoside Rb1 increases VEGF and
VEGFR expression
Western blotting was also used to determine whether
Ginsenoside Rb1 exerted protective effects on VEGFR and VEGF
expression (Fig. 6A). VEGF
(Fig. 6B) and VEGFR (Fig. 6C) protein expression was
significantly suppressed in the model group, compared with
controls. Administration of Ginsenoside Rb1 significantly induced
VEGFR and VEGF protein expression in SANFH rats, compared with the
untreated model group.
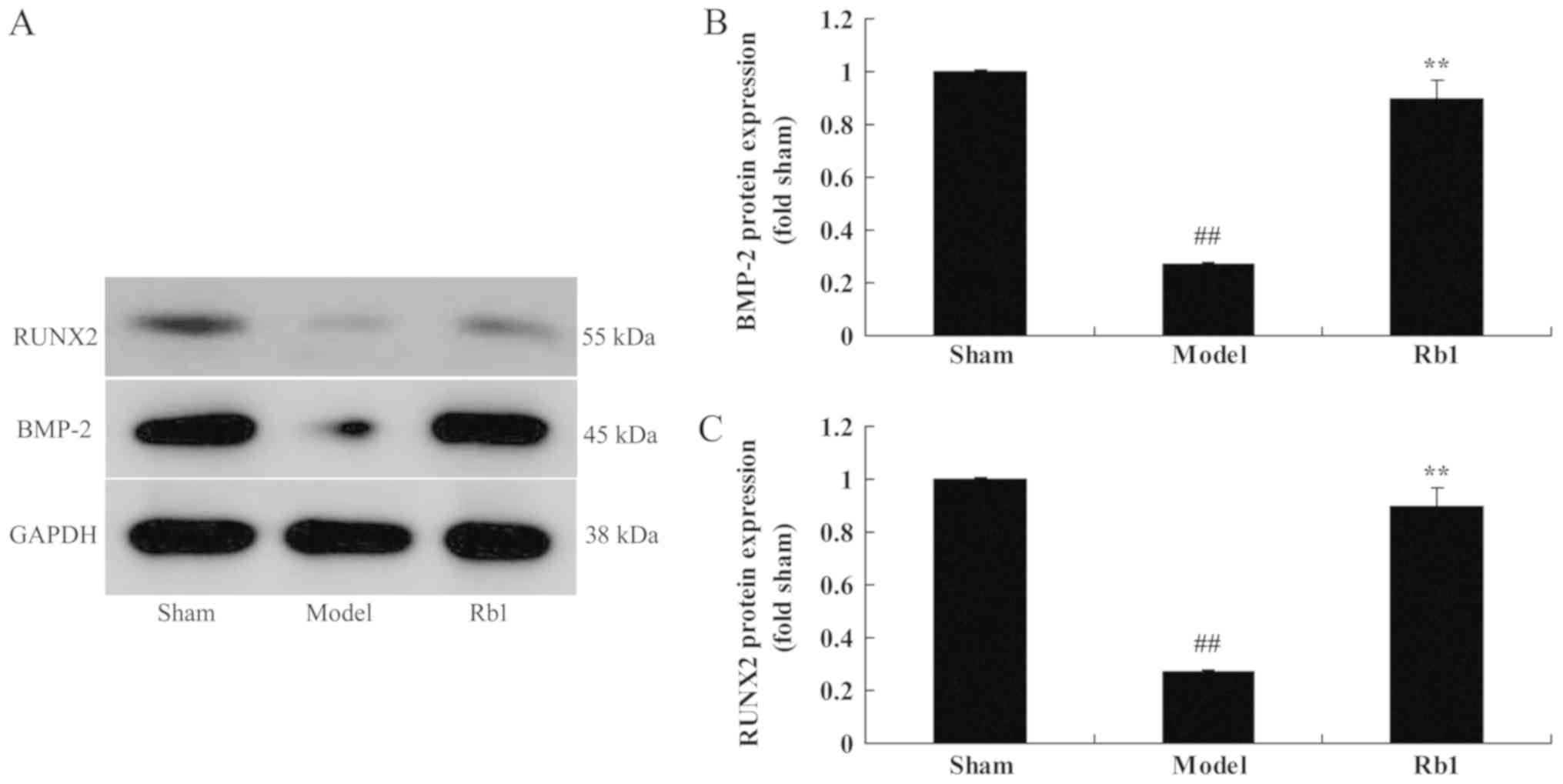
Ginsenoside Rb1 on RUNX2 and BMP-2
protein expression in a rat model
Finally, the protein expression of RUNX2 and BMP-2
was examined by western blot analysis (Fig. 7A). It was revealed that RUNX2
(Fig. 7B) and BMP-2 (Fig. 7C) protein expression in SANFH rats
was lower than that of control group. Treatment with Ginsenoside
Rb1 significantly induced RUNX2 and BMP-4 protein expression in
SANFH rats, compared with SANFH model group.
Discussion
Hyperlipidemia manifests in SANFH throughout the
whole course of the disease (12).
Reduced osteogenic differentiation slows the reparative process and
accelerates femoral head necrosis (13). In addition, hormone administration
results in hyperlipidemia, thrombosis and bone tissue ischemia
(14). Together, this leads to the
development of SANFH. In the present study, Ginsenoside Rb1 was
demonstrated to be protective against steroid-induced avascular
necrosis, which inhibited serum OST expression, reduced oxidative
stress and inhibited bone cell apoptosis in a rat model of
avascular necrosis. Similar to the results of the present study,
Xiang et al (15) reported
that Ginsenoside Rb1 inhibits osteoarthritis by downregulating
Notch signaling, and Zhu et al (16) demonstrated that Ginsenoside Rb1
alleviates aluminum chloride-induced rat osteoblast
dysfunction.
Organisms would induce a repair response following
SANFH (17). The head of the femur
begins to repair from the edge of the sequestrum and the
surrounding living tissues (18).
It is represented as revascularization, osteogenesis and absorption
of dead bones. VEGF plays an important role in osteogenesis by
promoting angiogenesis and inhibiting the apoptosis of chondrocytes
and osteoblasts to affect osteogenesis by means of promoting bone
turnover (19). These results
indicate that Ginsenoside Rb1 significantly induces VEGFR and VEGF
protein expression in SANFH rat. Lan et al (20) has reported that Ginsenoside Rb1
prevents homocysteine-induced dysfunction through via the
VEGF/p38MAPK pathway.
BMP plays an essential role in bone growth and wound
repair. Active mesenchymal cells are the target cells of BMP
(21). Under the induction of BMP,
muscular and perivascular mesenchymal cells can be differentiated
into osteocytes, a process that is specific and irreversible
(22). Recent studies have
suggested that BMP-2 may first combine with and activate Type II
and I receptors on the surface of the membrane (23). Secondly, intracellular signal
transduction pathways of Smad may then be activated to induce the
transcription of intranuclear target genes and protein expressions
(21). Novel research has
suggested that BMP-2 is the most representative in BMP family, with
relatively high contents (23).
Furthermore, osteogenic activity is relatively high and potent;
separation and purification are relatively easy. Taken together,
these findings confirm that Ginsenoside Rb1 significantly induced
BMP2 protein expression in SANFH rats. Zhu et al (16) reported that Ginsenoside Rb1
alleviates aluminum chloride-induced rat osteoblast dysfunction
through BMP-2 expression.
Runx2 is affected both by positive effects during
osteoblast differentiation from marrow stroma cells and by negative
modulation (24). The expression
of Runx2 is upregulated after activation, thereby promoting the
production of osteoblasts (25),
which is quite significant for the early repair of SANFH. However,
the activities of Runx2 are inhibited by peroxisome
proliferator-activated receptor γ, whose high expression can
inhibit the expression of Runx2 (26). Thus, the differentiation from bone
marrow stromal cells to osteoblasts is reduced while
differentiation to adipocytes is increased (27). This change will trigger worse
femoral head necrosis. Taken together, the above findings
illustrate that Ginsenoside Rb1 significantly induced RUNX2 protein
expression in SANFH rats.
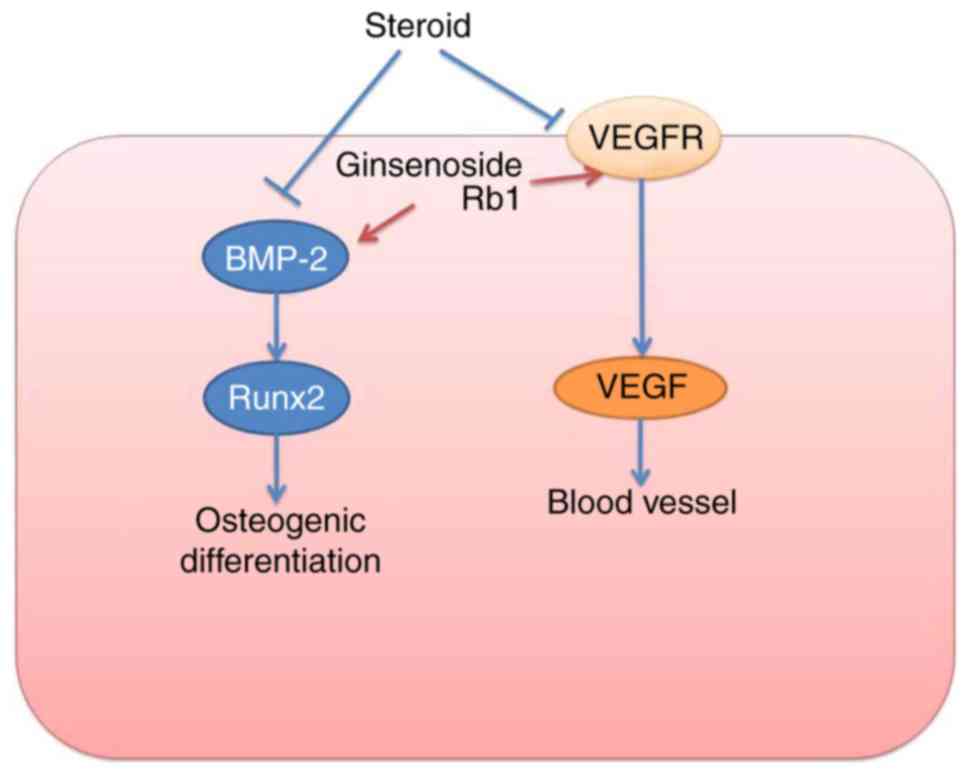
In conclusion, these outcomes indicate that
Ginsenoside Rb1 exerts a positive protective effect on SANFH that
is mediated by osteogenic differentiation targeted VEGFR/VEGF and
RUNX2/BMP-4 signaling pathways (Fig.
8). Ginsenoside Rb1 may be a useful and novel protective drug
for patients who require corticosteroid treatments and are at risk
of developing SANFH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TC designed the experiments. JY, DW and LP performed
the experiments. JY and TC analyzed the data. TC wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Ethical
Committee of Animal Experimentation at Southwest Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seldes RM, Tan V, Duffy G, Rand JA and
Lotke PA: Total knee arthroplasty for steroid-induced
osteonecrosis. J Arthroplasty. 14:533–537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moriya M, Uchiyama K, Takahira N,
Fukushima K, Yamamoto T, Hoshi K, Itoman M and Takaso M: Evaluation
of bipolar hemiarthroplasty for the treatment of steroid-induced
osteonecrosis of the femoral head. Int Orthop. 36:2041–2047. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagasawa K, Tada Y, Koarada S, Tsukamoto
H, Horiuchi T, Yoshizawa S, Murai K, Ueda A, Haruta Y and Ohta A:
Prevention of steroid-induced osteonecrosis of femoral head in
systemic lupus erythematosus by anti-coagulant. Lupus. 15:354–357.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khatami PG, Soleimani A, Sharifi N,
Aghadavod E and Asemi Z: The effects of high-dose vitamin E
supplementation on biomarkers of kidney injury, inflammation, and
oxidative stress in patients with diabetic nephropathy: A
randomized, double-blind, placebo-controlled trial. J Clin Lipidol.
10:922–929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bouchi R, Nakano Y, Fukuda T, Takeuchi T,
Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T and
Ogawa Y: Reduction of visceral fat by liraglutide is associated
with ameliorations of hepatic steatosis, albuminuria, and
micro-inflammation in type 2 diabetic patients with insulin
treatment: A randomized control trial. Endocr J. 64:269–281. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sulaj A, Kopf S, Grone E, Gröne HJ,
Hoffmann S, Schleicher E, Häring HU, Schwenger V, Herzig S, Fleming
T, et al: ALCAM a novel biomarker in patients with type 2 diabetes
mellitus complicated with diabetic nephropathy. J Diabetes
Complications. 31:1058–1065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bahmani F, Kia M, Soleimani A, Mohammadi
AA and Asemi Z: The effects of selenium supplementation on
biomarkers of inflammation and oxidative stress in patients with
diabetic nephropathy: A randomised, double-blind,
placebo-controlled trial. Br J Nutr. 116:1222–1228. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen L, Xiong Y, Wang DQ, Howles P,
Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC and Liu M:
Ginsenoside Rb1 reduces fatty liver by activating AMP-activated
protein kinase in obese rats. J Lipid Res. 54:1430–1438. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang M, Chen Y, Xiong Z, Yu S, Zhou B,
Ling Y, Zheng Z, Shi G, Wu Y and Qian X: Ginsenoside Rb1 inhibits
free fatty acidsinduced oxidative stress and inflammation in 3T3L1
adipocytes. Mol Med Rep. 16:9165–9172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou F, Zhang P and Chen X, Yan J, Yao J,
Yu Z and Chen X: Ginsenoside Rb1 protects the intestinal mucosal
barrier following peritoneal air exposure. Exp Ther Med.
12:2563–2567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Powell C, Chang C and Gershwin ME: Current
concepts on the pathogenesis and natural history of steroid-induced
osteonecrosis. Clin Rev Allergy Immunol. 41:102–113. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian L, Wen Q, Dang X, You W, Fan L and
Wang K: Immune response associated with Toll-like receptor 4
signaling pathway leads to steroid-induced femoral head
osteonecrosis. BMC Musculoskelet Disord. 15:182014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Fan L, Yu Z, Dang X and Wang K: The
effect of deferoxamine on angiogenesis and bone repair in
steroid-induced osteonecrosis of rabbit femoral heads. Exp Biol Med
(Maywood). 240:273–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang Y, Zhao J, Zhao M and Wang K:
Allicin activates autophagic cell death to alleviate the malignant
development of thyroid cancer. Exp Ther Med. 15:3537–3543.
2018.PubMed/NCBI
|
|
16
|
Zhu Y, Hu C, Zheng P, Miao L, Yan X, Li H,
Wang Z, Gao B and Li Y: Ginsenoside Rb1 alleviates aluminum
chloride-induced rat osteoblasts dysfunction. Toxicology.
368-369:183–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan HF, Pan JF, Li S, Guo CA, Liu SH and
Yan ZQ: Protective effects of total saponins of panax notoginseng
on steroid-induced avascular necrosis of the femoral head in vivo
and in vitro. Evid Based Complement Alternat Med. 2015:1656792015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patil AS, Sable RB and Kothari RM:
Occurrence, biochemical profile of vascular endothelial growth
factor (VEGF) isoforms and their functions in endochondral
ossification. J Cell Physiol. 227:1298–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seamon J, Wang X, Cui F, Keller T, Dighe
AS, Balian G and Cui Q: Adenoviral delivery of the VEGF and BMP-6
genes to rat mesenchymal stem cells potentiates osteogenesis. Bone
Marrow Res. 2013:7375802013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lan TH, Xu DP, Huang MT, Song JX, Wu HL
and Li M: Ginsenoside Rb1 prevents homocysteine-induced EPC
dysfunction via VEGF.p38MAPK and SDF-1/CXCR4 activation. Sci Rep.
7:130612017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumoto T, Yamada A, Aizawa R, Suzuki D,
Tsukasaki M, Suzuki W, Nakayama M, Maki K, Yamamoto M, Baba K and
Kamijo R: BMP-2 induced expression of Alx3 that is a positive
regulator of osteoblast differentiation. PLoS One. 8:e687742013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Johnson NR, Usas A, Lu A, Poddar M,
Wang Y and Huard J: Sustained release of bone morphogenetic protein
2 via coacervate improves the osteogenic potential of
muscle-derived stem cells. Stem Cells Transl Med. 2:667–677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geng S, Sun B, Lu R and Wang J: Coleusin
factor, a novel anticancer diterpenoid, inhibits osteosarcoma
growth by inducing bone morphogenetic protein-2-dependent
differentiation. Mol Cancer Ther. 13:1431–1441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariscal-Munoz E, Costa CA, Tavares HS,
Bianchi J, Hebling J, Machado JP, Lerner UH and Souza PP:
Osteoblast differentiation is enhanced by a nano-to-micro hybrid
titanium surface created by Yb:YAG laser irradiation. Clin Oral
Investig. 20:503–511. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin A, Xiong J, Koromila T, Ji JS,
Chang S, Song YS, Miller JL, Han CY, Kostenuik P, Krum SA, et al:
Estrogens antagonize RUNX2-mediated osteoblast-driven
osteoclastogenesis through regulating RANKL membrane association.
Bone. 75:96–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang S, Xu H, Yu S, Cao H, Fan J, Ge C,
Fransceschi RT, Dong HH and Xiao G: Foxo1 mediates insulin-like
growth factor 1 (IGF1)/insulin regulation of osteocalcin expression
by antagonizing Runx2 in osteoblasts. J Biol Chem. 286:19149–19158.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu L and Xu PC: Downregulated LncRNA-ANCR
promotes osteoblast differentiation by targeting EZH2 and
regulating Runx2 expression. Biochem Biophys Res Commun.
432:612–617. 2013. View Article : Google Scholar : PubMed/NCBI
|