Introduction
Brucellosis, caused by a facultative intracellular
parasite Brucella species, is the most common bacterial
zoonotic infection worldwide (1,2).
Brucella can survive and proliferate in several phagocytic and
non-phagocytic cell types, including macrophages, dendritic cells,
placental trophoblasts in vivo, and has the ability to
replicate in a variety of mammalian cells, such as microglia,
fibroblasts, epithelial and endothelial cells (3). In China, Brucellosis represents a
major challenge from the last decade, and a high incidence of human
brucellosis has been reported in northwestern China (4). Human brucellosis has similar clinical
symptoms with systemic diseases and can lead to delay in diagnosis
and may increase unexpected complications (5). Therefore, investigating the
proliferation of Brucella in host cells is important to
understand the pathogenesis of the disease. In addition, earlier
evidence indicates that Brucella inhibits apoptosis of host
cells to maintain an intracellular niche for its replication
(6). Therefore, induction of
apoptosis in the host cells may provide a useful therapeutic
strategy for the treatment of Brucellosis.
Dihydroartemisinin (DHA), a semi-synthetic
derivative of artemisin, has been recommended by World Health
Organization as an anti-malarial drug (7). Previous studies have documented that
DHA possesses the ability to prevent angiogenesis (8), cause cell cycle arrest (9), promote apoptosis, and inhibit cancer
invasion (10). However, there
have been few studies regarding its effectiveness against
bacteria.
In the present study, it was investigated whether
DHA inhibited the intracellular growth of Brucella suis
vaccine strain 2 (B. suis S2) in murine microglia BV2 cells,
and whether the inhibitory effect of DHA was associated with
stimulation of apoptosis of BV2 cells.
Materials and methods
Cell line and bacteria
Murine microglia BV2 cells were obtained from China
Center for Type Culture Collection and cultured with Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences), 2 mM glutamine, and 200 mM
streptomycin/penicillin and maintained in 5% CO2 at
37°C. B. suis S2 was provided by Professor Xu from Ningxia
Medical University (Yinchuan, China) and was grown in
Soybean-Casein Digest Agar Medium (TSA) at 37°C in a 5%
CO2 incubator. The single B. suis S2 colony was
inoculated in sterilized tryptic soy broth (TSB) solution at 37°C
in a 5% CO2 atmosphere until its use. Bacteria were
harvested by centrifugation for 20 min at 2,000 × g at 4°C and
washed twice with phosphate-buffered saline (PBS). Bacterial
density in the cultures were estimated by the McFarland standards
of bioMérieux, Inc. All bacterial experiments were carried out at
the Biosafety Level 2 Laboratory (Key Laboratory of Pathogenic
Microorganisms, The general hospital of Ningxia Medical
University).
In vitro infection and DHA
treatment
A total of 2×105 BV2 cells were seeded in
6-well culture plates. After reaching 60% confluence, the cells
were exposed to B. suis S2 at a multiplicity of infection
(MOI) of 100 for 2 h in medium without antibiotics. Subsequently,
BV2 cells were extensively washed to remove extracellular bacteria,
and infection was maintained for 24 h in the presence of 100 µg/ml
gentamicin to kill remaining extracellular bacteria (11). Two hours after infection, the cells
were treated with different concentrations of DHA (0, 10, 20, 30
and 40 µM).
Colony-forming unit assay (CFU
assay)
The TSA was prepared according to the manufacturer's
instructions and autoclaved at 120°C for 30 min. On a clean bench,
~25 ml of TSA was poured into a 90-mm2 sterile culture
plate, inverted after coagulation, and used for bacterial culture.
BV2 cells were infected with B. suis S2 at MOI 100 for 24 h,
washed with PBS twice and lysed in 0.3% Triton-X100 for 10 min. The
sample was inoculated on TSA media for 72 h, and the cell
suspension was diluted to count the number of colonies. The visible
colonies with a diameter greater than 1 mm were manually counted
with an optical microscope.
Cell viability assay
Cell viability was determined by Cell-counting Kit-8
(CCK-8; BestBio) assay according to the manufacturer's protocol
(12). In brief, cells
(1×104) were seeded in 96-well culture plates and
cultured overnight. The cells were treated with different
concentrations of DHA (0, 10, 20, 40 and 80 µM) for 24 h, and the
CCK-8 solution was added to each well and incubated for an
additional 4 h. The absorbance at 450 nm was measured by Microplate
reader (BioTek Instruments, Inc.) (13).
Lactate dehydrogenase (LDH) assay
The toxicity of BV2 cells after B. suis S2
infection was determined by the LDH assay using a commercially
available kit (Sigma-Aldrich; Merck KGaA) (14). Briefly, the BV2 cells were infected
with B. suis S2 at MOI 100 or exposure to different
concentrations of DHA (0, 10, 20, 30 and 40 µM) for 24 h. Finally,
the supernatant was collected for the LDH assay.
Flow cytometric assay
The BV2 cells were infected with B. suis S2
and then stained with Annexin V/PI according to manufacturer's
protocol (BestBio). The apoptosis in cells was analyzed by flow
cytometric analysis (FCM).
Western blot analysis
Cells were harvested, washed twice with PBS, and
lysed in RIPA buffer (Nanjing KeyGen Biotech Co., Ltd.). Protein
concentration in the lysates was determined by the bicinchoninic
acid (BCA) method according to the manufacturer's recommendations
(Nanjing KeyGen Biotech Co., Ltd.). Protein (30 µg) was separated
on 12% Mini-Protean TGX gels and subsequently transferred onto a
polyvinylidene difluoride (PVDF) membrane according to standard
protocols. Caspase-3 (ID product code ab184787; dilution in
1:1,000), cleaved caspase-3 (ID product code ab49822; dilution in
1:500) or GAPDH (ID product code ab181602; dilution in 1:5,000; All
from Abcam) antibodies were used for protein detection. Goat
anti-mouse polyclonal antibody (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.; OriGene Technologies; ZB-2305; dilution
1:5,000) was used as a secondary antibody. The blot was developed
using the Western Lightning Ultra chemiluminescent substrate from
Bio-Rad Laboratories, Inc., and detected using EpiChemi3 darkroom
(UVP BioImaging Systems).
Antimicrobial susceptibility
testing
The single clone was added to 5 ml TSB, incubated in
a 37°C incubator for 24 h, and the concentration of the bacteria
suspensions was adjusted to ~108 CFU/ml with TSB. TSB
(200 µl) and bacteria suspensions were used as a negative control
and positive control, respectively. After loading the drug and
bacteriostatic solution, the plate was incubated at 37°C in an
incubator for 24 h, and the absorbance at 580 nm was measured
(15).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD) from at least three independent experiments.
GraphPad Prism 7.0 (GraphPad Software) was used for statistical
analysis. Statistical differences between indicated groups were
performed using one-way analysis of variance (ANOVA) followed by
post hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Replication of B. suis S2 in murine
microglia BV2 cells
In order to investigate the growth of B. suis
S2 in BV2 cells, BV2 cells were infected in vitro with MOI
100. It was revealed that there was a significant intracellular
increase in CFUs 24 h post-infection (Fig. 1A), indicating the active growth of
B. suis S2 in the murine microglial cells. In addition,
there was no apoptosis of BV2 cells under light microscopy (data
not shown) after bacterial infection, and the levels of LDH were
not significantly altered 24 h post-infection (Fig. 1B).
Inhibition of caspase-dependent
apoptosis after B. suis S2 infection
Western blot analysis revealed that the expression
of cleaved caspase-3 was significantly decreased after B.
suis S2 infection, but gradually increased as the infection
period increased (Fig. 2). The
present data indicated that Brucella may inhibit apoptosis
of host cells for the infection.
Effects of DHA on BV2 cell
viability
To observe the biological effects of DHA on BV2
cells, the cells were treated with different concentrations of DHA
for 24 h. The results revealed that concentrations <40 µM DHA
exhibited no inhibitory effects on cell viability (Fig. 3A). Moreover, cells were treated
with 20 µM DHA for various intervals, and no significant changes in
cell viability were observed (Fig.
3B). The LDH assay demonstrated that a dose of DHA <20 µM
had no inhibitory effects on BV2 cells (Fig. 3B).
DHA reduces B. suis S2 growth in BV2
cells
The present data revealed that intracellular growth
of B. suis S2 could inhibit cell apoptosis of BV2 cells
(unpublished data). Furthermore, CFU assay results of the treated
BV2-infected cells with 10–40 µM DHA revealed a significant and
marked reduction in the B. suis S2 growth in the BV2 cells
(Fig. 4).
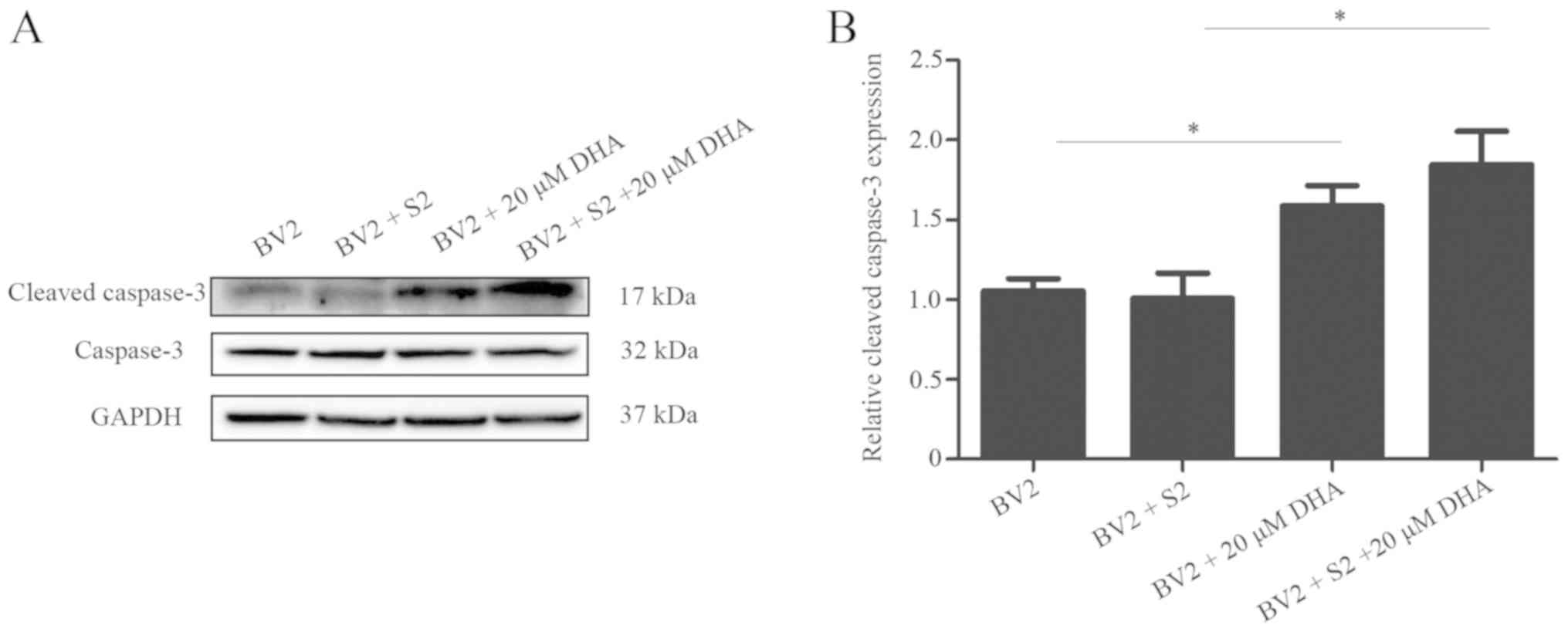
DHA (20 µM) inhibits bacterial
multiplication by inducing apoptosis
The effects of DHA on B. suis S2
multiplication in BV2 cells were analyzed by the flow cytometric
assay. The results revealed that 20 µM DHA treatment increased the
level of apoptosis in B. suis S2-infected BV2 cells
(Fig. 5). Furthermore, western
blot analysis revealed that 20 µM of DHA treatment increased the
expression of cleaved caspase-3 in B. suis S2-infected BV2
cells (Fig. 6). The present data
indicated that DHA inhibited bacterial multiplication through
induction of apoptosis in BV2 cells.
DHA does not directly inhibit or kill
B. suis S2
To confirm whether DHA affects B. suis S2
growth directly or indirectly through apoptosis, an antibacterial
susceptibility test was performed. The results revealed that
different concentrations of DHA had no effect on bacterial growth
(Fig. 7), indicating that DHA does
not directly inhibit or kill B. suis S2. DHA did not exhibit
any direct effects on bacterial growth, which supports our theory
that the mechanism involved in the reduction of intracellular
replication of BV2 cells by DHA may be achieved by promoting
apoptosis of BV2 cells.
Discussion
Brucella is an intracellular bacterium that
can cause chronic infections. It has been reported that infection
of Brucella inhibits apoptosis of host cells (16,17).
Therefore, it is necessary to investigate the mechanism underlying
infection and develop therapeutic strategies for Brucellosis.
In the present study, it was demonstrated that B.
suis S2 grew in BV2 cells without significant cytotoxicity,
which is consistent with the characteristics of intracellular
parasites. Previous studies revealed that Brucella outer
membrane protein OMP25 inhibited apoptosis of host cells (6). Similarly, this study confirmed that
the caspase-dependent apoptotic pathway of BV2 cells was
significantly inhibited after B. suis S2 infection, which
provides a favorable condition for the intracellular replication of
B. suis S2. This result is consistent with our findings on
the mechanism of sustained infection for intracellular parasites,
such as Mycobacterium tuberculosis (18).
Apoptosis, programmed cell death, is indispensable
for the development and homeostasis of multicellular organisms
(19). Caspase-3 is one of the
activated caspases that catalyzes the cleavage of many key cellular
proteins during apoptosis (20).
In the present study, western blot analysis revealed that the
expression of cleaved caspase-3 was significantly decreased in BV2
cells after B. suis S2 infection, but gradually increased as
the infection period increased. These observations indicated that
Brucella may inhibit apoptosis of host cells for the
infection. The present results are in agreement with the findings
of Brucella in other types of cells (21). Collectively, these data indicated
that inhibition of host cell apoptosis may be a common strategy for
Brucella-sustained infection. The underlying mechanisms of
the DHA effect on bacteria remain unknown. Some studies suggest
that the strategy of Brucella for infection establishment
includes the following: i) to avoid intracellular destruction by
limiting the fusion of the type IV secretion system-dependent
vacuole-containing vacuoles to lysosomes; ii) inhibiting apoptosis
of infected monocytes; and iii) inhibition of dendritic cell
maturation, inhibition of antigen presentation and activation of
naive T cells (22). It is our aim
to investigate the mechanisms in our future studies.
Conversely, DHA displays potent anti-viral and
anti-parasitic activities (23,24),
however, the molecular mechanisms are not fully understood yet.
Artemisinin was also effective on bacteria. For example, oral
administration of artesunate was revealed to reduce death from
sepsis caused by E. coli (25,26).
In combination with antibiotic oxacillin, oral administration of
artesunate revealed a high effectiveness against mouse sepsis
induced by methicillin-resistant aureus (MRSA). On the basis of
previous findings, the inhibitory effects of DHA were evaluated on
B. suis S2 in vitro. It was revealed that DHA
inhibited B. suis S2 growth in BV2 cells. Furthermore, DHA
increased the percentage of apoptosis and the
expression/proteolytic degradation of caspase-3 in B. suis
S2-infected cells. It is therefore concluded that DHA induces
caspase-dependent apoptotic pathway activation to inhibit
intracellular B. suis S2 growth.
Brucella is an intracellular parasitic
bacterium. In the present study, BV2 cells were infected with B.
suis S2, and CFU experiments revealed that B. suis S2
grows in BV2 cells. After treatment with DHA, apoptosis of BV2 cell
was increased. Additional experiments demonstrated that DHA had no
direct effects on B. suis S2, which is similar to the effect
of antibiotics on bacteria. Therefore, it is concluded that the
mechanism involved in the reduction of intracellular replication of
BV2 cells by DHA may be achieved by promoting apoptosis of BV2
cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Foundation of China (grant no. 31660030) and the
First-Class Discipline Construction Project in Colleges and
Universities of Ningxia (grant no. NXYLXK2017A05).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY, HL, ZhaW and ZheW conceived and designed the
experiments. JY, HL, ZhaW, LY and QL conducted all the experiments.
LY, QL, XN and TX contributed reagents, materials and analysis
tools. XN and TX performed the experiments and were involved in the
preliminary work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li YJ, Li XL, Liang S, Fang LQ and Cao WC:
Epidemiological features and risk factors associated with the
spatial and temporal distribution of human brucellosis in China.
BMC Infect Dis. 13:5472013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanodze L, Bautista CT, Garuchava N,
Chubinidze S, Tsertsvadze E, Broladze M, Chitadze N, Sidamonidze K,
Tsanava S, Akhvlediani T, et al: Expansion of brucellosis detection
in the country of Georgia by screening household members of cases
and neighboring community members. BMC Public Health. 15:4592015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tuon FF, Gondolfo RB and Cerchiari N:
Human-to-human transmission of Brucella-a systematic review. Trop
Med Int Health. 22:539–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng R, Xie S, Lu X, Sun L, Zhou Y, Zhang
Y and Wang K: A systematic review and meta-analysis of epidemiology
and clinical manifestations of human brucellosis in China. Biomed
Res Int. 2018:57129202018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elfaki MG, Alaidan AA and Al-Hokail AA:
Host response to Brucella infection: Review and future perspective.
J Infect Dev Ctries. 9:697–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma QL, Liu AC, Ma XJ, Wang YB, Hou YT and
Wang ZH: Brucella outer membrane protein Omp25 induces microglial
cells in vitro to secrete inflammatory cytokines and inhibit
apoptosis. Int J Clin Exp Med. 8:17530–17535. 2015.PubMed/NCBI
|
|
7
|
Zhang XG, Li GX, Zhao SS, Xu FL, Wang YH
and Wang W: A review of dihydroartemisinin as another gift from
traditional Chinese medicine not only for malaria control but also
for schistosomiasis control. Parasitol Res. 113:1769–1773. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong F, Zhou X, Li C, Yan S, Deng X, Cao
Z, Li L, Tang B, Allen TD and Liu J: Dihydroartemisinin targets
VEGFR2 via the NF-κB pathway in endothelial cells to inhibit
angiogenesis. Cancer Biol Ther. 15:1479–1488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Guo L, Zhou X, Dong F, Li L,
Cheng Z, Xu Y, Liang J, Xie Q and Liu J: Dihydroartemisinin induces
endothelial cell anoikis through the activation of the JNK
signaling pathway. Oncol Lett. 12:1896–1900. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Im E, Yeo C, Lee HJ and Lee EO:
Dihydroartemisinin induced caspase-dependent apoptosis through
inhibiting the specificity protein 1 pathway in hepatocellular
carcinoma SK-Hep-1 cells. Life Sci. 192:286–292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Lin P, Li Y, Xiang C, Yin Y, Chen
Z, Du Y, Zhou D, Jin Y and Wang A: Brucella suis vaccine strain 2
induces endoplasmic reticulum stress that affects intracellular
replication in goat trophoblast cells in vitro. Front Cell Infect
Microbiol. 6:192016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Yu X, Guo H, Sun L, Wang A, Liu Q,
Wang X and Li J: Bufalin exerts antitumor effects by inducing cell
cycle arrest and triggering apoptosis in pancreatic cancer cells.
Tumour Biol. 35:2461–2471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hang R, Zhang M, Ma S and Chu PK:
Biological response of endothelial cells to diamond-like
carbon-coated NiTi alloy. J Biomed Mater Res A. 100:496–506. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zahedifard M, Faraj FL, Paydar M, Yeng
Looi C, Hajrezaei M, Hasanpourghadi M, Kamalidehghan B, Abdul Majid
N, Mohd Ali H and Ameen Abdulla M: Synthesis, characterization and
apoptotic activity of quinazolinone Schiff base derivatives toward
MCF-7 cells via intrinsic and extrinsic apoptosis pathways. Sci
Rep. 5:115442015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Z, Xu X, Zhao S, Yang X, Guo J, Zhang
Q, Jing C, Chen S and He Y: Total synthesis and antimicrobial
evaluation of natural albomycins against clinical pathogens. Nat
Commun. 9:34452018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pujol M, Castillo F, Alvarez C, Rojas C,
Borie C, Ferreira A and Vernal R: Variability in the response of
canine and human dendritic cells stimulated with Brucella canis.
Vet Res. 48:722017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller CN, Smith EP, Cundiff JA, Knodler
LA, Bailey Blackburn J, Lupashin V and Celli J: A brucella type IV
effector targets the COG tethering complex to remodel host
secretory traffic and promote intracellular replication. Cell Host
Microbe. 22:317.e7–329.e7. 2017. View Article : Google Scholar
|
|
18
|
Fan L, Wu X, Jin C, Li F, Xiong S and Dong
Y: MptpB promotes mycobacteria survival by inhibiting the
expression of inflammatory mediators and cell apoptosis in
macrophages. Front Cell Infect Microbiol. 8:1712018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kopeina GS, Prokhorova EA, Lavrik IN and
Zhivotovsky B: Alterations in the nucleocytoplasmic transport in
apoptosis: Caspases lead the way. Cell Prolif. 51:e124672018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Julien O, Zhuang M, Wiita AP, O'Donoghue
AJ, Knudsen GM, Craik CS and Wells JA: Quantitative MS-based
enzymology of caspases reveals distinct protein substrate
specificities, hierarchies, and cellular roles. Proc Natl Acad Sci
USA. 113:E2001–E2010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui G, Wei P, Zhao Y, Guan Z, Yang L, Sun
W, Wang S and Peng Q: Brucella infection inhibits macrophages
apoptosis via Nedd4-dependent degradation of calpain2. Vet
Microbiol. 174:195–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Figueiredo P, Ficht TA, Rice-Ficht A,
Rossetti CA and Adams LG: Pathogenesis and immunobiology of
brucellosis: Review of Brucella-host interactions. Am J Pathol.
185:1505–1517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Efferth T, Romero MR, Wolf DG, Stamminger
T, Marin JJ and Marschall M: The antiviral activities of
artemisinin and artesunate. Clin Infect Dis. 47:804–811. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lam NS, Long X, Su XZ and Lu F:
Artemisinin and its derivatives in treating helminthic infections
beyond schistosomiasis. Pharmacol Res. 133:77–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Zhou H, Zheng J, Cheng J, Liu W,
Ding G, Wang L, Luo P, Lu Y, Cao H, et al: The antimalarial
artemisinin synergizes with antibiotics to protect against lethal
live Escherichia coli challenge by decreasing proinflammatory
cytokine release. Antimicrob Agents Chemother. 50:2420–2427. 2016.
View Article : Google Scholar
|
|
26
|
Jiang W, Li B, Zheng X, Liu X, Cen Y, Li
J, Pan X, Cao H, Zheng J and Zhou H: Artesunate in combination with
oxacillin protect sepsis model mice challenged with lethal live
methicillin-resistant Staphylococcus aureus (MRSA) via its
inhibition on proinflammatory cytokines release and enhancement on
antibacterial activity of oxacillin. Int Immunopharmacol.
11:1065–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|