Introduction
Peritoneal dialysis (PD) is an effective alternative
treatment for end-stage renal disease (1–4).
Peritoneal fibrosis is a serious complication during PD treatment
that affects the survival and prognosis of patients undergoing PD.
Peritoneal fibrosis is also one of the primary factors leading to
withdrawal from treatment (5–7). The
components and some bioincompatible properties of peritoneal
dialysates, such as low pH, lactate buffer, high sugar, low
calcium, plasticizer and glucose degradation products, cause loss
of peritoneal mesothelial cells (PMCs), subcutaneous dense zone
thickening, interstitial fibrosis, inflammation and
neovascularization (8–12). Damage to PMCs is a key initiating
factor that leads to peritoneal fibrosis (13–15).
After PMCs are damaged, extracellular matrix (ECM) components,
including collagen, fibronectin, laminin, proteoglycan and various
fibrogenic factors, such as transforming growth factor (TGF)-β1,
fibroblast growth factor, connective tissue growth factor, platelet
derived growth factor, toll-like receptors (TLRs), angiotensin II
receptor and receptor tyrosine kinases, are highly expressed or
secreted, which interferes with the normal metabolism of the ECM
and promoting its overdeposition, ultimately leading to peritoneal
fibrosis (10,16–23).
Peritoneal fibrosis can be delayed or inhibited by promoting PMC
survival and inhibiting PMC epithelial-to-mesenchymal transition
(EMT) (14,16–19,21,22,24).
Previous studies have shown that Astragalus
membranaceus inhibits peritoneal fibrosis in PD through
monocyte chemoattractant protein-1 and the TGF-β1 pathway (25), and ameliorates renal interstitial
fibrosis by inhibiting EMT, inflammation, TLR4/NF-κB and cyrillic B
(25–27). Astragalus inhibits PMC EMT
by downregulating β-catenin (28).
Astragaloside IV is a key compound extracted from Astragalus
membranaceus (27,29,30).
It has been shown that astragaloside IV inhibits TGF-β1-induced PMC
EMT through the upregulation of Smad7 in the TGF-β1/Smad signaling
pathway (31). However, the effect
of astragaloside IV on viability and apoptosis of PMCs remains
unclear.
Retinoid X receptor-α (RXRα) is a ligand-dependent
nuclear receptor expressed in various tissues and cells (32–34).
RXRα can form heterodimers with other nuclear receptors, including
peroxisome proliferator activated receptor (PPAR), vitamin D
receptor (VDR) and thyroid hormone receptors, resulting in the
involvement of RXRα in multiple signaling pathways (35–40).
Previous studies have shown that vitamin D/VDR can inhibit
peritoneal fibrosis and functional deterioration induced by
chlorhexidine gluconate by inhibiting PMC EMT (41–43).
Telmisartan inhibits peritoneal fibrosis through PPAR-γ activation
(44). The PPAR-β/δ agonist
GW501516 inhibits peritoneal inflammation in peritoneal fibrosis by
inhibiting the TGF-β-activated kinase 1/NF-κB pathway (45). The PPAR-γ agonists rosiglitazone
and pioglitazone protect rat PMCs against PD solution-induced
damage (46,47). These previous studies indicated
that the RXR signaling pathway is involved in regulating PMC EMT
and peritoneal fibrosis. However, the role of RXRα in PMC activity,
apoptosis and EMT in peritoneal fibrosis remains unclear.
In the present study, the human PMC HMrSV5 cell line
and high glucose-based PD fluids were used as a model (31) to study the effects of astragaloside
IV on PMC viability, apoptosis and EMT during PD. The role of RXRα
in PMC viability, apoptosis and EMT during PD was also
investigated. The findings of the present study may provide
important information for the prevention and treatment of
PD-induced fibrosis.
Materials and methods
Construction of RXRα short hairpin RNA
(shRNA) plasmid
The synthetic DNA fragment targeting RXRα
(GGATCCCGCACTATGGAGTGTACAGCTCAAGAGAGAGCTGTACACTCCAGTGCTTTTTTCCAAAAGCTT,
synthesized by Western Biomedical Technology, Ltd.) and the vector
SD1211 (Biovector Science Lab, Inc.) were modified with
BamHI and HindIII (Takara Bio, Inc.) at 37°C for 30
min. After gel purification, the digested DNA fragment and the
vector were ligated using T4 DNA ligase (Takara, Bio, Inc.) and
then transfected into DH5α competent cells (Tiangen Biotech Co.,
Ltd.) for plasmid amplification. After selection and screening,
plasmids were sequenced to confirm successful construction of the
shRNA plasmid.
Cell culture and grouping
The human PMC cell line HMrSV5 was obtained from the
Type Culture Collection of the Cell Bank of Chinese Academy of
Sciences. This cell line was established by Professor Pierre Ronco,
Hospital Tenon (Paris, France) (48) and had been used in a number of
previous studies (49–52). HMrSV5 cells were cultured in DMEM
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin in an incubator at 37°C supplemented with
5% CO2 and a saturated humidity. To investigate the
effects of astragaloside IV (Yuanye Bio-Technology Co., Ltd.) on
PMCs in high glucose-based PD fluids, HMrSV5 cells were divided
into four groups: i) Normal + vehicle control group, cells were
cultured in regular media and treated with DMSO; ii) PD model +
vehicle group, cells were cultured in PD fluids and treated with
DMSO; iii) normal + astragaloside IV group, cells were cultured in
regular media and treated with astragaloside IV; and iv) PD model
regular astragaloside IV group, cells were cultured in PD fluids
and treated with astragaloside IV. To investigate the role of RXRα
in maintaining PMCs in PD fluids, HMrSV5 cells were divided into
four groups: i) Blank + vehicle control group, cells were
transfected with the SD1211 empty plasmid, cultured in PD fluids
and treated with DMSO; ii) RXRα shRNA plasmid + vehicle group,
cells were transfected with the RXRα shRNA plasmid, cultured in PD
fluids and treated with DMSO; iii) blank + astragaloside IV group,
cells were transfected with the SD1211 empty plasmid, cultured in
PD fluids and treated with astragaloside IV; and iv) RXRα shRNA +
astragaloside IV group, cells were transfected with the SD1211 RXRα
shRNA plasmid, cultured in PD fluids and treated with astragaloside
IV. Cells in each group were first transfected with the appropriate
plasmid, for 6 h and then cultured in fresh media for 24 h. These
cells were then cultured in PD fluids and astragaloside IV or DMSO
was added. The PD fluids used for cell culture were made from
original PD fluids with the addition of 10% FBS. The original PD
fluids (Lactate-G4.25%; cat. no. 6AB9896) were purchased from
Guangzhou Baxter Medical Products Co., Ltd. Its components include
4.25 g glucose, 538 mg sodium chloride, 26 mg calcium chloride, 5.1
mg magnesium chloride and 448 mg sodium lactate/100 ml. The final
concentration of astragaloside IV in the normal media or PD fluids
was 40 µg/ml. Astragaloside IV was dissolved in DMSO to make a 40
mg/ml stock solution. The same volume of DMSO and astragaloside IV
stock solution was used to treat cells. All cells were cultured and
treated with pertinent chemicals at 37°C.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay was used to determine the viability
of HMrSV5 cells. To investigate the effects of astragaloside IV on
the viability of PMCs in PD fluids, HMrSV5 cells in the log phase
growth from each group were seeded in triplicate into 96-well
plates at a density 1×105 cells/cm2 and
cultured overnight. Cells were treated with astragaloside IV, PD
and their respective controls for 24, 48 or 72 h. To investigate
the role of RXRα in maintaining the viability of PMCs in PD fluids,
HMrSV5 cells from each group were seeded in triplicate into 96-well
plates at a density 1×105 cells/cm2 and
cultured overnight. Following overnight culture, cells were
transfected with SD1211 empty vector (0.4 µg/cm2) or
RXRα shRNA plasmid (0.4 µg/cm2) for 6 h and then
cultured in fresh media for 24 h. These cells were then treated
with astragaloside IV, PD and DMSO for 24, 48 or 72 h. Cell
viability was determined using a CCK-8 kit (Sigma-Aldrich; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The absorbance (A) at 450 nm was measured using a
microplate reader (Thermo Fisher Scientific, Inc.). A 96-well plate
with medium and CCK reagent was used as a blank control. Cell
viability (%)=[A (experiment)-A (blank plate)]/A (normal + vehicle
control group) ×100; or cell viability (%)=[A (experiment)-A (blank
plate)]/A (blank + vehicle control group) ×100.
Flow cytometry
Flow cytometry was used to examine the level of
apoptosis in HMrSV5 cells. To investigate the effects of
astragaloside IV on the level of apoptosis of PMCs in PD fluids,
HMrSV5 cells were seeded into 6-well plates at a density
1×105 cells/cm2, cultured overnight and
treated with astragaloside IV, PD and their respective controls for
48 h. To investigate the role of RXRα in apoptosis of PMCs in PD
fluids, HMrSV5 cells were seeded into 6-well plates at a density
1×105 cells/cm2. Following overnight culture,
cells were transfected with SD1211 empty vector (0.4
µg/cm2) or RXRα shRNA plasmid (0.4 µg/cm2)
for 6 h and then cultured in fresh media for 24 h. These cells were
then treated with PD and astragaloside IV or DMSO for 48 h. Cells
were collected and the rate of apoptosis was determined using a
EPICS XL flow cytometer (Beckman Coulter, USA) and Annexin V-PE
Apoptosis Detection Kit I (BD Biosciences, 559763) according to the
manufacturer's instructions. Briefly, cells were washed twice with
cold PBS and then resuspended in 1X Binding Buffer (BD Biosciences,
51–66121E) at a concentration of 1×106 cells/ml. Then,
100 µl of the solution (1×105 cells) was transferred to
a 5 ml culture tube and 5 µl of Annexin V-PE (BD Biosciences,
51-65875X) and 5 µl of 7-Amino-actinomycin D (7-AAD; BD
Biosciences, 51-68981E) added. The cells were gently vortexed and
incubated for 15 min at room temperature (25°C) in the dark. 1X
binding buffer (400 µl) was added to each tube. Flow cytometry
analysis was performed within one hour. The results were analyzed
using CytExpert 1.2 software (Beckman Coulter, Inc.).
Western blotting
To investigate the effects of astragaloside IV on
caspase-3 levels and EMT of PMCs in PD fluids, HMrSV5 cells were
seeded in 6-well plates at a density 1×105
cells/cm2. After overnight culture, cells were treated
with astragaloside IV, PD and their respective controls for 48 h.
To investigate the role of RXRα on the caspase-3 levels and EMT of
PMCs in PD fluids, HMrSV5 cells were seeded in 6-well plates at a
density 1×105 cells/cm2. After overnight
culture, cells were transfected with SD1211 empty vector (0.4
µg/cm2) or RXRα shRNA plasmid (0.4 µg/cm2)
for 6 h and then cultured in fresh media for 24 h. These cells were
then treated with astragaloside IV, PD and DMSO for 48 h. Cells
were collected and lysed using RIPA buffer [50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1% (v/v) NP40, 0.1% (w/v) SDS, 0.5% (w/v) sodium
deoxycholate] with protease inhibitor PMSF (100 mM; Beyotime
Institute of Biotechnology). Equal amounts of proteins (50 µg) were
separated by SDS-PAGE on 10% gels and then transferred onto PVDF
membranes. After blocking with 5% non-fat milk at room temperature
for 2 h, membranes were incubated overnight at 4°C with the
following primary antibodies: E-cadherin (1:500; Abcam, ab1416),
α-smooth muscle actin (α-SMA; 1:500; Abcam, ab32575), caspase-3
(1:500; Abcam, ab32351), β-actin (1:500; Abcam, ab179467) or GAPDH
(1:1,000; Cell Signaling Technologies, Inc., 2118). This was
followed by incubation with horseradish peroxidase-coupled
secondary antibodies (1:1,000; Cell Signaling Technologies, Inc.;
7074 and 7076). Bands were visualized using the Enhanced
Chemiluminescence Reagent kit (EMD Millipore) and analyzed using
the GDS8000 system GelDoc-It310 and software VisionWorks LS v6.5.2
(UVP, LLC).
Knockdown of RXRα expression in HMrSV5
cells and determination of relative RXRα mRNA levels using reverse
transcription-quantitative PCR (RT-qPCR)
HMrSV5 cells were transfected with SD1211 empty
vector (0.4 µg/cm2) or RXRα shRNA plasmid (0.4
µg/cm2) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) and Opti-MEM (Gibco;
Thermo Fisher Scientific, Inc.), which was added to cells,
incubated for 4–6 h and then cultured with regular media. After 24
h of culture, total RNA was extracted from cells using a MiniBEST
Universal RNA Extraction kit (Takara Bio, Inc., 9767), according to
the manufacturer's instructions, and reverse transcribed using
oligo dT primers and a PrimeScript II 1st Strand cDNA Synthesis kit
(Takara Bio, Inc., 6210A) according to the manufacturer's
instructions. The thermocycling conditions used for the reverse
transcription was as follows: 30°C 10 min, 42°C 30–60 min, 95°C 5
min, and then chilled in ice. Relative mRNA levels were analyzed by
RT-qPCR using a PowerUp SYBR Green Master Mix (Applied Biosystems)
and an ABI 7500 fast cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). GAPDH was used for normalization. The relative
RXRα mRNA levels were calculated using the 2−ΔΔCq method
after normalization (53). All
experiments were repeated three times. The following primers were
used: GAPDH forwards, AGATCCCTCCAAAATCAAGTGG and reverse,
GGCAGAGATGATGACCCTTTT; RXRα forward, CGGGAAGGTTCGCTAAGCT, and
reverse, TGTTCCAGGCATTTGAGCC. Primers were designed using Primer 5
according to reference sequences in NCBI and synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.).
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation for three experimental repeats. All the data
were analyzed using SPSS 17.0 software (SPSS, Inc.). Graphics for
quantitative data were processed using Prism 5 (GraphPad).
Statistical analysis of the difference among multiple groups was
performed by one-way ANOVA followed by the post hoc
Student-Newman-Keuls test. Statistical analysis of the difference
in RXRα levels in HMrSV5 cells transfected with SD1211 empty vector
and those transfected with RXRα shRNA plasmid was performed using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Astragaloside IV enhances the
viability of PMCs in PD fluid
To study the effect of astragaloside IV on the
viability of PMCs during high-glucose PD, HMrSV5 cells were treated
with PD fluid and astragaloside IV, and cell viability was
determined using a CCK-8 assay. The viability of HMrSV5 cells was
decreased in the PD model + vehicle groups compared with the normal
+ vehicle controls (Fig. 1).
Viability in the PD model group was increased significantly after
treatment with astragaloside IV compared with the PD model +
vehicle groups (Fig. 1). Under
normal conditions, the viability of HMrSV5 cells was not affected
by astragaloside IV (Fig. 1).
These results suggested that astragaloside IV increased viability
of PMCs cultured in PD fluid, but did not affect cell viability
under normal conditions.
Astragaloside IV reduces apoptosis of
PMCs cultured in PD fluid
To examine the effect of astragaloside IV on the
rate of apoptosis in PMCs during high glucose-based PD, PD
fluid-treated HMrSV5 cells were used as a PD model. These cells
were treated with astragaloside IV or vehicle control, and the rate
of apoptosis was determined using flow cytometry. Additionally, the
protein levels of caspase-3, a hallmark of apoptosis, were
determined using western blotting. The number of apoptotic HMrSV5
cells was significantly increased in PD fluids compared with the
normal control (Fig. 2A and B).
Apoptosis was significantly reduced in PD model cells after
treatment with astragaloside IV, while the HMrSV5 cells apoptosis
was not affected by astragaloside IV under normal conditions
(Fig. 2A and B). Caspase-3 protein
levels in HMrSV5 cells were significantly increased in the PD model
group compared with the normal control. Caspase-3 levels were
significantly decreased in the PD model cells following treatment
with astragaloside IV (Fig. 2C).
Caspase-3 protein levels were not affected by astragaloside IV
under normal conditions (Fig. 2C).
These results suggested that astragaloside IV inhibited apoptosis
of PMCs in the PD model, but that astragaloside IV did not affect
apoptosis under normal conditions.
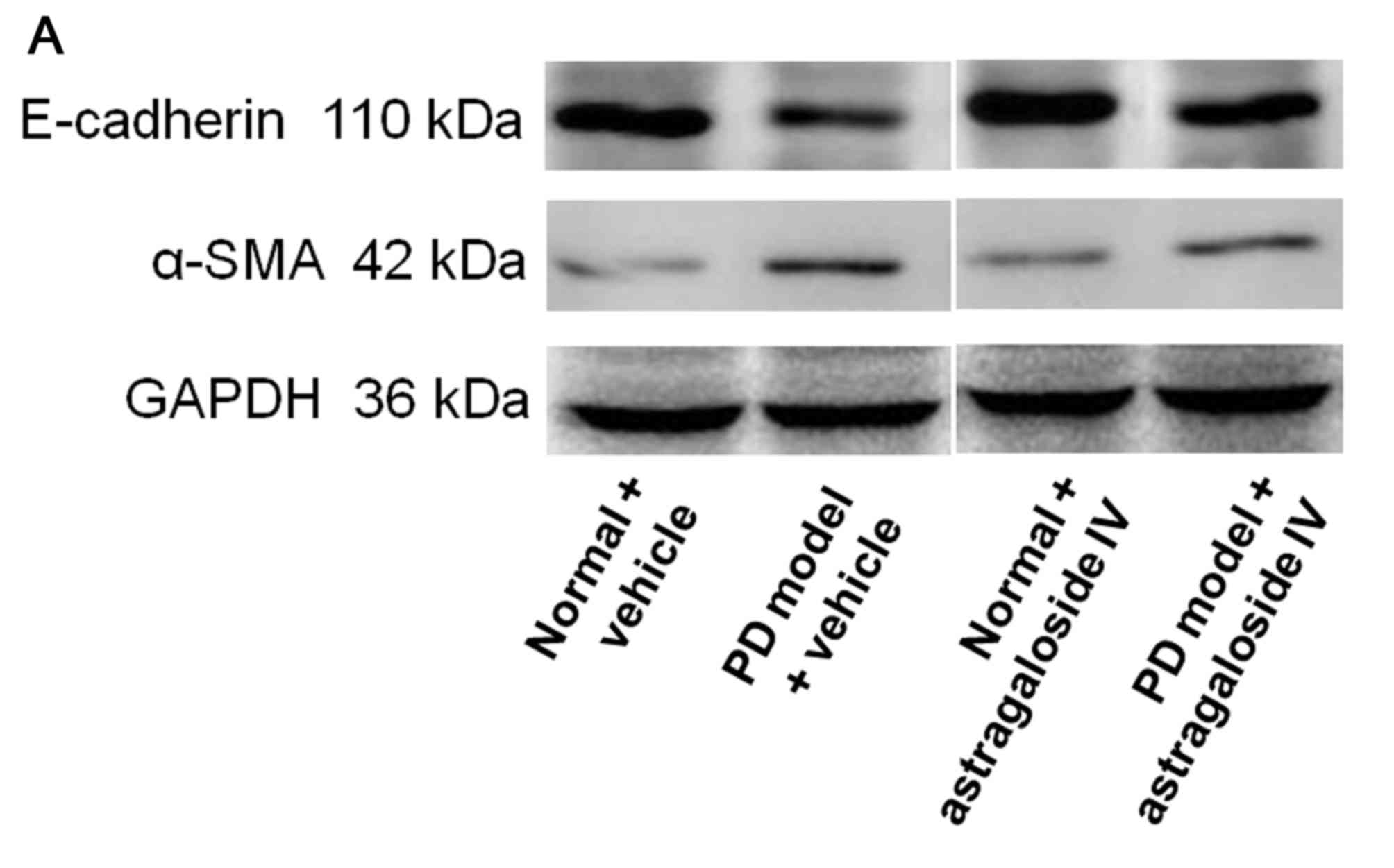
Astragaloside IV inhibits EMT of PMCs
cultured in PD fluid
To investigate the effect of astragaloside IV on EMT
of PMCs in PD, HMrSV5 were treated with high-glucose PD fluid and
astragaloside IV, and the levels of E-cadherin and α-SMA were
determined using western blot analysis. The level of E-cadherin was
decreased in HMrSV5 cells cultured in PD fluid compared with the
normal control (Fig. 3). The level
of E-cadherin in the PD model was significantly increased by
treatment with astragaloside IV (Fig.
3). The level of α-SMA was significantly increased in HMrSV5
cells cultured in PD fluid compared with the normal control
(Fig. 3). The level of α-SMA was
significantly decreased in the PD model after treatment with
astragaloside IV (Fig. 3). The
levels of both E-cadherin and α-SMA were not affected by
astragaloside IV under normal conditions (Fig. 3). These results suggested that
astragaloside IV inhibited PMC EMT induced by PD fluid, but did not
affect PMC EMT under normal conditions.
Knockdown of RXRα expression in HMrSV5
cells
To knockdown the expression of RXRα in HMrSV5 cells,
the RXRα shRNA plasmid was constructed using the SD1211 vector and
transfected into HMrSV5 cells. The relative expression level of
RXRα mRNA was determined using RT-qPCR to examine the effect and
efficiency of RXRα shRNA on RXRα expression in HMrSV5 cells. The
level of RXRα mRNA was decreased by ~80% in the HMrSV5 cells after
transfection with SD1211 RXRα shRNA compared with the empty vector
control (Fig. 4). These data
suggested that RXRα expression was significantly and efficiently
reduced in HMrSV5 cells by the SD1211 RXRα shRNA plasmid.
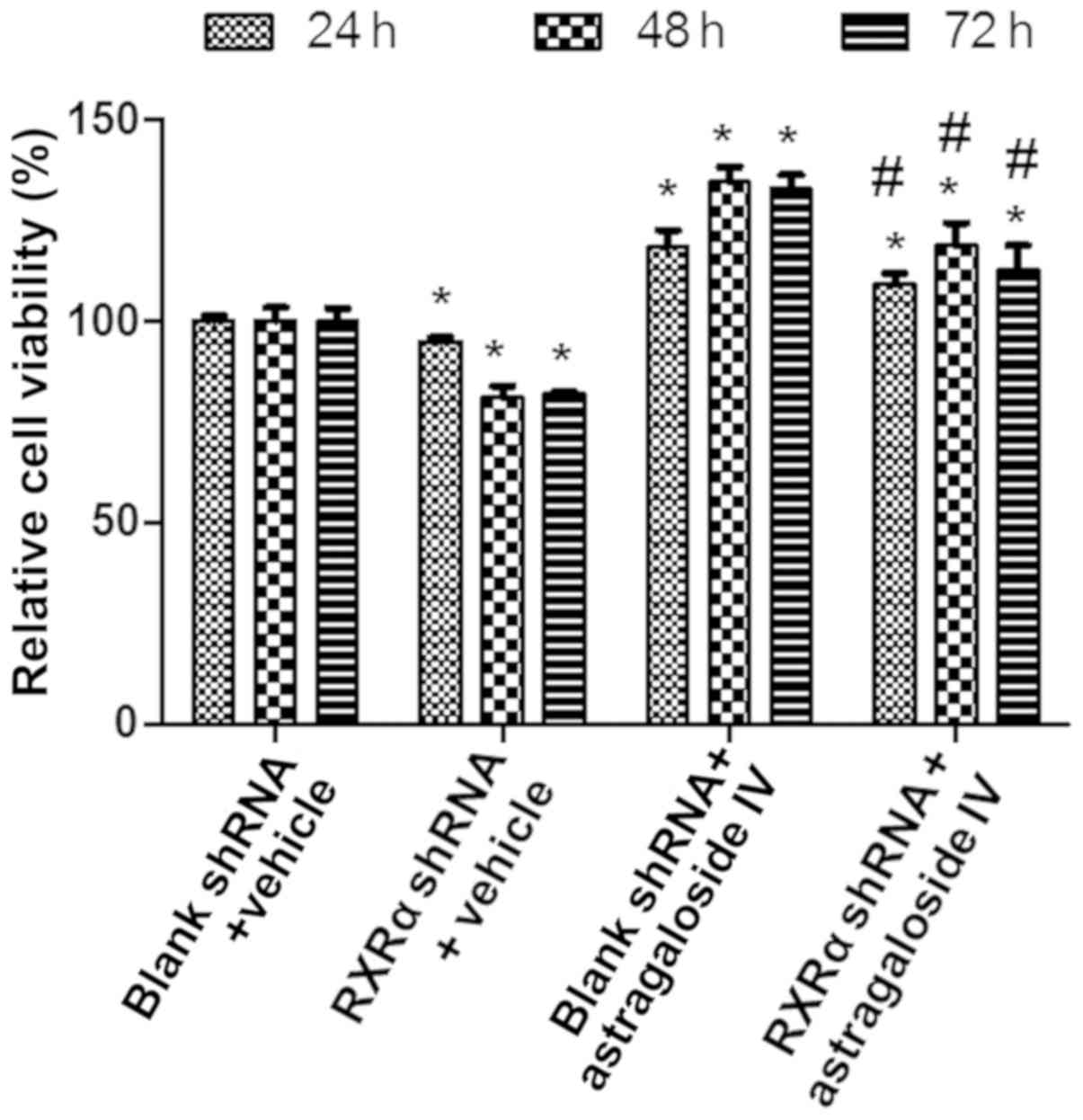
RXRα is required to maintain the
viability of PMCs in PD fluids
To examine the role of RXRα in maintaining the
viability of PMCs during high glucose-based PD, HMrSV5 cells were
transfected with SD1211 RXRα shRNA or empty SD1211, these cells
were treated with PD fluids and astragaloside IV or vehicle
control, and the cell viability was determined using the CCK-8
assay. The results showed that the viability of HMrSV5 cells
treated with vehicle or astragaloside IV was decreased after RXRα
shRNA transfection compared with the empty vector transfections
(Fig. 5). Treatment with
astragaloside IV resulted in increases in the viability of HMrSV5
cells after RXRα shRNA or blank vector transfection compared with
the vehicle control treatment (Fig.
5). These results suggested that a decrease in the level of
RXRα results in reduced viability of PMCs in PD fluid. Therefore,
RXRα is required to maintain the viability of PMCs in PD fluid.
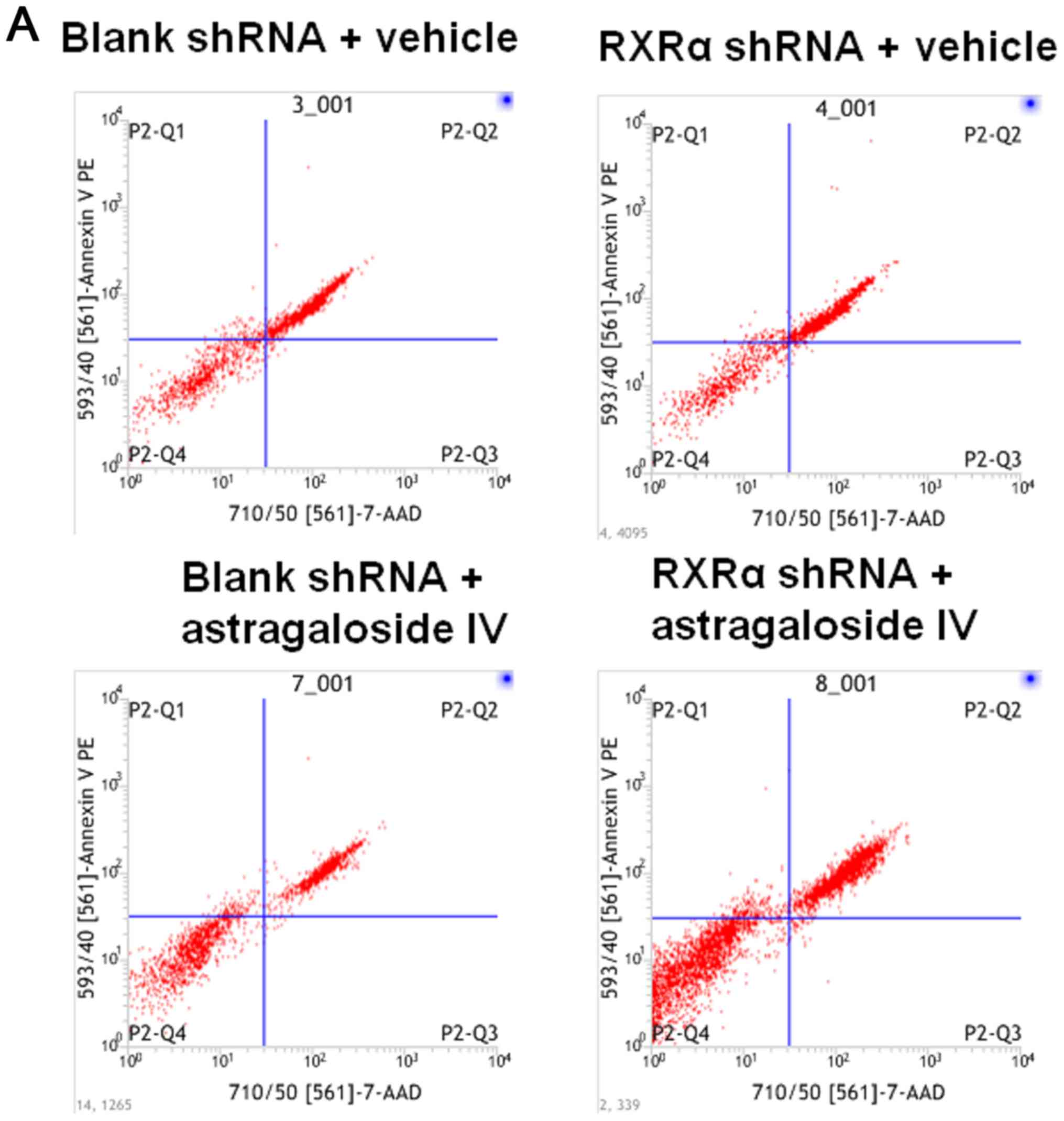
RXRα is required to reduce apoptosis
and the level of caspase-3 in PMCs cultured in PD fluid
To examine the role of RXRα in the apoptosis of PMCs
during high glucose-based PD, HMrSV5 cells were transfected with
SD1211 RXRα shRNA or SD1211 empty vector, these cells were treated
with PD fluid and astragaloside IV or vehicle control. The rate of
apoptosis was determined using flow cytometry and the level of
caspase-3 was determined using western blotting. The results showed
that the rate of apoptosis in HMrSV5 cells treated with vehicle or
astragaloside IV were increased after RXRα shRNA transfection
compared with the blank shRNA transfection (Fig. 6A and B). Treatment with
astragaloside IV resulted in decreased apoptosis of HMrSV5 cells
transfected with RXRα shRNA or blank vector compared with the
vehicle control treatments (Fig. 6A
and B). Similar changes in the levels of caspase-3 were
observed (Fig. 6C). These results
suggested that a decrease in the level of RXRα resulted in an
increase in apoptosis and the level of caspase-3 in PMCs cultured
in PD fluid. Therefore, RXRα may be involved in reducing the
apoptosis of PMCs in PD fluids.
RXRα is silencing increased EMT of
PMCs cultured in PD fluid
To examine the role of RXRα in EMT of PMCs during
high glucose-based PD, HMrSV5 cells were transfected with SD1211
RXRα shRNA or SD1211 empty vector, and cultured in PD fluid with
astragaloside IV or vehicle control. The levels of E-cadherin and
α-SMA were determined using western blot analysis. The level of
E-cadherin in HMrSV5 cells treated with vehicle or astragaloside IV
were decreased after RXRα shRNA transfection compared with the
blank shRNA transfections (Fig.
7). Treatment with astragaloside IV resulted in an increase in
the level of E-cadherin in HMrSV5 cells after RXRα shRNA or blank
vector transfections compared the vehicle control treatments
(Fig. 7). By contrast, the level
of α-SMA in HMrSV5 cells treated with vehicle or astragaloside IV
were increased after RXRα shRNA transfection compared with the
empty shRNA transfections (Fig.
7). Treatment with astragaloside IV resulted in a decrease in
the level of α-SMA in HMrSV5 cells after RXRα shRNA or empty vector
transfections compared with the vehicle control treatments
(Fig. 7). These results suggested
that a decrease in the level of RXRα resulted in an increase in EMT
in PMCs in PD fluid. Therefore, RXRα may be required inhibit EMT in
PMCs in PD fluid.
Discussion
Damage to PMCs is an initiating and important factor
in peritoneal fibrosis. A number of preclinical animal and in
vitro studies have revealed that the onset of peritoneal
fibrosis is delayed or inhibited by promoting PMC survival and
inhibiting PMC EMT (8–12,14,16–19,21,22,24).
Previous studies have revealed that several drugs can inhibit PMC
EMT and inhibit peritoneal fibrosis. Melatonin can reverse
lipopolysaccharide-induced EMT (54). Fluvastatin inhibits high
glucose-based PD-induced fibronectin expression in human PMCs via
the serum- and glucocorticoid-inducible kinase 1 pathway (55). The histone acetyltransferase
inhibitor C646 reverses EMT in human PMCs via the TGF-β/Smad3
signaling pathway (56). The
adenosine 5′-monophosphate (AMP)-activated protein kinase activator
HL156A protects against peritoneal fibrosis (57). Suramin inhibits the occurrence and
deterioration of peritoneal fibrosis (58). Selenium inhibits EMT by regulating
reactive oxygen species (ROS) and the ROS/matrix
metalloproteinase-9 signaling pathways and the PI3K/AKT pathways in
PMCs (59). Hydrogen sulfide can
improve peritoneal fibrosis by inhibiting inflammation and TGF-β
synthesis (60). Metformin
ameliorates the transition phenotype of PMCs and peritoneal
fibrosis via the modulation of oxidative stress (61). The data in the present study showed
that astragaloside IV increases cell viability and inhibits
apoptosis and EMT in PMCs cultured in high-glucose PD fluid,
without affecting PMCs under normal conditions. This is consistent
with a previous report by Zhang et al (31). Astragaloside IV may be a potential
drug that could be used for the inhibition of peritoneal
fibrosis.
Previous studies have shown that several signaling
pathways are involved in the protective effects of astragaloside IV
in different cell types during fibrosis and under high glucose
challenge. Astragaloside IV inhibits TGF-β1/PI3K/AKT-induced
forehead box O3a hyper-phosphorylation and downregulation to
reverse EMT during the progression of bleomycin-induced pulmonary
fibrosis (62). Astragaloside IV
has been reported to inhibit renal fibrosis and promote renal
function in diabetic KK-Ay mice through inhibition of
glucose-induced EMT in glomerular podocytes by activating autophagy
and Sirtuin-1 expression, which results in decreased acetylation of
NF-κB subunit p65 (63).
Astragaloside IV also downregulates the calcineurin/nuclear factor
of activated T cells/transient receptor potential channel 6 pathway
to prevent high glucose-induced podocyte apoptosis (64). Astragaloside IV prevents high
glucose-induced apoptosis and inflammatory reactions by inhibiting
the JNK pathway in human umbilical vein endothelial cells (65). Astragaloside IV ameliorates high
glucose-induced apoptosis and oxidative stress in the human
proximal tubular HK-2 cell line by regulating the nuclear factor
erythroid 2-related factor 2/antioxidant responsive element
(NFE2L2/ARE) signaling pathway (66). Astragaloside IV protects primary
cerebral cortical neurons from oxygen and glucose
deprivation/reoxygenation by activating the cyclic AMP
(cAMP)-dependent protein kinase/cAMP response element-binding
protein pathway (67).
Astragaloside IV inhibits cell viability, invasion, migration and
TGF-β1-induced EMT in gastric cancer cells through inhibition of
the PI3K/AKT/NF-κB pathway (68).
Astragaloside IV inhibits the invasion and migration of
hepatocellular carcinoma cells by reducing EMT via effect on the
AKT/glycogen synthase kinase-3β/β-catenin pathway (69), and suppressing long noncoding RNA
activated by TGF-β/interleukin-11/STAT3 signaling (70). Therefore, it is speculated that
similar signaling pathways may also be involved in the protective
effects of astragaloside IV in PMCs to prevent damage from high
glucose-based PD fluids, in addition to the upregulation of Smad7
in the TGF-β1/Smad signaling pathway during the inhibition of
TGF-β1-induced PMC EMT by astragaloside IV (31).
PMC homeostasis is important for resistance against
peritoneal fibrosis (13–15). Previous studies have identified
several molecular components that are essential for PMC
homeostasis. Heat shock protein 70 has been reported to protect
PMCs from late glycation end products-induced EMT through the
mitogen-activated protein kinase/ERK and TGF-β/Smad3 pathways
(71,72). NF-κB mediates the inhibition of
high glucose induced PMC extracellular matrix synthesis by
pioglitazone (46) and the effects
of chondroitin sulfate on peritoneal fibrosis (73). Twist promotes cell proliferation
and EMT-induced fibrosis by regulating Y-box binding protein 1 in
PMCs (74). Acidic organelles
mediate TGF-β1-induced cellular fibrosis via (pro)renin receptor
and vacuolar ATPase trafficking in human PMCs (75). MicroRNA-15a-5p suppressed PMC EMT
(76) and microRNA-21 promoted PMC
EMT (77). VDR, PPARγ and PPARβ/δ
are also involved in the regulation of PMC activity and homeostasis
during peritoneal dialysis (41–47).
As RXRα is a dimerization partner for VDR and PPAR, the role of
RXRα in PMC homeostasis during PD was investigated. The data from
the present study indicated that RXRα was required to maintain
viability, inhibit apoptosis and reduce EMT of PMCs in high
glucose-based PD fluid. Therefore, RXRα is an important factor for
PMC viability and the ability to resist apoptosis and EMT
induction.
It has previously been established that
Astragalus membranaceus inhibits peritoneal fibrosis during
PD (18–20,22).
The data from the present study, and the study by Zhang et
al (31), showed that
astragaloside IV, a component of Astragalus membranaceus,
increased cell viability and inhibited apoptosis and EMT in PMCs in
high glucose-based PD fluids without affecting PMCs under normal
conditions, suggesting that astragaloside IV is an active, key
component of Astragalus membranaceus that contributes to its
anti-fibrosis function. It was also shown that RXRα was required to
maintain viability, inhibit apoptosis and reduce EMT in PMCs
cultured in high-glucose PD fluids. A limitation of the present
study was that the cause-effect relationship between astragaloside
IV and RXRα was not investigated. It has been previously shown that
astragaloside IV can bind to glucocorticoid receptor (GR) with a
low affinity, modulating the GR-mediated signaling pathway,
including dephosphorylation of PI3K, AKT, inhibitor of κB and NF-κB
in microglia (78). Astragaloside
IV is a natural PPARγ agonist that suppresses the activity of
β-secretase 1 and amyloid β (Aβ) levels in SH-SY5Y cells, and
reduces neuritic plaque formation and Aβ levels in the brains of
APP/PS1 mice, a model of Alzheimer's disease (79). Whether and how the effect of
astragaloside IV on PMCs is mediated by RXRα remains to be
determined; this question requires further investigation in future
studies.
In conclusion, astragaloside IV increased cell
viability, and inhibited apoptosis and EMT of PMCs in high-glucose
PD fluid, but did not affect PMCs under normal condition. RXRα
silencing reduced viability, inhibited apoptosis and reduced EMT of
PMCs in high-glucose PD fluid. Astragaloside IV may be a potential
drug that could be used for the inhibition of peritoneal fibrosis.
RXRα was found to be an important factor involved in maintaining
the viability of PMCs, and in their ability to resist apoptosis and
EMT induction. The findings of the present study may provide
important information for the prevention and treatment of
PD-induced fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (NSFC; grant nos. 81673912 and
81873259).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ, XZ and KG performed experiments, and collected
and analyzed data. XW conceived the study and wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan S and Rosner MH: Peritoneal dialysis
for patients with end-stage renal disease and liver cirrhosis.
Perit Dial Int. 38:397–401. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vareldzis R, Naljayan M and Reisin E: The
incidence and pathophysiology of the obesity paradox: Should
peritoneal dialysis and kidney transplant be offered to patients
with obesity and end-stage renal disease? Curr Hypertens Rep.
20:842018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Javaid MM, Khan BA and Subramanian S:
Peritoneal dialysis as initial dialysis modality: A viable option
for late-presenting end-stage renal disease. J Nephrol. 32:51–56.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang WN, Zhang WL, Sun T, Ma FZ, Su S and
Xu ZG: Effect of peritoneal dialysis versus hemodialysis on renal
anemia in renal in end-stage disease patients: A meta-analysis. Ren
Fail. 39:59–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krediet RT, Abrahams AC, de Fijter CWH,
Betjes MGH, Boer WH, van Jaarsveld BC, Konings CJAM and Dekker FW:
The truth on current peritoneal dialysis: State of the art. Neth J
Med. 75:179–189. 2017.PubMed/NCBI
|
|
6
|
Krediet RT and Struijk DG: Peritoneal
changes in patients on long-term peritoneal dialysis. Nat Rev
Nephrol. 9:419–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bargman JM: Advances in peritoneal
dialysis: A review. Semin Dial. 25:545–549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davies SJ: Unraveling the mechanisms of
progressive peritoneal membrane fibrosis. Kidney Int. 89:1185–1187.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witowski J, Kawka E, Rudolf A and Jörres
A: New developments in peritoneal fibroblast biology: Implications
for inflammation and fibrosis in peritoneal dialysis. Biomed Res
Int. 2015:1347082015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raby AC and Labéta MO: Preventing
peritoneal dialysis-associated fibrosis by therapeutic blunting of
peritoneal Toll-like receptor activity. Front Physiol. 9:16922018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Jiang N and Ni Z: Strategies for
preventing peritoneal fibrosis in peritoneal dialysis patients: New
insights based on peritoneal inflammation and angiogenesis. Front
Med. 11:349–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Q, Bajo MA, Del Peso G, Yu X and
Selgas R: Preventing peritoneal membrane fibrosis in peritoneal
dialysis patients. Kidney Int. 90:515–524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HB and Ha H: Mechanisms of
epithelial-mesenchymal transition of peritoneal mesothelial cells
during peritoneal dialysis. J Korean Med Sci. 22:943–945. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Vriese AS, Tilton RG, Mortier S and
Lameire NH: Myofibroblast transdifferentiation of mesothelial cells
is mediated by RAGE and contributes to peritoneal fibrosis in
uraemia. Nephrol Dial Transplant. 21:2549–2555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Xing C, Zhang L, Mao H, Chen X,
Liang M, Wang F, Ren H, Cui H, Jiang A, et al: Autophagy promotes
fibrosis and apoptosis in the peritoneum during long-term
peritoneal dialysis. J Cell Mol Med. 22:1190–1201. 2018.PubMed/NCBI
|
|
16
|
Dobbie JW: Pathogenesis of peritoneal
fibrosing syndromes (sclerosing peritonitis) in peritoneal
dialysis. Perit Dial Int. 12:14–27. 1992.PubMed/NCBI
|
|
17
|
Strippoli R, Moreno-Vicente R, Battistelli
C, Cicchini C, Noce V, Amicone L, Marchetti A, Del Pozo MA and
Tripodi M: Molecular mechanisms underlying peritoneal EMT and
fibrosis. Stem Cells Int. 2016:35436782016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Liu N, Xiong C, Xu L, Shi Y, Qiu
A, Zang X, Mao H and Zhuang S: Inhibition of EGF receptor blocks
the development and progression of peritoneal fibrosis. J Am Soc
Nephrol. 27:2631–2644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morinelli TA, Luttrell LM, Strungs EG and
Ullian ME: Angiotensin II receptors and peritoneal dialysis-induced
peritoneal fibrosis. Int J Biochem Cell Biol. 77:240–250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartosova M, Schaefer B, Vondrak K, Sallay
P, Taylan C, Cerkauskiene R, Dzierzega M, Milosevski-Lomic G,
Büscher R, Zaloszyc A, et al: Peritoneal dialysis vintage and
glucose exposure but not peritonitis episodes drive peritoneal
membrane transformation during the first years of PD. Front
Physiol. 10:3562019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi SY, Ryu HM, Choi JY, Cho JH, Kim CD,
Kim YL and Park SH: The role of Toll-like receptor 4 in
high-glucose-induced inflammatory and fibrosis markers in human
peritoneal mesothelial cells. Int Urol Nephrol. 49:171–181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L and Zhuang S: The role of tyrosine
kinase receptors in peritoneal fibrosis. Perit Dial Int.
35:497–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomino Y: Mechanisms and interventions in
peritoneal fibrosis. Clin Exp Nephrol. 16:109–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duan S, Yu J, Liu Q, Wang Y, Pan P, Xiao
L, Ling G and Liu F: Epithelial-to-mesenchymal transdifferentiation
of peritoneal mesothelial cells mediated by oxidative stress in
peritoneal fibrosis rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
36:34–43. 2011.PubMed/NCBI
|
|
25
|
Li Z, Zhang L, He W, Zhu C, Yang J and
Sheng M: Astragalus membranaceus inhibits peritoneal
fibrosis via monocyte chemoattractant protein (MCP)-1 and the
transforming growth factor-β1 (TGF-β1) pathway in rats submitted to
peritoneal dialysis. Int J Mol Sci. 15:12959–12971. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shan G, Zhou XJ, Xia Y and Qian HJ:
Astragalus membranaceus ameliorates renal interstitial
fibrosis by inhibiting tubular epithelial-mesenchymal transition
in vivo and in vitro. Exp Ther Med. 11:1611–1616.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou X, Sun X, Gong X, Yang Y, Chen C,
Shan G and Yao Q: Astragaloside IV from Astragalus
membranaceus ameliorates renal interstitial fibrosis by
inhibiting inflammation via TLR4/NF-кB in vivo and in vitro. Int
Immunopharmacol. 42:18–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu M, Shi J, Sheng M, Gao K, Zhang L, Liu
L and Zhu Y: Astragalus inhibits Epithelial-to-Mesenchymal
transition of peritoneal mesothelial cells by Down-regulating
β-catenin. Cell Physiol Biochem. 51:2794–2813. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang WD, Chen H, Zhang C, Liu RH, Li HL
and Chen HZ: Astragaloside IV from Astragalus membranaceus
shows cardioprotection during myocardial ischemia in vivo and in
vitro. Planta Med. 72:4–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W and Fitzloff JF: Determination of
astragaloside IV in Radix astragali (Astragalus membranaceus
var. monghulicus) using high-performance liquid chromatography with
evaporative light-scattering detection. J Chromatogr Sci.
39:459–462. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Li Z, He W, Xu L, Wang J, Shi J
and Sheng M: Effects of Astragaloside IV Against the TGF-β1-induced
Epithelial-to-Mesenchymal transition in peritoneal mesothelial
cells by promoting Smad 7 expression. Cell Physiol Biochem.
37:43–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yue F, Cheng Y, Breschi A, Vierstra J, Wu
W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, et al: A
comparative encyclopedia of DNA elements in the mouse genome.
Nature. 515:355–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bourguet W, Ruff M, Chambon P, Gronemeyer
H and Moras D: Crystal structure of the ligand-binding domain of
the human nuclear receptor RXR-alpha. Nature. 375:377–382. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shulman AI and Mangelsdorf DJ: Retinoid ×
receptor heterodimers in the metabolic syndrome. N Engl J Med.
353:604–615. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rastinejad F: Retinoid X receptor and its
partners in the nuclear receptor family. Curr Opin Struct Biol.
11:33–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Watanabe M and Kakuta H: Retinoid X
receptor antagonists. Int J Mol Sci. 19(pii): E23542018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reitzel AM, Macrander J, Mane-Padros D,
Fang B, Sladek FM and Tarrant AM: Conservation of DNA and ligand
binding properties of retinoid X receptor from the placozoan
Trichoplax adhaerens to human. J Steroid Biochem Mol Biol.
184:3–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morishita KI and Kakuta H: Retinoid X
receptor ligands with anti-type 2 diabetic activity. Curr Top Med
Chem. 17:696–707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Menéndez-Gutiérrez MP and Ricote M: The
multi-faceted role of retinoid X receptor in bone remodeling. Cell
Mol Life Sci. 74:2135–2149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee YC, Hung SY, Liou HH, Lin TM, Tsai CH,
Lin SH, Tsai YS, Chang MY, Wang HH, Ho LC, et al: Vitamin D can
ameliorate chlorhexidine gluconate-induced peritoneal fibrosis and
functional deterioration through the inhibition of
Epithelial-to-mesenchymal transition of mesothelial cells. Biomed
Res Int. 2015:5950302015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang L, Wu L, Zhang X, Hu Y, Fan Y and Ma
J: 1,25(OH)2D3/VDR attenuates high glucoseinduced
epithelialmesenchymal transition in human peritoneal mesothelial
cells via the TGFβ/Smad3 pathway. Mol Med Rep. 15:2273–2279. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang L, Wu L, Du S, Hu Y, Fan Y and Ma J:
1,25(OH)2D3 inhibits high glucose-induced apoptosis and ROS
production in human peritoneal mesothelial cells via the MAPK/P38
pathway. Mol Med Rep. 14:839–844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su X, Yu R, Yang X, Zhou G, Wang Y, Li L
and Li D: Telmisartan attenuates peritoneal fibrosis via peroxisome
proliferator-activated receptor-gamma activation in rats. Clin Exp
Pharmacol Physiol. 42:671–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Su X, Zhou G, Wang Y, Yang X, Li L, Yu R
and Li D: The PPARβ/δ agonist GW501516 attenuates peritonitis in
peritoneal fibrosis via inhibition of TAK1-NFκB pathway in rats.
Inflammation. 37:729–737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou G, Su X, Ma J, Wang L and Li D:
Pioglitazone inhibits high glucose-induced synthesis of
extracellular matrix by NF-κB and AP-1 pathways in rat peritoneal
mesothelial cells. Mol Med Rep. 7:1336–1342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang YF, Wang Q, Su YY, Wang JL, Hua BJ,
Yang S, Feng JX and Li HY: PPAR-γ agonist rosiglitazone protects
rat peritoneal mesothelial cells against peritoneal dialysis
solution-induced damage. Mol Med Rep. 15:1786–1792. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rougier JP, Moullier P, Piedagnel R and
Ronco PM: Hyperosmolality suppresses but TGF beta 1 increases MMP9
in human peritoneal mesothelial cells. Kidney Int. 51:337–347.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao JL, Guo MZ, Zhu JJ, Zhang T and Min
DY: Curcumin suppresses epithelial-to-mesenchymal transition of
peritoneal mesothelial cells (HMrSV5) through regulation of
transforming growth factor-activated kinase 1 (TAK1). Cell Mol Biol
Lett. 24:322019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang P, Dai H and Peng L: Involvement of
STAT3 signaling in high glucose-induced epithelial mesenchymal
transition in human peritoneal mesothelial cell line HMrSV5. Kidney
Blood Press Res. 44:179–187. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chu Y, Wang Y, Zheng Z, Lin Y, He R, Liu J
and Yang X: Proinflammatory effect of high glucose concentrations
on HMrSV5 cells via the autocrine effect of HMGB1. Front Physiol.
8:7622017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tani H, Sato Y, Ueda M, Miyazaki Y,
Suginami K, Horie A, Konishi I and Shinomura T: Role of versican in
the pathogenesis of peritoneal endometriosis. J Clin Endocrinol
Metab. 101:4349–4356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shi S, Zhang Y, Wen W, Zhao Y and Sun L:
Molecular mechanisms of melatonin in the reversal of LPS-induced
EMT in peritoneal mesothelial cells. Mol Med Rep. 14:4342–4348.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang L, Liu J, Liu Y, Xu Y, Zhao X, Qian
J, Sun B and Xing C: Fluvastatin inhibits the expression of
fibronectin in human peritoneal mesothelial cells induced by
high-glucose peritoneal dialysis solution via SGK1 pathway. Clin
Exp Nephrol. 19:336–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang Y, Liu K, Liang Y, Chen Y, Chen Y and
Gong Y: Histone acetyltransferase inhibitor C646 reverses
epithelial to mesenchymal transition of human peritoneal
mesothelial cells via blocking TGF-β1/Smad3 signaling pathway in
vitro. Int J Clin Exp Pathol. 8:2746–2754. 2015.PubMed/NCBI
|
|
57
|
Ju KD, Kim HJ, Tsogbadrakh B, Lee J, Ryu
H, Cho EJ, Hwang YH, Kim K, Yang J, Ahn C and Oh KH: HL156A, a
novel AMP-activated protein kinase activator, is protective against
peritoneal fibrosis in an in vivo and in vitro model of peritoneal
fibrosis. Am J Physiol Renal Physiol. 310:F342–F350. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xiong C, Liu N, Fang L, Zhuang S and Yan
H: Suramin inhibits the development and progression of peritoneal
fibrosis. J Pharmacol Exp Ther. 351:373–382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu J, Zeng L, Zhao Y, Zhu B, Ren W and Wu
C: Selenium suppresses lipopolysaccharide-induced fibrosis in
peritoneal mesothelial cells through inhibition of
epithelial-to-mesenchymal transition. Biol Trace Elem Res.
161:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lu Y, Gao L, Li L, Zhu Y, Wang Z, Shen H
and Song K: Hydrogen sulfide alleviates peritoneal fibrosis via
attenuating inflammation and TGF-β1 synthesis. Nephron.
131:210–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shin HS, Ko J, Kim DA, Ryu ES, Ryu HM,
Park SH, Kim YL, Oh ES and Kang DH: Metformin ameliorates the
phenotype transition of peritoneal mesothelial cells and peritoneal
fibrosis via a modulation of oxidative stress. Sci Rep. 7:56902017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Qian W, Cai X, Qian Q, Zhang W and Wang D:
Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal
transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med.
22:4354–4365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang X, Gao Y, Tian N, Wang T, Shi Y, Xu J
and Wu B: Astragaloside IV inhibits glucose-induced
epithelial-mesenchymal transition of podocytes through autophagy
enhancement via the SIRT-NF-κB p65 axis. Sci Rep. 9:3232019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yao XM, Liu YJ, Wang YM, Wang H, Zhu BB,
Liang YP, Yao WG, Yu H, Wang NS, Zhang XM and Peng W: Astragaloside
IV prevents high glucose-induced podocyte apoptosis via
downregulation of TRPC6. Mol Med Rep. 13:5149–5156. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
You L, Fang Z, Shen G, Wang Q, He Y, Ye S,
Wang L, Hu M, Lin Y, Liu M and Jiang A: Astragaloside IV prevents
high glucoseinduced cell apoptosis and inflammatory reactions
through inhibition of the JNK pathway in human umbilical vein
endothelial cells. Mol Med Rep. 19:1603–1612. 2019.PubMed/NCBI
|
|
66
|
Wang J and Guo HM: Astragaloside IV
ameliorates high glucose-induced HK-2 cell apoptosis and oxidative
stress by regulating the Nrf2/ARE signaling pathway. Exp Ther Med.
17:4409–4416. 2019.PubMed/NCBI
|
|
67
|
Xue B, Huang J, Ma B, Yang B, Chang D and
Liu J: Astragaloside IV protects primary cerebral cortical neurons
from oxygen and glucose deprivation/reoxygenation by activating the
PKA/CREB pathway. Neuroscience. 404:326–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhu J and Wen K: Astragaloside IV inhibits
TGF-β1-induced epithelial-mesenchymal transition through inhibition
of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother
Res. 32:1289–1296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH,
Wang YC, Ye BG, Cao MQ, Gao DM and Tang ZY: Astragaloside IV
inhibits metastasis in hepatoma cells through the suppression of
epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin
pathway. Oncol Rep. 37:1725–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Y, Ye Y and Chen H: Astragaloside IV
inhibits cell migration and viability of hepatocellular carcinoma
cells via suppressing long noncoding RNA ATB. Biomed Pharmacother.
99:134–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang J, Zhu T, Liu X, Zhang L, Yang Y,
Zhang J and Guο M: Heat shock protein 70 protects rat peritoneal
mesothelial cells from advanced glycation end-products-induced
Epithelial-to-mesenchymal transition through mitogen-activated
protein Kinases/extracellular signal-regulated kinases and
transforming growth factor-β/Smad pathways. Mol Med Rep.
11:4473–4481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu J, Bao J, Hao J, Peng Y and Hong F:
HSP70 inhibits high glucose-induced Smad3 activation and attenuates
epithelial-to-mesenchymal transition of peritoneal mesothelial
cells. Mol Med Rep. 10:1089–1095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Abe S, Obata Y, Oka S, Koji T, Nishino T
and Izumikawa K: Chondroitin sulfate prevents peritoneal fibrosis
in mice by suppressing NF-κB activation. Med Mol Morphol.
49:144–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He L, Che M, Hu J, Li S, Jia Z, Lou W, Li
C, Yang J, Sun S, Wang H and Chen X: Twist contributes to
proliferation and epithelial-to-mesenchymal transition-induced
fibrosis by regulating YB-1 in human peritoneal mesothelial cells.
Am J Pathol. 185:2181–2193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Oba-Yabana I, Mori T, Takahashi C, Hirose
T, Ohsaki Y, Kinugasa S, Muroya Y, Sato E, Nguyen G, Piedagnel R,
et al: Acidic organelles mediate TGF-β1-induced cellular fibrosis
via (pro)renin receptor and vacuolar ATPase trafficking in human
peritoneal mesothelial cells. Sci Rep. 8:26482018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shang J, He Q, Chen Y, Yu D, Sun L, Cheng
G, Liu D, Xiao J and Zhao Z: miR-15a-5p suppresses inflammation and
fibrosis of peritoneal mesothelial cells induced by peritoneal
dialysis via targeting VEGFA. J Cell Physiol. 234:9746–9755. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gao Q, Xu L, Yang Q and Guan TJ:
MicroRNA-21 contributes to high glucose-induced fibrosis in
peritoneal mesothelial cells in rat models by activation of the
Ras-MAPK signaling pathway via Sprouty-1. J Cell Physiol.
234:5915–5925. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu HS, Shi HL, Huang F, Peterson KE, Wu
H, Lan YY, Zhang BB, He YX, Woods T, Du M, et al: Astragaloside IV
inhibits microglia activation via glucocorticoid receptor mediated
signaling pathway. Sci Rep. 6:191372016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang X, Wang Y, Hu JP, Yu S, Li BK, Cui Y,
Ren L and Zhang LD: Astragaloside IV, a natural PPARgamma agonist,
reduces Aβ production in Alzheimer's disease through inhibition of
BACE1. Mol Neurobiol. 54:2939–2949. 2017. View Article : Google Scholar : PubMed/NCBI
|