Introduction
The critical role of chondrocytes in the etiology of
osteoarthritis (OA) was previously described (1). However, the molecular mechanism
underlying OA remains poorly understood. Chondrocytes are the only
functional cells in cartilage, and they regulate the synthesis,
deposition and modification of the extracellular matrix (ECM).
Normal human articular cartilage is primarily composed of ECM with
chondrocytes embedded in it (2).
In the past, cartilage was considered to be a tissue with limited
blood supply and low oxygen tension that relied on the glycolytic
pathway, rather than mitochondria, for energy production (3). However, previous studies demonstrated
that oxygen tension in cartilage is 5–10% at the surface and <1%
in deeper layers of the tissue (4,5).
Notably, the cartilaginous tissues facing the synovial fluid are
exposed to relatively normal oxygen pressure (4,5).
Therefore, cartilage can present aerobic respiration via the
mitochondrial pathway and the tricarboxylic acid (TCA) cycle
(6).
A number of previous studies have demonstrated that
chondrocytes are able to produce energy through aerobic metabolism
(6–8). Chondrocytes express mitochondrial
dehydrogenase, and isolated mitochondria from chondrocytes contain
enzymes for oxidative phosphorylation and electron transfer
(6–8). Additionally, mitochondria isolated
from chondrocytes can use substrates of the TCA cycle to produce
energy (6–8). Although aerobic respiration accounts
for the degradation of only 20% of the carbohydrates in
chondrocytes, the efficiency of oxidative phosphorylation in terms
of ATP production is higher than that of anaerobic glycolysis
(7). Therefore, mitochondrial
aerobic respiration is one of the primary pathways used to produce
energy in chondrocytes. Changes in chondrocyte function, including
the ability to secrete type II collagen, and in chondrocyte
apoptotic rates can directly affect the occurrence and development
of articular cartilage degeneration in OA (9). However, whether mitochondrial
dysfunction can affect chondrocyte function, leading to OA, remains
unclear.
Enzyme complexes involved in the electron transport
in the respiratory chain are present in the mitochondrial inner
membrane (10). Oxidative
phosphorylation and electron transport involve the enzyme complexes
I–IV, and ATP is synthesized from ADP by ATP synthase, at the end
of the electron transport chain (11). The electron transport chain
generates a potential difference in the mitochondrial inner
membrane, which is necessary for the synthesis of ATP (12). The mitochondrial membrane potential
(Δψm) plays an important role in maintaining mitochondrial membrane
permeability and mitochondrial function (13). Therefore, the integrity of the
mitochondrial respiratory chain (MRC) is important for the
synthesis of ATP and for the maintenance of Δψm, and defects in the
respiratory chain or in the process of ATP synthesis may lead to
mitochondrial dysfunction.
Rotenone (Ro) is a natural isoflavone produced by
leguminous plants, and it is a specific inhibitor of the
respiratory chain enzyme complex I (14). Various previous studies have used
Ro to block the respiratory chain (15,16).
Curcumin (Cur) is an active pharmacological component extracted
from plants in the genus Curcuma (17). Cur possesses strong
anti-inflammatory and antioxidant effects that can effectively
decrease the intracellular level of reactive oxygen species (ROS),
stabilize the mitochondrial membrane and protect mitochondrial
function (18,19).
Therefore, the present study investigated the
differences in mitochondrial morphology and function between OA and
normal cartilage, with the aim of examining mitochondrial function
in chondrocytes from OA cartilage. Furthermore, treatments with Ro
and Cur were performed to affect mitochondrial function and to
examine chondrocyte apoptosis and collagen secretion.
Materials and methods
Sample data
A total of 30 cartilage samples were collected from
the femoral and tibial plateau of patients who underwent total knee
arthroplasty due to osteoarthritis at The Peking University First
Hospital between April and October 2016. Patients undergoing knee
arthroplasty for other reasons were not included. In total, 7 male
and 23 female patients (age 72±6 years, age range 59–78) were
enrolled in the present study. According to the Outerbridge grading
system (20), the samples ranged
from Outerbridge grade 0 to III. In total, 50% of the samples were
immersed in neutral formalin decalcification solution containing
10% EDTA at a volume ratio of 1:100. The decalcification solution
was replaced every week. After the subchondral bone softened, the
samples were able to be embedded in paraffin. The remaining samples
were digested with the modified Klagsbrun method to harvest
chondrocytes (21). Briefly, the
cartilage was cut into small pieces (1 mm3) and digested
with 0.25% trypsin (Nanjing KeyGen Biotech Co., Ltd.) solution for
30 min. Then it was digested with sterile 0.2% collagenase (Nanjing
KeyGen Biotech Co., Ltd.) for 5 h. The resulting cell suspension
was filtered twice, and isolated chondrocytes were seeded in
ventilated 75 cm2 Costar polystyrene monolayer cell
culture flasks (Corning, Inc.). The flasks were maintained at 37°C,
5% CO2 and 100% humidity. The present study was approved
by The Ethics Committee of Peking University First Hospital, and
all participants provided informed consent.
Histological observation
The embedded samples were cut into 4 µm-thick
paraffin sections. The overall chondrocyte morphology was evaluated
by hematoxylin (10 min, 37°C) and eosin (3 min, 37°C) staining, the
apoptosis of the chondrocytes was observed by TUNEL (60 min, 37°C)
staining (Roche Diagnostics) and light microscopy was used to image
the samples (magnification, ×100). Moreover, cartilage from the
operation room was cut into 3×5×5 mm pieces. The specimen was
quickly placed in 3% glutaraldehyde solution for 24 h and 1% citric
acid for 1 h at 4°C. It was then dehydrated in an ascending
gradient of ethanol and acetone (50, 70, 90 and 100%). The tissue
was embedded with Epon 812 resin overnight at 37°C and localized by
semi-thin section (thickness, 0.5–2.0 µm). Ultra-thin sections
(thickness, <0.1 µm) were stained with uranium acetate and lead
citrate. The sections were subjected to transmission electron
microscopy (TEM).
Chondrocyte culture
Normal and OA chondrocytes were harvested from
normal (Outerbridge grade 0) and OA (Outerbridge grade I–III)
cartilage samples following digestion, according to the
aforementioned procedure. Chondrocytes were cultured in DMEM
(HyClone; GE Healthcare Life Sciences) supplemented with 10% FBS
(HyClone; GE Healthcare Life Sciences) and 1%
penicillin-streptomycin (Nanjing KeyGen Biotech Co., Ltd.). The
cells were maintained in a humidified atmosphere containing 5%
CO2 and at 37°C.
Mitochondrial function assessment in
chondrocytes
Normal and OA chondrocytes were harvested as
mentioned above to prepare a monoplast suspension of
>1×107 cells/ml. Mitochondria were extracted using a
mitochondrial extraction kit (Nanjing KeyGen Biotech Co., Ltd.)
according to the manufacturer's protocol, and the activities of the
mitochondrial enzymes were calculated at 25°C using the initial
velocity equation for pseudo-first-order reactions, as previously
described (5,22).
Δψm measurement of chondrocytes
Normal and OA chondrocytes were harvested to prepare
a monoplast suspension of >1×105 cells/ml.
Chondrocytes were stained with JC-1 using a Δψm assay kit
(Sigma-Aldrich; Merck KGaA) at 37°C for 20 min, according to the
manufacturer's protocol. JC-1 is a dye that is highly sensitive to
the membrane potential (23). In
healthy chondrocytes, Δψm was normal, and JC-1 was able to form
polymers, exhibiting bright red fluorescence emission. By contrast,
in apoptotic chondrocytes, Δψm decreased and JC-1 acquired a
monomeric form, exhibiting green fluorescence emission. The Δψm was
measured by flow cytometry within 30 min of staining. An excitation
wavelength (Ex) at 488 nm and an emission wavelength (Em) at 530 nm
were used to detect the JC-1 monomer. An Ex at 490 nm and an Em at
590 nm were used to detect the JC-1 polymer. Flow cytometry was
performed using an INFLUX flow cytometer (BD Biosciences) and the
data was analyzed using FlowJo (version 7.6.5, FlowJo LLC).
TEM observation of chondrocytes
Chondrocytes were digested to prepare a monoplast
suspension of >1×105 cells/ml and were treated
according to the aforementioned procedures. The morphology of the
chondrocytes was then observed by TEM as described.
Drug treatment
Monoplast suspensions (1×105 cells/ml) of
normal chondrocytes were divided into three groups: i) Normal
control group, consisting of untreated chondrocytes; ii) Ro
(Sigma-Aldrich; Merck KGaA) group, consisting of chondrocytes
treated with 1.0 µmol/l Ro for 12 h; and iii) Cur (Sigma-Aldrich;
Merck KGaA) + Ro group, consisting of chondrocytes treated with 2.0
µmol/l Cur for 4 h followed by treatment with 1.0 µmol/l Ro for 12
h. All chondrocytes were cultured in an incubator with 5%
CO2 and saturated humidity at 37°C.
Measurement of cell proliferation and
apoptosis
The cultured primary chondrocytes were digested to
prepare a monoplast suspension of >1×104 cells/ml.
Cellular proliferation was assessed using a Cell Counting Kit-8
(CCK-8; Applygen Technologies, Inc.). Optical density values of the
three groups of cultured cells were measured and compared with the
control group and Ro group, Ro group and Cur + Ro group
(Independent-Samples T Test with subsequent Bonferroni correction,
with P<0.025 considered significant) every day. Chondrocytes
were stained by Annexin V-EGFP and propidium iodide at 37°C for 15
min, and cellular apoptosis was then assessed by flow cytometry
(INFLUX flow cytometer; BD Biosciences) using an Annexin
V-fluorescein isothiocyanate kit (Nanjing KeyGen Biotech Co.,
Ltd.), and the data was analyzed using FlowJo (version 7.6.5,
FlowJo LLC). Moreover, the morphological features of apoptotic
chondrocytes were observed via TEM as described.
Δψm measurement of cultured
chondrocytes
Normal and OA chondrocytes were harvested. Following
drug treatment, mitochondria were extracted using a mitochondrial
extraction kit (Nanjing KeyGen Biotech Co., Ltd.), according to the
manufacturer's protocol. The Δψm was then measured by flow
cytometry, as described.
Quantitative detection of type II
collagen
Total protein was extracted from cultured and
treated chondrocytes (1×106 cells/ml) with the total
protein extraction kit (Nanjing KeyGen Biotech Co., Ltd.),
following the manufacturer's protocol, and the levels of type II
collagen were assessed via a double antibody sandwich avidin-biotin
complex-ELISA method [cat. no. (Col II) h143; Applygen
Technologies, Inc.] (24). A
standard curve was drawn in a semi-logarithmic coordinate paper by
plotting the OD values from different concentrations of collagen
II. Then, according to the OD value of the sample, the
corresponding human collagen II content was found on the curve
(24).
Statistical analysis
SPSS software (version 23.0; IBM Corp.) was used to
perform statistical analyses. All data are reported as the mean ±
standard deviation. Comparisons between two groups were performed
using a Student's t-test with subsequent Bonferroni correction,
with P<0.025 considered significant. Statistical differences
among multiple groups were analyzed using one-way ANOVA followed by
Bonferroni's post hoc test, with P<0.05 considered
significant.
Results
Detection of apoptotic cells in the
cartilage tissue
The cartilage tissue was stained using a TUNEL
assay. The nuclei of normal cells were stained blue and exhibited a
normal shape (Fig. 1). Apoptotic
chondrocytes were distributed unevenly in the articular cartilage;
more apoptotic chondrocytes were identified in proximity to the
tide line, and fewer apoptotic chondrocytes were present in the
superficial and middle layers. The nuclei of apoptotic cells were
pyknotic and lost their ellipse shape. The chromatin was condensed
and the nuclei stained with the TUNEL assay were brown. The number
of apoptotic cells in the cartilage tissue increased and the number
of normal cells decreased with increasing Outerbridge grade
(Fig. 1).
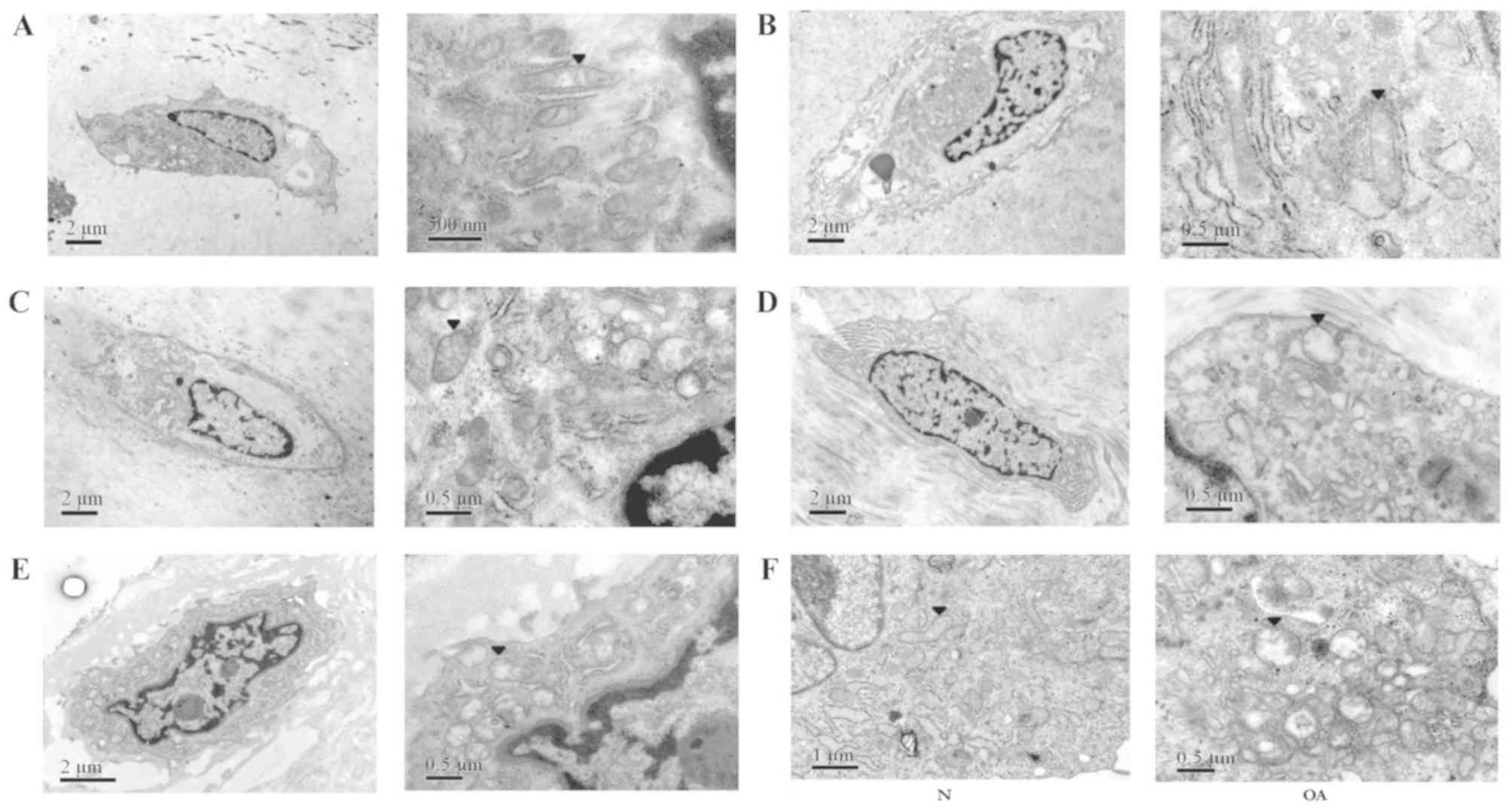
Chondrocytes in the superficial and transitional
cell layers were selected for observation by TEM, as they are
exposed to relatively normal oxygen pressure (25). The shape of the normal chondrocyte
was oval, and the cell membrane forms an elliptical boundary
(Fig. 2). Various organelles were
visible in the cytoplasm, including a large number of mitochondria,
the Golgi apparatus and the endoplasmic reticulum. The electron
density of the outer mitochondrial membrane was high, even and
continuous. The cristae were arranged in a regular pattern.
Moreover, morphological alterations in the mitochondria of OA
chondrocytes in the transitional cell layer were observed.
Chondrocytes from patients with Outerbridge grades I, II and III
(Fig. 2B, C and D, respectively)
exhibited a decreased number of organelles in the chondrocytes, and
the mitochondria were more swollen with increasing Outerbridge
grade. The mitochondrial shape was altered from oblong to round.
Additionally, the regular pattern of the cristae was impaired and,
in certain cases, cristae were not detected. The electron density
of the outer mitochondrial membrane became asymmetrical and
discontinuous.
The morphology of OA chondrocytes in the superficial
layer was reminiscent of fibroblasts (Fig. 2E); however, OA chondrocytes
exhibited fewer organelles in the cytoplasm compared with normal
chondrocytes. Notably, the morphology of the mitochondria was
similar to the transitional layer cells; the mitochondria were
swollen, the outer membrane electron density was uneven, the
structure of the cristae was impaired, and the number of cristae
was decreased. The results of cultured chondrocytes suggested that,
compared with normal cells, the mitochondria of OA chondrocytes
were swollen and abnormal, the electron density was uneven and
discontinuous, the cristae pattern was impaired and the number of
cristae was reduced (Fig. 2F).
Mitochondrial function in
chondrocytes
Detection of respiratory chain complex
activity
To investigate whether OA impaired mitochondrial
function in chondrocytes, quantification of the activities of the
MRC complexes 1–4 and ATP synthase was performed. The results for
these five enzymes in normal and OA chondrocytes showed constant
variance (P=0.645, 0.696, 0.157, 0.230 and 0.170). The activities
of the MRC complexes 1, 2, 2+3 and 4 and ATP synthase in normal
chondrocytes were 207.77±38.26, 161.60±22.66, 288.38±17.30,
218.80±29.63 and 193.94±43.66 nmol/min/mg, respectively. The
activities of the MRC complexes 1, 2, 2+3 and 4, and ATP synthase
in OA chondrocytes were 180.18±34.80, 141.68±22.10, 261.54±27.01,
194.52±20.29 and 158.44±22.72 nmol/min/mg, respectively. The
enzymatic activities in normal chondrocytes were significantly
increased compared with OA chondrocytes (Table I).
 | Table I.Activities of the mitochondrial
respiratory chain enzyme complexes 1, 2, 2+3 and 4, and ATP
synthase. |
Table I.
Activities of the mitochondrial
respiratory chain enzyme complexes 1, 2, 2+3 and 4, and ATP
synthase.
| Enzyme complex | Normal,
nmol/min/mg | Osteoarthritis,
nmol/min/mg | P-value |
|---|
| Complex 1 | 207.77±38.26 | 180.18±34.80 | 0.009a |
| Complex 2 | 161.60±22.66 | 141.68±22.10 | 0.027a |
| Complex 2+3 | 288.38±17.30 | 261.54±27.01 | 0.018a |
| Complex 4 | 218.80±29.63 | 194.52±20.29 | 0.034a |
| ATP synthase | 193.94±43.66 | 158.44±22.72 | 0.005a |
Δψm measurement of chondrocytes
JC-1, a carbocyclic lipophilic fluorescent dye, is a
dye that is highly sensitive to the membrane potential (23). In chondrocytes collected from
healthy tissues, Δψm was normal, and JC-1 was able to form polymers
in the mitochondrial matrix, exhibiting bright red fluorescence
emission (Fig. 3A). By contrast,
in apoptotic chondrocytes, Δψm decreased and JC-1 acquired a
monomeric form, exhibiting green fluorescence emission (Fig. 3B). During the late phases of
apoptosis, the green fluorescence intensity increased (Fig. 3C). Compared with normal
chondrocytes, the red fluorescence detected in OA chondrocytes was
decreased, and the green fluorescence was increased. The ratio of
red/green fluorescent signal in normal chondrocytes was 2.58±0.26
(Fig. 4A), whereas the signal in
OA chondrocytes was 1.50±0.35 (Fig.
4B). Notably, the difference between OA and normal chondrocytes
was statistically significant (P<0.01). The present result
suggested that the mitochondrial membranes were depolarized in OA
chondrocytes and that the mitochondrial function in OA chondrocytes
was impaired. The present JC-1 results were consistent with the
aforementioned TEM results.
 | Figure 4.Δψm results. Compared with (A) normal
chondrocytes, JC-1 staining suggested a decreased Δψm in (B) OA
chondrocytes, which exhibited a lower red/green fluorescence ratio.
Red/green fluorescence ratios in the normal and OA groups were
2.58±0.26 and 1.50±0.35, respectively. (C) Δψm decreased in the Ro
group, which presented a red/green fluorescence ratio of 1.78±0.24.
(D) Δψm in the Cur + Ro group was 2.23±0.15, lower than that in the
normal condition, but higher than that in the Ro group. There was a
significant difference between control and Ro groups (P<0.01)
and between Ro and Ro + Cur groups (P<0.01). Statistical
differences among multiple groups were analyzed using one-way ANOVA
followed by Bonferroni's post-hoc test. N, normal; OA,
osteoarthritis; Ro, rotenone; Cur, curcumin; Δψm, mitochondrial
membrane potential; FITC, fluorescein isothiocyanate. |
Drug treatment
Cellular viability assay
To investigate the potential role of mitochondrial
dysfunction in OA, a CCK-8 assay and Annexin-V staining were
performed on chondrocytes cultured with Ro or Cur. The cell
proliferation assay suggested that the proliferative ability of
chondrocytes in the Ro group was significantly decreased compared
with the control group from day 1 to day 5 (P<0.01). By
contrast, the proliferative ability of chondrocytes in the Ro + Cur
group significantly increased compared with the Ro group from day 1
to 5. The protective effects of Cur increased in the slow growth
and stationary phases (Fig. 5A).
Moreover, the early, late and total apoptosis rates in the normal
group were 0.58±0.14, 3.80±0.26 and 4.38±0.40%, respectively
(Fig. 5B). By contrast, the early,
late and total apoptosis rates in the Ro group were 1.38±0.52,
6.15±0.32 and 7.53±0.84%, respectively (Fig. 5C). Following Cur + Ro treatment,
the apoptosis rates were 0.62±0.10, 4.72±0.97 and 5.34±1.07%
(Fig. 5D). Notably, there was a
significant difference between OA and Ro groups (P<0.01) and
between Ro and Ro + Cur groups (P<0.01). The present results
suggested that Ro increase the apoptosis rate in chondrocytes and
that Cur was able to reduce the apoptosis rate induced by Ro.
 | Figure 5.Drug treatment results. (A) The
proliferative ability of chondrocytes in the Ro group was decreased
compared with the normal group from day 1 to 5. There was a
significant difference between control and Ro groups and between Ro
and Ro + Cur groups. The proliferative ability of chondrocytes in
the Ro + Cur group was increased compared with the Ro group. The
effects of Cur appear to be more evident during the slow growth
phase (0–2 days) and possibly during the stationary phase (4–5
days). (B) Early, late and total cellular apoptotic rates in the
normal group were 0.58±0.14, 3.80±0.26 and 4.38±0.40%,
respectively. (C) Early, late and total cellular apoptotic rates in
the Ro group were 1.38±0.52, 6.15±0.32 and 7.53±0.84%,
respectively. (D) Early, late and total cellular apoptotic rates in
the Cur + Ro group were 0.62±0.11, 4.72±0.97 and 5.34±1.07%,
respectively. Compared with (E) normal chondrocytes, the
mitochondria in the (F) Ro group were swollen and deformed. The
electron density of the outer membrane became heterogeneous and the
lumen of the cristae were expanded and collapsed. (G) Mitochondrial
morphology of the Cur + Ro group was not as severe as in the Ro
group; however, it appeared affected compared with the control
group, and a subset of mitochondria exhibited defects. Arrowheads
indicate mitochondria. *P<0.01 between treatments. N, normal;
Ro, rotenone; Cur, curcumin; OD, optical density; FITC, fluorescein
isothiocyanate. |
TEM of cultured chondrocytes
The mitochondria in the Ro group were swollen and
abnormal compared with the control group (Fig. 5E). The electron density of the
outer membrane became heterogeneous, and the lumen of the cristae
was expanded and collapsed (Fig.
5F). The mitochondrial morphology of the Cur + Ro group was not
as severe as in the Ro group; however, it appeared affected
compared with the control group, and a subset of mitochondria
exhibited defects (Fig. 5G).
Δψm measurement of cultured
chondrocytes
The ratio of red/green fluorescence was 2.58±0.26 in
the control group, 1.78±0.24 in the Ro group, and 2.23±0.14 in the
Cur + Ro group. Notably, there was a significant difference between
control and Ro groups (P<0.01) and between Ro and Ro + Cur
groups (P<0.01). The mitochondrial membranes were more
depolarized in chondrocytes treated with Ro, whereas Cur reversed
the defects in Δψm induced by Ro (Fig.
4C and D).
Quantitative detection of type II
collagen
A standard curve was drawn using standard optical
density (OD) values. The concentration of type II collagen was
calculated based on the OD value detected. Compared with the Ro
group (44.6±7.1 µg/l), the concentrations of collagen in the
control (72.9±24.3 µg/l; P=0.044) and Cur + Ro (100.25±4.50 µg/l;
P<0.01) groups were significantly higher. Although the Cur + Ro
group exhibited a higher collagen content than the control group,
there was no significant difference (P=0.05). Additionally, the
difference between the three groups was statistically significant
(Table II).
 | Table II.Collagen II content in
chondrocytes. |
Table II.
Collagen II content in
chondrocytes.
| Group | Collagen II,
ng/ml |
|---|
| Normal
chondrocytes | 72.88±24.3 |
| Rotenone | 44.63±7.11 |
| Curcumin +
Rotenone | 100.25±4.50 |
| P-value | 0.007a |
Discussion
TEM imaging allows for the visualization of
mitochondria. Notably, important mitochondrial morphological
features are detectable using TEM. The outer membrane is a
double-membrane structure with high permeability and the inner
membrane contains the respiratory chain and enzymes for ATP
synthesis, but is permeable only to ions and small molecules
(10). Selective permeability is
important to maintain the potential difference between the two
sides of the membrane, and it is crucial to create the
transmembrane proton gradient that is necessary for oxidative
phosphorylation (10). The cristae
are formed by folded inner membrane layers that increase the
surface area of the inner membrane of the mitochondria. Notably,
the morphology and number of the cristae are associated with cell
metabolism. The more energy the cell consumes, the larger the total
area of the mitochondrial inner membrane is expected to be, with
mitochondria presenting a higher number of cristae (12).
The present TEM images suggested that structural and
morphological impairments of the mitochondria were increased with
increasing Outerbridge grade in the transitional layer of the
cartilage. In the early degenerative stage, various factors can
cause the dysfunction of the MRC, including the opening of the
mitochondrial permeability transition pore (PTP), the disruption of
Δψm and the collapse of the outer membrane (26). Water molecules can enter the
mitochondria through the PTP, causing the loss of normal
mitochondrial morphology and mitochondrial swelling (26). Dysfunctional mitochondria exhibit
swollen and wider cristae, which may collapse. Beregi and Regius
(6) identified that, with aging,
the mitochondria in human peripheral lymphocytes and skeletal
muscle cells exhibit fewer cristae, and these are partially
replaced by myeloid lamellar structures. These morphological
changes are indicators of mitochondrial dysfunction. In fact, the
number of morphological defects observed in mitochondria are
directly associated with defects in the mitochondrial oxidative
phosphorylation (27).
The present study suggested that the activities of
the complexes of the MRC in OA chondrocytes were decreased compared
with normal chondrocytes. Maneiro et al (11) examined the activity of the same
complexes in patients with OA and found that OA chondrocytes
exhibited decreased activity of MRC-complex II and III, which
decreased by 35% and 13%, respectively, and this decrease was not
compensated by the increased activity of complex I. Lee et
al (28) demonstrated that the
number of mitochondria in OA chondrocytes was increased compared
with normal chondrocytes, as measured by citrate synthase content.
The results of Lee et al (28) suggested that the decrease in MRC
complexes, the subsequent impairment of electron transfer and the
insufficient generation of ATP may be compensated, at least in
part, by an increase in the total number of mitochondria in the
cartilage of patients with OA. Almeida et al (29) demonstrated that the activities of
MRC complexes were decreased in OA chondrocytes compared with
normal cells and that the decreased activity of MRC-complex I and
II in OA chondrocytes was irreversible. This effect may be due to
high concentrations of ROS or nitric oxide in the synovial fluid.
Both transient and irreversible defects are able to severely affect
mitochondrial function in the cartilage (30,31).
The present study suggested that the activities of the MRC
complexes in abnormal OA chondrocytes were significantly decreased
compared with normal cells.
Δψm plays an important role in maintaining
mitochondrial membrane permeability and mitochondrial function.
JC-1, a carbocyclic lipophilic fluorescent dye, is highly sensitive
to the mitochondrial membrane potential (23). Although JC-1 presents certain
limitations, such as poor sensitivity in case of a Δψm <100 mV,
it is considered to be the gold standard for detecting Δψm
(23). In the present study, Δψm
was decreased in OA chondrocytes compared with normal cells, as
suggested by the decreased ratio of red/green fluorescence
following JC-1 staining. The present results suggested that the
majority of mitochondria in OA chondrocytes were dysfunctional and
Δψm was decreased. In addition, the inhibition of electron
transport and oxidative phosphorylation, the opening of the PTP and
the decrease in Δψm could directly cause mitochondrial swelling and
collapse of the outer membrane (26). This phenotype was consistent with
the present TEM results, suggesting that the mitochondrial
dysfunction in OA chondrocytes was associated with defects in
mitochondrial morphology.
MRC complex I is the first enzyme of the respiratory
chain and it is the largest and most complex enzyme in the MRC
(32). Therefore, complex I is
very important in energy metabolism. Ro is a natural isoflavone
produced by leguminous plants, and it is a specific inhibitor of
the enzymatic activity of MRC complex I (14). Various previous studies used Ro to
inhibit the respiratory chain (15,16).
In the present study, TEM results suggested that the mitochondria
of chondrocytes treated with Ro exhibited morphological defects
that were similar to the ones observed in mitochondria of
chondrocytes isolated from Outerbridge grade III samples. Δψm was
decreased in the Ro group, as detected using the JC-1 method.
Additionally, cell proliferation was decreased, the apoptotic rate
was increased and the expression of type II collagen was
significantly decreased. The present results suggested that
inhibition of the MRC and reduction of the mitochondrial function
following Ro treatment could induce pathological changes in
chondrocytes similar to those observed in OA. In the present study,
these pathological changes were found to be associated with
metabolic dysfunction, increased ROS and impaired Δψm.
Furthermore, Cur was used in the present study to
attenuate the effects of Ro. After pre-treatment with Cur for 4 h,
the chondrocytes were protected against Ro-induced mitochondrial
dysfunction. The ratio of red/green fluorescence intensity
increased from 1.78 to 2.23, cell proliferation increased and the
apoptotic rate was reduced from 9.01% to 4.44%, a similar level
that that of the normal group (3.98%). The ability of chondrocytes
to secrete type II collagen in the Cur + Ro group was increased
compared with the Ro group, and it was similar to the control
group. Reddy and Lokesh (33)
demonstrated that the antioxidant effects of Cur were mediated by a
stabilization of antioxidant enzymes, such as superoxide dismutase
(SOD), catalase and glutathione peroxidase, and by a reduction in
lipid peroxidation. Koiram et al (34) showed that Cur had protective
effects on the activity of SOD and other antioxidant enzymes from
radiation, leading to reduced cell damage. Therefore, the
antioxidant effects of Cur could neutralize the ROS produced by
mitochondria following Ro-mediated MRC enzyme I inhibition,
preventing the mitochondrial damage caused by oxidative stress,
stabilizing the mitochondrial membrane, protecting mitochondrial
function, increasing the secretion of type II collagen and reducing
the apoptotic rate of chondrocytes.
The present study had some limitations. For example,
all factors affecting the metabolism of chondrocytes have effects
on both the superficial and deep layers of the cartilage. Previous
studies demonstrated that oxygen and nutrients diffusing through
the synovial fluid may have an important role in chondrocytes in
the deeper cartilage layers (2,25).
However, in the present study, chondrocytes from different layers
were not examined. Notably, in the cartilage, aerobic respiration
and anaerobic glycolysis occur in parallel, also under aerobic
conditions (3). Therefore, lactic
acid and pyruvic acid generated by anaerobic glycolysis may act as
free radical scavengers, increasing the synthesis of ATP despite a
decreased enzymatic activity of the MRC (35,36).
Additionally, adequate oxygen and glucose supply may partially
restore the function of chondrocyte mitochondria, thus affecting
the experimental results. Moreover, the chondrocytes were cultured
under standard oxygen conditions. Therefore, the morphology and
function of chondrocytes in vitro may not reflect the
physiological in vivo state.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, HL and YoC designed the experiments. ZL, HL,
YuC, XY, ZM and RW performed the experiments and analyzed the data.
ZL, HL and ZM wrote the manuscript and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Peking University First Hospital, and all participants
provided informed consent [approval no. 2012 (532)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blanco FJ, Guitian R, Vázquez-Martul E, de
Toro FJ and Galdo F: Osteoarthritis chondrocytes die by apoptosis:
A possible pathway for osteoarthritis pathology. Arthritis Rheum.
41:2841998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckwalter JA and Mankin HJ: Articular
cartilage: Degeneration and osteoarthritis, repair, regeneration,
and transplantation. Instr Course Lect. 47:487–504. 1998.PubMed/NCBI
|
|
3
|
Otte P: Basic cell metabolism of articular
cartilage. Manometric studies. Z Rheumatol. 50:304–312.
1991.PubMed/NCBI
|
|
4
|
Falchuk KH, Goetzl EJ and Kulka JP:
Respiratory gases of synovial fluids. An approach to synovial
tissue circulatory-metabolic imbalance in rheumatoid arthritis. Am
J Med. 49:223–231. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morgan-Hughes JA, Darveniza P, Kahn SN,
Landon DN, Sherratt RM, Land JM and Clark JB: A mitochondrial
myopathy characterized by a deficiency in reducible cytochrome b.
Brain. 100:617–640. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beregi E and Regius O: Comparative
morphological study of age related mitochondrial changes of the
lymphocytes and skeletal muscle cells. Acta Morphol Hung.
35:219–224. 1987.PubMed/NCBI
|
|
7
|
Takamiya S, Yanamura W, Capaldi RA,
Kennaway NG, Bart R, Sengers RC, Trijbels JM and Ruitenbeek W:
Mitochondrial myopathies involving the respiratory chain: A
biochemical analysis. Ann N Y Acad Sci. 488:33–43. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishikawa Y, Chin JE, Hubbard HL and
Wuthier RE: Utilization and formation of amino acids by chicken
epiphyseal chondrocytes: Comparative studies with cultured cells
and native cartilage tissue. J Cell Physiol. 123:79–88. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kühn K, D'Lima DD, Hashimoto S and Lotz M:
Cell death in cartilage. Osteoarthritis Cartilage. 12:1–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perkins G, Renken C, Martone ME, Young SJ,
Ellisman M and Frey T: Electron tomography of neuronal
mitochondria: Three-dimensional structure and organization of
cristae and membrane contacts. J Struct Biol. 119:260–272. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maneiro E, Martín MA, de Andres MC,
López-Armada MJ, Fernández-Sueiro JL, del Hoyo P, Galdo F, Arenas J
and Blanco FJ: Mitochondrial respiratory activity is altered in
osteoarthritic human articular chondrocytes. Arthritis Rheum.
48:700–708. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ernster L and Schatz G: Mitochondria: A
historical review. J Cell Biol. 91:227s–255s. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chauvin C, De Oliveira F, Ronot X,
Mousseau M, Leverve X and Fontaine E: Rotenone inhibits the
mitochondrial permeability transition-induced cell death in U937
and KB cells. J Biol Chem. 276:41394–41398. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Navarro A, Bández MJ, Gómez C, Repetto MG
and Boveris A: Effects of rotenone and pyridaben on complex I
electron transfer and on mitochondrial nitric oxide synthase
functional activity. J Bioenerg Biomembr. 42:405–412. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DiDonato S, Zeviani M, Giovannini P,
Savarese N, Rimoldi M, Mariotti C, Girotti F and Caraceni T:
Respiratory chain and mitochondrial DNA in muscle and brain in
Parkinson's disease patients. Neurology. 43:2262–2268. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa
B, Melendez JA and Robinson JP: Mitochondrial complex I inhibitor
rotenone induces apoptosis through enhancing mitochondrial reactive
oxygen species production. J Biol Chem. 278:8516–8525. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jackson JK, Higo T, Hunter WL and Burt HM:
The antioxidants curcumin and quercetin inhibit inflammatory
processes associated with arthritis. Inflamm Res. 55:168–175. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cole GM, Teter B and Frautschy SA:
Neuroprotective effects of curcumin. Adv Exp Med Biol. 595:197–212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asai A and Miyazawa T: Dietary
curcuminoids prevent high-fat diet-induced lipid accumulation in
rat liver and epididymal adipose tissue. J Nutr. 131:2932–2935.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Outerbridge RE: The etiology of
chondromalacia patellae. J Bone Joint Surg Br. 43:752–757. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klagsbrun M: Large-scale preparation of
chondrocytes. Methods Enzymol. 58:560–564. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgan-Hughes JA, Hayes DJ, Clark JB,
Landon DN, Swash M, Stark RJ and Rudge P: Mitochondrial
encephalomyopathies: Biochemical studies in two cases revealing
defects in the respiratory chain. Brain. 105:553–582. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shapiro HM: Membrane potential estimation
by flow cytometry. Methods. 21:271–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kongtawelert P and Ghosh P: A new
sandwich-ELISA method for the determination of keratan sulphate
peptides in biological fluids employing a monoclonal antibody and
labelled avidin biotin technique. Clin Chim Acta. 195:17–26. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aigner T, Kurz B, Fukui N and Sandell L:
Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr
Opin Rheumatol. 14:578–584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma Q, Fang H, Shang W, Liu L, Xu Z, Ye T,
Wang X, Zheng M, Chen Q and Cheng H: Superoxide flashes: Early
mitochondrial signals for oxidative stress-induced apoptosis. J
Biol Chem. 286:27573–27581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higuchi M, Proske RJ and Yeh ET:
Inhibition of mitochondrial respiratory chain complex I by TNF
results in cytochrome c release, membrane permeability transition,
and apoptosis. Oncogene. 17:2515–2524. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee HC, Yin PH, Lu CY, Chi CW and Wei YH:
Increase of mitochondria and mitochondrial DNA in response to
oxidative stress in human cells. Biochem J. 348:425–432. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Almeida A, Almeida J, Bolaños JP and
Moncada S: Different responses of astrocytes and neurons to nitric
oxide: The role of glycolytically generated ATP in astrocyte
protection. Proc Natl Acad Sci USA. 98:15294–15299. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goossens V, Stangé G, Moens K, Pipeleers D
and Grooten J: Regulation of tumor necrosis factor-induced,
mitochondria- and reactive oxygen species-dependent cell death by
the electron flux through the electron transport chain complex I.
Antioxid Redox Signal. 1:285–295. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yook YH, Kang KH, Maeng O, Kim TR, Lee JO,
Kang KI, Kim YS, Paik SG and Lee H: Nitric oxide induces BNIP3
expression that causes cell death in macrophages. Biochem Biophys
Res Commun. 321:298–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Distelmaier F, Koopman WJ, van den Heuvel
LP, Rodenburg RJ, Mayatepek E, Willems PH and Smeitink JA:
Mitochondrial complex I deficiency: From organelle dysfunction to
clinical disease. Brain. 132:833–842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reddy AC and Lokesh BR: Studies on spice
principles as antioxidants in the inhibition of lipid peroxidation
of rat liver microsomes. Mol Cell Biochem. 111:117–124.
1992.PubMed/NCBI
|
|
34
|
Koiram PR, Veerapur VP, Kunwar A, Mishra
B, Barik A, Priyadarsini IK and Mazhuvancherry UK: Effect of
curcumin and curcumin copper complex (1:1) on radiation-induced
changes of anti-oxidant enzymes levels in the livers of Swiss
albino mice. J Radiat Res (Tokyo). 48:241–245. 2007. View Article : Google Scholar
|
|
35
|
Le Goffe C, Vallette G, Jarry A, Bou-Hanna
C and Laboisse CL: The in vitro manipulation of carbohydrate
metabolism: A new strategy for deciphering the cellular defence
mechanisms against nitric oxide attack. Biochem J. 3:643–648. 1999.
View Article : Google Scholar
|
|
36
|
Aulwurm UR and Brand KA: Increased
formation of reactive oxygen species due to glucose depletion in
primary cultures of rat thymocytes inhibits proliferation. Eur J
Biochem. 267:5693–5698. 2000. View Article : Google Scholar : PubMed/NCBI
|