Introduction
Previous studies have shown that certain patients
with idiopathic cytopenia of undetermined significance can respond
well to treatment with adrenocortical hormone and/or intravenous
immunoglobulin (IVIG) (1,2). Autoantibodies on the membrane of bone
marrow (BM) haemopoietic cells have been detected (1–5).
Additional studies have indicated that autoantibodies inhibit the
function of BM or reduce the number of BM haemopoietic cells by
macrophage phagocytosis or by activating complement and inhibiting
functional antigen formation (6–8).
These findings indicate that persisting haemocytopenia may be a
type of autoimmune disease, termed immune-related haemocytopenia
(IRH). The production of autoantibodies in IRH may occur due to the
abnormal production of B lymphocytes, notably B1 lymphocytes
(CD5+) (9,10).
The function of B lymphocytes is regulated by T
lymphocytes, including T helper (Th)1, Th2, Th9 and Th17 cells and
regulatory T (Treg) cells. Usually, naïve CD4+ T cells
mature into Th1, Th2, Th9, Th17 or Treg cell subsets in response to
innate immune signals, costimulatory interactions with
antigen-presenting cells (APCs), paracrine cytokine signals, and
due to mTOR-mediated changes in energy metabolism (11,12).
Th1 cells are the quintessential cell type involved in
cell-mediated inflammation and delayed-type hypersensitivity
reactions, which are considered important for immunity against
intracellular pathogens (12). Th2
cells were initially described as anti-inflammatory cells based on
their ability to suppress cell-mediated immunity in Th1 models of
autoimmune disease (11). Th17
cells have been shown to serve an important role in promoting and
enhancing inflammation, including autoimmune tissue injury
(13,14). The mechanisms of Treg-mediated
immune suppression include the secretion of anti-inflammatory
cytokines, the expression of inhibitory receptors and cytokine
deprivation (15).
Previous research has shown that the percentage of
Th17 cells is increased in IRH (16). The quantity and function of Treg
cells in IRH were significantly lower than those of normal controls
(17), whereas the quantity and
function of T follicular helper cells (Tfh cells) were
significantly increased in patients with IRH, which were positively
associated with the presence of BM mononuclear cell antibodies,
disease activity and the response to treatment (18). These preliminary studies indicated
that Th cells maybe involved in the pathogenesis of IRH. To date,
the number of studies that have examined the function and quantity
of T lymphocytes in IRH is limited. The present study focused on
the evaluation of several T cell subsets in patients with IRH,
including Th1/2, Th17, Th9 cells and Tregs, and their secreted
regulatory cytokines in order to elucidate their pathogenic
mechanism of action. The relative telomere length (RTL) was also
detected in patients with untreated IRH and control subjects in
order to assess the degree and number of abnormal CD4+ T
cells. The number of CD19+ B cells was also measured to
observe the potential association between these cells and the
incidence of IRH.
Materials and methods
Patients
A total of 44 (25 women and 19 men) patients with
IRH, with a median age of 36 years (range 11–69 years), were
enrolled in the present study, including 18 patients who did not
receive therapy (untreated patients; 11 women and seven men, median
age 44.5 years, range 16–68 years) and 26 patients in remission (14
women and 12 men, median age 31 years, range 11–69 years). All
patients were inpatients of the Department of Haematology, Tianjin
medical University General Hospital, who were admitted between
October 2015 and October 2016 and were diagnosed with IRH according
to He et al (2). The
patients received corticosteroids (prednisone, 0.5 mg/kg/day),
cyclosporine (CsA; 3 mg/kg/day), and high-dose IVIG (Chengdu
Institute of Biological Products, Sichuan, China, 0.4 g/kg/day for
5 days) if they were dependent on blood transfusion. Complete blood
count and BM examination were performed regularly. The response
criteria were measured according to those used for aplastic anaemia
(AA) (19). The median follow-up
time was 16 months (range 3–60 months) for all patients and 32
months (19–60 months) for patients in remission. A total of 15/26
patients in remission received CsA immunosuppressive therapy for
>1 year following remission, whereas therapy administered in the
remaining 11 patients was terminated within 1 year.
A total of 20 healthy volunteers (10 women and 10
men) with a median age of 32 years (range 22–48 years) were
enrolled in the study as control subjects. A total of 10 ml of
peripheral blood (PB) was obtained from the patients and the
control subjects. The present study was approved by the Ethics
Committee of Tianjin Medical University. Written informed consent
was obtained from the patients and/or their parents in case the
participants <16 years old.
Flow cytometry
Several T cell subsets, including Th1
[CD4+ interferon (IFN)-γ+], Th2
[CD4+interleukin (IL)-4+], Treg
[CD4+CD25+ forkhead box P3
(FoxP3+)], regulatory B (Breg;
CD19+IL-10+), Th9
(CD4+IL-9+) and Th17
(IL-17+CD4+) cells and the
CD5+CD19+ B cell subset were detected by flow
cytometry. For the Th1, Th2, Th9, Th17 and Breg cells, peripheral
blood mononuclear cells (PBMCs) were incubated with 50 ng/ml of
phorbol ester (Beyotime Institute of Biotechnology, Jiangsu,
China), 1 µg/ml of Brefeldin A (Beyotime Institute of
Biotechnology) and 1 µg/ml of ionomycin (Beyotime Institute of
Biotechnology) at 37°C for 5 h.
Briefly, fresh PB (400 µl) was collected and
separated into four tubes with EDTA-anticoagulant. A total of 20 µl
of mouse IgG1-FITC (cat. no. 551954), mouse IgG1-PE (cat. no.
555749) and mouse IgG1-APC (cat. no. 555751) antibodies (all BD
Pharmingen, San Diego, CA, USA) were added into the negative tube.
A total of 20 µl of antibody against CD4-FITC (cat. no. 561842; BD
Biosciences, Franklin Lakes, NJ, USA), CD25-APC (cat. no. 560987;
BD Pharmingen) and CD5-FITC (cat.no. 555352; BD Biosciences),
CD19-APC (cat. no. 561742; BD Biosciences) were separately added
into different test tubes. Following incubation in the dark at 4°C
for 30 min, the red blood cells were lysed with 5 ml of
erythrocytolysin solution (BD Biosciences) and subsequently
centrifuged at 150 × g for 5 min at room temperature. Following
washing with PBS, the cells were permeabilised using a
Cytofix/Cytoperm Buf kit (BD Pharmingen) in the dark for 10 min and
further washed with PBS. A total of 20 µl of antibody against
FoxP3-PE (cat. no. 560852), IL-17-PE (cat. no. 560436), IL-11-APC
(cat. no. 560228), IL-10-APC (cat. no. 558458), IL-9-PE (cat. no.
560807), IFN-γ-APC (cat. no. 551385) and IL-4-PE (cat. no. 559333;
all BD Biosciences) were added separately into three test tubes and
incubated for 30 min in the dark. Subsequently, the cells were
washed twice and resuspended with PBS. At least 300,000 counts were
obtained using a BD FACSCalibur flow cytometer (BD Biosciences).
The results were analysed using CellQuest software 5.2.1. (BD
Biosciences).
The fresh heparinized BM samples (400 µl) were
washed with PBS three times, separated into four tubes and stained
with either 20 µl of mouse IgG1-FITC (cat. no. 551954), 20 µl of
mouse IgG1-PE (cat. no. 555749), or 20 µl of mouse IgG1-APC (cat.
no. 555751) as a negative control, or stained separately with 20 µl
of CD15-FITC (cat. no. 555401), 20 µl of GlyCoA-FITC (cat. no.
565234) and 20 µl of CD34-FITC (cat. no. 560942; all BD
Pharmingen). A total of 20 µl of anti-human IgG-PE (cat. no.
555787) and anti-human IgM-APC (cat. no. 551062; BD Pharmingen)
were added to each tube. Following incubation in the dark for 30
min at 4°C, the cells were incubated with 2 ml erythrocyte lytic
solution (BD Pharmingen) for 10 min at room temperature and washed
three times with PBS. Finally, at least 30,000–100,000 cells were
acquired and analysed on a FACSCalibur flow cytometer.
Enzyme-linked immunosorbent assay
(ELISA)
The serum levels of IL-2, IL-4, IL-6, IL-17, IL-23,
transforming growth factor (TGF)-β and IFN-γ in the patients with
untreated IRH, remission and control subjects were measured using
ELISA reagent kits for IL-2 (cat. no. 171B5003M) and TGB-β (cat.
no. 171W4001M; Bio-Rad Laboratories, Inc., Hercules, CA, USA), IL-6
(cat. no. D6050) and IL-23 (cat. no. D2300B; R&D Systems, Inc.,
Minneapolis, MN, USA) and IL-4 (cat. no. SEA077Hu), IL-17 (cat. no.
SEA063Hu), IL-35 (cat. no. SEC008Hu) and IFN-γ (cat. no. SEA033Hu;
USCNLIFE, Inc., Wuhan, China).
Briefly, diluted standards and patient serum (100
µl) were added in duplicate and incubated at 37°C for 2 h.
Following five washes with 1X wash buffer concentrate, 100 µl of
antibody was added to each well and incubated at room temperature
for 90 min. Subsequently, HRP was added to each well. Following
incubation at 37°C for 30 min, the wells were washed five times.
Subsequently, TMB solution was added to each well, and the samples
were incubated in the dark at room temperature for 20 min. Finally,
a stop solution was added, and the optical density (OD) was read at
450 nm within 15 min.
Sorting of CD4+ and
CD19+ lymphocytes by magnetic-activated cell sorting
(MACS)
The PBMCs were isolated from the heparinized
anticoagulant venous blood of the IRH and control subjects using
Ficoll-Hypaque density gradient centrifugation at room temperature
for 20 min at 600 × g. The CD4+T and CD19+B
lymphocytes were purified using the respective anti-CD4 or
anti-CD19 mAb-conjugated microbeads (Miltenyi Biotec, Bergisch
Gladbach, Germany) according to the manufacturer's instructions. A
total of 10,000,000 cells were resuspended in 90 µl of buffer.
Subsequently, 20 µl of CD4 or CD19 microbeads (Miltenyi Biotec)
were added and incubated at 4°C in the dark for 20 min. Following
washing with 2 ml of buffer, the cells were centrifuged at 300 g
for 5 min and resuspended in 500 µl of buffer. The MS column was
placed in the magnetic field of a suitable MACS separator (Miltenyi
Biotec). Following preparation of the column by rinsing with 1.5 ml
of buffer, the cells were added to the column. The column was
washed with 1.5 ml of buffer and all flow-through unlabelled cells
were collected. The magnetically-labelled cells were immediately
flushed out by firmly pushing the plunger into the column. The
purity of the enriched isolated CD4+ and
CD19+ lymphocytes was evaluated by flow cytometry and
was typically >90%.
Non-adherent cell culture of
MOLT-4
MOLT-4 cells (National Infrastructure of Cell Line
Resource, Beijing, China) were used as control cells and were
cultured in RPMI 1640 medium containing 10% FBS and 1% penicillin
(Gibco BRL; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C in a humidified atmosphere containing 5% CO2. The
cells were grown for 2–3 days in liquid.
FLOW-fluorescence in situ
hybridization (FISH)
The analysis was conducted according to the telomere
PNA kit/FITC used for flow cytometry (Dako, Carpinteria, CA, USA).
A total of 1 and/or 2×106 sorted cells and control cells
were diluted in PBS and divided into two tubes, namely A and B. The
DNA was denatured at 82°C for 10 min in an Eppendorf tube in the
presence of hybridization solution with or without a
fluorescein-conjugated PNA telomere probe. Subsequently,
hybridization was performed in the dark at room temperature
overnight. The samples were washed twice in washing solution at
40°C for 10 min each. Finally, the cells were resuspended in
DNA-staining solution and stored in the dark at 2–8°C for 2–3 h
prior to flow cytometric analysis. The specific fluorescence
activity was proportional to the telomere staining and was detected
in FL1, whereas the fluorescence derived from DNA staining was
detected in FL3. Finally, at least 20,000 cells were acquired and
analysed using the fluorescence-activated cell sorter (FACSCalibur)
flow cytometer (BD Biosciences). The DNA index of the cells was
determined as follows: RTL = (mean FL1 sample cells with probe-mean
FL1 sample cells without probe) × DNA index of control cells ×
100/(mean FL1 control cells with probe-mean FL1 control cells
without probe) × DNA index of sample cells.
Statistical analysis
SPSS 21.0 software (IBM Corp.) was used for
statistical analysis. Data are presented as the mean ± standard
deviation. The significance of the differences was assessed by
one-way ANOVA and the independent sample t-test. Relapse rates were
assessed using the chi-square test (Fisher's exact test) in the two
groups. Data that exhibited correlation were assessed using
Spearman's rank correlation. P<0.05 was considered to indicate a
statistically significant difference.
Results
CD4+ T cells exhibit
abnormal number in untreated patients with IRH, as demonstrated by
increased percentages of Th2, Th9, Th17 and Breg cells and
decreased percentages of Treg cells
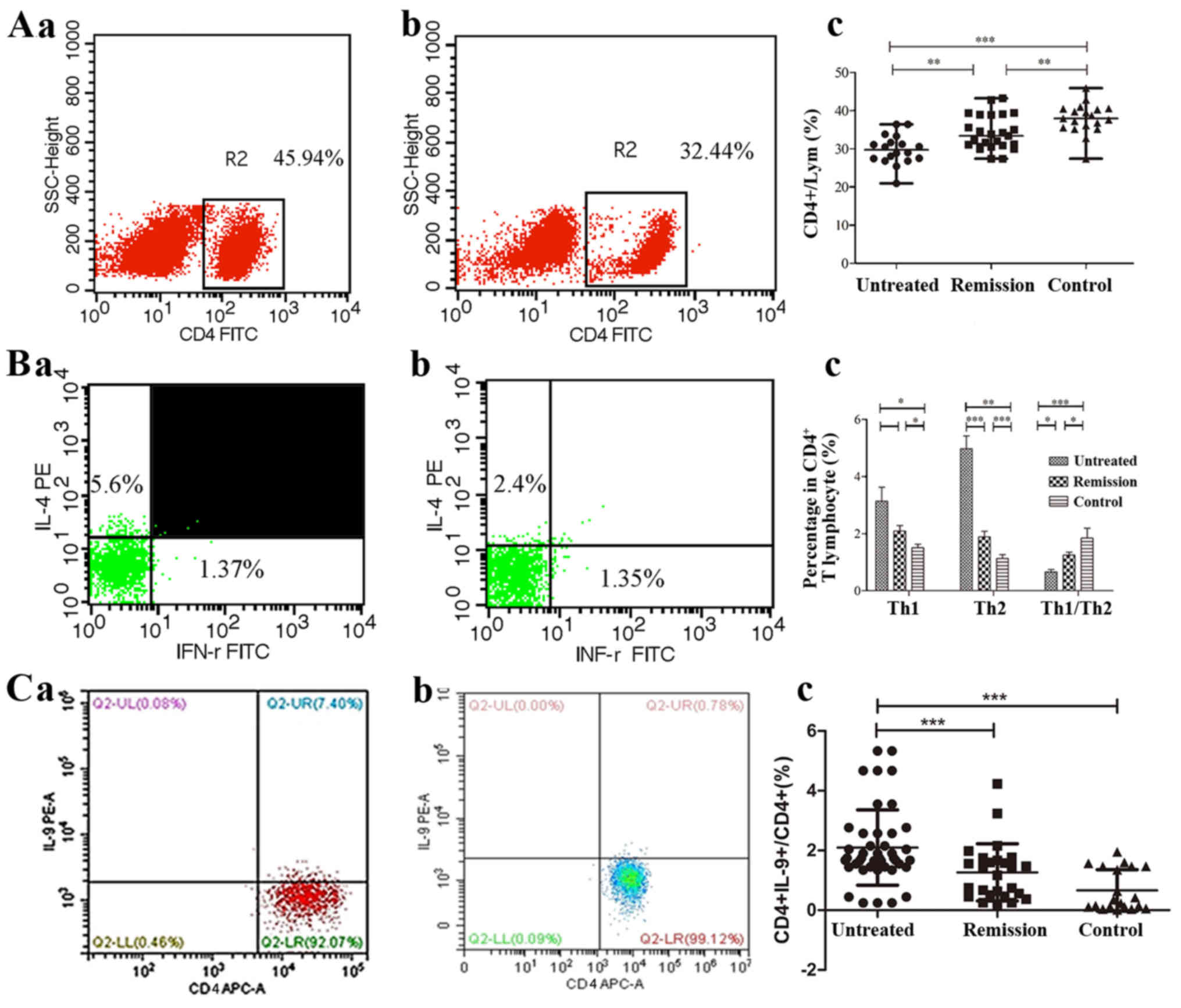
The percentages of CD4+ T cells, Th1,
Th2, Th9, Th17, Treg and Breg cells are shown in Table I. It was found that the percentages
of the CD4+ T cell lymphocyte populations were decreased
in the untreated patients with IRH compared with those noted in the
patients of the remission group (P=0.004) and control group
(P<0.001, Fig. 1A). The
percentages of Th1 and Th2 cells in the CD4+ T
lymphocyte population were increased significantly in the untreated
patients with IRH compared with those noted in the control subjects
(P=0.013 and P<0.001, respectively). In addition, the percentage
of Th1 cells was higher in patients in the remission group than
that in patients in the control group (P=0.042). The percentage of
Th2 cells was reduced significantly following treatment
(P<0.001), although it was higher than the percentage noted in
the control subjects (P=0.008). The ratio of Th1/Th2 lymphocytes
was further evaluated, and the results indicated that the ratio in
the untreated IRH group was significantly lower than the ratios in
the IRH remission group and control group (P=0.043 and P<0.001,
respectively, Fig. 1B). These
results indicated that Th2 cells may be important in the
development of IRH. The percentage of Th9 cells in the untreated
patient group was significantly increased compared with that noted
in the remission group and control group (P<0.001, Fig. 1C).
 | Table I.Percentages of different
CD4+ T lymphocyte subsets. |
Table I.
Percentages of different
CD4+ T lymphocyte subsets.
|
| CD4+ T
cell (CD4+/Lym) % | Th1
(CD4+IFN-γ+/CD4+) % | Th2
(CD4+IL-4+/CD4+) % | Th1/Th2 | Th9
(CD4+IL-9+/CD4+) % | Th17
(CD4+IL-17+/CD4+) % | Treg
(CD4+CD25+ FoxP3+/CD4+)
% | Breg
(CD19+IL-10+/CD4+) % |
|---|
| Untreated |
29.8039±3.8688a,b |
3.1367±2.0143b |
4.9783±1.8487a,b |
0.6630±0.345a,b |
2.73±1.96a,b |
4.8311±2.5255a,b |
1.4333±0.7255a,b |
26.5020±6.9294b |
| Remission |
34.1265±4.3872c |
2.0973±0.9592c |
1.8927±0.9811c |
1.2527±0.496c | 1.17±0.79 |
2.6134±1.1236c |
1.9127±0.8079c |
22.5186±9.8210c |
| Control | 38.1215±3.9399 | 1.2527±0.4963 | 1.1345±0.5741 | 1.8525±1.488 | 0.67±0.40 | 1.8035±0.9225 | 2.7095±0.7158 | 12.0657±4.2323 |
| Ab% (≥10) | 29.7370±3.4924 | 3.2300±1.6574 | 4.4664±1.5671 | 1.0366±0.814 | 2.42±1.50 | 4.6517±2.1363 | 1.9238±0.4042 | 28.0968±4.6957 |
| Ab% (<10) | 30.5481±4.1705 | 2.1257±1.5139 | 4.4603±1.5581 | 1.5122±0.212 | 1.86±1.44 | 3.6513±1.5129 | 2.7241±1.0677 | 25.9168±7.8114 |
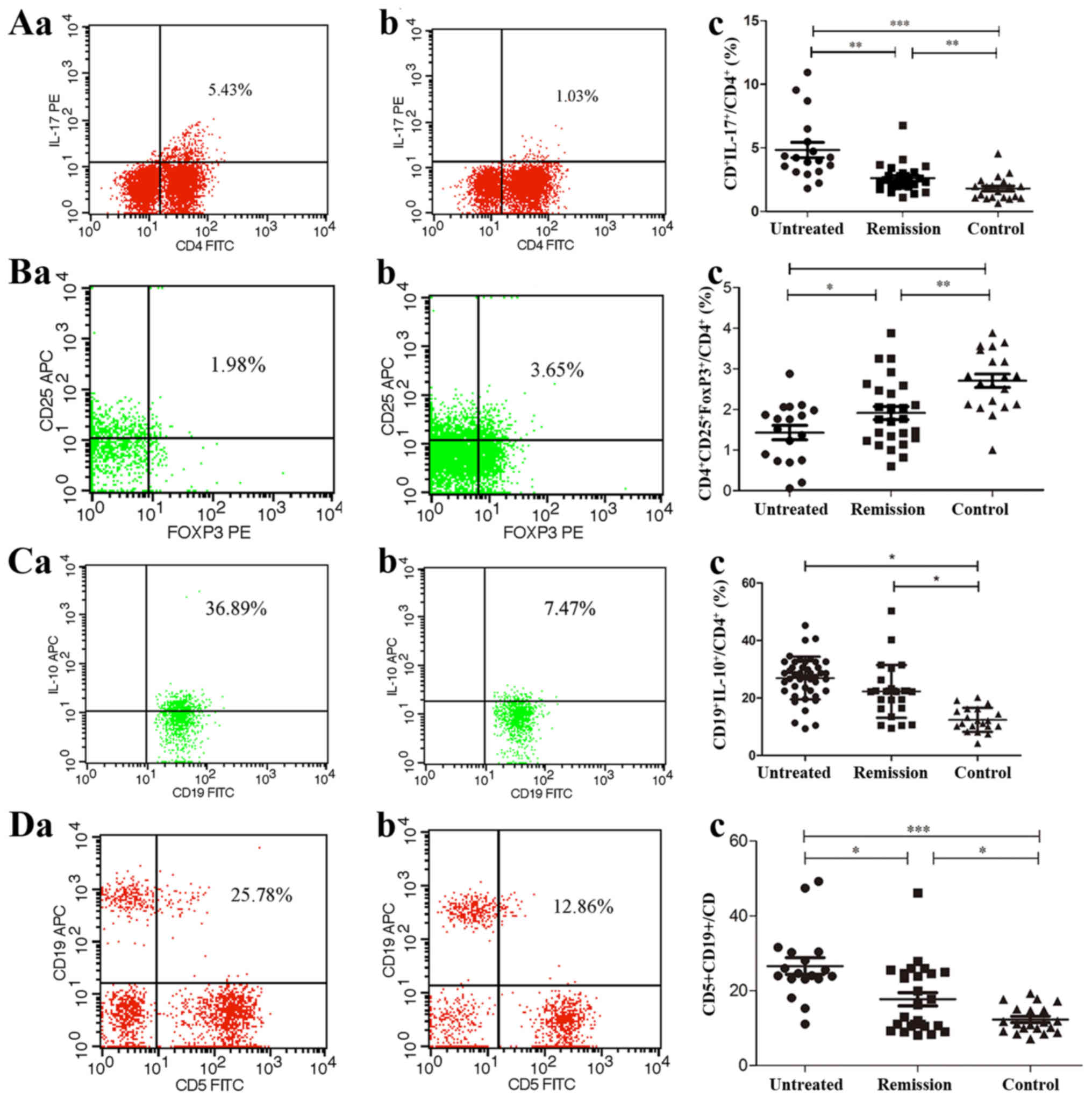
The percentage of Th17 cells was also significantly
increased in the untreated patients compared with that in the
control subjects (P<0.001). Following treatment, the percentage
of Th17 cells decreased significantly (P=0.007), but remained at a
higher level than that in the control group (P=0.006, Fig. 2A). The percentage of Treg cells in
the untreated patients was significantly decreased compared with
that in the remission patients (P=0.048) and control subjects
(P<0.001). In addition, the levels of Treg cells in the
remission group was significantly lower than that of the control
group (P=0.001, Fig. 2B). In
contrast to the Treg cells, the percentages of Breg cells in the
untreated and remission patients were significantly increased
compared with that in the control subjects (P=0.018 and P=0.032,
respectively, Fig. 2C). No
significant differences were noted with regard to these parameters
between the untreated and remission groups.
A value of autoantibodies >4.0% was defined as
positive in IRH. From these findings, the IRH patients were divided
into two groups: i) IRH patients with a value of ≥10%; ii) IRH
patients with a value of <10%. There was no statistical
significance between the two groups in terms of an abnormal
percentage of CD4+ T cell subsets or regulatory
cytokines regulated B1 lymphocytes, but the trend in accordance
with the present results (Table
I).
The correlation analysis between clinical data and
the percentages of the different T lymphocyte subsets is shown in
Table II. A negative correlation
was noted between the percentages of Th1 and Th2 lymphocytes and
the levels of haemoglobin (Hb) in patients with IRH. A negative
correlation was also noted between the percentages of Th1 and Th2
lymphocytes and the white blood cells (WBC) and platelet (Plt)
numbers in the patients with IRH. Furthermore, the percentage of
Th17 cells exhibited a negative correlation with the levels of Hb
and Plt numbers. In contrast to Th17 cells, the percentage of Treg
cells exhibited a positive correlation with the levels of Hb and
the WBC and Plt numbers. However, the percentage of neutrophils and
reticulocytes showed no significant correlation with the percentage
of T lymphocyte subsets.
 | Table II.Correlation analysis between PB
cells, RTLs of lymphocytes and levels of T lymphocytes. |
Table II.
Correlation analysis between PB
cells, RTLs of lymphocytes and levels of T lymphocytes.
| Factor | n | Mean ± SD | Cell | +/− | P-value | r |
|---|
| Hb (g/l) | 44 |
94.2954±27.6023 | Th1 | − | 0.012a | −0.377 |
|
|
|
| Th2 | − | 0.009b | −0.387 |
|
|
|
| Th17 | − |
<0.001c | −0.504 |
|
|
|
| Treg | + | 0.001b | 0.501 |
| WBC
(×109) | 44 | 4.4843±2.3569 | Th1 | − | 0.015a | −0.364 |
|
|
|
| Th2 | − | 0.012a | −0.374 |
|
|
|
| Th17 | N | 0.084 | −0.263 |
|
|
|
| Treg | + | 0.037a | 0.316 |
| N (%) | 44 |
50.1046±19.6863 | Th1 | N | 0.379 | −0.136 |
|
|
|
| Th2 | N | 0.350 | 0.144 |
|
|
|
| Th17 | N | 0.261 | 0.173 |
|
|
|
| Treg | N | 0.877 | 0.024 |
| Plt
(×109) | 44 |
54.4545±37.5143 | Th1 | − | 0.001b | −0.474 |
|
|
|
| Th2 | − | 0.034a | −0.32 |
|
|
|
| Th17 | − | 0.001b | −0.484 |
|
|
|
| Treg | + | 0.001b | 0.481 |
| Ret (%) | 44 | 1.8959±0.8766 | Th1 | N | 0.375 | −0.137 |
|
|
|
| Th2 | N | 0.211 | −0.192 |
|
|
|
| Th17 | N | 0.387 | −0.134 |
|
|
|
| Treg | N | 0.098 | 0.253 |
|
CD5+CD19+/CD19+
(%) | 44 |
21.3447±10.1374 | Th1 | + | 0.046 | 0.262 |
|
|
|
| Th2 | + | 0.002b | 0.447 |
|
|
|
| Th17 | + | 0.035a | 0.318 |
|
|
|
| Treg | − | 0.024a | −0.341 |
| RTLs of
CD19+B cells | 12 |
22.1360±15.3903 | Th1 | − | 0.002b | −0.79 |
|
|
|
| Th2 | − | 0.016a | −0.676 |
|
|
|
| Th17 | − | 0.020a | −0.657 |
|
|
|
| Treg | + | 0.005b | 0.748 |
| RTLs of
CD4+T cells | 12 | 7.2725±3.2566 | Th1 | − | 0.013a | −0.692 |
|
|
|
| Th2 | − | 0.021a | −0.655 |
|
|
|
| Th17 | N | 0.075 | −0.531 |
|
|
|
| Treg | + | 0.015a | 0.678 |
CD5+ B cell numbers are
increased in patients with untreated IRH
The percentage of CD5+CD19+ B
lymphocytes in the CD19+ B lymphocyte population was
significantly increased in the untreated IRH patient group
(26.6006±9.1446%) compared with that in the remission group
(17.7075±8.9295%, P=0.011) and that in the control subjects
(12.2995±3.5353%, P<0.001, Fig.
2D). The percentage of CD5+CD19+ B
lymphocytes in the IRH patients exhibited a positive correlation
with the percentage of Th1 (r=0.262, P=0.046), Th2 (r=0.447,
P=0.002) and T17 (r=0.318, P=0.035) cells, and a negative
correlation with that of Treg lymphocytes (r=−0.341, P=0.024;
Table II).
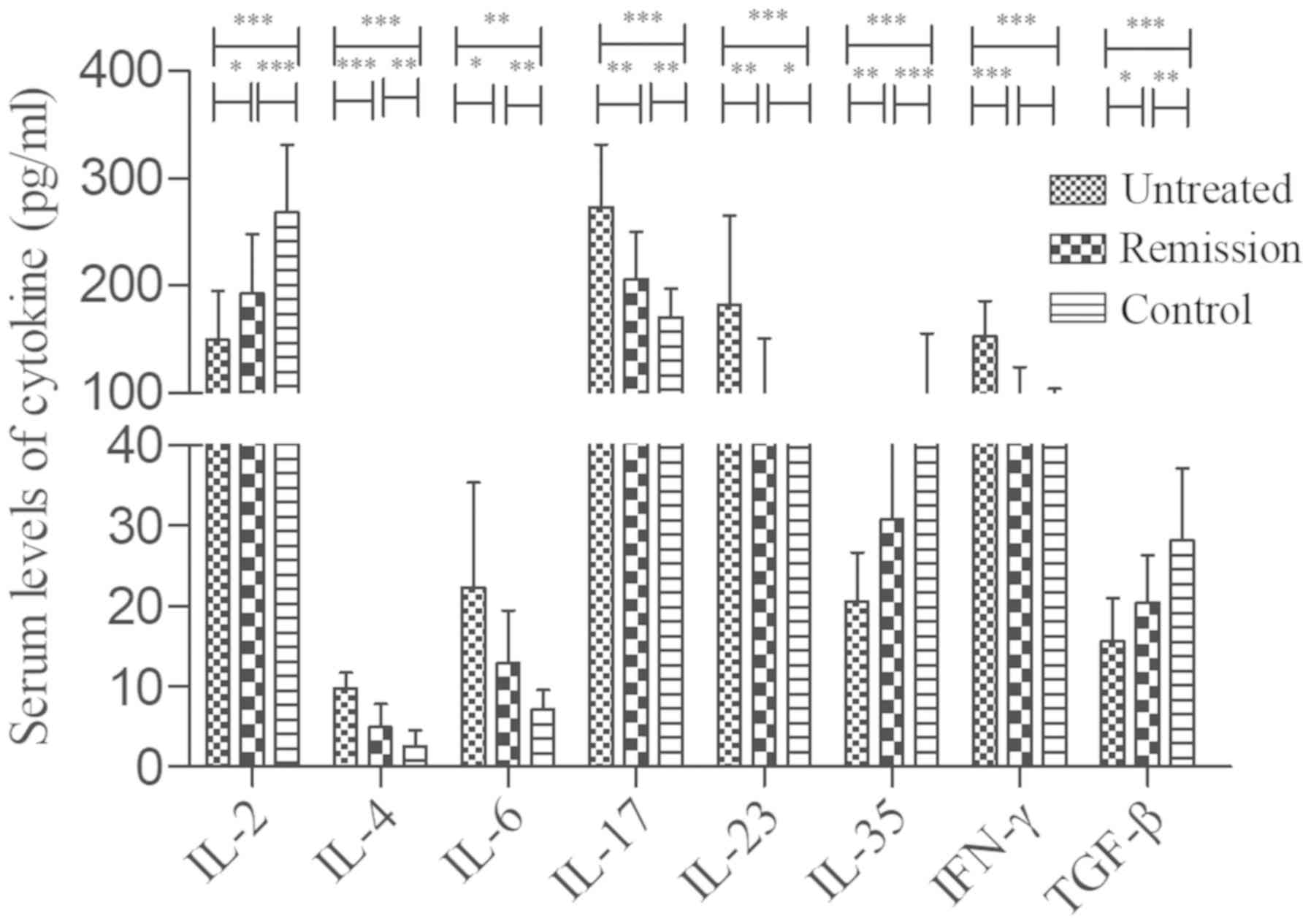
Secreted levels of abnormal cytokines
are associated with the percentage of CD4+ T cells in
patients with untreated IRH
In order to examine the regulation of
CD4+ T cells in the present study, the levels of several
cytokines were measured in the patients with IRH, including IL-2,
IL-4, IL-6, IL-17, IL-23, IL-35, TGF-β and IFN-γ, which are shown
in Table III. It was found that
the levels of IL-4, IL-6, IL-17 and IL-23 in the untreated patients
were increased compared with those in the remission patients and
control subjects. The levels of these cytokines in the remission
group were significantly higher than those in the control subjects
(Fig. 3). The levels of IL-2,
IL-35 and TGF-β were decreased significantly in the untreated
patients compared with those in the remission patients and control
subjects, while the corresponding levels in the remission patients
were significantly lower than those in the control subjects
(Fig. 3). Furthermore, the levels
of IFN-γ were increased in the untreated patients compared with
those in the remission patients and control subjects, whereas no
significant difference was noted between the latter two groups.
 | Table III.Serum levels of cytokines measured by
enzyme-linked immunosorbent assay. |
Table III.
Serum levels of cytokines measured by
enzyme-linked immunosorbent assay.
| Cytokine
(pg/ml) | Untreated | Remission | Control |
|---|
| IL-2 |
149.2868±45.8402 |
192.5027±55.0910c |
268.2111±62.8435 |
| IL-4 |
9.7976±1.9017a,b |
4.9491±2.8450c | 2.5726±2.0220 |
| IL-6 |
22.2948±13.0507a |
12.8473±6.5653c | 7.1145±2.4324 |
| IL-17 |
273.4800±58.0620a |
206.1831±44.0075c |
170.5465±26.4704 |
| IL-23 |
182.4770±82.8248a |
96.6372±53.6834c |
56.2377±32.1117 |
| IL-35 |
20.5905±6.0470a,b |
30.7670±10.6000c |
98.4509±57.0162 |
| IFN-γ |
152.9567±32.8728 |
93.0603±30.7645 |
63.7026±40.2122 |
| TGF-β |
15.6375±5.2789a,b |
20.4485±5.8137c | 28.1787±8.9327 |
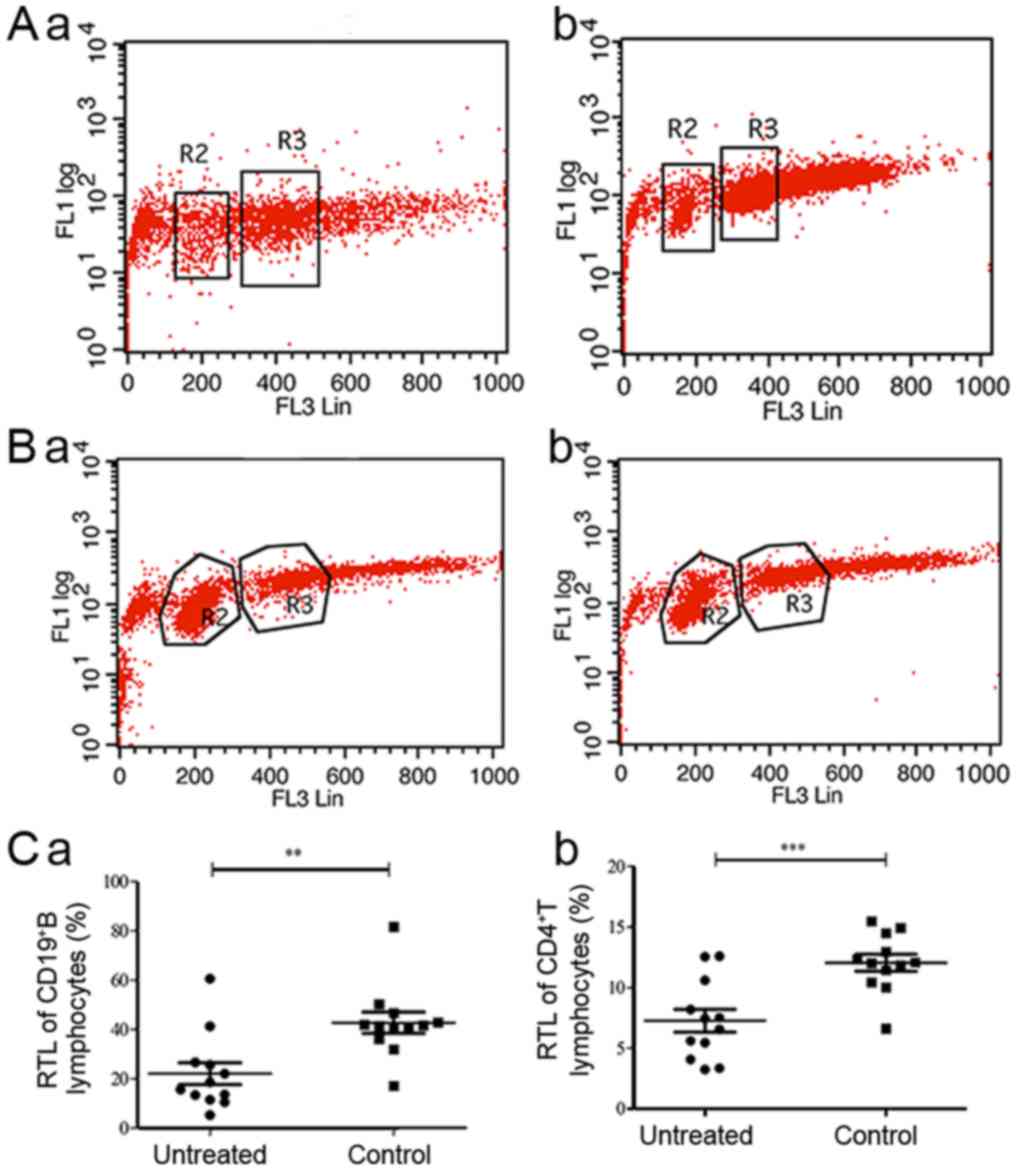
RTLs of CD4+ T cells and
CD19+B cells are shortened in patients with untreated
IRH
The RTLs of the CD19+ B and
CD4+ T lymphocytes of the untreated IRH group and of the
control group were measured using flow cytometry (Fig. 4A and B). The RTLs of
CD19+ B and CD4+ T lymphocytes in the
untreated IRH patient group were 22.1360±15.3903 and
7.2725±3.2566%, respectively, which were shorter than those in the
control group (42.7313±14.7974%, P=0.003, Fig. 4C-a, vs. 12.0657±2.4007%,
P<0.001, Fig. 4C-b). The RTLs
of the CD19+ B and CD4+ T lymphocytes of the
IRH patient group were negatively correlated with the percentages
of Th1 cells (r=−0.79, P=0.002 and r=−0.602, P=0.013,
respectively), Th2 cells (r=−0.676, P=0.016 and r=−0.655, P=0.021,
respectively) and Th17 cells (r=−0.657, P=0.02 and r=−0.531,
P=0.075, respectively). These percentages were also positively
correlated with those of Treg lymphocytes (r=0.748, P=0.005 and
r=0.678, P=0.015, respectively; Table
II).
Relapse rates of patients in
remission
All patients in remission were followed up in order
to observe their treatment and relapse rates. Of 26 patients in
remission, 15 patients received CsA as immunosuppressive therapy
for >1 year following remission, whereas the remaining 11
patients underwent therapy termination within 1 year. The median
follow-up time for the patients in remission was 32 months (19–60
months). Subsequently, the relapse rates in the 2-year follow-up
period were compared between the two groups. The results showed
that 45.5% (5/11) of the patients relapsed in the intermittent
treatment group, which was higher than that noted in the continuous
group (20%, 3/15, P=0.218).
Discussion
IRH is one type of autoimmune BM failure disease,
which is mediated by autoantibody secretion on the membrane of BM
haemopoietic cells. Autoantibodies can inhibit haemopoiesis and
thereby induce haemocytopenia (5).
In the present study, it was demonstrated that the
percentages of Th1 and Th2 cells were increased in the patients
with untreated IRH, whereas the Th1/Th2 ratio was decreased,
indicating that Th2 cells serve a major role in the development of
this disease. It is well known that Th2 cells are responsible for
humoral-mediated immunity, while Th1 cells are responsible for
cell-mediated immunity. In addition, Th2 cells secrete IL-4, which
is closely associated with the production of IgG1 by B cells
(20). In the present study, the
levels of IL-4 were upregulated in patients with untreated IRH and
were higher than those in control subjects following remission.
Therefore, the present study indicated that IRH is a type of
autoantibody-mediated autoimmune disease.
The data revealed a significant decrement in the
levels of CD4+CD25+Foxp3+ Treg
cells in patients with IRH compared with those in control subjects.
Naturally occurring CD4+CD25+ Treg cells are
key in immune tolerance. Foxp3 serves a central role in the
differentiation and maintenance of Treg cells. It has been shown
that Foxp3 gene transfer can convert naïve
CD4+CD25− T cells into a functional
regulatory population (21). In
addition, Treg cells exert suppressive effects on the effector T
cells by secreting IL-10, which can inhibit APC maturation and
exert direct suppressive effects on effector T cells (22). Tregs can further inhibit the
proliferation and cytokine production of Th1 and Th2 cells in
vitro and in vivo (23)
and possibly suppress B cell activation (24). IL-2 has been shown to regulate the
expression of CD25 (25) and was
critically required for the peripheral maintenance of natural
CD4+CD25+ Treg cells, which consequently
sustained immunologic self-tolerance (25,26).
TGF-β serves a key role in the differentiation and function of Treg
cells in mice by inducing the expression of Foxp3 in vivo
and in vitro (27).
Furthermore, TGF-β produced by Treg cells suppresses immune
responses in different target cells (28). A newly discovered cytokine that is
secreted by CD4+CD25+ Tregs has been
identified as IL-35. IL-35 can promote the suppressive function of
Treg cells and restrict the differentiation and function of
Th1/Th17 cells (29). In patients
with untreated IRH, the levels of IL-2, IL-35 and TGF-β were
decreased compared with those in control subjects, indicating that
these cells serve a protective role in the progression of IRH.
It has already been shown that the development of
Th17 cells is prompted by a combination of IL-6 and TGF-β, which
require the expression of STAT3 and the retinoic acid-related
orphan receptor γt (30,31). Th17 cells are not terminally
differentiated, as they are able to switch to Th1 cells and are
therefore implicated in several inflammatory reactions (32–34).
The main secretory cytokine of Th1 cells is IL-17. In the present
study, the percentage of Th17 cells and the serum levels of IL-17
were significantly higher than those of control subjects. IL-17
acts as a pleiotropic cytokine and as a chemoattractant for
neutrophils (34). It is also a
suppressor of Th1 and Treg cells (34). IL-23 is known to be a survival and
proliferative factor for Th17 cells (35). Emerging data suggest that not all
Th17 cells are pathogenic, and that the pathogenic state is induced
only following exposure to APCs that secrete IL-23. IL-23 is
crucial for the ability of Th17 cells to induce autoimmunity. It
acts on newly generated IL-23R-expressing Th17 cells and causes a
shift in function in order to produce IFN-γ (36,37).
In addition, the present study found that serum levels of IL-23
were increased in patients with IRH and that they activated Th17
cells to induce the autoimmune status of the disease. Th9 cells
require the combination of TGF-β and IL-4 for their development.
Th9 cells are important in certain autoimmune diseases through the
production of IL-9. IL-9 induces CD4+ T cells to
differentiate into Th17 cells. IL-9 enhances the suppressive the
function of Treg cells. TGF-β promotes Treg maturation, whereas
IL-4 induces Th2 cell activation (38). The results of the present study
indicated that the levels of Th9 and Th17 were increased.
The balance between suppressive Treg cells and
pathogenic Th17 cells is critical in orchestrating autoimmune
responses (39). IL-6 serves a
major role in determining this balance (39). In the present study, a significant
increase in the plasma levels of IL-6 was noted in patients with
IRH. This pleiotropic inflammatory cytokine is produced by T cells,
monocytes, macrophages and synovial fibroblasts, and mediates
various functions by binding to its receptor IL-6R (40). As a potent activator of STAT3, IL-6
has the capacity to switch immune responses from the induction of
suppressive Treg cells to the development of pathogenic Th17 cells
(41). Furthermore, the
IL-6-induced B cell help requires the expression of the signal
transducer and activator STAT3, suggesting that the ability to
provide help to B cells is not restricted to a well-defined Th1 or
Th2 effector population (31).
The hyperfunction and increased levels of B1
lymphocytes may be caused by the imbalance of Th1/Th2 cells. It has
been shown that the abnormal number and function of B1 lymphocytes
may be one factor causing the production of autoantibodies in
patients with IRH (42). In the
present study, the percentage of abnormal T lymphocytes and
CD5+ B lymphocytes gradually returned to normal in
patients with IRH who were treated with immunotherapy and presented
with disease remission and/or curative effects. No significant
difference was noted between the cured patients and control
subjects. Therefore, the levels of abnormal T lymphocytes and
CD5+ B lymphocytes can be used to evaluate the treatment
of patients with IRH and the prognosis of the disease. B1 cells,
notably B1a cells, have been shown to contribute to autoimmune
pathogenesis by the production of autoantibodies (43), antigen presentation, activation of
CD4+ T cells (44,45)
and cytokine production (46). The
results of the present study further revealed a significant
increase in the levels of CD5+CD19+ B1
lymphocytes in patients with IRH. Our previous studies showed a
positive correlation between the levels of IgG1 and the proportion
of CD5+ B1 lymphocytes, and that haematopoietic cells
may be targeted by IgG1 autoantibodies in certain patients with IRH
(47). Furthermore, B1 cells can
induce T cells to express IL-17 and IFN-γ to a greater extent than
that observed with either B2 cells or dendritic cells (44). Accumulating evidence has shown the
multifaceted functions of B1 cells, which include several processes
other than antibody production. These functions are implicated in
the development of autoimmune diseases, including autoimmune
arthritis and systemic lupus erythematosus (SLE) (48,49).
The main cytokines secreted by CD4+ T cells that are
involved in IRH are presented in Fig.
5.
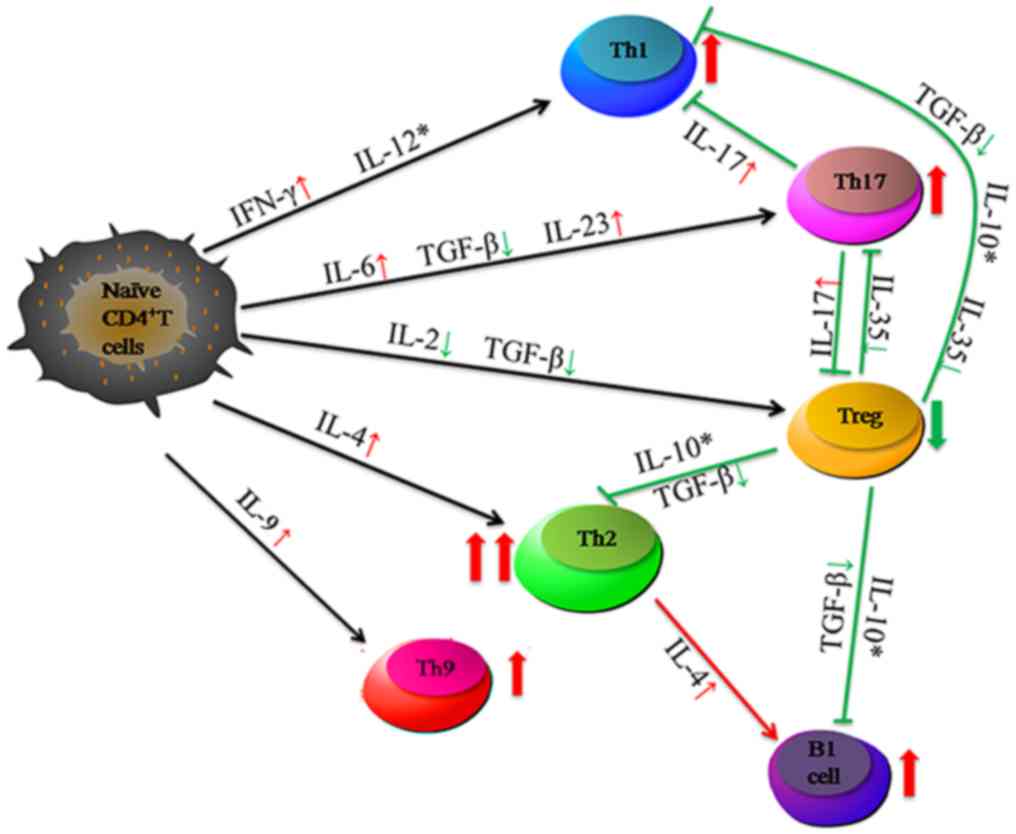 | Figure 5.Abnormal percentage of
CD4+ T cell subsets and abnormal expression levels of
regulatory cytokines in patients with untreated IRH. The
percentages of Th1, Th2, Th9 and Th17 cells were increased, whereas
the percentage of Treg cells was decreased in patients with IRH.
Several cytokines were abnormal and were involved in the regulation
of CD4+ T lymphocytes. These CD4+ T cells and
related cytokines further regulated CD5+ B lymphocytes
to produce autoantibodies. Thin red arrows indicate a small
increase, while large red arrows indicate a large increase. The
green arrows indicate a decrease. *, cytokines not detected in the
present study. IRH, immune-related haemocytopenia; Th, T helper;
Treg, regulatory T cell; IL, interleukin; IFN, interferon; TGF,
transforming growth factor. |
Breg cells induce IL-10, which suppresses the
release of IFN-γ and TNF-α. Breg cells can inhibit naïve T cell
differentiation into Th1 and Th17 cells and the activation of
CD4+CD25− T cells to Tregs. In rheumatoid
arthritis, Breg cells are suppressed and do not produce IL-10,
which in turn leads to reduced Th17 development (50). In SLE, the percentages of Breg
cells have been shown to be increased, although these cells were
refractory to CD40 stimulation and produced less IL-10, thus
failing to suppress T cell proliferation (51). The present study indicated that the
number of Breg cells was increased in IRH. Breg cells are increased
in order to inhibit the activity of abnormal T lymphocytes.
Concomitantly, the present study examined whether the function of
Breg cells was defective.
Telomeres are heterochromatic structures with tandem
DNA repeats of 5′-TTAGGG-3′ at the chromosomal ends. With each cell
division, telomeres shorten progressively due to the
‘end-replication problem’. Various studies have documented that
accelerated telomere loss in different lymphocyte subsets is a
common feature of autoimmune disease, including SLE, RA and
psoriasis (52–55). The present study demonstrated for
the first time, to the best of our knowledge, that the telomere
length of several lymphocyte subgroups (CD19+ B and
CD4+ T lymphocytes) was significantly shortened in
patients with IRH compared with that of control subjects.
Furthermore, it was shown that the RTL significantly correlated
with the percentage of T lymphocyte subgroups. Telomere shortening
is an important suppressive mechanism that limits cellular
proliferative capacity via regulating senescence checkpoint
activation. The immune system is in constant self-renewal and is
consequently dependent on efficient telomere maintenance. As
telomeres protect chromosomes and genetic information from damage
and erosion, their shortening mainly depends on antigen irritation
and various stimulatory factors (56). Telomere shortening in IRH may be
due to the chronic activation/proliferation occurring in autoimmune
conditions and therefore could lead to premature immune senescence,
involving exit from the mitotic cycle and/or cell death.
Furthermore, the present study examined the
association between the abnormal CD4+ T cell subtypes
and the clinical data of the patients; the results showed that the
increased percentages of Th17, Th1 and Th2 cells correlated
negatively with Hb levels and Plt count, whereas the decreased
percentages of Treg cells and Th1/Th2 cells correlated positively
with Hb levels and Plt count (Table
II). This indicated that these markers can be used for
evaluation of the severity of the disease. In addition, abnormal
percentages of CD4+ T cell subtypes and abnormal
expression of corresponding cytokine levels, including reduced
levels of IL-2, TGF-β and IL-35, were noted following successful
application of immunotherapy (normal blood cell counts). Although
the levels of IL-4, IL-6, IL-17, IL-23 and IFN-γ were
simultaneously increased in these patients, they did not reach the
normal baseline levels, which illustrated that clinical
immunosuppressive therapy should be prolonged. Finally, patient
treatment and disease relapse rates were examined. It was found
that 45.5% (5/11) of the patients relapsed in the intermittent
treatment group, which was higher than the number in the continuous
treatment group (20%, 3/15) (P=0.218). Therefore, it was concluded
that it is necessary for patients in remission to undergo extension
of their immunosuppressive therapy.
Additional T cell subsets are present that were not
detected in the present study. For example, Tfh cells provide a
helper function to B cells and represent one of the most numerous
and important subsets of effector T cells (57). Tfh cells can express Bcl-6 and
CXCR5, providing assistance to B-cells via the production of IL-21
(58). It has already been
demonstrated in a previous study that the increased frequency and
hyperfunction of Tfh cells in patients with IRH is associated with
disease progression, which includes the presence of autoantibodies,
disease activity and response to treatment (18). Th9 cells undergo a maturation
program similar to that of Th2 cells, with IL-4 inducing the
activation of STAT6 and producing IL-9 and IL-10. However, unlike
Th2 cells, they require TGF-β for maturation. This cytokine
provides protection against intestinal helminth infections
(59). Th22 cells are
phenotypically and functionally related to Th17 cells, which are
involved in wound repair and protection against bacterial, viral
and fungal infections on epithelial surfaces, including the skin
and gastrointestinal tract (60).
Th25 cells can produce IL-25 and stimulate non-lymphoid cells in
order to secrete effector cytokines in response to extracellular
pathogens (61). However, the
effects of Th9, Th22 and Th25 cells on IRH remain to be fully
elucidated. The pathogenesis of IRH has been associated with the
cytokine network, notably the B and the T lymphocyte subsets.
Further investigations are required to elucidate the exact
mechanism of IRH.
In conclusion, the present study demonstrated
increased percentages of Th17 cells, decreased percentages of Treg
cells and regulatory cytokines and a decreased ratio of Th1/Th2 in
patients with IRH. These immune mediators regulated CD5+
B lymphocyte function in order to produce autoantibodies in IRH.
The abnormal percentage of CD4+ T subtypes and the
abnormal levels of their corresponding cytokines may be used as
indicators for evaluating the severity of this disease and for
extension of the immunosuppressive therapy used in the clinic.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570106, 81600088,
81600093, 81400085 and 81770110), the Tianjin Municipal Natural
Science Foundation (grant nos. 14JCYBJC25400, 15JCYBJC24300 and
16ZXMJSY00180) and the Science and Technology Foundation of Tianjin
Municipal Health Bureau (grant nos. 2011kz115 and 2014KZ120).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RF designed the research plan and revised the
manuscript. JC, HL and LiyL performed the experiments, analysed the
data and wrote the manuscript. HW, YL, YW, KD, SH and YS
contributed to the experimental work. LijL, JS, GW and ZS recorded
the clinical characteristics of the patients with IRH. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Medical University. Written informed consent
was obtained for all patients included in the clinical trial.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fu R, Liu H, Wang Y, Liu H, He H, Chen J,
Wang H, Yu H, Ding K, Huang L, et al: Distinguishing immunorelated
haemocytopenia from idiopathic cytopenia of undetermined
significance (ICUS): A bone marrow abnormality mediated by
autoantibodies. Clin Exp Immunol. 177:412–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He H, Shao Z and Cao Z: Bone marrow
mononuclear Coombs test. Chin J Hematol. 21:550–551. 2000.
|
|
3
|
Sun J, Cao Z and Tu F: Modified direct
antiglobulin test. Chin J Laborat Med. 26:12–14. 2006.
|
|
4
|
Wang YH, Fu R, Dong SW, Liu H and Shao ZH:
Erythroblastic islands in the bone marrow of patients with
immune-related pancytopenia. PLoS One. 9:e951432014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu H, Fu R, Wang Y, Liu H, Li L, Wang H,
Chen J, Yu H and Shao Z: Detection and analysis of autoantigens
targeted by autoantibodies in immunorelated pancytopenia. Clin Dev
Immunol. 2013:2976782013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YH, Fu R, Shao ZH, Wang HQ, Xing LM,
Liu H, Wu YH, Li LJ, Liu H, Wang J, et al: Study on quantity and
function of bone marrow macrophages in patients with BMMNC-Coombs
Test(+) pancytopenia. Zhonghua Xue Ye Xue Za Zhi. 30:538–542.
2009.(In Chinese). PubMed/NCBI
|
|
7
|
Chen J, Fu R, Li LJ, Liu H, Wang YH, Wang
HL and Shao ZH: Variation in complement level and its significance
in cytopenia patients with positive BMMNC-Coombs. Zhonghua Xue Ye
Xue Za Zhi. 30:454–457. 2009.(In Chinese). PubMed/NCBI
|
|
8
|
Fu R, Liu H, Wang J, Li LJ, Wang HL, Wang
YH and Shao ZH: Preliminary study of autoantigens on the membrane
of erythropoietic cells of the patients with BMMNC-Coomb's test(+)
hemocytopenia. Zhonghua Yi Xue Za Zhi. 92:2689–2693. 2012.(In
Chinese). PubMed/NCBI
|
|
9
|
Fu R, Shao Z, He H, Liu H, Jia H, Sun J,
Zhao M, He G, Shi J, Bai J, et al: Quantity and apoptosis-related
protein level of B lymphocyte in patients with immunorelated
pancytopenia. Zhonghua Xue Ye Xue Za Zhi. 23:236–238. 2002.(In
Chinese). PubMed/NCBI
|
|
10
|
Wang Y, Fu R, Liu H, Wang H, Zhang T, Ding
S, Zhang J, Gao S, Liu C, Wang J, et al: Memory B (CD5+
CD19+ CD27+) lymphocyte in patients with
immune-related pancytopenia. Zhonghua Xue Ye Xue Za Zhi.
35:719–723. 2014.(In Chinese). PubMed/NCBI
|
|
11
|
Raphael I and Forsthuber TG: Stability of
T-cell lineages in autoimmune diseases. Expert Rev Clin Immunol.
8:299–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raphael I, Nalawade S, Eagar TN and
Forsthuber TG: T cell subsets and their signature cytokines in
autoimmune and inflammatory diseases. Cytokine. 74:5–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage
distinct from the T helper type 1 and 2 lineages. Nat Immunol.
6:1123–1132. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyara M and Sakaguchi S: Natural
regulatory T cells: Mechanisms of suppression. Trends Mol Med.
13:108–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu R, Wang HL, Chen J, Wang J, Li LJ, Liu
H, Wang YH, Ren Y and Shao ZH: Study of the quantity and function
of Th17 cells in the blood cytopenic patients with positive
BMMNC-Coombs test. Zhonghua Xue Ye Xue Za Zhi. 31:684–687. 2010.(In
Chinese). PubMed/NCBI
|
|
17
|
Fu R, Chen J, Wang HL, Wang J, Li LJ, Liu
H, Wang YH, Ren Y and Shao ZH: Quantity and function of regulatory
T cells in hemocytopenic patients with positive BMMNC-Coombs test.
Zhonghua Yi Xue Za Zhi. 90:2989–2993. 2010.(In Chinese). PubMed/NCBI
|
|
18
|
Yu H, Zhang J, Fu R, Liu H, Wang H, Ding
K, Wang Y, Li L, Wang H and Shao Z: Increased frequency of bone
marrow T follicular helper cells in patients with immune-related
pancytopenia. Clin Dev Immunol. 2013:7304502013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu X, Guan J, Xu J, Wei J, Jiang L, Yin
J, Zhao L and Zhang Y: Pilot study using tacrolimus rather than
cyclosporine plus antithymocyte globulin as an immunosuppressive
therapy regimen option for severe aplastic anemia in adults. Blood
Cells Mol Dis. 53:157–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heinzel FP, Sadick MD, Holaday BJ, Coffman
RL and Locksley RM: Reciprocal expression of interferon gamma or
interleukin 4 during the resolution or progression of murine
leishmaniasis. Evidence for expansion of distinct helper T cell
subsets. J Exp Med. 169:59–72. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mattozzi C, Salvi M, D'Epiro S,
Giancristoforo S, Macaluso L, Luci C, Lal K, Calvieri S and
Richetta AG: Importance of regulatory T cells in the pathogenesis
of psoriasis: Review of the literature. Dermatology. 227:134–145.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu D, Liu H, Komai-Koma M, Campbell C,
McSharry C, Alexander J and Liew FY:
CD4+CD25+ regulatory T cells suppress
differentiation and functions of Th1 and Th2 cells, Leishmania
major infection, and colitis in mice. J Immunol. 170:394–399.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakaguchi S, Ono M, Setoguchi R, Yagi H,
Hori S, Fehervari Z, Shimizu J, Takahashi T and Nomura T:
Foxp3+ CD25+ CD4+ natural
regulatory T cells in dominant self-tolerance and autoimmune
disease. Immunol Rev. 212:8–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HP, Kelly J and Leonard WJ: The basis
for IL-2-induced IL-2 receptor alpha chain gene regulation:
Importance of two widely separated IL-2 response elements.
Immunity. 15:159–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Setoguchi R, Hori S, Takahashi T and
Sakaguchi S: Homeostatic maintenance of natural Foxp3(+) CD25(+)
CD4(+) regulatory T cells by interleukin (IL)-2 and induction of
autoimmune disease by IL-2 neutralization. J Exp Med. 201:723–735.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Zhang P, Li J, Kulkarni AB,
Perruche S and Chen W: A critical function for TGF-beta signaling
in the development of natural
CD4+CD25+Foxp3+ regulatory T
cells. Nat Immunol. 9:632–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakamura K, Kitani A and Strober W: Cell
contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T
cells is mediated by cell surface-bound transforming growth factor
beta. J Exp Med. 194:629–644. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakano S, Morimoto S, Suzuki S, Tsushima
H, Yamanaka K, Sekigawa I and Takasaki Y: Immunoregulatory role of
IL-35 in T cells of patients with rheumatoid arthritis.
Rheumatology (Oxford). 54:1498–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mangan PR, Harrington LE, O'Quinn DB,
Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR and
Weaver CT: Transforming growth factor-beta induces development of
the T(H)17 lineage. Nature. 441:231–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eddahri F, Denanglaire S, Bureau F,
Spolski R, Leonard WJ, Leo O and Andris F: Interleukin-6/STAT3
signaling regulates the ability of naive T cells to acquire B-cell
help capacities. Blood. 113:2426–2433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin FJ, Jiang GR, Shan JP, Zhu C, Zou J
and Wu XR: Imbalance of regulatory T cells to Th17 cells in IgA
nephropathy. Scand J Clin Lab Invest. 72:221–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ooi JD, Kitching AR and Holdsworth SR:
Review: T helper 17 cells: Their role in glomerulonephritis.
Nephrology (Carlton). 15:513–521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stangou M, Bantis C, Skoularopoulou M,
Korelidou L, Kouloukouriotou D, Scina M, Labropoulou IT, Kouri NM,
Papagianni A and Efstratiadis G: Th1, Th2 and Treg/T17 cytokines in
two types of proliferative glomerulonephritis. Indian J Nephrol.
26:159–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aggarwal S, Ghilardi N, Xie MH, de Sauvage
FJ and Gurney AL: Interleukin-23 promotes a distinct CD4 T cell
activation state characterized by the production of interleukin-17.
J Biol Chem. 278:1910–1914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Croxford AL, Mair F and Becher B: IL-23:
One cytokine in control of autoimmunity. Eur J Immunol.
42:2263–2273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olewicz-Gawlik A, Danczak-Pazdrowska A,
Kuznar-Kaminska B, Gornowicz-Porowska J, Katulska K, Trzybulska D,
Batura-Gabryel H, Silny W, Poplawski D and Hrycaj P: Interleukin-17
and interleukin-23: importance in the pathogenesis of lung
impairment in patients with systemic sclerosis. Int J Rheum Dis.
17:664–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ouyang H, Shi Y, Liu Z, Feng S, Li L, Su
N, Lu Y and Kong S: Increased interleukin-9 and
CD4+IL-9+ T cells in patients with systemic
lupus erythematosus. Mol Med Rep. 7:1031–1037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Masuda M, Matsumoto M, Tanaka S, Nakajima
K, Yamada N, Ido N, Ohtsuka T, Nishida M, Hirano T and Utsumi H:
Clinical implication of peripheral CD4+CD25+
regulatory T cells and Th17 cells in myasthenia gravis patients. J
Neuroimmunol. 225:123–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishihara K and Hirano T: IL-6 in
autoimmune disease and chronic inflammatory proliferative disease.
Cytokine Growth Factor Rev. 13:357–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aricha R, Mizrachi K, Fuchs S and
Souroujon MC: Blocking of IL-6 suppresses experimental autoimmune
myasthenia gravis. J Autoimmun. 36:135–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu R and Shao Z: Immuno-related
pancytopenia: A newly recognized disease (part 1). Chin J Med.
40:5–8. 2005.
|
|
43
|
Enghard P, Humrich J, Chu VT, Grussie E,
Hiepe F, Burmester GR, Radbruch A, Berek C and Riemekasten G: Class
switching and consecutive loss of dsDNA-reactive B1a B cells from
the peritoneal cavity during murine lupus development. Eur J
Immunol. 40:1809–1818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhong X, Gao W, Degauque N, Bai C, Lu Y,
Kenny J, Oukka M, Strom TB and Rothstein TL: Reciprocal generation
of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol.
37:2400–2404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhong X, Lau S, Bai C, Degauque N,
Holodick NE, Steven SJ, Tumang J, Gao W and Rothstein TL: A novel
subpopulation of B-1 cells is enriched with autoreactivity in
normal and lupus-prone mice. Arthritis Rheum. 60:3734–3743. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maseda D, Candando KM, Smith SH,
Kalampokis I, Weaver CT, Plevy SE, Poe JC and Tedder TF: Peritoneal
cavity regulatory B cells (B10 cells) modulate
IFN-γ+CD4+ T cell numbers during colitis
development in mice. J Immunol. 191:2780–2795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shao Y, Fu R, Liu H, Wang Y, Ding S, Wang
H, Li L and Shao Z: IgG autoantibody subclasses altered in
immuno-related hemocytopenia. Cell Immunol. 294:13–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu YY, Georg I, Díaz-Barreiro A, Varela N,
Lauwerys B, Kumar R, Bagavant H, Castillo-Martín M, El Salem F,
Marañón C and Alarcón-Riquelme ME: Concordance of increased B1 cell
subset and lupus phenotypes in mice and humans is dependent on BLK
expression levels. J Immunol. 194:5692–5702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deng J, Wang X, Chen Q, Sun X, Xiao F, Ko
KH, Zhang M and Lu L: B1a cells play a pathogenic role in the
development of autoimmune arthritis. Oncotarget. 7:19299–19311.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Flores-Borja F, Bosma A, Ng D, Reddy V,
Ehrenstein MR, Isenberg DA and Mauri C:
CD19+CD24hiCD38hi B cells maintain regulatory T cells
while limiting TH1 and TH17 differentiation. Sci Transl Med.
5:173ra232013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Blair PA, Noreña LY, Flores-Borja F,
Rawlings DJ, Isenberg DA, Ehrenstein MR and Mauri C:
CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in
healthy individuals but are functionally impaired in systemic Lupus
Erythematosus patients. Immunity. 32:129–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Georgin-Lavialle S, Aouba A, Mouthon L,
Londono-Vallejo JA, Lepelletier Y, Gabet AS and Hermine O: The
telomere/telomerase system in autoimmune and systemic
immune-mediated diseases. Autoimmun Rev. 9:646–651. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fujii H, Shao L, Colmegna I, Goronzy JJ
and Weyand CM: Telomerase insufficiency in rheumatoid arthritis.
Proc Natl Acad Sci USA. 106:4360–4365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hoffecker BM, Raffield LM, Kamen DL and
Nowling TK: Systemic lupus erythematosus and vitamin D deficiency
are associated with shorter telomere length among African
Americans: A case-control study. PLoS One. 8:e637252013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu K, Higashi N, Hansen ER, Lund M, Bang K
and Thestrup-Pedersen K: Telomerase activity is increased and
telomere length shortened in T cells from blood of patients with
atopic dermatitis and psoriasis. J Immunol. 165:4742–4747. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Son NH, Murray S, Yanovski J, Hodes RJ and
Weng N: Lineage-specific telomere shortening and unaltered capacity
for telomerase expression in human T and B lymphocytes with age. J
Immunol. 165:1191–1196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
King C, Tangye SG and Mackay CR: T
follicular helper (TFH) cells in normal and dysregulated immune
responses. Annu Rev Immunol. 26:741–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lüthje K, Kallies A, Shimohakamada Y, Belz
GT, Light A, Tarlinton DM and Nutt SL: The development and fate of
follicular helper T cells defined by an IL-21 reporter mouse. Nat
Immunol. 13:491–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jabeen R, Goswami R, Awe O, Kulkarni A,
Nguyen ET, Attenasio A, Walsh D, Olson MR, Kim MH, Tepper RS, et
al: Th9 cell development requires a BATF-regulated transcriptional
network. J Clin Invest. 123:4641–4653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Eyerich S, Eyerich K, Pennino D, Carbone
T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann
C, et al: Th22 cells represent a distinct human T cell subset
involved in epidermal immunity and remodeling. J Clin Invest.
119:3573–3585. 2009.PubMed/NCBI
|
|
61
|
Fallon PG, Ballantyne SJ, Mangan NE,
Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE and McKenzie
AN: Identification of an interleukin (IL)-25-dependent cell
population that provides IL-4, IL-5, and IL-13 at the onset of
helminth expulsion. J Exp Med. 203:1105–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|