Introduction
Prostate cancer is one of the most commonly
diagnosed cancers in men, resulting in significant mortality and
morbidity (1). A previous study
reported that a number of signaling pathways, including androgen
receptor, phosphoinositide 3-kinase, mitogen-activated protein
kinase and Wnt, may be involved in the progression of prostate
cancer (2); however, the molecular
mechanisms that control this progression requires further study.
MicroRNAs (miRNAs) are small, non-coding RNAs that target the
3′-untranslated region (UTR) of target mRNA transcripts to suppress
translation or to induce mRNA degradation (3). miRNAs serve crucial roles in various
cellular processes, including proliferation, apoptosis,
differentiation, migration and invasion (4). Previous studies have reported that
dysregulated miRNA expression may be a factor in various types of
cancer (5). A previous study has
also suggested that miRNAs may serve a number of roles in prostate
cancer pathogenesis (6); however,
their precise roles in the pathogenesis and the possible mechanisms
remain unclear.
miRNAs function by regulating the expression of
target genes. Runt-related transcription factor 3 (RUNX3) was
previously predicted to be a target gene of miR-301a-3p (7). Downregulated RUNX3 expression has
been implicated in gastric cancer, lung adenocarcinoma and
hepatocellular carcinoma (7–9), and
was associated with increased chemotherapy resistance (10).
A previous study reported that miR-301a-3p was
overexpressed in hepatocellular carcinoma, pancreatic tumor tissues
and small cell lung cancer compared with adjacent benign tissues
(11); however, the role of
miR-301a-3p in prostate cancer remains unknown. The present study
aimed to investigate the expression levels and biological roles of
miR-301a-3p in prostate cancer progression. The data demonstrated
that miR-301a-3p expression levels were significantly upregulated
in human prostate cancer tissues and cell lines and suggested that
overexpression of miR-301a-3p may promote prostate cancer cell
proliferation and invasion. RUNX3 was verified as a direct target
of miR-301a-3p. Therefore, miR-301a-3p may promoted prostate cancer
cell proliferation and invasion by targeting RUNX3.
Materials and methods
Clinical specimens
Paired prostate malignant cancer tissues and
adjacent normal prostate tissues (n=15) were obtained from patients
with prostate cancer who underwent resection surgery at the
China-Japan Union Hospital of Jilin University (Changchun, China).
Written informed consent was obtained from the participating
patients prior to the start of the study, and the study was
approved by The Institutional Human Experiment and Ethics Committee
of China-Japan Union Hospital of Jilin University.
Cell lines and cell culture
Human prostate cancer cell lines (PC-3, LNCaP and
DU-145), normal prostate epithelial cells (RWPE-1) and 293T cells
were purchased from the American Type Culture Collection (Manassas,
VA, USA). Prostate cancer cell lines and 293T cells were grown in
RPMI-1640 and Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), respectively, both
supplemented with 10% fetal bovine serum (FBS, Gibco, Thermo Fisher
Scientific, Inc.), 100 mg/ml streptomycin and 100 U/ml penicillin
(Gibco; Thermo Fisher Scientific, Inc.). RWPE-1 cells were grown in
keratinocyte serum-free media supplemented with 5 ng/ml human
recombinant epidermal growth factor, 0.05 mg/ml bovine pituitary
extract, 100 mg/ml streptomycin and 100 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc.). All cells were cultured in a
humidified incubator containing 5% CO2 at 37°C.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from 6 mm tissue
sample or 1×107 cell from PC-3, LNCaP and DU-145 cell
lines respectively, according to the manufacturer's protocol. The
primers were synthesized by the Shanghai Sangon Biological
Engineering and Technology Service (Shanghai, China). For gene
expression detection, cDNA was synthesised from total RNA, RT-qPCR
mixture system contained the cDNA, primers and SYBR-Green qPCR
Master Mix. The thermocycling conditions for RT-qPCR were: 94°C 5
min, followed by 45 cycles of 94°C for 45 sec, 59°C for 45 sec and
72°C for 1 min. miRNA expression levels were assessed by the TaqMan
stem-loop RT-PCR method according to the manufacturer's protocol
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Reverse
transcription was performed using the one-step PrimerScript miRNA
cDNA synthesis kit to synthesize cDNA from total RNA (Takara
Biotechnology Co., Ltd., Dalian, China). In brief, 10 µl qPCR mix,
0.4 µl forward primer, 0.4 µl reverse primer, 2 µl cDNA and dd
H2O were mixed. The thermocycling conditions for RT-qPCR
were 95°C for 5 min, followed by 30 cycles of 95°C for 5 sec, 60°C
for 10 sec and 72°C for 1 min. The U6 small nuclear RNA (RNU6B) was
used for miRNA expression normalization. mRNA expression level of
RUNX3 was normalized to β-actin. Relative gene or miRNA expression
was quantified by 2−ΔΔCq method (12). Primers were as follows: RUNX3,
forward 5′-GCTGTTATGCGTATTCCCGTAG-3′ and reverse,
5′-TGAAGTGGCTTGTGGTGCTGAGTGA-3′; cyclin D1 forwards,
5′-AACTACCTGGACCGCTTCCT-3′ and reverse, 5′-CCACTTGAGCTTGTTCACCA-3′;
c-myc, forward, 5′-TCTTAGTCTTTTTCTTAATAGGG-3′ and reverse
5′-GGTATCTGGACCTCACTGACAAG-3′; β-actin, forward
5′-TTAATAGTCATTCCAAATATGA-3′ and reverse,
5′-GGGACAAAAAAGGGGGAAGG-3′; miR-301a-3p, forward
5′-CGTGCGAAGCTCAGGAGGG-3′ and reverse, 5′-TGGCTGTCGTGGACTGCG-3′;
RNU6B, 5′-GGACATCCGATAAAATTGGAACGATACAG-3′ and reverse,
5′-AATTTGGACCATTTCTCGATTTATGCGTGT-3′. The experiment was repeated
three times.
Cell transfection
miR-301a-3p mimics (cat. no. 4464066), antisense
nucleotides of miR-301a-3p (anti- miR-301a-3p; cat. no. AM17000)
and control oligonucleotides (miR-NC; cat. no. 4464058) were
purchased from Thermo Fisher Scientific, Inc. Prostate cancer cells
(LNCaP and DU-145) at 1×108 were transiently transfected
with 50 nM miRNA [as determined by dose response analysis (data not
shown)] using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Normal prostate cancer cells were used as a control. Following 48 h
incubation at 37°C, transfection efficiency was detected by RT-qPCR
analysis, as aforementioned.
Cell proliferation assay
Cell proliferation was measured by MTT assay. LNCaP
and DU-145 cells (1×104 cells/well) were plated in
96-well plates and cultured for 24 h at 37°C, respectively. The
cells were transfected with 50 nM miR-301a-3p mimics or
anti-miR-301a-3p for 1, 3, 5 and 7 days at 37°C. An untransfected
control was also used. Following each transfection period, 20 µl
MTT solution (5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to each well and incubated at 37°C for 4 h. The
medium was discarded, and the formazan crystals were dissolved by
adding 200 µl dimethyl sulfoxide per well. The absorbance was
measured at a wavelength of 490 nm using a microplate
spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
The experiment was repeated three times.
Colony formation assay
LNCaP and DU-145 cells (200 cells/well) were seeded
into 6-well plates and transfected with 50 nM miR-301a-3p mimic,
anti-miR-301a-3p or miR-NC at 37°C for 48 h. Subsequently, the
transfected cells were cultured at 37°C for 14 days in growth
medium containing 0.3% noble agar to allow formation of natural
colonies. The cells were stained with 0.1% crystal violet in 70%
ethanol at room temperature for 20 min. The number of stained
colonies was counted under an inverted microscope. The experiment
was repeated three times.
Cell invasion assay
Cell invasive ability was examined using a Transwell
filter precoated with Matrigel (BD Bioscience, San Jose, CA, USA).
Transfected cells (1×104 cells) in 200 µl serum-free
medium were added to the upper chamber, and 500 µl of growth medium
containing 10% FBS was added to the lower chamber as a
chemoattractant. Cells were incubated for 24 h at 37°C, and the
invading cells on in the lower filter side were stained with 0.1%
crystal violet for 20 min at room temperature and counted under an
inverted fluorescence microscope (Olympus Corporation). The
experiment was repeated three times.
Plasmid construction and
dual-luciferase assay
The target reaction between miRNA and RUNX3 was
predicted using targetscan (http://www.targetscan.org/vert_72/). Wild-type (wt)
RUNX3 3′-UTR containing the predicted miR-301a-3p binding site was
synthesized as described above using the following sequences:
Forward
5′-CTAGTATGGAGCTGGGTGGAAACTGCTTTGCACTATCGTTTGCTTGGTGTTTGTTTTA3′,
reverse
5′-CGCGTAAAACAAACACCAAGCAAACGATAGTGCAAAGCAGTTTCCACCCAGCTCCATA-3′.
The mutant mut) RUNX3 3′-UTR construct was designed to mutate three
intermittent nucleotides that are complementary to the miR-301a-3p
seed-region. The mutant primer sequences were: Forward
5′-CTAGTATGGAGCTGGGTGGAAACTGCTTAGgAgTATCGTTTGCTTGGTGTTTGTTTTA-3′,
reverse
5′-CGCGTAAAACAAACACCAAGCAAACGATACTCCTAAGCAGTTTCCACCCAGCTCCATA-3′.
The strands were annealed and cloned into the pMIR-REPORT miRNA
expression reporter vector (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using BamHI and EcoRI. 293T cells
were co-transfected with either pMIR-RUNX3 wt or pMIR-RUNX3 mut
plasmid containing firefly luciferase together with the pRL-TK
vector (Promega Corporation, Madison, WI, USA) containing
Renilla luciferase and miR-301a-3p mimic or miR-NC using
Lipofectamine 2000. Relative luciferase activity was calculated 36
h post-transfection by the Dual-Luciferase Reporter Assay (Promega
Corporation), according to the manufacturer's protocol. The
experiment was repeated three times. Luciferase expression was
normalized to Renilla luciferase.
Western blot analysis
A total of 30 µg protein was extracted from 6 mm
tissue sample or 1×107 cell from LNCaP and DU-145 cancer
cell line. The protein was separated by 8% SDS-PAGE, followed by
electrophoretic transfer to nitrocellulose membranes for 1.5 h at
100 V. Membranes were blocked with 5% BSA for 1 h at room
temperature and probed with primary antibodies (Cell Signaling
Technology, Inc., Danvers, MA, USA), including mouse anti-human
RUNX3 (cat. no. 13089; 1:1,000) and mouse anti-human β-actin (cat.
no. 3700; 1:2,000) overnight at 4°C. Membranes were washed with PBS
and incubated with a horseradish peroxidase-conjugated anti-mouse
secondary antibody (cat. no. 7076; 1:2,000; Cell Signaling
Technology, Inc.) for 1 h at room temperature. Blots were developed
using Enhanced Chemiluminescence kit (EMD Millipore, Billerica, MA,
USA). Proteins were detected using a ChemiDoc XRS imaging system
and Quantity One® 1-D analysis software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Relative protein expression
was normalized to β-actin. The experiment was repeated three
times.
Wnt signaling activity assay
pcDNA3.0-RUNX3 overexpression vector were
constructed using pcDNA3.0 plasmid (Invitrogen; Thermo Fisher
Scientific, Inc.). The expression sequences encoding RUNX3 were
amplified from cDAN, which was synthesized from prostate cancer
cells, then sub-cloned into pcDNA3.0 plasmid. A total of
1×108 LNCaP or DU-145 cancer cells were transfected with
10 ng TOPFlash firefly luciferase reporter vector (Addgene, Inc.,
Cambridge, MA, USA) and 4 ng phRL-TK Renilla luciferase
vectors (Promega Corporation) in the presence of 50 nM miR-301a-3p
mimics alone or combined with 50 nM pcDNA3.0-RUNX3 overexpression
vectors. Cells were incubated at 37°C for 48 h, and the relative
luciferase activity was determined using the Dual-Glo luciferase
assay system (Promega Corporation). Luciferase expression was
normalized to Renilla luciferase. The experiment was
repeated three times.
Statistical analysis
All numerical data were expressed as the mean ±
standard deviation. Statistical analyses were performed using the
commercial statistical software SPSS version 11.5 (SPSS Inc.,
Chicago, IL, USA). Differences between two groups were analyzed by
paired two-tailed Student's t-test. Data of more than two groups
were analyzed using one-way analysis of variance followed by a
Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-301a-3p expression is increased
and RUNX3 mRNA expression is decreased in prostate cancer tissues
and cell lines
To investigate the role of miR-301a-3p and RUNX3 in
prostate cancer progression, miR-301a-3p and RUNX3 mRNA expression
levels were determined in clinical prostate cancer tissues and
paired adjacent normal tissues by RT-qPCR analysis. The results
demonstrated that miR-301a-3p expression was significantly higher,
whereas RUNX3 mRNA expression was significantly lower in prostate
cancer tissues compared with the respective expression levels in
paired adjacent normal tissues (Fig.
1A and B). In addition, the expression levels of miR-301a-3p
and RUNX3 mRNA were examined in several prostate cancer cell lines,
with the non-tumorigenic prostate epithelial cell line RWPE-1 used
as the control. The data revealed that miR-301a-3p was
significantly increased, whereas RUNX3 mRNA was significantly
decreased in the prostate cancer cell lines compared with the
respective expression levels in RWPE-1 cells (Fig. 1C and D). These results demonstrated
that miR-301a-3p is upregulated and RUNX3 is downregulated in
prostate cancer, and this may be involved in the pathogenesis of
prostate cancer.
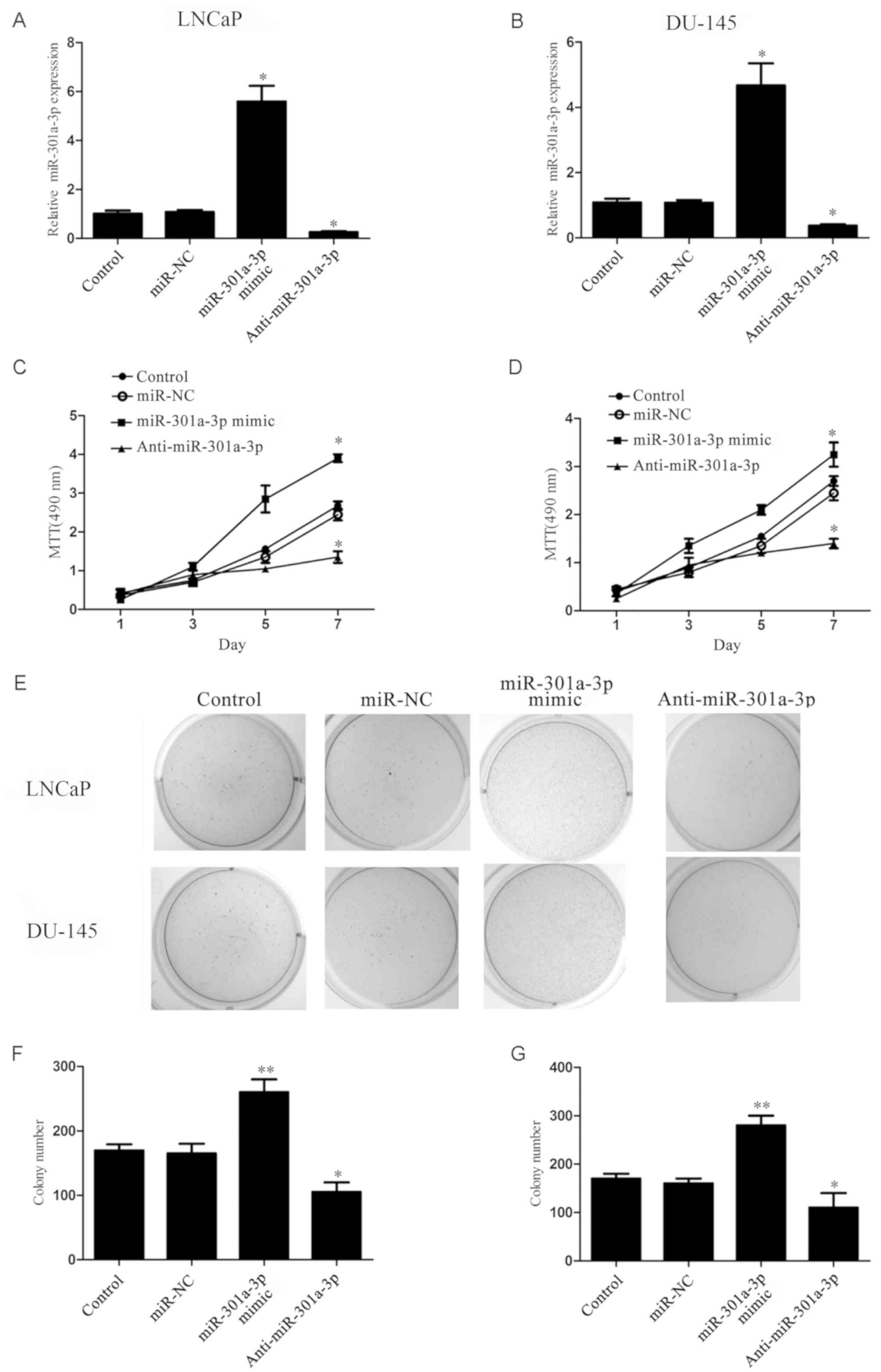
miR-301a-3p promotes the proliferative
and colony-forming capacity of prostate cancer cells
To investigate the function of miR-301a-3p in
prostate cancer, LNCaP and DU-145 prostate cancer cells transfected
with miR-301a-3p mimics or anti-miR-301a-3p. LNCaP, an miR-301
highly expressing cell line and DU-145, in which miR-301 expression
is relatively low was chosen out of the three prostate cancer cell
line. These cell lines were chosen to examine effect the difference
in expression had on the result. RT-qPCR results demonstrated that
the expression of miR-301a-3p was significantly increased in
miR-301a-3p mimics-treated cells and significantly decreased in
anti-miR-301-3p-treated cells compared with untransfected Control
and miR-NC-transfected groups (Fig. 2A
and B); no significant differences were identified between the
Control and miR-NC groups. MTT assay revealed that the
overexpression of miR-301a-3p significantly promoted prostate
cancer cell proliferation, whereas the inhibition of miR-301a-3p
expression significantly reduced prostate cancer cell proliferation
(P<0.05; Fig. 2C and D).
Furthermore, the colony-forming capacity of prostate cancer cells
was significantly increased by miR-301a-3p overexpression, whereas
suppression of miR-301a-3p exhibited the opposite effects (Fig. 2E-G). No significant differences in
proliferation and colony formation were indicated between the
Control and miR-NC groups. These results indicated that miR-301a-3p
may serve an oncogenic role in prostate cancer.
miR-301a-3p promotes the invasion of
prostate cancer cells
To further investigate the functions of miR-301a-3p
in prostate cancer, cell migration (Fig. 3A and B) was performed on the
transfected cells. LNCaP and DU-145 cells transfected with
miR-301a-3p mimics exhibited a significantly increased number of
invading cells compared with untransfected or miR-NC transfected
cells. Conversely, inhibition of miR-301a-3p expression
significantly reduced cell invasion. No significant differences in
the numbers of invading cells were identified between the Control
cells and the miR-NC-transfected cells.
miR-301a-3p targets RUNX3 in prostate
cancer cells
To investigate the underlying mechanism by which
miR-301a-3p controls the pathogenesis of prostate cancer,
bioinformatics analysis was performed to predict target genes of
miR-301a-3p. Of the predicted target genes (all illustrated in
Fig. 4A) the tumor suppressor
RUNX3 was selected for further analysis. RUNX3 was chosen because
it is a tumor-related gene, and its function has been confirmed in
various cancers.
To verify that RUNX3 is a target of miR-301a-3p,
dual-luciferase reporter assays were performed; LNCaP and DU-145
cells were co-transfected with either wt or mut RUNX3 3′-UTR and
either miR-301a-3p mimics or miR-NC. Luciferase activity in cells
co-transfected with miR-301a-3p mimics and wt RUNX3 3′UTR was
significantly reduced compared with cells resulted into a
significant decrease in luciferase activity (Fig. 4B and C).
The effects of miR-301a-3p on RUNX3 expression were
investigated in vitro. The data demonstrated that RUNX3
protein expression levels were significantly decreased in LNCaP and
DU-145 cells transfected with miR-301a-3p mimics compared with
untransfected Control cells (Fig. 4D
and E). To further investigate the role of RUNX3, rescue
experiments were conducted by co-transfecting cells with
miR-301a-3p mimics and RUNX3 overexpression vector, which exhibited
a significant increase in RUNX3 protein expression compared with
cells transfected with miR-301a-3p mimics alone (Fig. 4D and E). Taken together, these
results suggested that miR-301a-3p directly targets the 3′-UTR of
RUNX3 and regulates RUNX3 expression in prostate cancer cells.
miR-301a-3p regulates Wnt signaling pathway. To
further elucidate the molecular role of miR-301a-3p in regulating
prostate cancer, the effects of miR-301a-3p on the Wnt signaling
pathway were examined by using a luciferase assay. The Wnt pathway
was chosen for investigation as RUNX3 was proved to modulate the
Wnt pathway in a number of types of cancer and studies of the Wnt
pathway are more prevalent. The data revealed that miR-301a-3p
overexpression significantly increased Wnt activity, whereas
miR-301a-3p inhibition reduced Wnt activity in LNCaP and DU-145
cells (Fig. 5A). To verify these
results, the mRNA expression levels of downstream Wnt signaling
genes c-myc and cyclin D1 were examined; the expression levels of
c-myc and cyclin D1 were significantly increased by miR-301a-3p
overexpression in LNCaP and DU-145 cells (Fig. 5B and C), whereas miR-301a-3p
inhibition decreased the expression of c-myc and cyclin D1. These
results indicated that miR-301a-3p may serve a role in regulating
the Wnt signaling pathway. To investigate whether miR-301a-3p
regulates Wnt pathway through RUNX3, a rescue assay was conducted
by overexpressing RUNX3 in miR-301a-3p mimics-transfected cells.
The data indicated that the promotional effects of miR-301a-3p on
the Wnt signaling pathway was significantly reversed by RUNX3
overexpression (Fig. 5D).
Discussion
Prostate cancer is the second leading cause of
cancer-related mortality in men worldwide (13). Although hormone therapy may
initially be effective, the tumor may still enter the insensitive
period and metastasize (14).
Previous studies suggested that miRNAs may serve important roles in
regulating the progression of prostate cancer (15,16);
therefore, it is of great importance to identify prostate
cancer-associated miRNAs that may be used as biomarkers for
prostate cancer diagnosis and treatment. Results from the present
study revealed that miR-301a-3p expression was significantly
increased in prostate cancer tissues and cell lines. In addition,
functional analysis indicated that miR-301a-3p may serve a role in
regulating prostate cancer cell proliferation and invasion and may
serve an important role in prostate cancer progression.
Previous studies have reported that miR-301a-3p is
highly expressed in various types of human cancers, including
pancreatic tumors, hepatocellular carcinoma, breast cancer and
small cell lung cancer (17).
Results from the present study demonstrated that miR-301a may
function as a novel oncogene in prostate cancer and contribute to
tumor progression of prostate cancer. The biological role of miRNAs
is dependent on its target genes. The present study demonstrated
that miR-301a-3p directly targets the 3′-UTR of RUNX3 and regulates
RUNX3 expression, which was consistent with a former study
(7). RUNX3 expression was
previously reported to be reduced in many types of human cancers,
including melanoma, renal cell carcinoma, colorectal cancer and
breast cancer (18,19). RUNX3 was also reported to regulate
migration and proliferation of human colorectal cancer (20). The present study results
demonstrated that RUNX3 is downregulated in prostate cancer. A
previous study reported that targeted restoration of RUNX3
expression in hepatocellular cancer cells inhibits tumor
development (21). Another study
suggested that RUNX3 controls the differentiation and growth of
gastric cancer cells (22). The
present study suggested that miR-301a-3p-induced invasion and
proliferation may depend on the direct post-transcriptional
downregulation of RUNX3 expression in prostate cancer.
Aberrant activation of the Wnt signaling pathway is
frequently associated with prostate cancer (23). Given the regulatory effects of
RUNX3 on the Wnt signaling pathway (24), it was hypothesized that miR-301a-3p
may also influence the Wnt signaling pathway. The present study
results indicated that miR-301a-3p may promote the Wnt signaling
pathway by affecting RUNX3 expression.
In conclusion, the present study demonstrated that
miR-301a-3p was upregulated and affected the proliferation and
invasive ability of prostate cancer cells by regulating of RUNX3
expression and Wnt signaling. Therefore, miR-301a-3p may act as an
oncogene through targeting RUNX3 in prostate cancer. These results
may aid in the further elucidation of potential molecular
mechanisms of prostate cancer development, and may have therapeutic
value in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LF designed the present study and wrote the
manuscript. YW and WH performed the experiments. WHW participated
in design of the present study and helped revise the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
participating patients prior to the start of the study, and the
study was approved by The Institutional Human Experiment and Ethics
Committee of China-Japan Union Hospital of Jilin University.
Patient consent for publication
Written informed consent was obtained from the
participating patients prior to the start of the study.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNA/miR
|
microRNA
|
|
RUNX3
|
runt-related transcription factor
3
|
|
UTR
|
untranslated region
|
References
|
1
|
Prorok PC, Wright P, Riley TR, Kramer BS,
Berg CD and Gohagan JK: Overall and multiphasic findings of the
prostate, lung, colorectal and ovarian (PLCO) randomized cancer
screening trial. Rev Recent Clin Trials. Apr 9–2018.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devlin HL and Mudryj M: Progression of
prostate cancer: Multiple pathways to androgen independence. Cancer
Lett. 274:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manikandan J, Aarthi JJ, Kumar SD and
Pushparaj PN: Oncomirs: The potential role of non-coding microRNAs
in understanding cancer. Bioinformation. 2:330–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang YL, Wu S, Jiang B, Yin FF, Zheng SS
and Hou SC: Role of MicroRNAs in prostate cancer pathogenesis. Clin
Genitourin Cancer. 13:261–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aakula A, Kohonen P, Leivonen SK, Mäkelä
R, Hintsanen P, Mpindi JP, Martens-Uzunova E, Aittokallio T,
Jenster G, Perälä M, et al: Systematic identification of MicroRNAs
that impact on proliferation of prostate cancer cells and display
changed expression in tumor tissue. Eur Urol. 69:1120–1128. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang M, Li C, Yu B, Su L, Li J, Ju J, Yu
Y, Gu Q, Zhu Z and Liu B: Overexpressed miR-301a promotes cell
proliferation and invasion by targeting RUNX3 in gastric cancer. J
Gastroenterol. 48:1023–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu N, Shen C, Luo Y, Xia L, Xue F, Xia Q
and Zhang J: Upregulated miR-130a increases drug resistance by
regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell.
Biochem Biophys Res Commun. 425:468–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Lu Q and Cai X: MicroRNA-106a
induces multidrug resistance in gastric cancer by targeting RUNX3.
FEBS Lett. 587:3069–3075. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Y, Wang R, Song HZ, Pan BZ, Zhang YW
and Chen LB: Epigenetic downregulation of RUNX3 by DNA methylation
induces docetaxel chemoresistance in human lung adenocarcinoma
cells by activation of the AKT pathway. Int J Biochem Cell Biol.
45:2369–2378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miko E, Czimmerer Z, Csanky E, Csánky E,
Boros G, Buslig J, Dezso B and Scholtz B: Differentially expressed
microRNAs in small cell lung cancer. Exp Lung Res. 35:646–664.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakabayashi M, Hayes J, Taplin ME,
Lefebvre P, Lafeuille MH, Pomerantz M, Sweeney C, Duh MS and
Kantoff PW: Clinical predictors of survival in men with
castration-resistant prostate cancer: Evidence that Gleason score 6
cancer can evolve to lethal disease. Cancer. 119:2990–2998. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao J, Xu C, Fang Z, Li Y, Liu H, Wang Y,
Xu C and Sun Y: Androgen receptor regulated microRNA miR-182-5p
promotes prostate cancer progression by targeting the ARRDC3/ITGB4
pathway. Biochem Biophys Res Commun. 474:213–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Li S, Yang X, Qiao B, Zhang Z and
Xu Y: miR-539 inhibits prostate cancer progression by directly
targeting SPAG5. J Exp Clin Cancer Res. 35:602016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi W, Gerster K, Alajez NM, Tsang J,
Waldron L, Pintilie M, Hui AB, Sykes J, P'ng C, Miller N, et al:
MicroRNA-301 mediates proliferation and invasion in human breast
cancer. Cancer Res. 71:2926–2937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Chen G, Cheng Y, Martinka M and
Li G: Prognostic significance of RUNX3 expression in human
melanoma. Cancer. 117:2719–2727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen F, Bai J, Li W, Mei P, Liu H, Li L,
Pan Z, Wu Y and Zheng J: RUNX3 suppresses migration, invasion and
angiogenesis of human renal cell carcinoma. PLoS One. 8:e562412013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee CW, Ito K and Ito Y: Role of RUNX3 in
bone morphogenetic protein signaling in colorectal cancer. Cancer
Res. 70:4243–4252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Wang X, Cheng J, Wang Z, Jiang T,
Hou N, Liu N, Song T and Huang C: MicroRNA-20a-5p targets RUNX3 to
regulate proliferation and migration of human hepatocellular cancer
cells. Oncol Rep. 36:3379–3386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang
L, Le X, Yao J, Wu TT, Huang S and Xie K: Loss of RUNX3 expression
significantly affects the clinical outcome of gastric cancer
patients and its restoration causes drastic suppression of tumor
growth and metastasis. Cancer Res. 65:4809–4816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukamachi H, Ito K and Ito Y:
Runx3-/-gastric epithelial cells differentiate into intestinal type
cells. Biochem Biophys Res Commun. 321:58–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ju X, Ishikawa TO, Naka K, Ito K, Ito Y
and Oshima M: Context-dependent activation of Wnt signaling by
tumor suppressor RUNX3 in gastric cancer cells. Cancer Sci.
105:418–424. 2014. View Article : Google Scholar : PubMed/NCBI
|