Introduction
Hypertension, defined as systolic blood pressure
(BP) ≥140 mmHg or diastolic BP ≥90 mmHg, has been a longstanding
and common chronic disease in modern society due to the
improvements in people's living standards and changes in their
lifestyles (1). Sustained
hypertension is an important risk factor for the development of
cardiovascular disorders, such as transient ischemic attacks or
stroke (2–4), which is the leading cause of death
worldwide (5,6), imposing a heavy economic burden on
family and society (7). Therefore,
how to prevent hypertension-associated stroke has become a major
public health problem.
Spontaneously hypertensive rats (SHR) and
stroke-prone spontaneously hypertensive rats (SHRSP) are widely
used animal models for studying the molecular mechanisms of severe
hypertension and associated stroke (8) to provide potential therapeutic
strategies (9). Previous studies
have utilized high-throughput microarray technology to investigate
gene expression in the brain (10), adrenal gland (11), kidneys (12), mesenteric artery (13) and liver (14) of SHR and SHRSP rats compared with
the normotensive control strain, Wistar-Kyoto (WKY) rats. In these
studies, obvious overlaps were identified in different tissue
samples as SHRSP-specific genes, including angiotensinogen (Agt),
which was found to be crucial for SHRSP in the adrenal gland
(11) and kidneys (12), and angiotensin II
receptor-associated protein (Agtrap), which was found to be crucial
for SHRSP in the brain (10) and
kidneys (12). These analyses
indicated the common genes that may be underlying targets for
treatment of hypertension-associated stroke. However, to the best
of the authors' knowledge, no studies have been performed to date
to investigate the shared genes in all studied samples
previously.
Furthermore, increasing evidence has reported that
microRNAs (miRNAs), small non-coding RNA molecules of 18–25
nucleotides in length, are also essential in the development of
hypertension and associated stroke. They function by negatively
regulating the expression of their target genes at the
post-transcriptional level. For example, Rubattu et al
(15) demonstrated that uncoupling
protein 2 (UCP2)-targeted rno-miR-503 was significantly upregulated
in the brain of SHRSP rats. Downregulation of miR-503 level
protected SHRSP from stroke occurrence. In vitro
overexpression of miRNA-503 in endothelial cells suppressed UCP2
expression and led to a significant increase in cell mortality,
with decreased cell viability (15). Matsuoka et al (16) observed that the miR-124 level was
markedly lower in brains of SHRSP compared with WKY rats, whereas
claudin domain-containing 1 (Cldnd1) mRNA and protein levels were
significantly higher. Human brain endothelial cells transfected
with a miR-124 mimic exhibited a significantly decreased mRNA
expression level of Cldnd1 (16).
However, the mechanism underpinning miRNA-mRNA interaction for
SHRSP rats has yet to be fully elucidated (13).
The present study aimed to further screen for
crucial miRNA-mRNA interactions in order to explain the etiology of
SHRSP, and to explore novel treatment modalities by integrating all
the mRNA and miRNA expression profiles associated with SHRSP that
were obtained from the public databases using a serial of
bioinformatic methods.
Materials and methods
Collection of microarray data
The microarray datasets of SHRSP were available at
the National Center for Biotechnology Information (NCBI) Gene
Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). The GSE41452
(10), GSE31457 (11) and GSE41453 datasets (12) were analyzed by the research team of
Watanabe et al (12) to
respectively investigate the mRNA expression profiles in whole
brains, adrenal glands and kidneys of 3 rat strains: Normotensive
WKY, SHR, and SHRSP, at 3 and 6 weeks of age. GSE53363 and GSE53361
datasets were provided by Palao et al (13) to respectively analyze the mRNA and
miRNA expression profiles in mesenteric arteries of normotensive
WKY (including 3 sublines, WKY/NCrl, WKY/NHsd, WKY/NTac), SHR
(including 2 sublines, SHR/NCrL and SHR/NHsd) and SHRSP rats at 6
weeks and 5 months of age. The characteristics of the microarray
datasets are shown in Table I.
 | Table I.Microarray datasets collected from
the GEO database. |
Table I.
Microarray datasets collected from
the GEO database.
|
|
|
|
| Sample size |
|
|---|
|
|
|
|
|
|
|
|---|
| Accession
number | Type |
| Platform | WKY | SHR | SHRSP | Tissue source |
|---|
| GSE41452 | mRNA | GPL14745 | Agilent-028282
Whole Rat Genome microarray 4×44K v3 | 6 | 6 | 6 | Whole brains |
| GSE31457 | mRNA | GPL14745 | Agilent-028282
Whole Rat Genome microarray 4×44K v3 | 6 | 6 | 6 | Adrenal glands |
| GSE41453 | mRNA | GPL14745 | Agilent-028282
Whole Rat Genome microarray 4×44K v3 | 6 | 6 | 6 | Kidneys |
| GSE53363 | mRNA | GPL15084 | Agilent-028279
SurePrint G3 Rat GE 8×60K microarray | 6 | 4 | 2 | Mesenteric
artery |
| GSE53361 | miRNA | GPL18115 | Agilent-046066
Unrestricted Rat miRNA V19.0 | 6 | 6 | 4 | Mesenteric
artery |
Differential expression analysis
The normalized series matrix files of each dataset
were downloaded from GEO. Data from the GSE41452, GSE31457 and
GSE41453 datasets were merged into one in order to use the same
model samples. The series matrix data were extracted and
quantile-normalized using the Linear Models for Microarray Data
(Limma) package (version 3.38.3; http://bioconductor.org/packages/release/bioc/html/limma.html)
in R (version 3.5.2; http://www.R-project.org/) (17). The differentially expressed genes
(DEGs) and miRNAs (DEMs) between SHR (or SHRSP) and WKY were
identified using the Limma empirical Bayes analysis pipeline.
P-values were adjusted to the false discovery rate (FDR) using
Benjamini and Hochberg multiple testing (18). DEGs and DEMs were defined as |log2
fold change (FC)|>0.5 and P<0.05, since the number of DEGs
and DEMs would be fewer if FDR were to be considered, which may
have been disadvantageous in terms of performing the following
analyses. Hierarchical clustering of DEGs and DEMs, with results
visualized as heat-maps, was performed using the pheatmap package
(version 1.0.8; http://cran.r-project.org/web/packages/pheatmap)
based on Euclidean distances. Venn diagrams (http://bioinformatics.psb.ugent.be/webtools/Venn/)
were drawn to obtain the DEGs that were SHR-specific,
SHRSP-specific and SHR-SHRSP shared, respectively, in two datasets
(GSE41452-GSE31457-GSE41453 and GSE53363), as well as genes common
to both datasets.
Protein-protein interaction (PPI)
network
All genes for SHR-specific, SHRSP-specific and
SHR-SHRSP shared in the two datasets were used for constructing the
PPI network to reveal the possible interaction mechanisms of common
DEGs. Underlying PPI associations were collected from the Search
Tool for the Retrieval of Interacting Genes database, version 10.0
(STRING; http://string db.org/) (19), which is a comprehensive database
that provides >200 million interactions among 5 million
proteins, including experimental, predicted, transferred and
text-mining interactions. Only interaction pairs with a combined
score >0.4 (medium confidence) were used to establish the PPI
network, and this was accomplished using Cytoscape software
(version 3.4.0; www.cytoscape.org/) (20).
miRNA-target gene regulatory
network
The target genes of DEMs were predicted using the
miRwalk database (version 2.0; http://www.zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2)
(21), which is a comprehensive
database for integrating the predicted results of 12 algorithms
(miRWalk, Microt4, miRanda, mirbridge, miRDB, miRMap, miRNAMap,
Pictar2, PITA, RNA22, RNAhybrid and Targetscan). Subsequently, the
target genes of DEMs that were predicted by at least 5 of the
databases were overlapped with the common DEGs in two datasets to
obtain potentially new negative expression associations between
DEMs and DEGs, and this procedure was followed to construct the
miRNA-target gene regulatory network using Cytoscape software
(20).
Function enrichment analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway and Gene Ontology (GO) [including the categories of
‘biological process’ (BP), ‘molecular function’ (MF) and ‘cellular
component’ (CC)] enrichment analyses were performed to explore the
underlying functions of the DEGs in the SHR-specific,
SHRSP-specific and SHR-SHRSP shared PPI networks, as well as the
miRNA-mRNA network, The Database for Annotation, Visualization and
Integrated Discovery (DAVID) (version 6.8; http://david.abcc.ncifcrf.gov) (22). P<0.05 was considered to indicate
a statistically significant value.
Screening of small-molecule drugs
To better identify potential therapeutic drugs for
treatment of SHRSP, the common DEGs (upregulated or downregulated)
in two datasets were queried using the Connectivity Map online tool
(version 2.0; http://portals.broadinstitute.org/cmap/) (23). The query small molecules were
output with a connectivity score from +1 to −1. Small-molecule
drugs may be therapeutic when their connectivity scores are near to
−1; by contrast, small-molecule drugs may actually induce the
disease if their connectivity scores are near to +1. Candidate
small-molecule drugs were identified with a P-value <0.05 and a
|connectivity score|≥0.8.
Results
Differential expression analysis
In the merged dataset (GSE31457-GSE41452-GSE41453),
a total of 366 DEGs were identified between SHR and WHY rats,
including 177 upregulated and 189 downregulated DEGs. Three hundred
and ninety-three DEGs were screened between SHRSP and WHY,
including 151 upregulated and 242 downregulated DEGs. Venn diagrams
revealed that 75 upregulated DEGs were SHR-specific, 49 were
SHRSP-specific, and 102 were SHR-SHRSP shared; for the
downregulated DEGs, 44 were SHR-specific, 97 were SHRSP-specific,
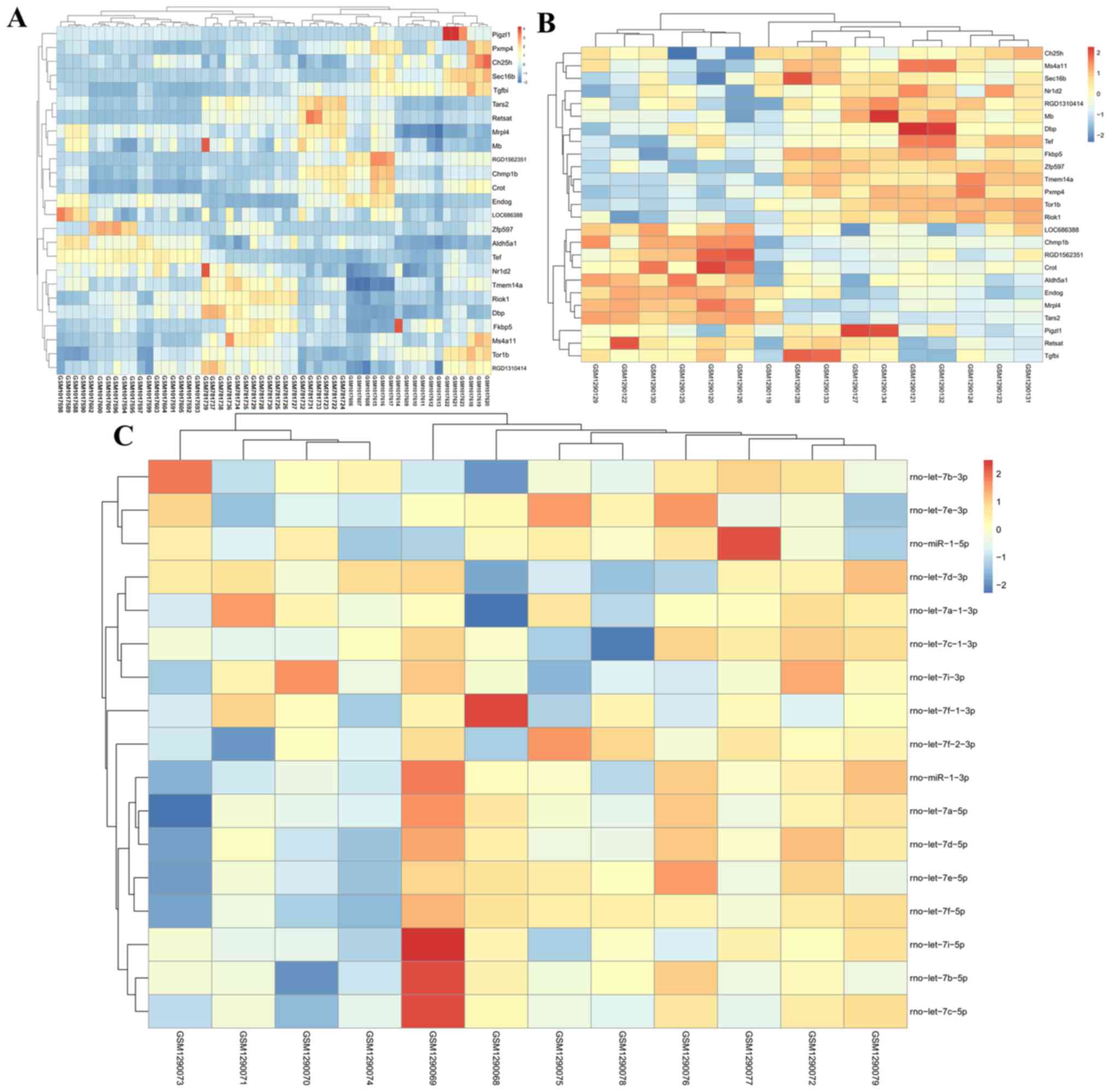
and 145 were SHR-SHRSP shared (Fig.
1A).
In the GSE53363 dataset, a total of 246 DEGs were
identified between SHR and WHY, including 145 upregulated and 101
downregulated DEGs, whereas 480 DEGs were screened between SHRSP
and WHY, including 300 upregulated and 180 downregulated DEGs. Venn
diagrams revealed that 55 upregulated DEGs were SHR-specific, 210
were SHRSP-specific, and 90 were SHR-SHRSP shared; for the
downregulated DEGs, 44 were SHR-specific, 123 were SHRSP-specific
and 57 were SHR-SHRSP shared (Fig.
1A).
In the GSE53361 dataset, a total of 11 DEMs were
identified between SHR and WHY, including 5 upregulated and 6
downregulated DEMs; 13 DEMs were screened between SHRSP and WHY,
including 6 upregulated and 7 downregulated DEMS (Table II). Venn diagrams revealed that 2
upregulated DEMs were SHR-specific (rno-miR-146b-5p and
rno-miR-132-3p), whereas 3 were SHRSP-specific (rno-miR-196b-5p,
rno-miR-21-5p and rno-miR-196a-5p) and 3 were SHR-SHRSP shared
(rno-miR-31a-5p, rno-miR-31a-3p and rno-miR-511-3p). For the
downregulated DEMs, 2 were SHR-specific (rno-miR-3593-3p and
rno-miR-150-3p), 3 were SHRSP-specific (rno-miR-126a-5p,
rno-miR-126a-3p and rno-miR-483-3p), and 4 were SHR-SHRSP shared
(rno-miR-1224, rno-miR-146a-5p, rno-miR-672-5p and rno-miR-150-5p)
(Fig. 1A). Among these DEMs,
rno-miR-31a-3p and rno-miR-31a-5p may be especially important,
since their FDR was <0.05 (Table
II).
 | Table II.Differentially expressed miRNAs
comparing between SHR (or SHRSP) and WKY rats. |
Table II.
Differentially expressed miRNAs
comparing between SHR (or SHRSP) and WKY rats.
| A, SHR vs. WKY |
|---|
|
|---|
|
| SHR vs. WKY |
|---|
|
|
|
|---|
| miRNA | logFC | P-value | FDR |
|---|
| rno-miR-31a-3p | 1.66 |
3.99E-06 |
2.87E-03 |
| rno-miR-31a-5p | 2.35 |
4.15E-05 |
1.49E-02 |
| rno-miR-150-5p | −0.51 |
2.87E-04 |
6.88E−02 |
| rno-miR-511-3p | 0.77 |
6.86E-04 |
9.87E−02 |
|
rno-miR-146b-5p | 0.84 |
5.78E-03 |
6.92E−01 |
|
rno-miR-146a-5p | −0.56 |
8.54E-03 |
7.62E−01 |
| rno-miR-672-5p | −0.74 |
1.67E-02 |
7.62E−01 |
| rno-miR-132-3p | 0.76 |
1.70E-02 |
7.62E−01 |
| rno-miR-150-3p | −0.68 |
2.63E-02 |
8.42E−01 |
|
rno-miR-3593-3p | −0.56 |
3.44E-02 |
8.42E−01 |
| rno-miR-1224 | −0.89 |
3.54E-02 |
8.42E−01 |
|
| B, SHRSP vs.
WKY |
|
|
| SHRSP vs.
WKY |
|
|
|
| miRNA | logFC | P-value | FDR |
|
| rno-miR-31a-3p | 1.65 |
1.27E-03 | 4.33
E−01 |
|
rno-miR-126a-5p | −0.58 |
1.91E-03 | 4.33
E−01 |
|
rno-miR-196a-5p | 2.11 |
2.52E-03 | 4.33
E−01 |
|
rno-miR-146a-5p | −0.85 |
2.98E-03 | 4.33
E−01 |
| rno-miR-150-5p | −0.51 |
3.05E-03 | 4.33
E−01 |
| rno-miR-31a-5p | 2.12 |
3.61E-03 | 4.33
E−01 |
|
rno-miR-126a-3p | −0.53 |
1.57E-02 |
1.00E−01 |
| rno-miR-511-3p | 0.73 |
2.10E-02 |
1.00E−01 |
| rno-miR-21-5p | 0.70 |
2.39E-02 |
1.00E−01 |
| rno-miR-1224 | −1.43 |
2.57E-02 |
1.00E−01 |
| rno-miR-672-5p | −1.01 |
2.60E-02 |
1.00E−01 |
|
rno-miR-196b-5p | 1.98 |
2.76E-02 |
1.00E−01 |
| rno-miR-483-3p | −0.66 |
4.14E-02 |
1.00E−01 |
Subsequently, the merged and GSE53363 datasets were
compared. This analysis identified 2 SHR-specific DEGs [cholesterol
25-hydroxylase (Ch25h) and SEC16 homolog B, endoplasmic reticulum
export factor (Sec16b)], 8 SHRSP-specific DEGs (7 with consistent
expression in the two datasets {RGD1310414, phosphatidylinositol
glycan anchor biosynthesis, class Z (Pigzl1), nuclear receptor
subfamily 1, group D, member 2 (Nr1d2), Tef, LOC686388, D-box
binding PAR bZIP transcription factor (Dbp) and transforming growth
factor β induced (Tgfbi); and one gene with differing expression
[Myoglobin (Mb)]} and 15 SHR-SHRSP shared DEGs (14 with consistent
expression between the two datasets: (aldehyde dehydrogenase 5
family, member A1 (Aldh5a1), transmembrane protein 243
(RGD1562351), zinc finger protein 597 (Zfp597), endonuclease G
(Endog), membrane-spanning 4-domains A6A (Ms4a11), torsin family 1
member B (Tor1b), retinol saturase (Retsat), carnitine
O-octanoyltransferase (Crot), FKBP prolyl isomerase (5Fkbp5),
charged multivesicular body protein 1B (Chmp1b), mitochondrial
ribosomal protein L4 (Mrpl4), threonyl-tRNA synthetase 2,
mitochondrial (Tars2), RIO kinase 1 (Riok1) and transmembrane
protein 14A (Tmem14a); and one gene with differing expression
[Peroxisomal membrane protein 4 (Pxmp4)]} that were common in these
two datasets (Fig. 1B; Table III). Among these DEGs, Tor1b and
Mrpl4 may be especially important, since their FDR was <0.05 in
all datasets as well as SHR and SHR-SP (Table III).
 | Table III.Differentially expressed genes
between SHR (or SHRSP) and WKY. |
Table III.
Differentially expressed genes
between SHR (or SHRSP) and WKY.
|
|
|
GSE31457-GSE41452-GSE41453 | GSE53363 |
|---|
|
|
|
|
|
|---|
|
| Gene | logFC | P-value | FDR | logFC | P-value | FDR |
|---|
| SHR-specific | Ch25h | 0.51 |
4.25E-03 |
3.40E−01 | 0.62 |
6.53E-03 |
3.82E−01 |
|
| Sec16b | 0.67 |
2.61E-02 |
9.16E−01 | 0.50 |
5.58E-03 |
3.71E−01 |
| SHRSP-specific | RGD1310414 | 0.57 |
1.44E-06 |
8.96E-04 | 0.53 |
2.70E-03 |
1.64E−01 |
|
| Nr1d2 | 0.58 |
6.35E-04 |
1.10E−01 | 0.54 |
2.69E-03 |
1.64E−01 |
|
| Dbp | 0.97 |
1.15E-03 |
1.63E−01 | 0.87 |
4.76E-03 |
2.05E−01 |
|
| LOC686388 | −0.53 |
1.41E-03 |
1.81E−01 | −0.60 |
6.61E-04 |
8.71E−02 |
|
| Mb | −0.53 |
3.18E-03 |
3.05E−01 | 0.54 |
4.04E-04 |
7.10E−02 |
|
| Pigzl1 | 1.23 |
4.50E-02 |
1.00E−01 | 0.63 |
3.13E-02 |
4.07E−01 |
|
| Tgfbi | −0.57 |
4.66E-02 |
1.00E−01 | −0.62 |
2.09E-02 |
3.59E−01 |
|
| Tef | 0.822 |
4.84E-02 |
1.00E−01 | 0.74 |
1.53E-02 |
3.20E−01 |
| SHR-SHRSP
(SHRSP) | Endog | −1.52 |
5.13E-09 |
7.06E-06 | −0.94 |
9.03E-06 |
6.20E-03 |
|
| Tor1b | 1.29 |
7.91E-09 |
1.02E-05 | 1.37 |
1.69E-08 |
7.75E-05 |
|
| Chmp1b | −1.08 |
1.16E-08 |
1.40E-05 | −0.70 |
8.21E-04 |
9.27E−02 |
|
| Riok1 | 0.96 |
2.01E-06 |
1.21E-03 | 0.66 |
4.15E-04 |
7.14E−02 |
|
| Fkbp5 | 1.25 |
1.15E-05 |
5.05E-03 | 1.76 |
7.83E-06 |
5.70E-03 |
|
| Mrpl4 | −0.74 |
4.99E-05 |
1.58E-02 | −0.87 |
5.28E-06 |
5.00E-03 |
|
| RGD1562351 | −0.89 |
3.21E-04 |
6.80E−02 | −0.50 |
7.14E-03 |
2.42E−01 |
|
| Tars2 | −0.66 |
5.01E-04 |
9.04E−02 | −0.93 |
4.27E-07 |
7.32E-04 |
|
| Crot | −0.77 |
1.27E-03 |
1.74E−01 | −0.52 |
9.78E-03 |
2.83E−01 |
|
| Zfp597 | 1.37 |
2.35E-03 |
2.52E−01 | 1.00 |
2.64E-05 |
1.32E-02 |
|
| Ms4a11 | 0.76 |
4.37E-03 |
3.72E−01 | 1.09 |
7.76E-03 |
2.52E−01 |
|
| Aldh5a1 | −0.59 |
5.85E-03 |
4.45E−01 | −0.51 |
2.75E-03 | 1.66
E−01 |
|
| Retsat | −1.07 |
1.10E-02 |
6.08E−01 | −0.66 |
4.09E-02 |
4.45E−01 |
|
| Pxmp4 | −0.68 |
1.42E-02 | 7.07
E−01 | 0.93 |
1.02E-05 |
6.67E-03 |
|
| Tmem14a | 0.67 |
1.75E-02 |
7.93E−01 | 0.80 |
6.04E-06 |
5.00E-03 |
| SHR-SHRSP
(SHR) | Endog | −1.54 |
1.30E-09 |
1.94E-06 | −0.71 |
4.88E-04 |
1.01E−01 |
|
| Tor1b | 1.27 |
3.43E-08 |
2.55E-05 | 1.04 |
1.01E-04 |
4.38E-02 |
|
| Chmp1b | −1.09 |
7.64E-09 |
7.37E-06 | −0.88 |
4.82E-05 |
2.81E-02 |
|
| Riok1 | 0.98 |
1.38E-07 |
6.17E-03 | 0.56 |
3.63E-03 |
3.00E−01 |
|
| Fkbp5 | 1.20 |
5.53E-06 |
2.32E-03 | 1.22 |
9.36E-04 |
1.67E−01 |
|
| Mrpl4 | −0.68 |
1.82E-05 |
6.17E-03 | −0.90 |
1.93E-06 |
4.40E-03 |
|
| RGD1562351 | −0.81 |
1.163E-03 |
1.45E−01 | −0.60 |
8.74E-04 |
1.60E−01 |
|
| Tars2 | −0.61 |
8.61E-04 |
1.18E−01 | −0.721 |
1.21E-04 |
4.54E-02 |
|
| Crot | −0.61 |
1.26E-02 |
6.10E−01 | −0.53 |
4.67E-03 |
0.3.48E−01 |
|
| Zfp597 | 1.35 |
5.63E-03 |
3.92E−01 | 0.94 |
6.49E-05 |
3.07E-02 |
|
| Ms4a11 | 0.80 |
1.34E-02 |
6.33E−01 | 0.71 |
2.24E-02 |
5.98E−01 |
|
| Aldh5a1 | −0.55 |
8.58E-03 |
5.12E−01 | −0.58 |
3.00E-04 |
7.34E−02 |
|
| Retsat | −0.96 |
2.42E-02 |
8.81E−01 | −0.61 |
3.19E-02 |
6.45E−01 |
|
| Pxmp4 | −0.79 |
9.60E-03 |
5.41E−01 | 0.77 |
2.78E-04 |
6.95E−02 |
|
| Tmem14a | 0.81 |
4.98E-03 |
3.72E−01 | 0.93 |
1.14E-03 |
1.84E−01 |
The expression of common DEGs in the
GSE31457-GSE41452-GSE41453 (Fig.
2A) and GSE53361 (Fig. 2B)
datasets, and all DEMs in the GSE53361 dataset (Fig. 2C) in the three sample groups, are
shown in the heat-map.
PPI network and function enrichment
analyses
PPI networks were respectively constructed for
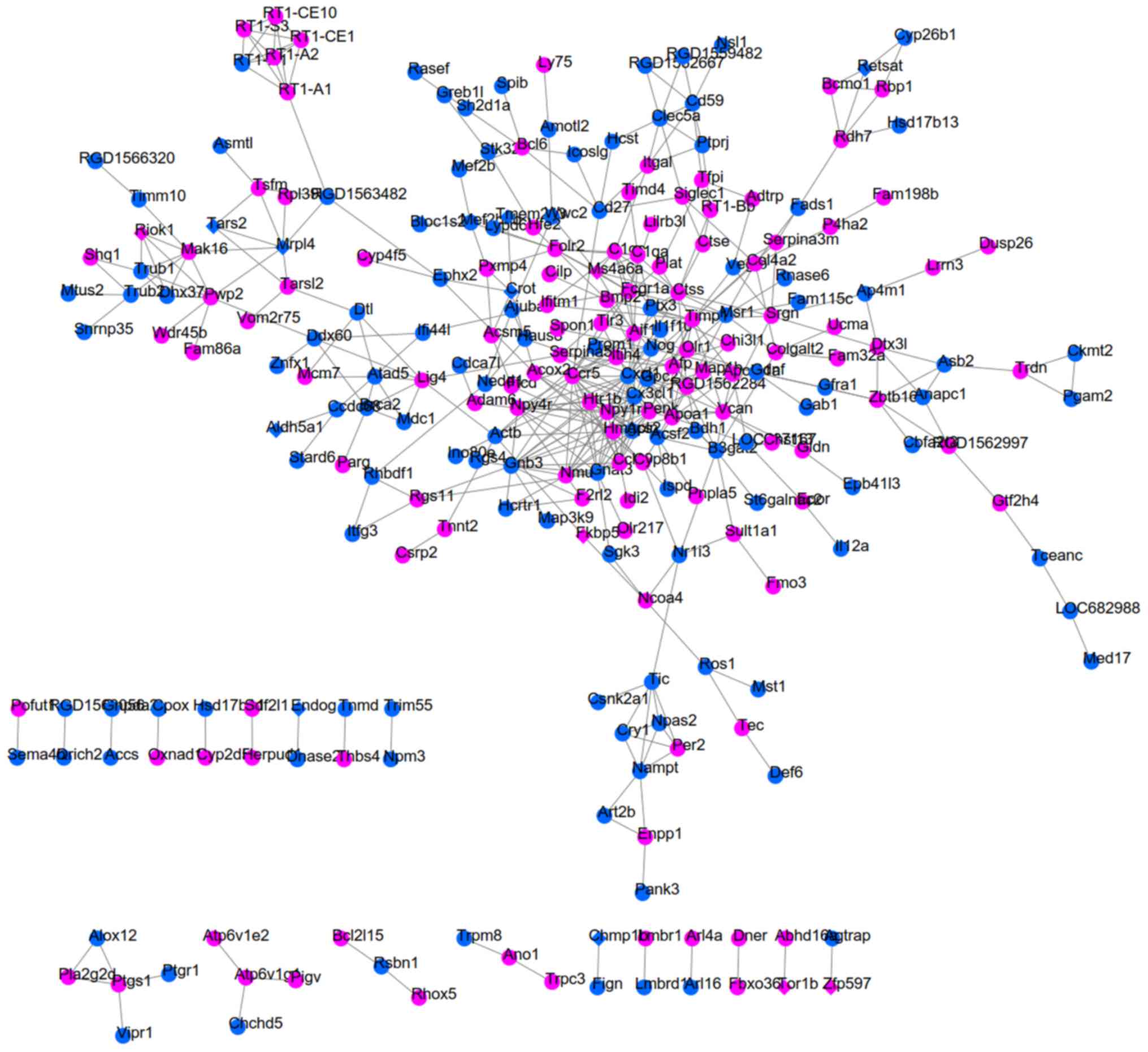
SHR-specific, SHRSP-specific and SHR-SHRSP shared DEGs by uniting
the DEGs in two datasets due to the small number of common DEGs
(Fig. 2). As a result, 123
interaction pairs between 115 DEGs (for example, Sec16b-Ccdc92)
were obtained for the SHR-specific group (Fig. 3); 828 interaction pairs between 345
DEGs (for example, Mb-Hsph1) were obtained for the SHRSP-specific
group (Fig. 4); and 454
interaction pairs between 254 DEGs (for example, Crot-Ephx2 and
Mrpl4-Tars2) were obtained for the SHR-SHRSP shared group (Fig. 5).
Function analysis revealed that 30 significant GO
terms (comprising 22 BP, 5 MF and 3 CC) were enriched for genes in
the SHR-specific group, including GO:0006955~immune response,
GO:0030174~regulation of DNA-dependent DNA replication initiation,
and GO:0033262~regulation of nuclear cell cycle DNA replication;
however, no common genes were involved (Table IV). In addition, 103 significant
GO terms (including 64 BP, 11 MF and 28 CC) and 9 KEGG pathways
were enriched for genes in the SHRSP-specific group, including
GO:0030198~extracellular matrix organization (TGFBI),
GO:0002062~chondrocyte differentiation (TGFBI),
GO:0007623~circadian rhythm (DBP), GO:0001525~angiogenesis (TGFBI)
and rno04141:Protein processing in endoplasmic reticulum [heat
shock protein family H (Hsp110) member 1 (HSPH1)] (Table V). Furthermore, 62 significant GO
terms (including 32 BP, 6 MF and 11 CC) and 13 KEGG pathways were
enriched for genes in the SHR-SHRSP shared group, including
GO:0006954~inflammatory response [epoxide hydrolase 2 (EPHX2)],
GO:0055114~oxidation-reduction process (ALDH5A1),
GO:0032355~response to estradiol (ENDOG), GO:0006631~fatty acid
metabolic process (CROT), GO:0007417~central nervous system
development (ALDH5A1), GO:0004829~threonine-tRNA ligase activity
(TARS2) and rno00650:Butanoate metabolism (ALDH5A1) (Table VI).
 | Table IV.Function enrichment analysis for
genes of the SHR-specific group of the PPI network. |
Table IV.
Function enrichment analysis for
genes of the SHR-specific group of the PPI network.
| Category | Term | P-value | Genes |
|---|
| GO BP | GO:0008584~male
gonad development |
2.69E−04 | TEX19.1, ADAM6,
CYP11A1, RXFP2, CST3, UBB, INSL6 |
| GO BP | GO:0050728~negative
regulation of inflammatory response |
1.76E−03 | CXCL17, IER3,
TNFAIP6, PPARD, SMAD3 |
| GO BP | GO:0043066~negative
regulation of apoptotic process |
4.11E−03 | IER3, PPARD, SGK1,
PLK3, DUSP1, RXFP2, SMAD3, PALB2, INSL6, HIGD1A |
| GO BP | GO:0031640~killing
of cells of other organism |
1.80E−02 | DEFA5, NP4 |
| GO BP | GO:0030513~positive
regulation of BMP signaling pathway |
2.00E−02 | HES1, TWSG1,
GDF5 |
| GO BP | GO:0006955~immune
response |
2.30E−02 | CXCL1, CD244, CD36,
SMAD3, COLEC12, CCL4 |
| GO BP | GO:0001562~response
to protozoan |
2.40E−02 | IER3, CYSS |
| GO BP |
GO:0030174~regulation of DNA-dependent DNA
replication initiation |
2.40E−02 | TEX10, CDT1 |
| GO BP | GO:0014070~response
to organic cyclic compound |
2.47E−02 | HES1, CYP2B2,
CYP11A1, CYSS, CST3, FOSL1 |
| GO BP | GO:0001701~in utero
embryonic development |
2.76E−02 | HES1, MAFF, SMAD3,
LIG4, PALB2, FOSL1 |
| GO BP | GO:0007431~salivary
gland development |
3.00E−02 | CYSS, CST3 |
| GO BP |
GO:0033262~regulation of nuclear cell
cycle DNA replication |
3.00E−02 | TIPIN, CDT1 |
| GO BP | GO:0010332~response
to gamma radiation |
3.10E−02 | CXCL1, CYP11A1,
LIG4 |
| GO BP | GO:0046677~response
to antibiotic |
3.28E−02 | CYP11A1, CYSS,
SKIL |
| GO BP | GO:0006952~defense
response |
3.41E−02 | CXCL1, NP4,
CST3 |
| GO BP |
GO:0006954~inflammatory response |
3.43E−02 | CXCL1, IL17B, KNG2,
LTB4R, CCL4, TLR7 |
| GO BP | GO:0032755~positive
regulation of interleukin-6 production |
3.67E−02 | CD36, IL33,
TLR7 |
| GO BP | GO:0042493~response
to drug |
4.10E−02 | CD36, CYP11A1,
CYSS, CST3, FABP3, NDUFA10, CDH3, FOSL1 |
| GO BP | GO:0000076~DNA
replication checkpoint |
4.16E−02 | TIPIN, CDT1 |
| GO BP |
GO:0044539~long-chain fatty acid
import |
4.16E−02 | CD36, FABP3 |
| GO BP |
GO:0001756~somitogenesis |
4.39E−02 | SMAD3, ZEB2,
PALB2 |
| GO BP | GO:0071356~cellular
response to tumor necrosis factor |
4.68E−02 | HES1, MAP3K5,
CYP11A1, CCL4 |
| GO MF | GO:0003677~DNA
binding |
6.91E−04 | BATF3, POLL, RCOR3,
PTF1A, TIPIN, SMAD3, ZEB2, LIG4, CDT1, HES1, POU2F1, H2AFX, SP7,
SKIL, PALB2, FOSL1 |
|
| GO:0070538~oleic
acid binding |
1.63E−02 | CD36, FABP3 |
|
|
GO:0004869~cysteine-type endopeptidase
inhibitor activity |
2.31E−02 | KNG2, CYSS,
CST3 |
|
|
GO:0001078~transcriptional repressor
activity, RNA polymerase II core promoter proximal region
sequence-specific binding |
2.69E−02 | BATF3, HES1, PRDM5,
SKIL |
|
|
GO:0004559~alpha-mannosidase activity |
4.29E−02 | MAN2B2, MANEA |
| GO CC |
GO:0005615~extracellular space |
7.23E−04 | CXCL1, TWSG1, KNG2,
DEFB14, GDF5, CST3, IL33, CCL4, CXCL17, TNFAIP6, CTSK, CD36, IL17B,
DEFA5, SERPINA4, NP4, CYSS, FABP3, UBB |
|
|
GO:0043231~intracellular membrane-bounded
organelle |
7.83E−03 | SEC16B, CYP2B2,
GLUL, ATP2B3, KNG2, CD36, POU2F1, TIPIN, TREM2, FOSL1, PKD2L1 |
|
|
GO:0000785~chromatin |
3.77E−02 | HES1, PLK3, MTBP,
H2AFX |
 | Table V.Function enrichment analysis for the
genes of the SHRSP group of the PPI network. |
Table V.
Function enrichment analysis for the
genes of the SHRSP group of the PPI network.
| Category | Term | P-value | Genes |
|---|
| GO BP | GO:0032570~response
to progesterone |
1.00E−05 | CCNE1, FOS, PTGER2,
NCF2, ERBB4, ADH1, HSPA8, JUNB, TGFB1 |
| GO BP | GO:0010033~response
to organic substance |
1.69E−05 | TNF, CYP2D1, MGP,
TGFB1, AMPD1, AFP, C1QB, PLA2G4A, BTG2, DUSP1, ALB, SQLE, SPP1 |
| GO BP | GO:0007067~mitotic
nuclear division |
7.62E−05 | ITGB3BP, SPC25,
CDK1, PLK1, NUF2, CENPW, MAPRE2, FABP1, CEP55, CCNG1, WEE1 |
| GO BP | GO:0000122~negative
regulation of transcription from RNA polymerase II promoter |
7.95E−04 | TNF, PRRX1, ZEB2,
AURKB, ZBTB16, TGFB1, CCNE1, EZR, NR1D1, SOX18, CRY1, TBX6, ISL1,
PROX2, JUNB, NKX6-1, SHOX2, HHEX, ATF3, BTG2, PLK1, IRF2BPL, TRPS1,
BCL6B, NELFE, NFIA, CRYM |
| GO BP | GO:0051301~cell
division |
1.34E−03 | ITGB3BP, SPC25,
CDK1, CCNE1, CHMP1B, DSN1, NUF2, CENPW, MAPRE2, CCNG1, WEE1 |
| GO BP | GO:0032496~response
to lipopolysaccharide |
1.50E−03 | FOS, SLC11A1,
TNFRSF11B, PLA2G4A, PTGER2, TNF, RELT, NCF2, SERPINE1, ACP5, C2,
LOXL1, JUNB, TRIB1 |
| GO BP |
GO:0030198~extracellular matrix
organization |
1.67E−03 | COL18A1, TNFRSF11B,
TNF, ADAMTSL2, OLFML2B, TGFBI, NID1, POSTN |
| GO BP | GO:0001755~neural
crest cell migration |
1.69E−03 | ERBB4, SEMA3D,
ZEB2, ISL1, HTR2B, GDNF |
| GO BP | GO:0045880~positive
regulation of smoothened signaling pathway |
1.83E−03 | SHOX2, SCUBE3,
PRRX1, GAS1, POR |
| GO BP |
GO:0030316~osteoclast differentiation |
2.07E−03 | TF, TNF, ACP5,
DCSTAMP, JUNB |
| GO BP |
GO:0002062~chondrocyte
differentiation |
8.72E−03 | SHOX2, TRPS1,
TGFBI, CYTL1, TGFB1 |
| GO BP | GO:0001889~liver
development |
1.47E−02 | AFP, CCNE1, HHEX,
COBL, BAAT, HMGCS2, DBP, TK1 |
| GO BP |
GO:0007623~circadian rhythm |
2.06E−02 | DHX9, TNF, NR1D1,
DBP, NTRK2, CRY1, TPH1 |
| GO BP | GO:0045944~positive
regulation of transcription from RNA polymerase II promoter |
2.50E−02 | TNF, HEXB, PRRX1,
CYTL1, FSTL3, ZEB2, GDNF, TGFB1, HSPH1, SLC11A1, FOS, TEF, SOX18,
TBX6, CCNH, NR4A1, ISL1, MECOM, PROX2, JUNB, SHOX2, HHEX, ATF3,
IRF2BPL, DBP, TRPS1, NELFE, NFIA |
| GO BP |
GO:0001525~angiogenesis |
4.50E−02 | COL18A1, SERPINE1,
TGFBI, APOLD1, SOX18, PLXND1, EPHB3, THY1 |
| GO BP | GO:0042542~response
to hydrogen peroxide |
4.94E−02 | CDK1, PLA2G4A,
DUSP1, ERBB4, MB |
| GO MF | GO:0042803~protein
homodimerization activity |
6.49E−04 | CADM3, ERBB4, HEXB,
ZBTB16, GREM2, GDNF, TGFB1, SLC11A1, ADH1, FAP, ALOX5AP, TEF, CIB2,
ZFP423, TESC, OLFML2B, ERP29, EPHX2, FZD1, NR4A1, MECOM, HHEX,
C1QB, PPP1R9A, ATF3, NTRK2, CTSE, CRYM |
|
| GO:0005515~protein
binding |
2.81E−03 | COBL, ERBB4,
KCNAB1, WASF1, KCNA2, CACNB3, ZBTB16, CDH2, RAB3IP, SDC3, FOS, EZR,
HSPA2, NR1D1, ARPC2, SCG3, GUCY1A2, DNAJA1, C2, VMP1, HSPA8,
SCN10A, NGEF, CDK1, RAB8B, KCNB1, ERP29, FZD1, NR4A1, NID1, ISL1,
THY1, SH3BP5, RT1-A2, EPHA4, PPP1R9A, BTG2, NTRK2, FABP1, ALOX5,
MAP6, HTR2B, NFIA |
|
| GO:0005525~GTP
binding |
5.84E−03 | TF, RAB8B, GBP5,
GIMAP6, GIMAP8, RRAD, NPR2, RRAGD, GNAT2, RND3, RAB19, RAC3,
GUCY1A2, RAB15, RAB26 |
|
|
GO:0050840~extracellular matrix
binding |
1.06E−02 | OLFML2B, TGFBI,
NID1, SPP1 |
|
| GO:0046982~protein
heterodimerization activity |
1.50E−02 | ZFP423, CD3G, CD3E,
KCNB1, HEXB, CRLF1, FZD1, NR4A1, RRAGD, TGFB1, FOS, ATF3, ALOX5AP,
TEF, GUCY1A2, ADRA1A, CENPW, SOX18 |
| GO CC |
GO:0005615~extracellular space |
9.32E−06 | TF, DHH, TNF,
LTBP2, IGFBP7, HEXB, PLBD1, SERPINB1A, CCL9, FSTL3, ACP5, POSTN,
GREM2, GDNF, TGFB1, CHIT1, MTHFD2, TNFRSF11B, PTGIS, EZR, ALB,
SERPINA5, FAP, SERPINE1, TGFBI, SEMA3D, C2, CES1C, HSPA8, SPP1,
DPT, WNT8B, COL18A1, CPA6, PRG4, IL9, MGP, CLIC1, SOD3, AFP, CBLN2,
F5, IRF2BPL, CPXM1, ALOX5, UBB |
|
|
GO:0031012~extracellular matrix |
1.48E−04 | COL18A1, LTBP2,
IGFBP7, CKAP4, MGP, NID1, POSTN, TGFB1, SOD3, CFP, TGFBI, SERPINE1,
LOXL1, CLEC14A, DPT |
|
|
GO:0070062~extracellular exosome |
1.59E−04 | LTBP2, IGFBP7,
HEXB, TSPAN6, TSPAN8, HSPH1, DES, SLC2A5, SERPINA5, SERPINE1,
TGFBI, SCN10A, MB, PTPRJ, DBNL, CDK1, PTPRF, ERP29, MGP, PRKCH,
CLIC1, THY1, C1QB, RND3, CHMP1B, DHRS4, BTG2, RELT, RAB19, CTSE,
RAB15, UBB, TNFAIP3, AKR1D1, TF, ECH1, SERPINB1A, ACP5, CDH2,
GIPC2, ITM2C, EZR, HSPA2, ARPC2, ALB, RAC3, DNAJA1, C2, NDRG2,
HSPA8, SPP1, ARHGDIB, DPT, COL18A1, RAB8B, CPPED1, CKAP4, EPHX2,
NID1, TMBIM1, RACGAP1, ANXA4, SOD3, NKX6-1, LYVE1, TMEM8A, HEBP1,
MYH11, FABP1, CRYM, CLEC14A |
|
| GO:0005604~basement
membrane |
2.12E−04 | COL18A1, TF, LAMC3,
ALB, TGFBI, NTN4, NID1, LOXL1, COL4A5 |
|
|
GO:0030027~lamellipodium |
3.81E−04 | DBNL, TESC,
PPP1R9A, MYO10, WASF1, KCNA2, FAP, CDH2, ARAP3, PLXND1, RAB3IP |
| KEGG | rno04010:MAPK
signaling pathway |
3.86E−03 | TNF, NR4A1, CACNB3,
FGF13, MECOM, TGFB1, FOS, PLA2G4A, HSPA2, DUSP1, NTRK2, GADD45A,
HSPA8 |
| KEGG | rno04610:Complement
and coagulation cascades |
1.22E−02 | C1QB, F10, F5,
SERPINA5, SERPINE1, C2 |
| KEGG | rno05142:Chagas
disease (American trypanosomiasis) |
1.69E−02 | C1QB, FOS, CD3G,
TNF, CD3E, SERPINE1, TGFB1 |
| KEGG | rno04110:Cell
cycle |
3.54E−02 | CDK1, CCNE1, CCNH,
PLK1, GADD45A, WEE1, TGFB1 |
| KEGG | rno04380:Osteoclast
differentiation |
3.78E−02 | FOS, TNFRSF11B,
TNF, NCF2, ACP5, JUNB, TGFB1 |
| KEGG | rno04612:Antigen
processing and presentation |
3.89E−02 | RT1-A2, TNF, HSPA2,
RT1-CE4, RT1-M2, HSPA8 |
| KEGG | rno04141:Protein
processing in endoplasmic reticulum |
4.30E−02 | HSPH1, HSPA2,
CKAP4, ERP29, DNAJA1, DDOST, SSR2, HSPA8 |
| KEGG | rno04115:p53
signaling pathway |
4.53E−02 | CDK1, CCNE1,
SERPINE1, CCNG1, GADD45A |
| KEGG | rno04514:Cell
adhesion molecules (CAMs) |
4.77E−02 | RT1-A2, CADM3,
OCLN, PTPRF, RT1-CE4, RT1-M2, CDH2, SDC3 |
 | Table VI.Function enrichment for the genes of
SHR-SHRSP shared group in the PPI network. |
Table VI.
Function enrichment for the genes of
SHR-SHRSP shared group in the PPI network.
| Category | Term | P-value | Genes |
|---|
| GO BP |
GO:0006954~inflammatory response |
9.20E−05 | CXCL1, BMP2, OLR1,
AIF1, PTGS1, CHI3L1, EPHX2, CCL19, TLR3, CX3CL1, CYP4F5, CCR5,
CYP26B1, CD27 |
| GO BP | GO:0006955~immune
response |
1.33E−04 | CXCL1, RT1-A2,
RT1-A1, RT1-CE1, ENPP1, CCR5, IL12A, RT1-CE10, IFI44L, CX3CL1,
CTSS, CD27, RT1-BB |
| GO BP | GO:0007631~feeding
behavior |
8.99E−04 | HCRTR1, HTR1B,
NPY4R, NPY1R, APLN |
| GO BP | GO:0002474~antigen
processing and presentation of peptide antigen via MHC class I |
1.00E−03 | RT1-A2, RT1-A1,
RT1-CE1, RT1-CE10, RT1-S3 |
| GO BP |
GO:0055114~oxidation-reduction
process |
1.33E−03 | HSD17B11, PTGR1,
BMP2, CYP2D5, ALDH5A1, FADS1, HSD17B13, PTGS1, RDH7, P4HA2, CYP4F5,
CPOX, CYP26B1, FMO3, OXNAD1, CYP8B1, BDH1, RETSAT, ALOX12 |
| GO BP | GO:0001916~positive
regulation of T cell mediated cytotoxicity |
1.59E−03 | RT1-A2, RT1-A1,
IL12A, RT1-S3 |
| GO BP | GO:0032355~response
to estradiol |
3.72E−03 | CXCL1, PENK,
FCGR1A, ENDOG, MAP1B, TFPI, BRCA2, NPY1R, BDH1 |
| GO BP | GO:0045785~positive
regulation of cell adhesion |
5.49E−03 | PTPRJ, DUSP26,
IL12A, CX3CL1, ALOX12 |
| GO BP |
GO:0007568~aging |
6.20E−03 | CCR5, PENK, FADS1,
ENDOG, PTGS1, GFRA1, EPOR, NPY1R, CX3CL1, TIMP1, ALOX12 |
| GO BP | GO:0006631~fatty
acid metabolic process |
6.58E−03 | ACOX2, PER2, ACSF2,
ACSM5, CROT |
| GO BP | GO:0007417~central
nervous system development |
8.57E−03 | NOG, ALDH5A1, DNER,
VCAN, LIG4, ZBTB16 |
| GO BP | GO:0001666~response
to hypoxia |
2.00E−02 | AJUBA, VEGFB, PLAT,
BMP2, PENK, EPOR, CX3CL1, CBFA2T3, AGTRAP |
| GO BP | GO:0009612~response
to mechanical stimulus |
4.08E−02 | ACTB, BMP2, ENDOG,
MAP1B, CHI3L1 |
| GO MF | GO:0042605~peptide
antigen binding |
1.09E−03 | RT1-A1, RT1-CE1,
RT1-CE10, RT1-S3, RT1-BB |
|
| GO:0005102~receptor
binding |
1.83E−03 | ACOX2, PLAT, BMP2,
RT1-CE1, HFE2, EPHX2, RT1-S3, HCST, TRDN, RT1-A1, RT1-CE10, CROT,
TEC |
|
| GO:0008083~growth
factor activity |
8.50E−03 | VEGFB, CXCL1, BMP2,
IL12A, GDNF, TIMP1, THBS4 |
|
|
GO:0004829~threonine-tRNA ligase
activity |
3.67E−02 | TARS2, TARSL2 |
|
|
GO:0001602~pancreatic polypeptide receptor
activity |
3.67E−02 | NPY4R, NPY1R |
| GO CC |
GO:0005615~extracellular space |
2.96E−06 | CXCL1, NAMPT, NOG,
HFE2, ENPP1, GLDN, MST1, CX3CL1, C1QC, GDNF, TIMP1, GPC2, APOA1,
SERPINA5, PTX3, APLN, SRGN, SPON1, THBS4, ACTB, PLAT, BMP2, UCMA,
IL1F10, CHI3L1, CILP, CCL19, CTSS, VEGFB, PROM1, AFP, SERPINA3M,
CD59, TFPI, IL12A, SEMA4B, GFRA1, VCAN |
|
| GO:0009986~cell
surface |
9.64E−06 | PLAT, PTPRJ, ITGAL,
BMP2, HFE2, ENPP1, TNMD, TLR3, RT1-S3, CX3CL1, CTSS, RT1-BB, HCST,
PROM1, APOA1, CCR5, FOLR2, CD59, TFPI, VCAN, ROS1, CLEC5A,
CD27 |
|
| GO:0009897~external
side of plasma membrane |
3.39E−05 | LY75, ITGAL, TRPM8,
ANO1, CCL19, RT1-BB, RT1-A2, CCR5, SERPINA5, FCGR1A, GFRA1, EPOR,
CD27, ICOSLG |
|
| GO:0042612~MHC
class I protein complex |
1.76E−04 | RT1-A2, RT1-A1,
RT1-CE1, RT1-CE10, RT1-S3 |
|
|
GO:0005576~extracellular region |
1.70E−03 | HSD17B11, COL4A2,
OLR1, ENPP1, HSD17B13, CTSS, CX3CL1, GDNF, C1QC, TIMP1, DNASE2B,
VEGFB, APOA1, PENK, TFPI, VCAN, APOL11A, PLA2G2D, NMU, THBS4 |
| KEGG |
rno04145:Phagosome |
1.97E−06 | ACTB, MSR1,
RT1-CE1, OLR1, CTSS, ATP6V1G1, RT1-S3, RT1-BB, RT1-A2, RT1-A1,
FCGR1A, ATP6V1E2, RT1-CE10, RT1-N1, THBS4 |
| KEGG | rno05416:Viral
myocarditis |
8.38E−05 | ACTB, RT1-A2,
ITGAL, RT1-A1, RT1-CE1, RT1-CE10, RT1-S3, RT1-N1, RT1-BB |
| KEGG | rno05330:Allograft
rejection |
1.12E−04 | RT1-A2, RT1-A1,
RT1-CE1, IL12A, RT1-CE10, RT1-S3, RT1-N1, RT1-BB |
| KEGG | rno04940:Type I
diabetes mellitus |
1.89E−04 | RT1-A2, RT1-A1,
RT1-CE1, IL12A, RT1-CE10, RT1-S3, RT1-N1, RT1-BB |
| KEGG | rno04514:Cell
adhesion molecules (CAMs) |
3.86E−04 | RT1-A2, SIGLEC1,
ITGAL, RT1-A1, RT1-CE1, RT1-CE10, VCAN, RT1-S3, RT1-N1, ICOSLG,
RT1-BB |
| KEGG |
rno05332:Graft-versus-host disease |
5.91E−04 | RT1-A2, RT1-A1,
RT1-CE1, RT1-CE10, RT1-S3, RT1-N1, RT1-BB |
| KEGG | rno04612:Antigen
processing and presentation |
8.43E−04 | RT1-A2, RT1-A1,
RT1-CE1, RT1-CE10, RT1-S3, CTSS, RT1-N1, RT1-BB |
| KEGG | rno05320:Autoimmune
thyroid disease |
1.34E−03 | RT1-A2, RT1-A1,
RT1-CE1, RT1-CE10, RT1-S3, RT1-N1, RT1-BB |
| KEGG | rno05168:Herpes
simplex infection |
2.21E−03 | RT1-A2, RT1-A1,
RT1-CE1, CSNK2A1, PER2, IL12A, RT1-CE10, TLR3, RT1-S3, RT1-N1,
RT1-BB |
| KEGG | rno04610:Complement
and coagulation cascades |
5.57E−03 | PLAT, C1QA, CD59,
SERPINA5, TFPI, C1QC |
| KEGG | rno00650:Butanoate
metabolism |
9.57E−03 | HMGCS2, ALDH5A1,
BDH1, ACSM5 |
| KEGG |
rno05150:Staphylococcus aureus
infection |
1.04E−02 | C1QA, ITGAL,
FCGR1A, C1QC, RT1-BB |
| KEGG |
rno05169:Epstein-Barr virus infection |
3.01E−02 | RT1-A2, ITGAL,
RT1-A1, RT1-CE1, RT1-CE10, RT1-S3, RT1-N1 |
miRNA-mRNA regulatory network
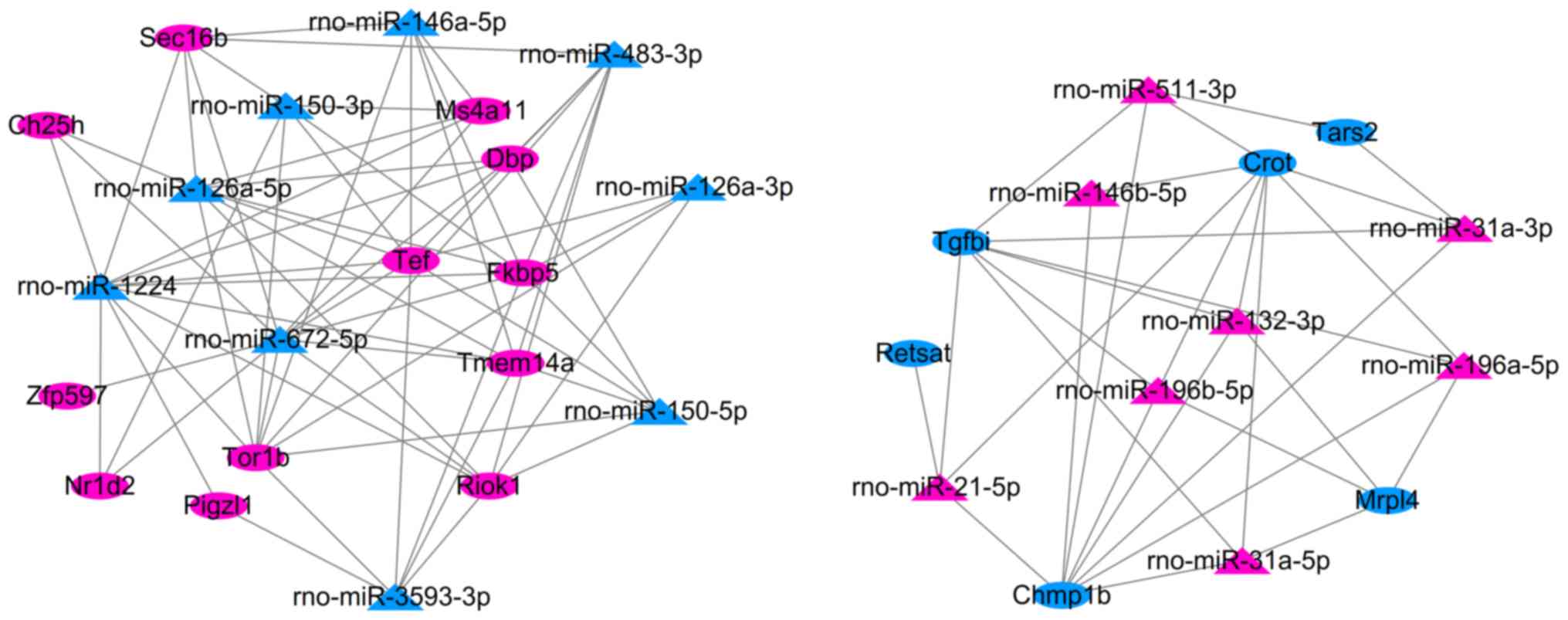
After integrating the target genes of DEMs with the
common DEGs (consistent expression trend) in two datasets, 97
negative interaction pairs between 17 miRNAs and 18 DEGs were
identified, including SHRSP-specific rno-miR-126a-5p-Dbp/Tor1b,
rno-miR-196a-5p/rno-miR-196b-5p/rno-miR-21-5p-Tgfbi, and SHR-SHRSP
shared rno-miR-672-5p-Zfp597 and rno-miR-31a-5p-Crot/Mrpl4, which
were used for constructing the miRNA-mRNA regulatory network
(Fig. 6).
Prediction of potential therapeutic
agents
After uploading the upregulated and downregulated
DEGs to the CMAP database, a serial of small-molecule drugs that
may exert therapeutic potential for SHR were predicted, including
botulin, Gly-His-Lys, benzathine benzylpenicillin, Prestwick-1103,
PF-00539745-00, quinostatin, 5279552 and podophyllotoxin (Table VII).
 | Table VII.Candidate small-molecule drugs. |
Table VII.
Candidate small-molecule drugs.
| CMAP name | Enrichment | P-value |
|---|
| Betulin | −0.96 |
2.20E−04 |
| Gly-His-Lys | −0.93 |
5.40E−04 |
| Benzathine
Benzylpenicillin | −0.90 |
1.60E−04 |
| Prestwick-1103 | −0.89 |
4.00E−04 |
| PF-00539745-00 | −0.88 |
3.43E−03 |
| Quinostatin | −0.88 |
2.99E−02 |
| 5279552 | −0.86 |
3.77E−02 |
|
Podophyllotoxin | −0.86 |
7.00E−04 |
| Cefuroxime | 0.80 |
3.00E−03 |
| STOCK1N-35215 | 0.81 |
1.37E−02 |
| NS-398 | 0.91 |
1.38E−02 |
Discussion
By integrating the results of the miRNA regulatory
network, PPI network and function enrichment, the present study has
demonstrated that changes in the expression of Dbp, Crot and Mrpl4
(all P<0.01, especially the latter one with FDR <0.05 in all
datasets) may possibly be important genetic changes associated with
SHRSP: Upregulated Dbp is associated with circadian rhythm and
regulated by rno-miR-126a-5p, whereas downregulated Crot may
participate in fatty acid metabolic processes or the inflammatory
response by interacting with EPHX2. Downregulated Mrpl4 may exert
roles by interacting with threonine-tRNA ligase TARS2. Expression
of Crot and Mrpl4 may both be modulated by rno-miR-31a.
Accumulating evidence has demonstrated abnormal BP
circadian rhythm is associated with the development of hypertension
(24) and stroke (25). Normal circadian BP is in a ‘dipper’
pattern, with a decline in nocturnal BP of 10–20% compared with the
day time (24). Patients with
non-dipper (i.e., a lack of nocturnal BP fall) patterns of
circadian BP rhythm are found to have a 4.222-fold increased risk
for the development of hypertension (P=0.011) (26). Furthermore, non-dipper (risk
ratio=1.42) or reverse-dipper (odds ratio=2.492) patterns of BP
were also shown to be stronger risk factors for the occurrence of
stroke in patients with essential hypertension (i.e., high BP that
lacks a known secondary cause) (25,27,28).
Therefore, genes that regulate the circadian rhythm may be
potentially associated with the pathogenesis of hypertension and
stroke. This hypothesis has been validated in a previously
published study (29). For
example, Leu et al (30)
identified genetic polymorphisms in five circadian clock genes
[neuronal PAS domain protein 2 (NPAS2), rs3888170; period circadian
regulator 2 (PER2), rs6431590; retinoic acid receptor-related
orphan receptor ββ (RORββ), rs1410225; brain and muscle ARNT-like 1
(BMAL1), rs3816358; and RORα, rs10519096], and these were
significantly associated with the non-dipper phenotype in 372 young
hypertensive patients. The study of Corella et al (31) revealed that CLOCK-rs4580704 single
nucleotide polymorphism (SNP) was associated with an increased risk
of stroke in type-2 diabetic subjects, with CC-carriers having a
higher risk (31). Kurbatova et
al (32) observed further that
the transcript expression levels of CLOCK, BMAL1 and PER were
differentially regulated in hypertension patients with various
genotypes (32). In the SHR rat,
small interfering RNA-mediated knockdown of Per1 was shown to
significantly reduce BP (33,34).
Bmal1-deficient female mice were also observed to exhibit a
significantly smaller infarct core volume compared with female
Bmal1+/+ mice at 14 days after the induction of
photothrombosis (35). In line
with these studies, the present study also revealed that Dbp, a
clock-controlled transcription factor, was significantly
upregulated in all analyzed tissues of SHRSP rats compared with WKY
rats. These results were dissimilar to a previous study performed
with SHR rats, in which the expression of Dbp occurred
differentially in heart (significant expression) and aortas (no
expression), although it was also relatively higher compared with
WKY rats (36). These findings
further verified that Dbp may be a specific target for treatment of
SHRSP, and that downregulation of Dbp may represent a potential
therapeutic approach.
Relative to direct knockout of the specific target
gene, the introduction of endogenous non-coding miRNAs that
negatively regulate this gene may be safer. Therefore, miRNAs that
could regulate the expression of Dbp were also investigated in our
study. Our results predicted that miR-126 could bind with Dbp at
the 3′-untranslated region, and thereby overexpression of miR-126
may represent a potential therapeutic approach for SHRSP via the
suppression of Dbp expression. Other studies have also reported on
the association of miR-126 with the development of stroke. Jin and
Xing (37,38) reported that the plasma miR-126
expression level was lower in patients with acute ischemic stroke
compared with those of controls, and its expression was negatively
correlated with National Institutes of Health Stroke Scale (NIHSS)
scores. Overexpression of miR-126 in the stem cells attenuated the
infarct volume, improved functional recovery, enhanced
neurogenesis, and inhibited neuroinflammation (39,40).
These studies indirectly indicated that a negative correlation
existed between miR-126 and Dbp in stroke. However, direct evidence
that may have been used to investigate the regulatory relationship
between them was lacking, and the present study may provide a novel
mechanism for explaining the pathogenesis of SHRSP.
The CROT gene encodes a member of the
carnitine/choline acetyltransferase family that converts
4,8-dimethylnonanoyl-CoA into its corresponding carnitine ester,
and facilitates the transport of medium- and long-chain acyl-CoA
molecules out of the peroxisome to the cytosol and mitochondria as
a feature of the fatty acid oxidation process. Downregulation of
CROT may contribute to impaired fatty acid oxidation and thus
promote fat accumulation, leading to the development of obesity
(41–43). Obesity is considered to be
important risk factors for hypertension (44) and stroke (45). Thus, CROT may also be expressed at
a lower level in SHR and SHRSP rats, a finding which was, for the
first time to the best of our knowledge, confirmed in our study.
Furthermore, it has also been reported that obesity may induce
hypertension and stroke according to a pro-inflammatory mechanism
(46,47). Therefore, CROT was also
hypothesized to inhibit inflammation. Although some experimental
evidence has been provided in support of this hypothesis (41), our hypothesis was that CROT may
indirectly exert an anti-inflammatory effect by interacting with
EPHX2, a member of epoxide hydrolase family, since it was enriched
in the inflammatory response process in our study. Furthermore, the
study of Ulu et al (48)
demonstrated that an epoxide hydrolase inhibitor enhanced the
anti-hypertensive and anti-inflammatory effects of omega-3
polyunsaturated fatty acids, providing further indirect support for
our hypothesis. Moreover, few previous studies have been concerned
with miRNAs that regulate the expression of CROT, other than miR-33
(41). The present study has, for
the first time, revealed that miR-31 may also regulate CROT, and
this process may be involved in the development of SHR and SHRSP.
Although no direct evidence has been provided to prove that miR-31
is important for stroke and hypertension, its roles in mediating
the production of inflammatory cytokines (49) may reveal the possible interaction
between miR-31 and CROT.
In addition, the present study has identified that
miR-31 regulates Mrpl4, and therefore Mrpl4 may also be associated
with inflammation in SHR and SHRSP. Although studies on Mrpl4 are
relatively rare, its potential roles in inflammation have been
investigated previously. For example, MRPL4 was reported to be a
downregulated downstream target of hypoxia-inducible factor-1α
(HIF-1α) (50). Accumulation of
HIF-1α was observed in neurons of 9-month-old SHR and SHRSP rats
(51). Deletion of HIF-1α was
shown to significantly reduce vascular high pressure and vascular
inflammation, attenuate atherosclerosis (52), and improve neuronal survival and
sensorimotor function in ischemic stroke (53). Furthermore, our prediction was also
that Mrpl4 could interact with TARS2. It was previously reported
that secreted TARS stimulated endothelial cell migration and
angiogenesis (54), which was
beneficial for neurogenesis and functional recovery in patients
with stroke (55). Accordingly,
the present study has suggested miR-31 may be involved in SHRSP by
regulating MRPL4 to mediate inflammation and angiogenesis
inhibition.
Furthermore, the analysis of small-molecule drugs in
the present study revealed that botulin, Gly-His-Lys and
podophyllotoxin may potentially be agents for treatment of SHR and
SHRSP. Although no evidence exists based on the study of their
therapeutic effects on SHR and SHRSP, their anti-inflammatory
activities may indirectly reveal their therapeutic potential. For
example, botulinum toxin type A treatment was demonstrated to
reduce persistent inflammatory hypernociception induced by
arthritis in the temporomandibular joint of rats by decreasing the
expression of the pro-inflammatory cytokine, interleukin-1β (IL-1β)
(56). Wang et al (57) also observed that intra-articular
botulinum toxin type A administration caused anti-neurogenic
inflammation by blocking the infiltration of inflammatory cells
(57). The tripeptide Gly-Gly-His
was shown to inhibit secretion of pro-inflammatory IL-6 in
fibroblasts (58). The study of
Kalita et al (59)
demonstrated that a combination of podophyllotoxin and rutin is a
safe and effective protective agent to attenuate radiation-induced
gastrointestinal injury by negatively regulating NF-κB/p53
signaling (59).
In conclusion, our study has provided some
preliminary evidence to suggest that Dbp, Crot and Mrpl4 may be
potential targets for treatment of SHRSP. Their expression may be
reversed by miRNAs (rno-miR-126a-5p and rno-miR-31a) or
small-molecule drugs (botulin, Gly-His-Lys and podophyllotoxin).
However, further in vitro and in vivo experiments are
required in order to confirm these conclusions.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The sequencing data GSE41452, GSE31457, GSE41453,
GSE53363 and GSE53361 were downloaded from the GEO database in NCBI
(http://www.ncbi.nlm.nih.gov/geo/).
Authors' contributions
QZ and LW conceived the design of the original
study. QZ and HS conducted the statistical analysis. LY was
involved with the interpretation of the data. QZ drafted the
manuscript. LW participated in critical revisions of the
manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim IG, So WY and Sung DJ: The
relationships between lifestyle factors and hypertension in
community-dwelling Korean adults. J Phys Ther Sci. 27:3689–3692.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kupferman JC, Zafeiriou DI, Lande MB,
Kirkham FJ and Pavlakis SG: Stroke and hypertension in children and
adolescents. J Child Neurol. 32:408–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alloubani A, Saleh A and Abdelhafiz I:
Hypertension and diabetes mellitus as a predictive risk factors for
stroke. Diabetes Metab Syndr. 12:577–584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shambesh MKA, Emahbes TM, Saleh ZE, Elosta
MAA and Shambesh IM: Role of hypertension as a major risk factor of
stroke in Africa; Libya: Community based survey. British J Medicine
Med Res. 9:1–11. 2015. View Article : Google Scholar
|
|
5
|
Adil MM, Beslow LA, Qureshi AI, Malik AA
and Jordan LC: Hypertension is associated with increased mortality
in children hospitalized with arterial ischemic stroke. Pediatr
Neurol. 56:25–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang
L, Wang L, Jiang Y, Li Y, Wang Y, et al: Prevalence, incidence, and
mortality of stroke in China: Results from a nationwide
population-based survey of 480 687 adults. Circulation.
135:759–771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGrath R, Markides K, Hall O and Peterson
M: The burden of health conditions for middle-aged and older adults
in the United States: Disability-adjusted life years. BMC Geriatr.
19:1002019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rubattu S, Cotugno M, Bianchi F, Sironi L,
Gelosa P, Stanzione R, Forte M, De Sanctis C, Madonna M, Marchitti
S, et al: A differential expression of uncoupling protein-2
associates with renal damage in stroke-resistant spontaneously
hypertensive rat/stroke-prone spontaneously hypertensive
rat-derived stroke congenic lines. J Hypertens. 35:1857–1871. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lan C, Chen X, Zhang Y, Wang W, Wang WE,
Liu Y, Cai Y, Ren H, Zheng S, Zhou L and Zeng C: Curcumin prevents
strokes in stroke-prone spontaneously hypertensive rats by
improving vascular endothelial function. BMC Cardiovasc Disord.
18:432018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshida M, Watanabe Y, Yamanishi K,
Yamashita A, Yamamoto H, Okuzaki D, Shimada K, Nojima H, Yasunaga
T, Okamura H, et al: Analysis of genes causing hypertension and
stroke in spontaneously hypertensive rats: gene expression profiles
in the brain. Int J Mol Med. 33:887–896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto H, Okuzaki D, Yamanishi K, Xu Y,
Watanabe Y, Yoshida M, Yamashita A, Goto N, Nishiguchi S, Shimada
K, et al: Genetic analysis of genes causing hypertension and stroke
in spontaneously hypertensive rats. Int J Mol Med. 31:1057–1065.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watanabe Y, Yoshida M, Yamanishi K,
Yamamoto H, Okuzaki D, Nojima H, Yasunaga T, Okamura H, Matsunaga H
and Yamanishi H: Genetic analysis of genes causing hypertension and
stroke in spontaneously hypertensive rats: Gene expression profiles
in the kidneys. Int J Mol Med. 36:712–724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palao T, Swärd K, Jongejan A, Moerland PD,
de Vos J, van Weert A, Arribas SM, Groma G, vanBavel E and Bakker
EN: Gene expression and MicroRNA expression analysis in small
arteries of spontaneously hypertensive rats. Evidence for ER
stress. PLoS One. 10:e01370272015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nemoto K, Ikeda A, Ito S, Miyata M,
Yoshida C and Degawa M: Comparison of constitutive gene expression
levels of hepatic cholesterol biosynthetic enzymes between
Wistar-Kyoto and stroke-prone spontaneously hypertensive rats. Biol
Pharm Bull. 36:1216–1220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rubattu S, Stanzione R, Bianchi F, Cotugno
M, Forte M, Ragione FD, Fioriniello S, D'Esposito M, Marchitti S,
Madonna M, et al: Reduced brain UCP2 expression mediated by
microRNA-503 contributes to increased stroke susceptibility in the
high-salt fed stroke-prone spontaneously hypertensive rat. Cell
Death Dis. 8:e28912017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuoka H, Tamura A, Kinehara M, Shima A,
Uda A, Tahara H and Michihara A: Levels of tight junction protein
CLDND1 are regulated by microRNA-124 in the cerebellum of
stroke-prone spontaneously hypertensive rats. Biochem Biophys Res
Commun. 498:817–823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diboun I, Wernisch L, Orengo CA and
Koltzenburg M: Microarray analysis after RNA amplification can
detect pronounced differences in gene expression using limma. BMC
Genomics. 7:2522006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Green GH and Diggle PJ: On the operational
characteristics of the benjamini and hochberg false discovery rate
procedure. Stat Appl Genet Mol Biol. 6:272007. View Article : Google Scholar
|
|
19
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: Software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dweep H and Gretz N: miRWalk2. 0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Subramanian A, Narayan R, Corsello SM,
Peck DD, Natoli TE, Lu X, Gould J, Davis JF, Tubelli AA, Asiedu JK,
et al: A next generation connectivity map: L1000 platform and the
first 1,000,000 Profiles. Cell. 171:1437–1452.e17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhixiang Y, Cheng W, Jibing X, Bisheng G,
Ming X and Deyu L: Ambulatory blood pressure monitoring in children
suffering from orthostatic hypertension. Biomed Eng Online.
17:1292018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kario K, Pickering TG, Matsuo T, Hoshide
S, Schwartz JE and Shimada K: Stroke prognosis and abnormal
nocturnal blood pressure falls in older hypertensives.
Hypertension. 38:852–857. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mateo-Gavira I, Vílchez-López FJ,
García-Palacios MV, Laureano CS, Jiménez-Carmona S and
Aguilar-Diosdado M: Nocturnal blood pressure is associated with the
progression of microvascular complications and hypertension in
patients with type 1 diabetes mellitus. J Diabetes Complications.
30:1326–1332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Castilla-Guerra L, Espino-Montoro A,
Fernández-Moreno MC and López-Chozas JM: Abnormal blood pressure
circadian rhythm in acute ischaemic stroke: Are lacunar strokes
really different? Int J Stroke. 4:257–261. 2010. View Article : Google Scholar
|
|
28
|
Yan B, Peng L, Dong Q, Zheng F, Yang P,
Sun L, Gong S, Zeng L and Wang G: Reverse-dipper pattern of blood
pressure may predict lacunar infarction in patients with essential
hypertension. Eur J Neurol. 22:1022–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Richards J, Diaz AN and Gumz ML: Clock
genes in hypertension: novel insights from rodent models. Blood
Press Monit. 19:249–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leu HB, Chung CM, Lin SJ, Chiang KM, Yang
HC, Ho HY, Ting CT, Lin TH, Sheu SH, Tsai WC, et al: Association of
circadian genes with diurnal blood pressure changes and non-dipper
essential hypertension: A genetic association with young-onset
hypertension. Hypertens Res. 38:155–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Corella D, Asensio EM, Coltell O, Sorlí
JV, Estruch R, Martínez-González MÁ, Salas-Salvadó J, Castañer O,
Arós F, Lapetra J, et al: CLOCK gene variation is associated with
incidence of type-2 diabetes and cardiovascular diseases in type-2
diabetic subjects: Dietary modulation in the PREDIMED randomized
trial. Cardiovasc Diabetol. 15:42016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kurbatova IV, Topchieva LV, Korneva VA,
Kolomeichuk SN and Nemova NN: Expression of circadian rhythm genes
CLOCK, BMAL1, and PER1 in buccal epithelial cells of patients with
essential arterial hypertension in dependence on polymorphic
variants of CLOCK and BMAL1 genes. Bull Exp Biol Med. 157:360–363.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Douma LG, Solocinski K, Holzworth MR,
Crislip GR, Masten SH, Miller AH, Cheng KY, Lynch IJ, Cain BD,
Wingo CS and Gumz ML: Female C57BL/6J mice lacking the circadian
clock protein PER1 are protected from nondipping hypertension. Am J
Physiol Regul Integr Comp Physiol. 316:R50–R58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alli AA, Yu L, Holzworth M, Richards J,
Cheng KY, Lynch IJ, Wingo CS and Gumz ML: Direct and indirect
inhibition of the circadian clock protein PER1: Effects on ENaC and
blood pressure. Am J Physiol Renal Physiol. 316:F807–F813. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lembach A, Stahr A, Ali AAH, Ingenwerth M
and von Gall C: Sex-dependent effects of bmal1-deficiency on mouse
cerebral cortex infarction in response to photothrombotic stroke.
Int J Mol Sci. 19(pii): E31242018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshiro N, Takeshi T, Daizo K, Takahiro O,
Shinji M, Miho M, Tsuyoshi S, Yoshio F, Mitsumasa O and Tadaaki I:
Circadian gene expression of clock genes and plasminogen activator
inhibitor-1 in heart and aorta of spontaneously hypertensive and
Wistar-Kyoto rats. J Hypertens. 21:1107–1115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin F and Xing J: Circulating miR-126 and
miR-130a levels correlate with lower disease risk, disease
severity, and reduced inflammatory cytokine levels in acute
ischemic stroke patients. Neurol Sci. 39:1757–1765. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin F and Xing J: Circulating
pro-angiogenic and anti-angiogenic microRNA expressions in patients
with acute ischemic stroke and their association with disease
severity. Neurol Sci. 38:2015–2023. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Geng W, Tang H, Luo S, Lv Y, Liang D, Kang
X and Hong W: Exosomes from miRNA-126-modified ADSCs promotes
functional recovery after stroke in rats by improving neurogenesis
and suppressing microglia activation. Am J Transl Res. 11:780–792.
2019.PubMed/NCBI
|
|
40
|
Pan Q, Zheng J, Du D, Liao X, Ma C, Yang
Y, Chen Y, Zhong W and Ma X: MicroRNA-126 priming enhances
functions of endothelial progenitor cells under physiological and
hypoxic conditions and their therapeutic efficacy in cerebral
ischemic damage. Stem Cells Int. 2018:29123472018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vega-Badillo J, Gutiérrez-Vidal R,
Hernández-Pérez HA, Villamil-Ramírez H, León-Mimila P,
Sánchez-Muñoz F, Morán-Ramos S, Larrieta-Carrasco E,
Fernández-Silva I, Méndez-Sánchez N, et al: Hepatic miR-33a/miR-144
and their target gene ABCA1 are associated with steatohepatitis in
morbidly obese subjects. Liver Int. 36:1383–1391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rottiers V and Näär AM: MicroRNAs in
metabolism and metabolic disorders. Nat Rev Mol Cell Biol.
13:239–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim S, Sohn I and Lee YS and Lee YS:
Hepatic gene expression profiles are altered by genistein
supplementation in mice with diet-induced obesity. J Nutr.
135:33–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roush GC: Obesity-induced hypertension:
Heavy on the accelerator. J Am Heart Assoc. 8:e0123342019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mitchell AB, Cole JW, McArdle PF, Cheng
YC, Ryan KA, Sparks MJ, Mitchell BD and Kittner SJ: Obesity
increases risk of ischemic stroke in young adults. Stroke.
46:1690–1692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Irwin DC, Garat CV, Crossno JT Jr, Maclean
PS, Sullivan TM, Erickson PF, Jackman MR, Harral JW, Reusch JEB and
Klemm DJ: Obesity-related pulmonary arterial hypertension in rats
correlates with increased circulating inflammatory cytokines and
lipids and with oxidant damage in the arterial wall but not with
hypoxia. Pulm Circ. 4:638–653. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maysami S, Haley MJ, Gorenkova N, Krishnan
S, McColl BW and Lawrence CB: Prolonged diet-induced obesity in
mice modifies the inflammatory response and leads to worse outcome
after stroke. J Neuroinflammation. 12:1402015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ulu A, Harris TR, Morisseau C, Miyabe C,
Inoue H, Schuster G, Dong H, Iosif AM, Liu JY, Weiss RH, et al:
Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and
soluble epoxide hydrolase inhibitors in angiotensin-II-dependent
hypertension. J Cardiovasc Pharmacol. 62:285–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu N, Meisgen F, Butler LM, Han G, Wang
XJ, Söderberg-Nauclér C, Ståhle M, Pivarcsi A and Sonkoly E:
MicroRNA-31 is overexpressed in psoriasis and modulates
inflammatory cytokine and chemokine production in keratinocytes via
targeting serine/threonine kinase 40. J Immunol. 190:678–688. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Benita Y, Kikuchi H, Smith AD, Zhang MQ,
Chung DC and Xavier RJ: An integrative genomics approach identifies
Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core
response to hypoxia. Nucleic Acids Res. 37:4587–4602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ritz MF, Fluri F, Engelter ST,
Schaeren-Wiemers N and Lyrer PA: Cortical and putamen age-related
changes in the microvessel density and astrocyte deficiency in
spontaneously hypertensive and stroke-prone spontaneously
hypertensive rats. Curr Neurovasc Res. 6:279–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu D, Lei L, Desir M, Huang Y, Cleman J,
Jiang W, Fernandez-Hernando C, Di Lorenzo A, Sessa WC and Giordano
FJ: Smooth muscle hypoxia-inducible factor 1α links intravascular
pressure and atherosclerosis-brief report. Arterioscler Thromb Vasc
Biol. 36:442–445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barteczek P, Li L, Ernst AS, Böhler LI,
Marti HH and Kunze R: Neuronal HIF-1α and HIF-2α deficiency
improves neuronal survival and sensorimotor function in the early
acute phase after ischemic stroke. J Cereb Blood Flow Metab.
37:291–306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Williams TF, Mirando AC, Wilkinson B,
Francklyn CS and Lounsbury KM: Secreted Threonyl-tRNA synthetase
stimulates endothelial cell migration and angiogenesis. Sci Rep.
3:13172013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhai ZY and Feng J: Constraint-induced
movement therapy enhances angiogenesis and neurogenesis after
cerebral ischemia/reperfusion. Neural Regen Res. 14:1743–1754.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lora VR, Clemente-Napimoga JT, Abdalla HB,
Macedo CG, Canales GT and Barbosa CM: Botulinum toxin type A
reduces inflammatory hypernociception induced by arthritis in the
temporomadibular joint of rats. Toxicon. 129:52–57. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang L, Wang K, Chu X, Li T, Shen N, Fan
C, Niu Z, Zhang X and Hu L: Intra-articular injection of Botulinum
toxin A reduces neurogenic inflammation in CFA-induced arthritic
rat model. Toxicon. 126:70–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gruchlik A, Jurzak M, Chodurek E and
Dzierzewicz Z: Effect of Gly-Gly-His, Gly-His-Lys and their copper
complexes on TNF-alpha-dependent IL-6 secretion in normal human
dermal fibroblasts. Acta Pol Pharm. 69:1303–1306. 2012.PubMed/NCBI
|
|
59
|
Kalita B, Ranjan R, Singh A, Yashavarddhan
MH, Bajaj S and Gupta ML: A combination of podophyllotoxin and
rutin attenuates radiation induced gastrointestinal injury by
negatively regulating NF-κB/p53 signaling in lethally irradiated
mice. PLoS One. 11:e01685252010. View Article : Google Scholar
|