Introduction
Lung cancer is one of the most common types of
cancer and has the highest mortality rates worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for >80% of cancer-associated mortalities (2). With the development of scientific
technology, notable progress has been achieved in the early
diagnosis and treatment of various tumor types. A tumor can be
completely removed in the early stage of disease, although it may
recur in half of the patients who undergo the process (1). According to statistics, the majority
of patients with lung cancer are diagnosed in the advanced stages,
at which the prognosis is very poor and the 5-year survival rate is
<16% (3). Platinum-based
chemotherapy is the primary treatment modality for NSCLC. However,
adverse treatment outcomes, including drug resistance, may result
in failure of chemotherapy (4).
MicroRNAs (miRNAs/miRs) are single-stranded
non-coding RNAs of 20–22 nucleotides, which participate in gene
expression and are involved in regulating post-transcriptional
expression (5). miRNAs influence
the proliferation, differentiation and apoptosis of cancer cells
(5). It has been reported that
miRNAs participate in tumor formation, which provides opportunities
for the optimization of cancer treatment (6). miRNAs function as tumor promotors or
inhibitors; thus, they serve critical roles in tumor occurrence and
development, which is important not only in the diagnosis and
prediction of prognosis, but also in developing novel therapeutic
strategies for tumor treatment (7,8). The
expression of miR-21 in plasma and tissue samples from patients
with NSCLC could be used to predict the survival index and
chemotherapy effect of platinum-based drugs (9).
miRNAs have also reported to be relevant to the
potential toxicity mechanism (10)
and the antitumor activity of drugs in several tumor types,
including breast cancer (6), lung,
colorectal (11) and esophageal
cancer (12). Blower et al
(13) identified that
overexpression of miR-7i, miR-16 and miR-21 miRNA improved the
chemotherapeutic effect of an anticancer drug in NSCLC cell lines.
miR-539 could increase the chemosensitivity of NSCLC cells to
cisplatin by directly targeting double cortin like kinase 1 (DCLK1)
(14). Zhang et al
(15) reported that by regulating
glutathione S-transferase pi gene expression, miR-513a-3p could
increase the sensitivity of a lung adenocarcinoma cell line to
cisplatin. These studies indicate that miRNAs serve important roles
in the anticancer activity of cisplatin.
Although it is understood that miRNAs serve
important roles in the chemotherapeutic effect of anticancer drugs,
the roles of miRNAs in cisplatin-induced suppression of lung cancer
proliferation require further investigation. Therefore, the present
study aimed to investigate the effects of cisplatin on the
expression of miRNAs and the proliferation of lung cancer
cells.
Materials and methods
Chemicals and supplements
The following drugs and reagents were used in the
present study: Fetal calf serum (Hyclone; GE Healthcare), trypsin
(Sigma-Aldrich; Merck KGaA), RPMI-1640 culture medium
(Sigma-Aldrich; Merck KGaA), DMSO (Sigma-Aldrich; Merck KGaA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich; Merck KGaA), penicillin/streptomycin (100 ×; Gibco;
Thermo Fisher Scientific, Inc.), cisplatin (Qilu Pharmaceutical
Co., Ltd.), SYBR Green PCR master mix quantification PCR kit
(Qiagen, Inc.), 5X poly A buffer, MgCl2, and dATP
(Promega Corporation), dNTP mixture and RNase inhibitor (Takara
Bio, Inc.).
Cell lines and cell culture
To investigate the roles of cisplatin in the
proliferation of lung cancer cells, the A549 cells were used in
this study, which is a lung cancer cell line and provided by the
Institute of Shanghai Cell Biology (16,17).
All cells were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum, 100 IU/ml penicillin, and 100 µg/ml
streptomycin sulfates at 37°C with 5% CO2. Cells
(1×104) seeded in the 96-well flat bottom microtiter
plates were respectively treated with 0, 3, 6 and 9 µg/ml of
cisplatin for 24–72 h at 37°C as our previous report (17). Then the cells were observed under a
microscope (with magnification 100 ×, BX43, Olympus, Inc., Japan)
at 48 h after treatment.
MTT assay
Cell proliferation assays were performed using a
modified colorimetric MTT assay, according to the manufacturer's
protocols. All procedures were repeated a minimum of three times,
as described in our previous studies (18,19).
Absorbance was measured at 570 nm using an ELISA reader (Multiskan
FC; Thermo Fisher Scientific, Inc).
Cell apoptosis assay
Apoptosis was measured using BD FACSDiva Software on
a flow cytometer (BD FACSCantoTMII, BD Bioscience) after
48 h of incubation with 0, 3, 6 or 9 µg/ml cisplatin. Briefly,
cells (1×105/well of a 12-well flat-bottom microtiter
plate) were stained with Annexin V-FITC/PI (Nanjing KeyGen Biotech.
Co. Ltd.), according to the manufacturer's protocols.
Cell migration assay
To monitor cell migration, cells (1×104
cells/well) were seeded onto the top chamber of a CIM plate
(xCELLigence). The cells were incubated at room temperature for 30
min, and CIM plates were placed on an RTCA station (xCELLigence
System; Roche, Diagnostics GmbH). Real-time monitoring of cell
migration was conducted for 24 h by detecting the changes of
electrical impedance at the electrode/cell interface.
Reverse transcription-quantitative PCR
(RT-qPCR)
A549 cells and tumor xenografts were harvested at 48
h following cisplatin treatment. miRNAs was isolated from the cells
using mirVana™ miRNA kit (Ambion; Thermo Fisher Scientific, Inc.)
and ploy (A) was added using poly (A) polymerase (2 U/µl; Ambion;
Thermo Fisher Scientific, Inc.). cDNA was synthesized by incubating
the reaction at 37°C for 1 h with a specific primer
(5′-AACATGTACAGTCCATGGATGd(T)30N(A,G,C or T)-3′). qPCR was
performed to detect miRNA levels using TB Green® Premix
Ex Taq™ kit (RR420A, Takara Bio Company), as described previously
(18) on a StepOnePlusTM Real-Time
PCR Systems (Version 2.3, life technologies). The following
thermocycling conditions were used: 95°C for 5 min followed by 40
cycles of 95°C for 30 sec, 60°C for 20 sec and 72°C for 20 sec. The
following forward primer sequences were used: miR-93,
5′-CAAAGTGCTGTTCGTGCAGGTA-3′; miR-26a, 5′-CAAGTAATCCAGGATAGGC-3′;
miR-26b, 5′-CAAGTAATCCAGGATAGGT-3′; miR-29a,
5′-GCACCATCTGAAATCGGTTA-3′; miR-29c, 5′-GCACCATTTGAAATCGGTTA-3′;
and miR-125b, 5′-TCCCTGAGACCCTAACTTGTG-3′. The reverse primer used
to amplify these miRNAs was: 5′-AACATGTACAGTCCATGGATAG-3′. Human 5S
rRNA was used as the control with the following primer sequences:
Forward 5′-GCCATACCACCCTGAACG-3′, and reverse
5′-AACATGTACAGTCCATGGATG-3′. The data was calculated using the
2−ΔΔCq method (20).
All assays were performed in three triplicates.
miRNA mimics and transfection
The human miR-93 duplex mimic (miR-93;
5′-caaagugcuguucgugcagguag-3′) and control oligos (Mock;
5′-ccuacgccaccaauuucgu-3′) were obtained from Shanghai GenePharma
Co., Ltd. Lung cancer cells (2×105) were transfected
with 1.5 µg miRNA using 2.5 µl Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. miRNAs and total protein were extracted
from cells 48 h after transfection.
miRNA target genes
TargetScanHuman Release 7.2 (http://www.targetscan.org/vert_72/) was used to
predict the biological targets of miRNAs. The target genes and
their miRNA binding sites were predicted using this online
tool.
Western blotting
The cells were lysed with radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology) at 4°C on ice
for 30 min. After centrifugation in 13,400 × g for 5 min at 4°C, 30
µg of protein was separated by 10% SDS-PAGE and transferred onto a
nitrocellulose membrane. The blots were incubated with rabbit
anti-human cyclin D2 (1:400; cat. no. 10934-1; Proteintech Group,
Inc.)/Bcl-2-associated X protein (Bax; 1:400; cat. no. BS2583;
Bioworld Technology, Inc.)/Bcl-2 (1:400; cat. no. 12789-1;
Proteintech Group, Inc.)/c-Myc (1:400; cat. no. BS2462; Bioworld
Technology, Inc.)/β-actin (1:400; cat. no. BS-0061R; Beijing
Biosynthesis Biotechnology Co., Ltd.) antibodies at 4°C overnight.
Membranes were washed with TBS and Tween-20 (TBST) three times.
HRP-labeled goat anti-rabbit IgG (1:6,000; cat. no. BS13278;
Bioworld Technology, Inc.) was then added for 1 h at room
temperature. Finally, signals were captured using enhanced
chemiluminescence (Wuhan Boster Biological Technology, Ltd.) after
three washes with 1X TBST. The assays were performed in triplicate.
Densitometry was performed using a Gel Image System 4.2 (Tanon
Science & Technology Co., Ltd.).
In vivo study
In total, 6 four-week old male athymic BALB/c mice
(15 g) were purchased from Beijing HFK Bioscence Co., Ltd. The mice
were kept at 22±2°C with a humidity of 50±5% in 20 ×40 ×60 cm
cages, with a 12 h light/dark cycle and were fed with a standard
diet and water. Each mouse was subcutaneously injected with an A549
cell suspensions (5×106 cells in 0.1 ml of PBS) into the
armpit area. The experimental group (n=3) was treated with
cisplatin (3 mg/kg) by intraperitoneal injection, while the control
group (n=3) was treated with an equal volume of saline. All mice
were sacrificed after 4 weeks and tumors were collected. All animal
experiments were approved by the Ethics Committee of Animal
Experiments of Binzhou Medical University.
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used to analyze
the data. Experiments were performed in triplicate and the data are
presented as the mean ± SD. The comparison between two groups was
analyzed using an unpaired two-sided Student's t-test. The
comparisons among three or more groups were analyzed by One-way
ANOVA. If the results had statistical significance, the comparison
between two groups was performed using Bonferonni's tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cisplatin suppresses the proliferation
of lung adenocarcinoma cells
In our previous study, it was identified that
cisplatin could inhibit A549 cell proliferation by upregulating the
expression of MutS homolog 2 through miR-21 (17). To further investigate the mechanism
of cisplatin-induced suppression of lung adenocarcinoma cell
growth, the present study treated A549 cells with different
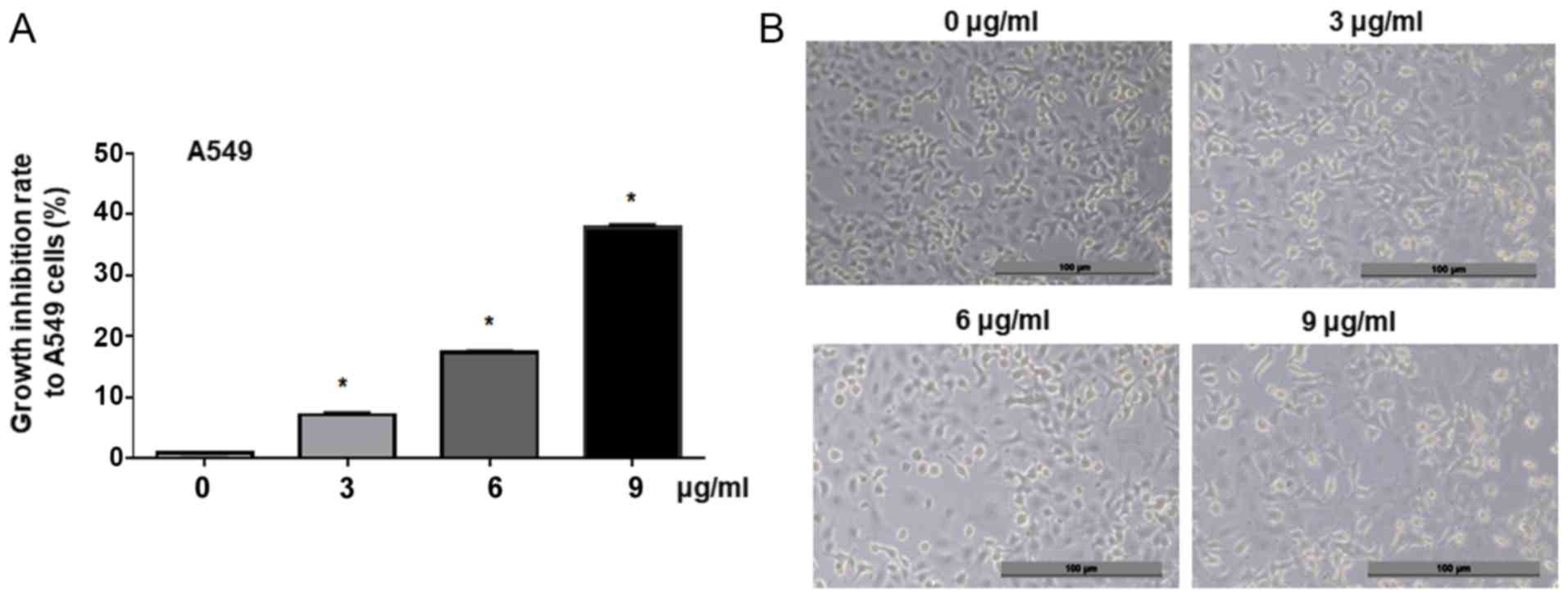
concentrations of cisplatin. MTT analysis results demonstrated that
cisplatin significantly inhibited the proliferation of A549 cells
in a dose-dependent manner compared with the control (0, 3, 6 and 9
µg/ml, Fig. 1A). Morphological
analysis revealed that the treatment with cisplatin (3, 6 and 9
µg/ml) for 24 h suppressed A549 cell viability, compared with the
untreated control cells (0 µg/ml, Fig.
1B). This indicated that cisplatin could effectively suppress
lung adenocarcinoma cell proliferation in a dose-dependent
manner.
Cisplatin induces apoptosis and
inhibits the migration of A549 cells
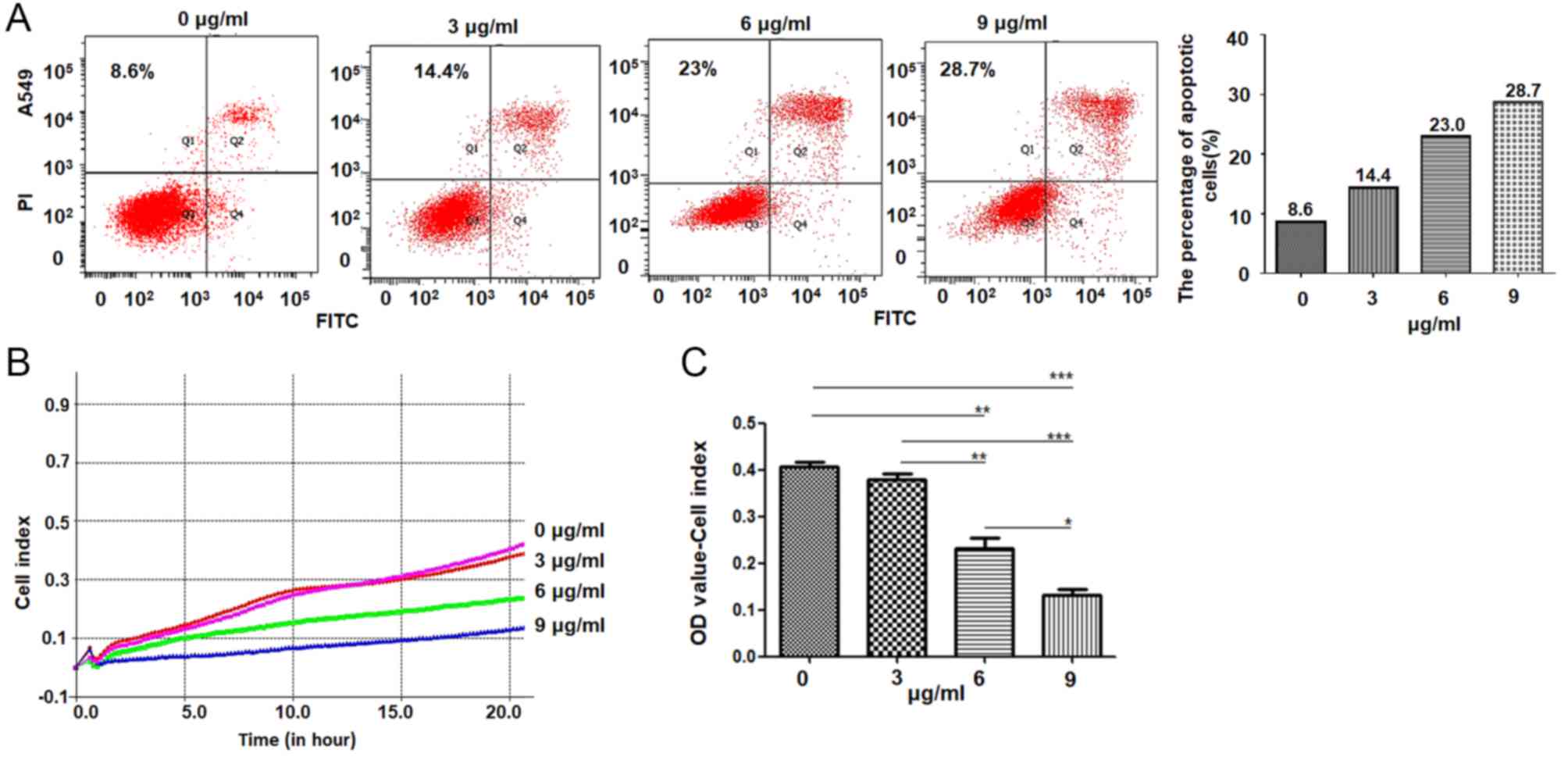
Next, the current study detected the roles of
cisplatin in regulating the apoptosis and migration of lung
adenocarcinoma cells. The results demonstrated that the percentage
of apoptotic cells in 3, 6 and 9 µg/ml cisplatin-treated cultures
was 14.4, 23 and 28.7%, respectively, higher than the 8.6%
determined in the control treatment. The percentage of apoptotic
cells was elevated in cisplatin-treated A549 cells in a
dose-dependent manner, particularly in the 9 µg/ml
cisplatin-treated cells (Fig. 2A).
The migration of A549 cells was analyzed using a RTCA station,
which demonstrated that treatment with 6 and 9 µg/ml cisplatin
treatment significantly suppressed A549 cell migration (Fig. 2B and C).
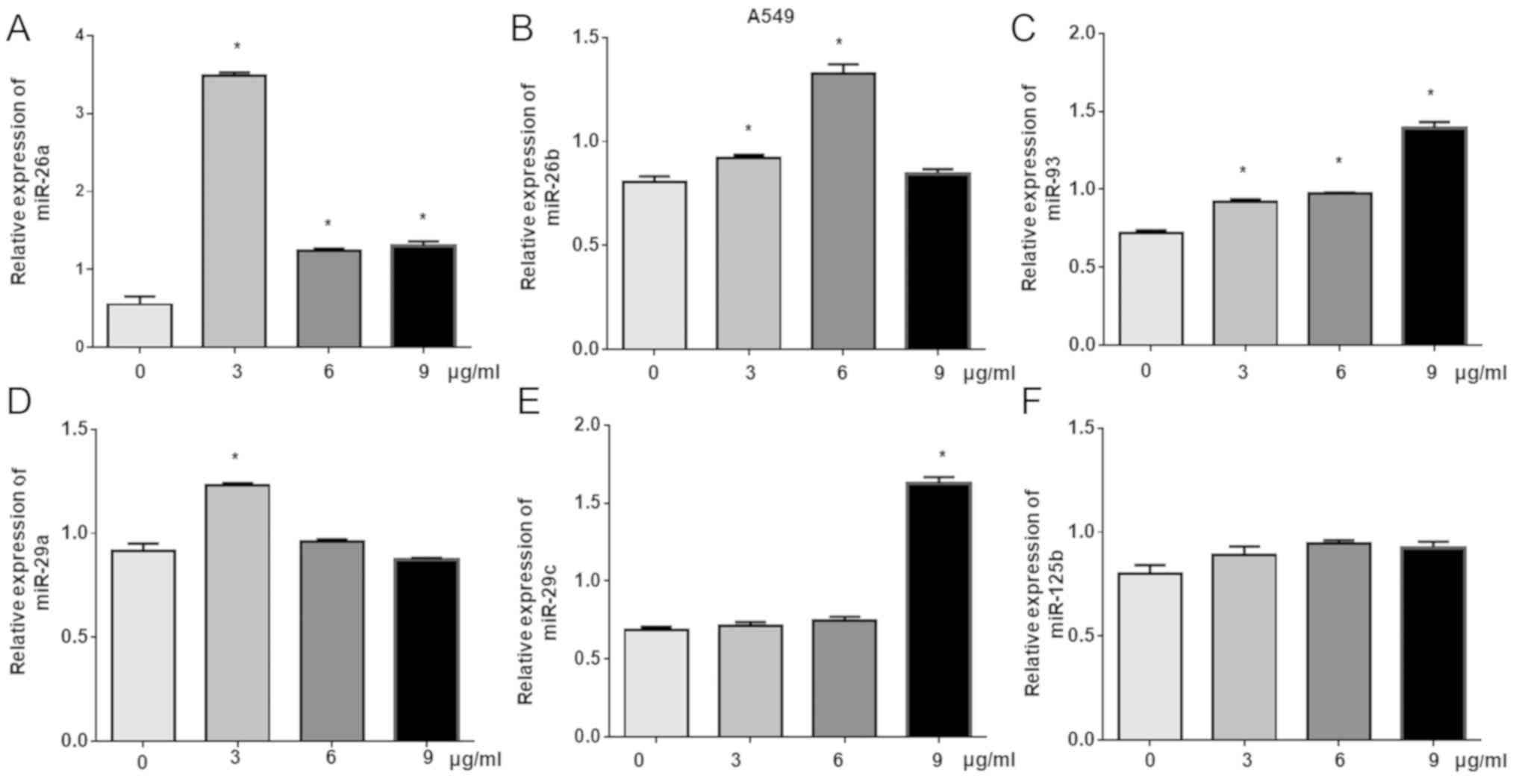
miR-93 is upregulated in
cisplatin-treated A549 cells
Previous studies have reported that miRNAs exhibit
important roles in the regulation of chemotherapy drugs and
molecule-targeted drugs (6,9–12,14,15).
miR-93, miR-26a, miR-26b, miR-29a, miR-29c and miR-125 have been
reported to exert important roles in cancers (21–24).
To further investigate the roles of these miRNAs in
cisplatin-induced inhibition of lung cancer cell proliferation,
RT-qPCR was used to detect changes in the expression of six miRNAs,
including miR-93, miR-26a, miR-26b, miR-29a, miR-29c and miR-125b,
in cisplatin-treated A549 cells. The results demonstrated that the
levels of miR-29c were significantly increased following treatment
with 9 µg/ml cisplatin; compared with the control, significant
increases in the expression of miR-26b were detected in response to
3 and 6 µg/ml cisplatin, while 3 µg/ml cisplatin significantly
induced miR-29a expression. Of note, treatment with all
concentrations resulted in the significant upregulation of miR-93
and miR-26a compared with the control (Fig. 3).
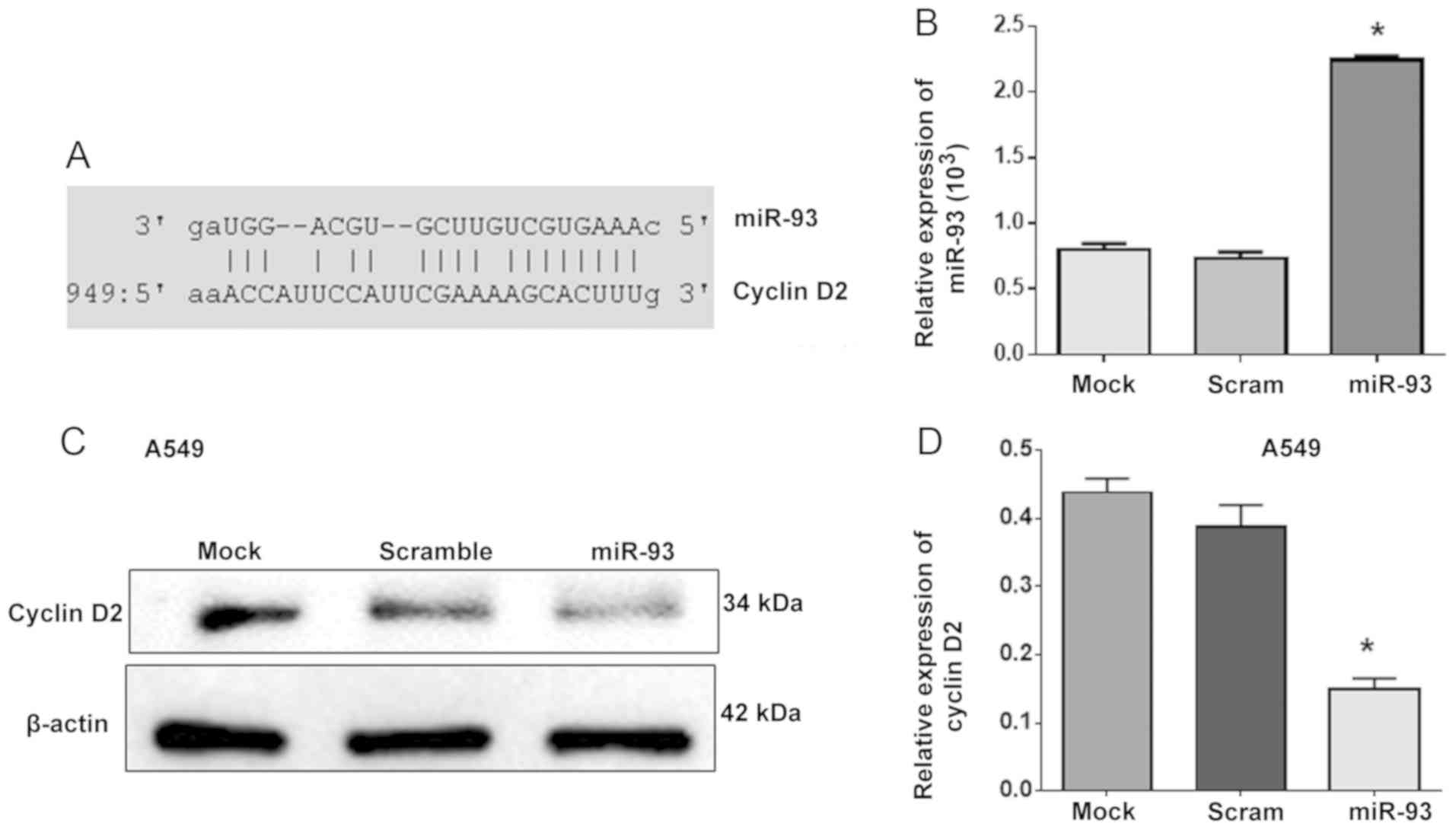
miR-93 regulates the expression of
cyclin D2
miR-93 has been reported to promote the apoptosis
and increase the percentage of human umbilical vein endothelial
cells in G1 phase by targeting angiopoietin 2 (25). The present study further revealed
that that cyclin D2 is a novel target gene of miR-93 using
TargetScanHuman 7.2 software online (Fig. 4A). Subsequently, A549 cells were
transfected with miR-93 to investigate whether miR-93 could
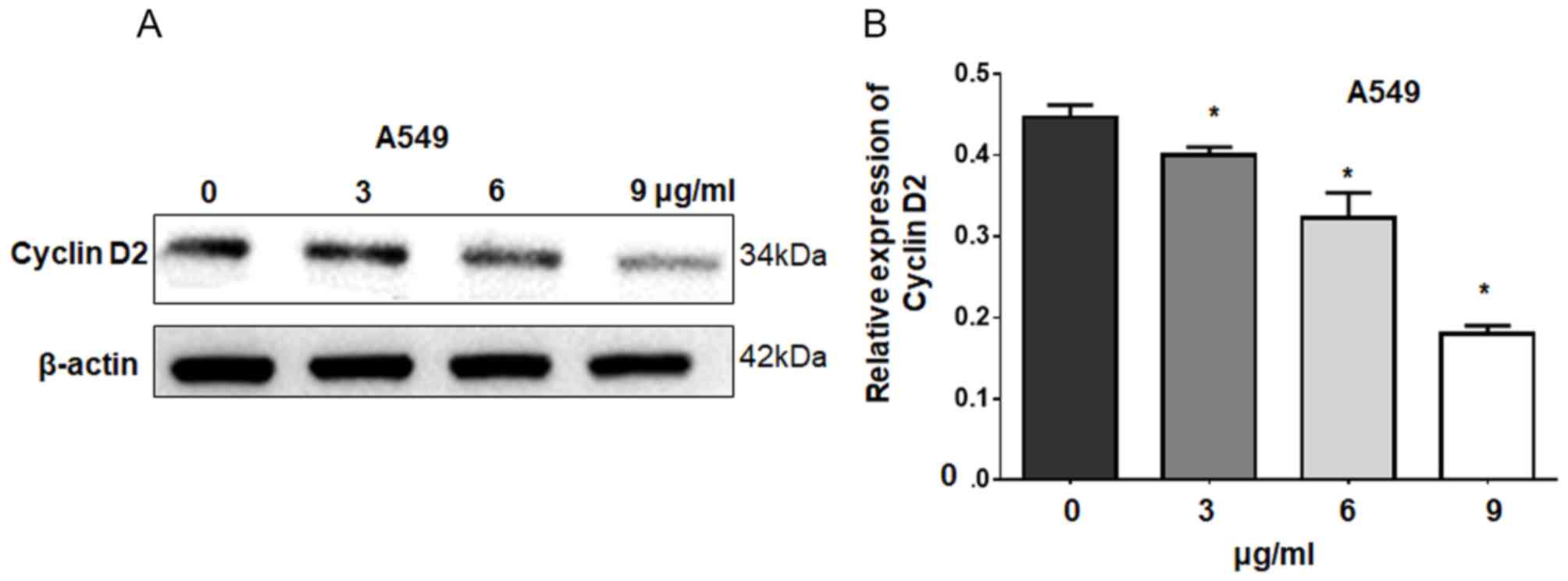
regulate cyclin D2 expression as an upstream factor (Fig. 4B). Western blot analysis showed
that overexpression of miR-93 significantly reduced the expression
of cyclin D2 in miR-93-transfected A549 cells compared with control
cells (Fig. 4C and D), which
indicated that miR-93 decreased the expression of cyclin D2 as an
upstream factor.
Cisplatin suppresses cell growth by
decreasing cyclin D2 expression via miR-93
The aforementioned results indicated that cisplatin
suppresses lung cancer cell growth by upregulating miR-93
expression, which negatively regulates cyclin D2 expression. To
further investigate whether cisplatin suppresses cell growth by
decreasing cyclin D2 expression via miR-93, cyclin D2 levels were
detected by western blotting following cisplatin treatment. Our
results revealed that cisplatin significantly downregulated cyclin
D2 expression in A549 cells in a dose-dependent manner compared
with the control (Fig. 5). These
results suggest that cisplatin suppresses cell growth by decreasing
cyclin D2 expression via miR-93.
Cisplatin affects the expression of
apoptosis-associated proteins
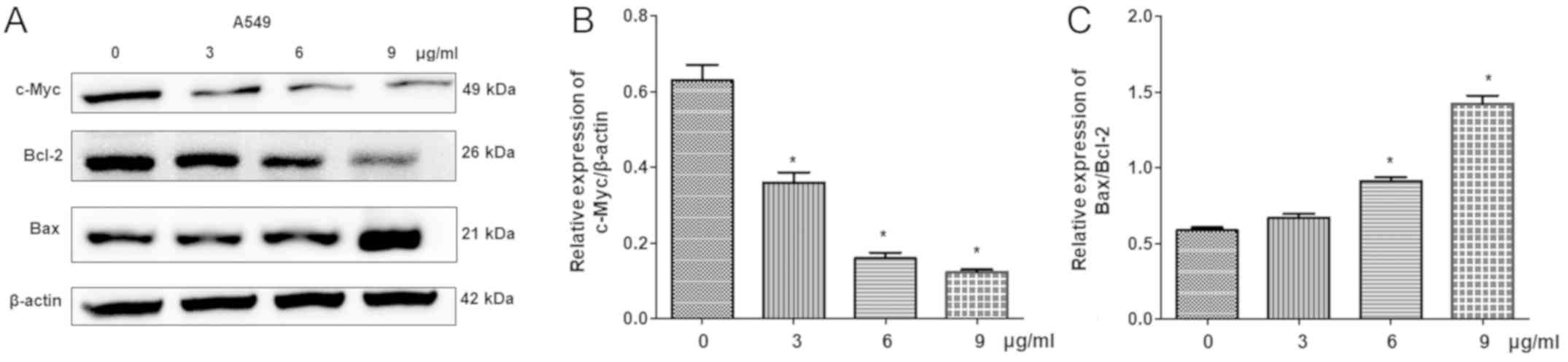
Additionally, the expression levels of
apoptosis-associated proteins, including Bcl-2, Bax and c-Myc, were
detected by western blot analysis. The results demonstrated that
cisplatin significantly reduced the expression of c-Myc and Bcl-2,
but increased Bax levels in a dose-dependent manner compared with
the control (Fig. 6). This
indicated that cisplatin-induced cell apoptosis is associated with
the regulation of c-Myc, Bcl-2 and Bax expression.
Cisplatin suppresses A549 cell growth
in vivo
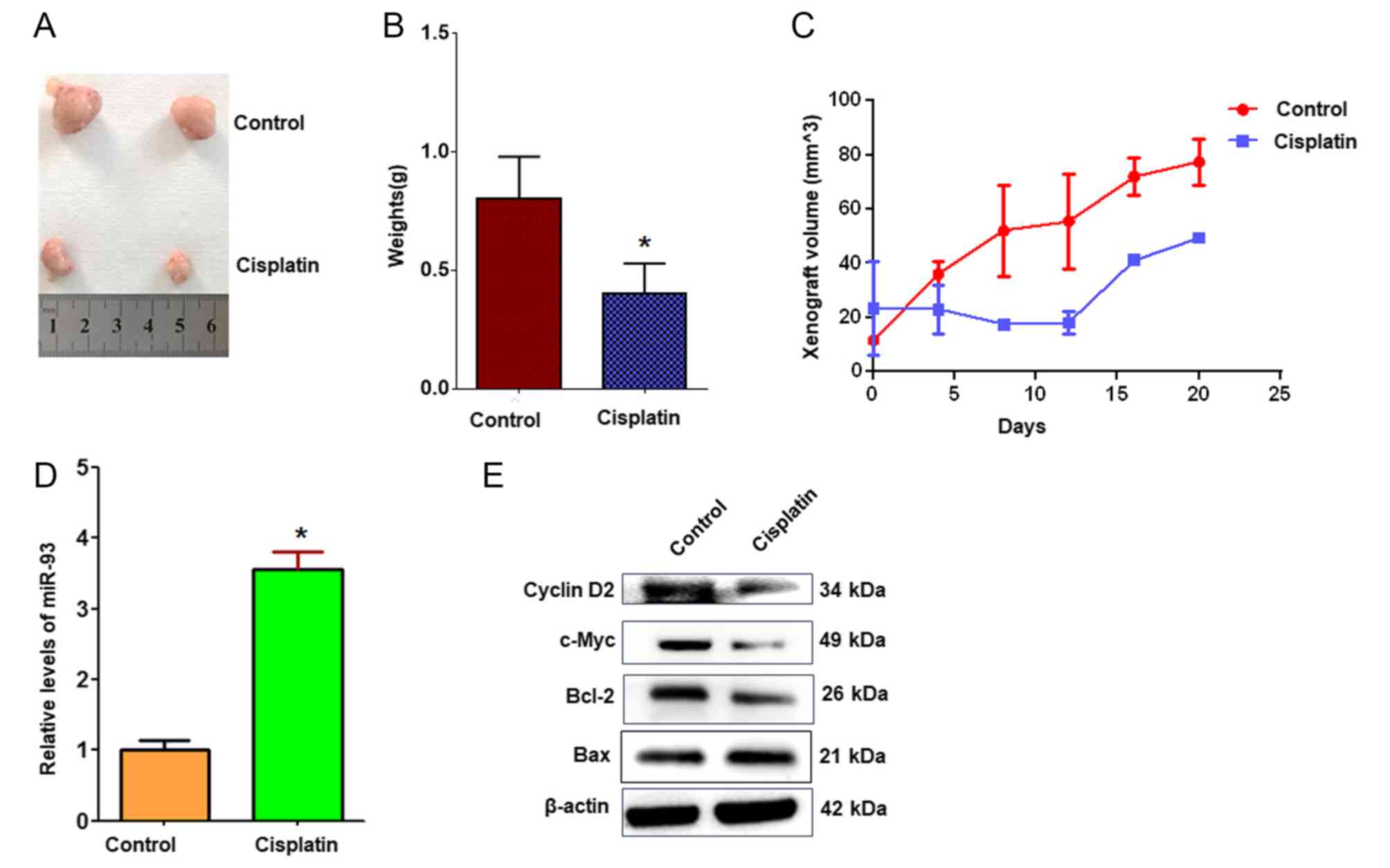
To evaluate the roles of cisplatin and miR-93 in
regulating cell proliferation in vivo, A549 lung cancer
xenografts were established in BALB/c nude mice. The results
demonstrated that tumor volumes and weights were markedly decreased
in cisplatin-treated xenografts compared with controls (Fig. 7A-C). RT-qPCR revealed that miR-93
expression was significantly increased in cisplatin-treated
xenografts compared with the control treatment (Fig. 7D). Cyclin D2, the predicted target
of miR-93, was notably downregulated in tumors treated with
cisplatin compared with in control tumors (Fig. 7E), which supports the hypothesis
that cisplatin suppresses A549 cell growth in vivo via
miR-93 and cyclin D2.
Discussion
Noncoding RNAs, including miRNAs, are involved in a
number of pathological conditions of cancer (26). miRNAs are responsible for the
development of resistance to anticancer drugs as they affect drug
resistance-associated genes, and induce alternative signaling
pathways and the DNA damage response (26). miR-205 can significantly induce
apoptosis and enhance chemotherapeutic effects in prostate cancer
cells (27). Furthermore, ethanol
extract of Antrodia cinnamomea can inhibit the growth of
breast cancer cells by increasing the expression levels of
miR-21-5p, miR-26-5p and miR-30-5p (28). The interaction of phosphoinositide
3-kinase with seven in absentia homolog 2 is regulated by the
miRNA-30-5p family and is considered as a potential treatment
target in NSCLC (29). The
aforementioned miRNAs serve important roles in enhancing tumor
sensitivity toward targeted therapies. The present study selected
miR-93, which was significantly increased in lung adenocarcinoma
cells after cisplatin treatment. Additionally, it was demonstrated
that cyclin D2 is a direct target of miR-93, and miR-93 and cyclin
D2 were reported to serve important roles in cisplatin-induced lung
adenocarcinoma cell apoptosis.
Platinum-based drugs, particularly cisplatin, are
first-line chemotherapy drugs (30). miRNAs are involved into the
mechanism of cisplatin for the treatment of cancer (14,31,32).
The epigenetic regulation of insulin like growth factor 1 receptor
via miR-1294 is important for cisplatin resistance in ovarian
cancer (32). miR-539 can increase
the chemosensitivity of lung cancer cells to cisplatin treatment by
directly targeting DCLK1 (14).
miR-363 may be a biomarker for predicting responsiveness to
cisplatin-based chemotherapy by snail-induced
epithelial-mesenchymal transition in epithelial ovarian cancer
cells (31). Although these
studies support that miRNAs participate in the anticancer roles of
cisplatin for the treatment of cancer, the effects of cisplatin on
the proliferation of lung cancer cells require further
investigation. Previous studies found that miR-93, miR-26a,
miR-26b, miR-29a, miR-29c and miR-125 play important roles in
cancer (22–24). The present study investigated the
roles of these miRNAs in the cisplatin-induced suppression of lung
cancer and demonstrated that cisplatin could effectively inhibit
lung adenocarcinoma cell proliferation in a dose-dependent manner.
The mechanism by which cisplatin inhibits lung adenocarcinoma cell
proliferation is proposed to be associated with the expression of
miR-93 and miR-26a.
miRNAs are involved in numerous biological processes
associated with cancer, including carcinogenesis, cell
proliferation, invasion and migration, which serve crucial roles in
the regulation of tumor development and progression (33,34).
Through binding with the 3′-untranlasted region of target mRNAs,
miRNAs inhibit targeted gene expression to a certain degree
(33). miR-9600, a novel molecule,
has been identified to impair its target expression, which inhibits
the growth of lung cancer cells (35). miR-93 has been reported to promote
the apoptosis and the percentage of cells in the G1 peak of human
umbilical vein endothelial cells by regulating its target gene,
angiopoietin 2 (25). As miR-93
levels were significantly increased in cisplatin-treated A549
cells, the present study further investigated the targeted gene of
this miRNA to evaluate the mechanism of cisplatin-induced lung
cancer cell apoptosis. It was proposed that cyclin D2 is a novel
target of miR-93, which serves important roles in cisplatin-induced
apoptosis and migration.
D-type cyclins, including D1, D2 and D3, serve
important roles in the G1 to S phase transition (36). Cyclin D2 acts as a proto-oncogene
in several types of cancer (37).
Cyclin D2 cytoplasmic localization may reflect an important
physiological role in tumor progression (38). Cyclin D2 is overexpressed in 53% of
colon tumors, and correlates to the high metastatic degree of
tumors (39). Overexpression of
cyclin D2 also correlates with progression and poor prognosis in
gastric cancer (38). Aberrant
cyclin D2 expression has been demonstrated in human ovarian
granulosa cell tumors and testicular germ cell tumor cell lines
(40). As a cell cycle regulator,
cyclin D2 is regulated by let-7 in lung cancer (41). Similarly, the present study
demonstrated that cisplatin inhibited lung cancer cell
proliferation by decreasing cyclin D2 levels.
In summary, the current study further investigated
the mechanism of cisplatin in the inhibition of lung cancer cell
proliferation, which demonstrated that cisplatin could inhibit lung
adenocarcinoma cell proliferation and migration in a dose-dependent
manner. Furthermore, cisplatin was proposed to effectively inhibit
lung adenocarcinoma cell growth by downregulating cyclin D2 via
miR-93.
Acknowledgements
The authors would like to thank Dr Lixia Zhang
(Experimental Central Lab of Binzhou Medical University) for help
with the analysis of the flow cytometry data.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81772281 and
31371321), the Shandong Science and Technology Committee (grant
nos. ZR2019MH022, 2017GSF221011 and 2018GSF118056), the Yantai
Science and Technology Committee (grant nos. 2016ws044 and
2018XSCC051), Health and Family Planning Commission of Shandong
(grant no. 2015WS0499), and the Shandong Province Taishan Scholar
Project (grant no. ts201712067).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SYX conceived and designed the study. NX, YRL, YML,
YNY, LP, YBW, PYW, and YJL performed the experiments. NX, YRL and
SYX wrote the paper. PYW and SYX reviewed and edited the
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Committee on the Ethics of Animal Experiments of Binzhou Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith W and Khuri FR: The care of the lung
cancer patient in the 21st century: A new age. Semin Oncol 31 (2
Suppl 4). S11–S15. 2004.
|
|
3
|
Testa U, Castelli G and Pelosi E: Lung
cancers: Molecular characterization, clonal heterogeneity and
evolution, and cancer stem cells. Cancers (Basel). 10:E2482018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rizvi NA, Hellmann MD, Brahmer JR,
Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie
SA, Goldman JW, et al: Nivolumab in combination with platinum-based
doublet chemotherapy for first-line treatment of advanced
non-small-cell lung cancer. J Clin Oncol. 34:2969–2979. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mens MMJ and Ghanbari M: Cell cycle
regulation of stem cells by MicroRNAs. Stem Cell Rev. 14:309–322.
2018. View Article : Google Scholar :
|
|
6
|
Lou W, Liu J, Gao Y, Zhong G, Ding B, Xu L
and Fan W: MicroRNA regulation of liver cancer stem cells. Am J
Cancer Res. 8:1126–1141. 2018.PubMed/NCBI
|
|
7
|
Qadir MI and Faheem A: miRNA: A diagnostic
and therapeutic tool for pancreatic cancer. Crit Rev Eukaryot Gene
Expr. 27:197–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shomali N, Mansoori B, Mohammadi A,
Shirafkan N, Ghasabi M and Baradaran B: MiR-146a functions as a
small silent player in gastric cancer. Biomed Pharmacother.
96:238–245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao W, Lu X, Liu L, Xu J, Feng D and Shu
Y: MiRNA-21: A biomarker predictive for platinum-based adjuvant
chemotherapy response in patients with non-small cell lung cancer.
Cancer Biol Ther. 13:330–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma JG and Li XY: MicroRNAs are involved in
the toxicity of microcystins. Toxin Rev. 36:165–175. 2017.
View Article : Google Scholar
|
|
11
|
Wu QB, Sheng X, Zhang N, Yang MW and Wang
F: Role of microRNAs in the resistance of colorectal cancer to
chemoradiotherapy. Mol Clin Oncol. 8:528–532. 2018.PubMed/NCBI
|
|
12
|
Hong L, Yang Z, Ma J and Fan D: Function
of miRNA in controlling drug resistance of human cancers. Curr Drug
Targets. 14:1118–1127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blower PE, Chung JH, Verducci JS, Lin S,
Park JK, Dai Z, Liu CG, Schmittgen TD, Reinhold WC, Croce CM, et
al: MicroRNAs modulate the chemosensitivity of tumor cells. Mol
Cancer Ther. 7:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng H, Qianqian G, Ting J and Aimin Y:
miR-539 enhances chemosensitivity to cisplatin in non-small cell
lung cancer by targeting DCLK1. Biomed Pharmacother. 106:1072–1081.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Zhu J, Xing R, Tie Y, Fu H, Zheng
X and Yu B: miR-513a-3p sensitizes human lung adenocarcinoma cells
to chemotherapy by targeting GSTP1. Lung Cancer. 77:488–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ju J, Chen A, Deng Y, Liu M, Wang Y, Wang
Y, Nie M, Wang C, Ding H, Yao B, et al: NatD promotes lung cancer
progression by preventing histone H4 serine phosphorylation to
activate slug expression. Nat Commun. 8:9282017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YX, Yue Z, Wang PY, Li YJ, Xin JX,
Pang M, Zheng QY and Xie SY: Cisplatin upregulates MSH2 expression
by reducing miR-21 to inhibit A549 cell growth. Biomed
Pharmacother. 67:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang PY, Sun YX, Zhang S, Pang M, Zhang
HH, Gao SY, Zhang C, Lv CJ and Xie SY: Let-7c inhibits A549 cell
proliferation through oncogenic TRIB2 related factors. FEBS Lett.
587:2675–2681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YX, Yan YF, Liu YM, Li YJ, Zhang HH,
Pang M, Hu JX, Zhao W, Xie N, Zhou L, et al: Smad3-related miRNAs
regulated oncogenic TRIB2 promoter activity to effectively suppress
lung adenocarcinoma growth. Cell Death Dis. 7:e25282016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao Y, Deng K, Liu X, Dai M, Chen X and
Chen J and Chen J, Huang Y, Dai S and Chen J: Molecular mechanism
and role of microRNA-93 in human cancers: A study based on
bioinformatics analysis, meta-analysis, and quantitative polymerase
chain reaction validation. J Cell Biochem. 120:6370–6383. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwon Y, Kim Y, Eom S, Kim M, Park D, Kim
H, Noh K, Lee H, Lee YS, Choe J, et al:
MicroRNA-26a/-26b-COX-2-MIP-2 loop regulates allergic inflammation
and allergic inflammation-promoted enhanced tumorigenic and
metastatic potential of cancer cells. J Biol Chem. 290:14245–14266.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan M, Wu J and Cai Y: Suppression of Wnt
signaling by the miR-29 family is mediated by demethylation of
WIF-1 in non-small-cell lung cancer. Biochem Biophys Res Commun.
438:673–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang PY, Li YJ, Zhang S, Li ZL, Yue Z, Xie
N and Xie SY: Regulating A549 cells growth by ASO inhibiting miRNA
expression. Mol Cell Biochem. 339:163–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian Q, Sun W, Zhu W, Liu Y, Ge A, Ma Y,
Zhang Y, Zeng X and Huang M: The role of microRNA-93 regulating
angiopoietin2 in the formation of malignant pleural effusion.
Cancer Med. 6:1036–1048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hahne JC and Valeri N: Non-Coding RNAs and
resistance to anticancer drugs in gastrointestinal tumors. Front
Oncol. 8:2262018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagesh PKB, Chowdhury P, Hatami E, Boya
VKN, Kashyap VK, Khan S, Hafeez BB, Chauhan SC, Jaggi M and Yallapu
MM: miRNA-205 nanoformulation sensitizes prostate cancer cells to
chemotherapy. Cancers (Basel). 10:E2892018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin YS, Lin YY, Yang YH, Lin CL, Kuan FC,
Lu CN, Chang GH, Tsai MS, Hsu CM, Yeh RA, et al: Antrodia
cinnamomea extract inhibits the proliferation of
tamoxifen-resistant breast cancer cells through apoptosis and
skp2/microRNAs pathway. BMC Complement Altern Med. 18:1522018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan LW, Wang F, Meng F, Wang L, Wong SC,
Au JS, Yang S and Cho WC: MiR-30 family potentially targeting
PI3K-SIAH2 predicted interaction network represents a novel
putative theranostic panel in non-small cell lung cancer. Front
Genet. 8:82017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Li R and Gan YH: Knockdown of Yin
Yang 1 enhances anticancer effects of cisplatin through protein
phosphatase 2A-mediated T308 dephosphorylation of AKT. Cell Death
Dis. 9:7472018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao L, Wan Q, Li F and Tang CE: MiR-363
inhibits cisplatin chemoresistance of epithelial ovarian cancer by
regulating snail-induced epithelial-mesenchymal transition. BMB
Rep. 51:456–461. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Huang S, Guo Y and Li L: MiR-1294
confers cisplatin resistance in ovarian Cancer cells by targeting
IGF1R. Biomed Pharmacother. 106:1357–1363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Markou A, Sourvinou I, Vorkas PA, Yousef
GM and Lianidou E: Clinical evaluation of microRNA expression
profiling in non small cell lung cancer. Lung Cancer. 81:388–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Price C and Chen J: MicroRNAs in cancer
biology and therapy: Current status and perspectives. Genes Dis.
1:53–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun CC, Li SJ, Zhang F, Zhang YD, Zuo ZY,
Xi YY, Wang L and Li DJ: The novel miR-9600 suppresses tumor
progression and promotes paclitaxel sensitivity in non-small-cell
lung cancer through altering STAT3 expression. Mol Ther Nucleic
Acids. 5:e3872016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang P: The cell cycle and development:
Redundant roles of cell cycle regulators. Curr Opin Cell Biol.
11:655–662. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blanco L and Tirado CA: Testicular germ
cell tumors: A cytogenomic update. J Assoc Genet Technol.
44:128–133. 2018.PubMed/NCBI
|
|
38
|
Takano Y, Kato Y, van Diest PJ, Masuda M,
Mitomi H and Okayasu I: Cyclin D2 overexpression and lack of p27
correlate positively and cyclin E inversely with a poor prognosis
in gastric cancer cases. Am J Pathol. 156:585–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mermelshtein A, Gerson A, Walfisch S,
Delgado B, Shechter-Maor G, Delgado J, Fich A and Gheber L:
Expression of D-type cyclins in colon cancer and in cell lines from
colon carcinomas. Br J Cancer. 93:338–345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sicinski P, Donaher JL, Geng Y, Parker SB,
Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers
JD, et al: Cyclin D2 is an FSH-responsive gene involved in gonadal
cell proliferation and oncogenesis. Nature. 384:470–474. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Johnson CD, Esquela-Kerscher A, Stefani G,
Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J,
Shingara J, et al: The let-7 microRNA represses cell proliferation
pathways in human cells. Cancer Res. 67:7713–7722. 2007. View Article : Google Scholar : PubMed/NCBI
|